Abstract
Aging is associated with a reduction in metabolic functions, increased incidence of neurodegenerative diseases, and memory or cognitive dysfunction. With aging, a decrease in plasma estrogen levels, related to loss of gonadal function, occurs in females. Estrogens have neuroprotective effects and estradiol treatment improves some aspects of neuronal homeostasis affected by aging. In other way, recent studies show that apo D can play a neuroprotective role in some neuropathologies and during aging. The possible relation between estradiol treatment and the expression of apo D, during aging in the CNS, was investigated in female rats. Our results confirm an expression of apo D zone-dependent, in relation with aging, and an overexpression of apo D related to ovariectomy and estradiol treatment. This overexpression strengthens the idea that apo D plays a neuroprotective role in the CNS.
Keywords: Apolipoprotein D, Estrogens, Estradiol, Aging, Rat, Female, Telencephalon, Diencephalon, Frontal cortex, Western blot
Introduction
Apolipoprotein D is a small 29–32 kDa glycoprotein primarily associated with high-density lipoproteins in human plasma. According to its primary structure, apo D belongs to lipocalin superfamily, whose members bind and transport hydrophobic molecules including cholesterol, bilirubin, porphyrin, steroid hormones, and arachidonic acid (Rassart et al. 2000). Apo D expression has been identified in humans as well as in other mammalian species (Boyles et al. 1990b; Patel et al. 1995; Provost et al. 1991; Seguin et al. 1995; Smith et al. 1990). In humans, apo D is expressed in corporal fluids (Balbin et al. 1990; Terrisse et al. 1998), some systemic tissues (Drayna et al. 1986; Rassart et al. 2000) as well as in the peripheral nervous system (PNS) and the central nervous system (CNS) (Boyles et al. 1990a; De Jonge et al. 2003; Ganfornina et al. 2010; Navarro et al. 1998b, 2010). In the CNS, apo D expression has been described in astrocytes, oligodendrocytes, perivascular cells, and neurons (Boyles et al. 1990b; Del Valle et al. 2003; Hu et al. 2001; Navarro et al. 1998b, 2004, 2010; Patel et al. 1995).
Aging is a multidimensional dynamic process affecting in a continuous and non-reversible way all the organisms. Although some authors consider that aging just begins in the moment of birth, other researchers take as the starting point of aging process once the individual has a complete development. At this moment, catabolic processes start to be more intense than the anabolic ones, so the well-known “loss of vitality” begins. CNS is widely affected by aging at macroscopic, microscopic, and molecular levels. Macroscopic and microscopic changes include a noticeable reduction in brain volume and weight, the modification of the plasmatic membrane integrity, neuronal dead, etc. The increase in the expression of apo D, related with aging, has been described by several authors (Kalman et al. 2002; Loerch et al. 2008; Navarro et al. 2010). Variations in the apo D levels were described in tumors (Diez-Itza et al. 1994; Rassart et al. 2000; Søiland et al. 2007), neurodegenerative diseases, as Alzheimer’s (Belloir et al. 2001; Navarro et al. 2001, 2003, 2004; Ordoñez et al. 2011; Terrisse et al. 1998; Thomas et al. 2003) and Parkinson’s diseases (Ordoñez et al. 2006), as well as in different chemical and mechanical brain injuries (Montpied et al. 1999; Trieu and Uckum 2000).
Although the main role of apo D in CNS is still unknown, a neuroprotective role for this apolipoprotein has been postulated by different authors (Muffat et al. 2008; Navarro et al. 2008, 2010; Ordoñez et al. 2006; Sanchez et al. 2006; Thomas et al. 2001a, b). The increased apo D expression in the PNS has been related to the repairing and remodeling processes secondary to neurodegeneration (Boyles et al. 1990a; Ganfornina et al. 2010). Apo D may serve as a lipid carrier to and from cells and/or as a scavenger to deleterious molecules or free radicals like lipid peroxidation products (Ganfornina et al. 2008, 2010; Navarro et al. 2008; Sanchez et al. 2006).
The expression of Apo D is regulated by many biological factors. There are several response elements in the Apo D promoter (Lambert et al. 1993), so a great number of molecules such as sexual hormones, glucocorticoids, interleukins, retinoic acid, are able to modify the apolipoprotein synthesis (Do Carmo et al. 2002; Khan et al. 2003; Rassart et al. 2000).
In the mammalian brain, experimental studies and clinical observations have demonstrated the importance of estrogens in the preservation of brain cognitive functions, highlighting its protective effects against neuronal damage (Alonso et al. 2008; Garcia-Segura et al. 2001; Wise et al. 2001). When cellular damage is induced on neuronal cultures (by serum or growth factor deprivation, anoxia, excitotoxic or oxidative damage), the cellular homeostasis is protected by estrogens (Bae et al. 2000; Green and Simpkins 2000; Saravia et al. 2007). On the other hand, estrogen replacement therapy appears to decrease the risk and/or severity of neurodegenerative damages that accompany the gonadal loss, and improve memory and cognition in experimental subjects (Alonso et al. 2008; Resnick and Maki 2001).
In the present paper, we studied the expression of apo D during brain aging in ovariectomized rats and in ovariectomized rats treated with17β-estradiol replacement therapy. Two different encephalic regions were selected in function of their contrasting resistance to ageing: telencephalon and diencephalon. Telencephalic region is more affected by ageing (neuronal death, glial proliferation, neuropil, etc.) while diencephalic region is apparently unaffected. The possible neuroprotective role of apo D in CNS, during aging, and its relation with hormonal therapy is also discussed.
Materials and methods
Animals
Virgin female Wistar rats (from the bioterium of the Faculty of Medicine, University of Oviedo), weighting 250–280 g (age 8–10 weeks), and maintained under standard conditions of temperature (23 ± 3°C) and humidity (65 ± 1%), and a regular lighting schedule of 12-h light/dark cycle (0800–2,000 h) were used. The animals were fed with a standard diet (Panlab A04, Barcelona, Spain) and had free access to water. All experimental manipulations were performed between 0930 and 1230 h. All experimental procedures carried out with animals were approved by a local veterinary committee from the University of Oviedo vivarium, and subsequent handling strictly followed the European Communities Council Directive of 24 November 1986 (86/609/ECC).
Experimental design
Rats were ovariectomized through a midline incision under light anesthesia by inhalation of halothane. Ovariectomized rats were separated randomly into three groups: ovariectomized animals (O), ovariectomized animals treated with 17β-estradiol (E) and sham surgery animals (intact) (C), and were housed individually throughout the experiment.
Following surgery, all rats began the experimental treatment exactly 1 week after ovariectomy to ensure a uniform time of estrogen depletion before replacement, and to recover from surgery stress. After this, the rats were implanted subcutaneously in the posterior neck with 90-day-release 17β-estradiol pellets (25 μg/pellet; Innovative Research of America, Sarasota, FL, USA) or placebos containing no estradiol. The pellets were replaced every 90 days. This dosing regimen results in physiological levels of plasma estradiol that has been shown to be neuroprotective in rats (Harukuni et al. 2001).
Groups O, E and C were divided randomly into four subgroups (seven animals per subgroup): 6, 12, 18, and 24 (according to the month of the experimental period on which the animals were killed). Therefore, the animals were killed when they were approximately 8, 14, 20, and 26 months old. Moreover, 14 animals (7O and 7C), were sacrificed 1 week after ovariectomy (age 9–11 weeks). Therefore, the animals included in this group did not receive any treatment. These animals were considered O-0 month and C-0 month groups.
The stage of the estrous cycle in intact rats was determined by daily examination of fresh vaginal smears. Intact animals in diestrous phase were selected for subgroups 0 and 6. After month 12 of the experiment, none of the intact rats showed repetitive estrous cycles; instead, 87.26% of animals showed persistent diestrous phase.
Blood samples for the determination of 17β-estradiol plasma concentrations were collected from the jugular vein into heparinized tubes, centrifuged at 3,000 rpm for 20 min at 4°C and plasma was immediately drawn off and stored frozen at −20°C until assayed. Plasma 17β-estradiol was measured by RIA using Immunochen kits of cover tubes (ICN Biomedicals). The assay sensitivity was 10 pg/ml, and the intra-assay coefficient of variation was 9.45%. All samples were measured on the same day. The sample was assayed in triplicate.
Once the animals were killed by bleeding, the brains were quickly removed and the telencephalon and diencephalon were dissected and immediately frozen in liquid nitrogen for future experiments.
Crude extracts preparation, immunoprecipitation, and western blot analysis
Telencephalon and diencephalon previously stored were homogenized in lysis buffer (50 mM Tris–HCl [pH 7.5], 150 mM NaCl, 1% Nonidet P-40 [Roche Diagnostics, Indianapolis, IN], 0.05% sodium deoxycholate, 0.1% sodium orthovanadate, 5 mM EDTA, 10% glycerol) at 4°C. The extracts were centrifuged at 21,000 × g at 4°C for 10 min and the protein content of the supernatant was determined by the Bradford dye-binding method (Bradford 1976).
Proteins in the crude homogenate were resolved by SDS-PAGE (10% Tris-acrylamide-Bis) and electrotransferred from the gel to nitrocellulose membranes (Hybond-ECL, Amersham Pharmacia Biotech, Little Chalfont, UK) as described by Towbin et al. (1979). Non-specific protein binding to the nitrocellulose membranes was reduced by preincubating the filter in blocking buffer (TNT, 7% BSA), and the membrane was then incubated overnight at 4°C with the primary antibody against apo D (diluted 1:10,000), antibody was a gift from Dr. Carlos López Otín, Departamento de Bioquímica y Biología Molecular, Universidad de Oviedo (see Diez-Itza et al. 1994; Lopez-Boado et al. 1994; Navarro et al. 1998b, 2003, 2008, 2010). After incubation with the primary antibody, the membranes were washed and incubated with an antirabbit antibody coupled to HRP (sc-2004, Santa Cruz Biotechnology; diluted 1:20,000). Finally, all membranes were stripped and probed with a monoclonal anti-β-actin antibody (sc-1615, Santa Cruz Biotechnology Inc., diluted 1:2,500). Inmunoreactive bands were detected using an enhanced chemiluminescence system (ECL, Amersham Pharmacia Biotech, UK). Films were analyzed using a digital scanner (Nikon AX-110) and NIH Image 1.57 software. The density of each band was normalized to its respective loading control (β-actin) and represented as a percentage of control values (intact rats of 0 month, group C 0). In order to minimize inter-assay variations, in each experiment samples from all animal groups were processed in parallel.
Statistical analysis
Data are expressed as mean ± standard error of mean (SEM). First, we evaluated the Gaussian distribution of each variable. Thereafter, data were statistically analyzed using an analysis of variance (ANOVA) design followed by between-group comparison using the Tukey honestly significant difference (HSD) test. The data for month 0 were analyzed by impaired Student’s t-test. Values of P ≤ 0.05 were considered significant. Statistical analysis was performed using SPSS version15.0 for Windows.
Results
General characteristics of experimental animals
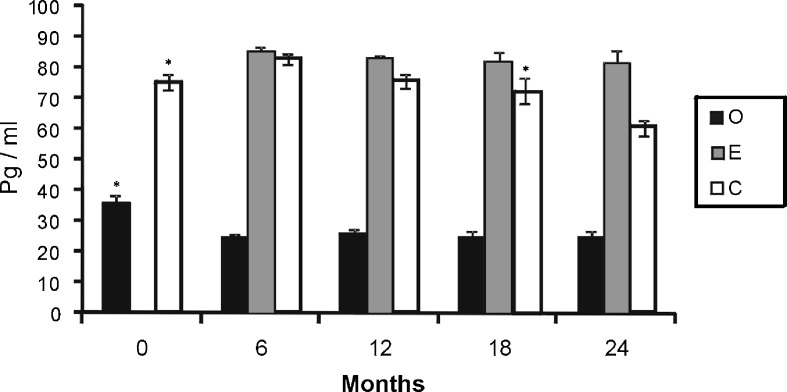
Plasma 17β-estradiol values obtained during the study were previously published in a recent paper of our group (Moreno et al. 2010). The plasma level of 17β-estradiol was significantly higher in groups E and C than in group O at all time points. In addition, plasma levels of 17β-estradiol were significantly higher in group E than in group C at 18 and 24 months. In the C group animals, plasma levels of 17β-estradiol increased significantly at 6 months and then decreased significantly at 24 months. In the E group, the estradiol level did not change significantly during the study. A significant decrease in the estradiol level was found in group O during the first 6 months (Fig. 1).
Fig. 1.
Levels of 17β-stradiol of ovariectomized animals (O; black bars), sham surgery animals (intact) (C; white bars) and ovariectomized animals treated with 17β-estradiol (E; grey bars). Mean ± standard error of the mean for seven animals. Significant differences are shown. *p ≤ 0.05 (month vs. next month)
Expression of apo D
The Western blot studies clearly demonstrated a band of approximately 29 kDa in all cerebral extracts analyzed, which corresponds to the molecular weight of apo D previously described in many reports (Balbin et al. 1990; Drayna et al. 1986; Navarro et al. 2010).
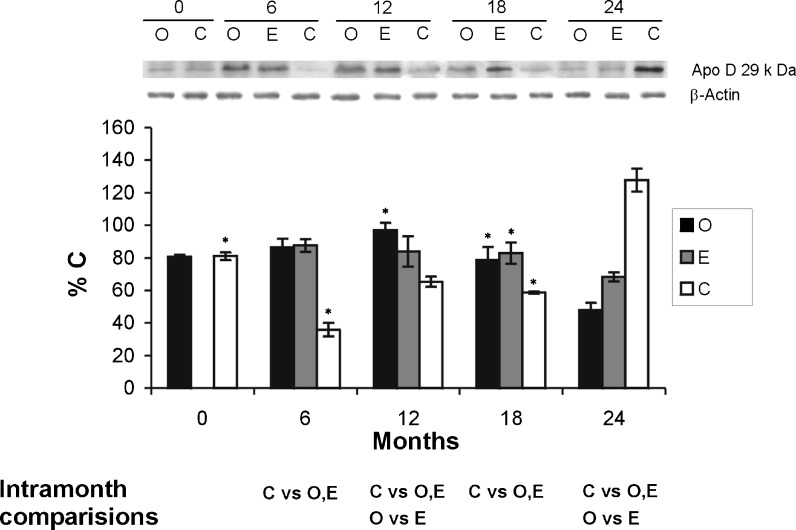
The effects of aging on the apo D levels in the cortex and diencephalon of the control and experimental animals were studied. In the cortex of intact rats, a significant reduction in apo D values was noted between 0 and 6 months; the apo D level then increased from 6 months until 12 months, and finally a significant increase occurs from 12 months until the end of the study at 24 months (Fig. 2). Therefore, our data show a significant increase in the level of apo D related with aging from the beginning to the conclusion of the experimental procedure in group C.
Fig. 2.
Apo D total protein content in the cortex of ovariectomized animals (O; black bars), sham surgery animals (intact) (C; white bars) and ovariectomized animals treated with estradiol (E; grey bars). The histogram shows the densitometric analysis of the Western blots. Values are means ± SEM (n = 7) and represented as the percentage of control values (rats of 0 month from group C). Only significant differences are shown. *p ≤ 0.05 (month vs. next month)
An increase in the apo D level was noted in the cortex of O group between 0 and 12 months. This increase was statistically significant and was followed by a significant diminution, in the expression of apo D, from 12 months to the end of the study at 24 months (Fig. 2). In this group of animals, a significant reduction for apo D level was observed from the beginning to the conclusion of the experimental procedure.
In the E group, the expression of apo D shows high values from the beginning of the sampling (6 months) but its level of expression decreases from this point to the end of the study at 24 months (Fig. 2).
The amount of apo D in the cortex, between 6 and 18 months, was significantly lower in the C group than in O and E groups but at 24 months of ageing the expression of apo D in group C was higher than in the other groups (O and E). The O group showed, at 12 months, the higher value for apo D but at 24 months, this group showed the lower value of expression. In the E group, the level of apo D, at 6 and 12 months, was similar to which was obtained in group O, but its level was increased at 18 and 24 months and showed a higher level than in O group.
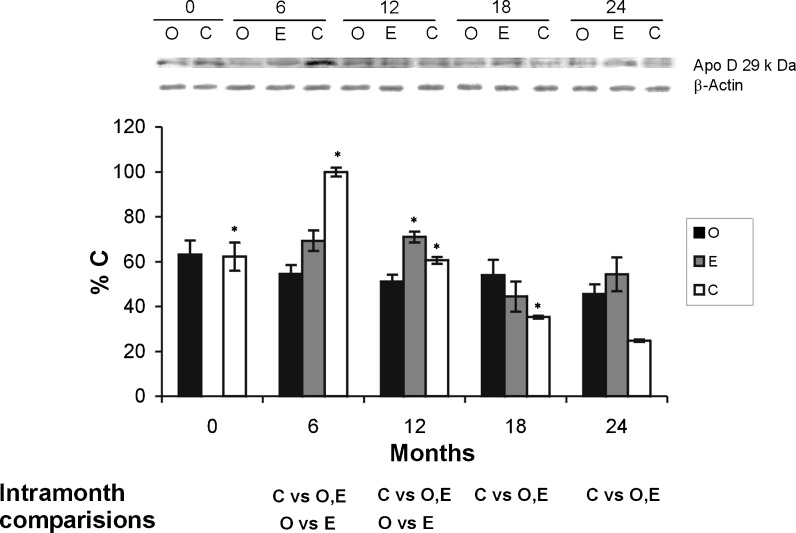
In the diencephalon of intact rats, a significant increase in apo D values was noted between 0 and 6 months. This increase was statistically significant and was followed by a significant diminution, in the expression of apo D, from 12 months to the end of the study at 24 months (Fig. 3). Therefore, our data show a significant decrease in the level of apo D related with aging from the beginning to the conclusion of the experimental procedure in group C (Fig. 3). The diminution in the expression of apo D was also observed in the other both groups (O and E) from the beginning to the conclusion of the experimental procedure (Fig. 3). This diminution, related with ageing, was statistically significant in both groups.
Fig. 3.
Apo D total protein content in diencephalon of ovariectomized animals (O; black bars), sham surgery animals (intact) (C; white bars) and ovariectomized animals treated with estradiol (E; grey bars). The histogram shows the densitometric analysis of the Western blots. Values are means ± SEM (n = 7) and represented as the percentage of control values (rats of 0 month from group C). Only significant differences are shown. *p ≤ 0.05 (month vs. next month)
The amount of apo D in the diencephalon, at the 6 months, was significantly higher in the C group than in the E and O groups. The expression of apo D is higher in the E group than in the O along all the study. Only at 18 months, a higher level for apo D in group O was obtained, although the difference was not statistically significant. In conclusion, in the diencephalon of all groups of our study a general decrease in the levels of apo D during ageing was observed, and the lowest values were obtained in the group C (Fig. 3).
Discussion
Adult rats are a suitable animal model for studying the onset of aging, as has been pointed out in previous studies. From 6 months of age, rats begin to display age-induced metabolic disturbances and various studies have shown that estradiol administration in postmenopausal or ovariectomized females has positive effects in the CNS (Alonso et al. 2008; Foster et al. 2003; Garcia-Segura et al. 2001). These observations indicate that treatment with estradiol may be able to prevent age-related effects and neurodegenerative diseases. On the other hand, the expression of apo D increases with normal aging in all species studied (Kalman et al. 2002; Loerch et al. 2008; Navarro et al. 1998b, 2010) as well as with neuropathological diseases, like Alzheimer’s dementia (Belloir et al. 2001; Ordoñez et al. 2011; Terrisse et al. 1998) or schizophrenia (Mahadik et al. 2002; Thomas et al. 2001a, b). Although the specific role of this apolipoprotein is unknown, studies in Drosophila (Muffat et al. 2008; Sanchez et al. 2006) and mouse (Ganfornina et al. 2008) ascribe to apo D a key role in longevity regulation and higher survival rate, taking part in the control of lipoperoxidation. It is worthy to note the presence of estrogen response elements (EREs) in the promoter of apo D gene (Lambert et al. 1993) and the fact that, in breast cancer cell cultures, estrogens administration leads to a decrease in apo D synthesis concomitant with an increase in cellular proliferation (Simard et al. 1991). All these previous data make it very interesting to study in vivo evaluation of estrogen effect on the amount of apo D.
Our study shows a diminution of apo D levels from 0 to 6 months in the telencephalon of control animals. During ontogenetic development, an overexpression of apo D, at encephalic level, has been described (Ganfornina et al. 2005; Sánchez et al. 2002). The final processes of maturation in the cerebral cortex of these animals can cause the apo D overexpression in the younger animals, because the final reorganization of this encephalic region is achieved after birth. From 6 months, an increase of apo D expression, especially at 12 and 24 months, was found. At stage of 12 months occurs a progressive loss of gonadal function (Iossa et al. 1999). With aging, a proliferation of reactive glia has been also described (Nichols 1999), which can be responsible for most of the increase in apo D expression. Glial cells are the main cellular type involved in apo D synthesis (Boyles et al. 1990b; Del Valle et al. 2003; Kalman et al. 2002; Navarro et al. 2004; Patel et al. 1995), but the number of neurons expressing apo D, also increases with aging (Belloir et al. 2001; Navarro et al. 1998b, 2010; Rassart et al. 2000). Control animals of 24 months show an overexpression of apo D similar to which is observed in aged humans (Kalman et al. 2002; Loerch et al. 2008; Navarro et al. 1998b, 2010). In the past years, it has been proven that the absence of apo D reduces life span and, in contrast, its overexpression induces the opposite effect and increases the stress resistance (Ganfornina et al. 2008; Muffat et al. 2008; Sanchez et al. 2006). Other studies show that apo D can protect nervous cells from damage (Montpied et al. 1999; Navarro et al. 2008; Walker et al. 2006). In our opinion, apo D can be included in a compensatory mechanism against physiological and structural changes inherent to aging process.
In the telencephalon of ovariectomized animals, a similar amount of Apo D was found from 0 to 12 months, which can be caused by the alteration of the neuroendocrine axis induced by ovariectomy. A noticeable decrease in the level of apo D is observed from 12 to 24 months. As discussed above, at this stage (12 months) a progressive loss of gonadal function occurs in normal animals (Iossa et al. 1999). With senescence, an important cognitive decline occurs in human and other mammalian species (Biessels et al. 2002) that is accompanied by the decay of many physiological systems, like glucose metabolism (De Santi et al. 1995). This process is aggravated by the absence of steroid sexual hormones (Dueñas et al. 1996; Sherwin 2003). Our study shows that ovariectomy affects the ability of apo D synthesis in the telencephalon, which is clearly observed in aged animals (24 months). Early gonadectomy leads to a deterioration in cerebral glucose metabolism and memory processes (Alonso et al. 2008; Garcia-Segura et al. 2001). Therefore, it is possible that the lack of gonads can affect, in aged animals, to brain homeostasis in a wide range of processes, such as neuronal feeding or apo D synthesis.
In the cerebral cortex, the 17β-estradiol treatment mainly affects the expression of apo D from 12 months to the end of the study, revealing a positive effect of the continuous hormone supply on apo D expression, during aging. Exogenous administration of estrogens partially mitigates the deleterious effect of the ovariectomy, improving moderately the ability of apo D synthesis by the nervous cells of cerebral cortex.
In the diencephalon of control animals, a decrease in the expression of apo D, related with aging, was observed. These results are clearly different from those obtained for the cerebral cortex, in the present and other studies where an increment of apo D expression related with aging was described (Belloir et al. 2001; Hu et al. 2001; Navarro et al. 1998b, 2010). One explanation for the results found on diencephalon can be related to the particular importance of the different CNS regions in the regulation of corporal homeostasis. The diencephalon plays a fundamental role in the control of different physiological functions, which are basic for life, and an alteration of some of its neuronal nuclei can cause the death of the animal at any time. Some ultrastructural studies perform on hamster diencephalon demonstrate the neuronal preservation of different hypothalamic nuclei during aging (Navarro et al. 1997, 1998a). This neuronal protection along aging process is also present in other primitive, but very important neuronal regions, as the vestibular nuclear system of human and other mammals (Álvarez et al. 2000; Fernández et al. 2007). Finally, recent studies of our group show that apo D is expressed in all the neurons of different neuronal nuclei of the human brainstem, from birth to senescence (observations not published). In these nuclei, there is not overexpression of apo D associated to ageing but a constant expression of it exists along all the life of the person.
In the diencephalon of ovariectomized animals, the amount of apo D is lower than in control animals from the beginning up to 12 months, when the irregular estrous cycle appears in the last group. This difference could be due to a multifactorial hormonal regulation, being involved, among other, estrogens and progesterone, as has been demonstrated in cellular cultures (Balbin et al. 1990; Simard et al. 1992). However, although ovariectomized animals have scarce hormonal changes and apo D levels do not suffer a sharp increase (with the exception of that of aging-related) from the middle to the end of the study the O group shows higher apo D expression than normal aged animals. This could imply an adaptive response of the diencephalon, directly related with hormonal regulation, of ovariectomized animals to the constant lack of estrogens along their lives.
The diencephalon of ovariectomized and estradiol-treated rats shows slight differences in apo D expression along aging, but in both groups, from 12 months to the end of the experiment, apolipoprotein levels are higher than in the controls. It is probable that this increase in apo D synthesis is triggered in response to the hormonal imbalance induced by ovariectomy, supporting the idea of apo D as a neuroprotective compensatory protein.
According to the results of this work, the estradiol effect on apo D levels is focused in the cerebral cortex, more than in the diencephalon. The cerebral cortex is remodeled along the entire life of animals and with aging can be affected by different processes of neurodegeneration, which do not directly cause death, but only a progressive diminution of the cerebral functions. On the contrary, the diencephalon, with the hypophysis, constitutes the main hormonal regulatory system of the organism (the hypothalamic–hypophysary axis), so a very strict system of neuroprotective mechanisms is necessary to support its functionality during aging because a minimal alteration of some neuronal nuclei of this region can achieve a lethal alteration of homeostasis. Therefore, this brain area has such an effective mechanism of homeostasis maintenance that an increase in apo D, as an injury-response protein (Ganfornina et al. 2008; Sanchez et al. 2006), is not necessary. However, ovariectomy and 17β-estradiol treatment involve an alteration of the hypothalamic–hypophysary hormonal balance, disturbing this perfect equilibrium.
In conclusion, our data confirm that in the CNS apo D age-related expression is zone-dependent, and that the hormonal treatment of ovariectomized rats affects the expression of apo D, in a different manner in the brain areas under study. Some particular regions, as cerebral cortex, that suffer great remodeling during aging are more sensitive to hormones being the 17β-estradiol treatment able to restores partially the apo D expression diminished by ovariectomy. On the contrary, regions of the CNS that show a great resistance to alterations induced by ageing, such as the diencephalon, are also less affected by hormonal treatment. However, both ovariectomized and 17β-estradiol-treated rats show apo D levels higher than the control group. This overexpression could be aimed to correct the alterations in the homeostasis caused by ovariectomy.
Acknowledgements
This work was supported by MEC and FEDER SAF2007-64076 grants; FIS PI020324 grant and MEC grants AP20040854, AP20053815 and FICYT grant BP05-064 to CP, EM and CO.
References
- Alonso A, Moreno M, Ordoñez P, Fernández R, Pérez C, Diaz F, Navarro A, Tolivia J, Gonzalez C. Chronic estradiol treatment improves brain homeostasis during aging in female rats. Endocrinology. 2008;149(1):57–72. doi: 10.1210/en.2007-0627. [DOI] [PubMed] [Google Scholar]
- Álvarez JC, Diaz C, Suárez C, Fernández JA, González del Rey C, Navarro A, Tolivia J. Aging and the human vestibular nuclei: morphometric analysis. Mech Ageing Dev. 2000;114:149–172. doi: 10.1016/S0047-6374(00)00098-1. [DOI] [PubMed] [Google Scholar]
- Bae YH, Hwang JY, Kim YH, Koh JY. Anti-oxidative neuroprotection by estrogens in mouse cortical cultures. J Korean Med Sci. 2000;15:327–336. doi: 10.3346/jkms.2000.15.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbin M, Freije JM, Fueyo A, Sanchez LM, Lopez-Otin C. Apolipoprotein D is the major protein component in cyst fluid from women with human breast gross cystic disease. Biochem J. 1990;271:803–807. doi: 10.1042/bj2710803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloir B, Lovari E, Surini-Demiri M, Savioz A. Altered apolipoprotein D expression in the brain of patients with Alzheimer disease. J Neurosci Res. 2001;64:61–69. doi: 10.1002/jnr.1054. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Van Der Heide LP, Kamal A, Bleys RL, Hispen WH. Ageing and diabetes: implications for brain function. Eur J Pharmacol. 2002;441:1–14. doi: 10.1016/S0014-2999(02)01486-3. [DOI] [PubMed] [Google Scholar]
- Boyles JK, Notterpek LM, Anderson LJ. Accumulation of apolipoproteins in the regenerating and remyelinating mammalian peripheral nerve Identification of apolipoprotein D apolipoprotein A-IV apolipoprotein E and apolipoprotein. J Biol Chem. 1990;265:17805–17815. [PubMed] [Google Scholar]
- Boyles JK, Notterpek LM, Wardell MR, Rall SC., Jr Identification characterization and tissue distribution of apolipoprotein D in the rat. J Lipids Res. 1990;31:2057–2065. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- De Jonge RR, Vreijling JP, Meintjes A, Kwa MS, van Kampen AH, van Schaik IN, Baas F. Transcriptional profile of the human peripheral nervous system by serial analysis of gene expression. Genomics. 2003;82:97–108. doi: 10.1016/S0888-7543(03)00124-1. [DOI] [PubMed] [Google Scholar]
- De Santi S, De Leon MJ, Convit A, Tarshish C, Rusinek H, Tsui WH, Sinaiko E, Wang GJ, Bartlet E, Volkow N. Age-related changes in brain: II. Positron emission tomography of frontal and temporal lobe glucose metabolism in normal subjects. Psychiatr Q. 1995;66:357–370. doi: 10.1007/BF02238755. [DOI] [PubMed] [Google Scholar]
- Del Valle E, Navarro A, Astudillo A, Tolivia J. Apolipoprotein D expression in human brain reactive astrocytes. J Histochem Cytochem. 2003;51:1285–1290. doi: 10.1177/002215540305101005. [DOI] [PubMed] [Google Scholar]
- Diez-Itza I, Vizoso F, Merino AM, Sanchez LM, Tolivia J, Fernández J, Rubial A, Lopez-Otin C. Expression and prognostic significance of apolipoprotein D in breast cancer. Am J Pathol. 1994;144:310–320. [PMC free article] [PubMed] [Google Scholar]
- Do Carmo S, Seguin D, Milne R, Rassart E. Modulation of apolipoprotein D and apolipoprotein E mRNA expression by growth arrest and identification of key elements in the promoter. J Biol Chem. 2002;277:5514–5523. doi: 10.1074/jbc.M105057200. [DOI] [PubMed] [Google Scholar]
- Drayna D, Fielding C, McLean J, Baer B, Castro G, Chen E, Comstock L, Henzel W, Kohr W, Rhee L, Wion K, Lawn R. Cloning and expression of human apolipoprotein D cDNA. J Biol Chem. 1986;261:16535–16539. [PubMed] [Google Scholar]
- Dueñas M, Torres-Aleman I, Naftolín F, Garcia-Segura LM. Interaction of insulin-like growth factor-I and estradiol signalling pathways on hypothalamic neuronal differentiation. Neuroscience. 1996;74:531–539. doi: 10.1016/0306-4522(96)00142-X. [DOI] [PubMed] [Google Scholar]
- Fernández JA, Suárez C, Navarro A, Diaz C, Álvarez JC, Gonzalez del Rey C, Tolivia J. Aging in the vestibular nuclear complex of the male golden hamster (Mesocricetus auratus): anatomic and morphometric study. Histol Histopathol. 2007;22:855–868. doi: 10.14670/HH-22.855. [DOI] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiol Aging. 2003;24:839–852. doi: 10.1016/S0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Ganfornina MD, Sánchez D, Pagano A, Tonachini L, Descalzi-Cancedda F, Martínez S. Molecular characterization and developmental expression pattern of the chicken apolipoprotein D gene: implications for the evolution of vertebrate lipocalins. Dev Dyn. 2005;232(1):191–199. doi: 10.1002/dvdy.20193. [DOI] [PubMed] [Google Scholar]
- Ganfornina MD, Do Carmo S, Lora JM, Torres-Schumann S, Vogel M, Allhorn M, González C, Bastiani MJ, Rassart E, Sánchez D. Apolipoprotein D is involved in the mechanisms regulating protection from oxidative stress. Aging Cell. 2008;7:506–515. doi: 10.1111/j.1474-9726.2008.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina MD, Do Carmo S, Martinez E, Tolivia J, Navarro A, Rassart E, Sanchez D. Apo D, a glia-derived apolipoprotein, is required for peripheral nerve functional integrity and a timely response to injury. Glia. 2010;58:1320–1334. doi: 10.1002/glia.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoitia I, Doncarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/S0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Green PS, Simpkins JW. Neuroprotective effects of estrogens: potential mechanisms of action. Int J Dev Neurosci. 2000;18:347–358. doi: 10.1016/S0736-5748(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Harukuni I, Hurn PD, Crain BJ. Deleterious effect of β-estradiol in a rat model of transient forebrain ischemia. Brain Res. 2001;900:137–142. doi: 10.1016/S0006-8993(01)02278-8. [DOI] [PubMed] [Google Scholar]
- Hu CY, Ong WY, Sundaram RK, Chan C, Patel SC. Immunocytochemical localization of apolipoprotein D in oligodendrocyte precursor-like cells perivascular cells and pericytes in the human cerebral cortex. J Neurocytol. 2001;30:209–218. doi: 10.1023/A:1012797623620. [DOI] [PubMed] [Google Scholar]
- Iossa S, Lionetti L, Mollica MP, Barletta A, Liverini G. Energy intake and utilization vary during development in rats. J Nutr. 1999;129:1593–1596. doi: 10.1093/jn/129.8.1593. [DOI] [PubMed] [Google Scholar]
- Kalman J, McConathy W, Araoz C, Kasa P, Lacko AG. Apolipoprotein D in the aging brain and in Alzheimer’s dementia. Neurol Res. 2002;22:330–336. doi: 10.1080/01616412.2000.11740678. [DOI] [PubMed] [Google Scholar]
- Khan MM, Parikh VV, Mahadik SP. Antipsychotic drugs differentially modulate apolipoprotein D in rat brain. J Neurochem. 2003;86:1089–1100. doi: 10.1046/j.1471-4159.2003.01866.x. [DOI] [PubMed] [Google Scholar]
- Lambert J, Provost PR, Marcel YL, Rassart E. Structure of the human apolipoprotein D gene promoter region. Biochim Biophys Acta. 1993;1172:190–192. doi: 10.1016/0167-4781(93)90292-L. [DOI] [PubMed] [Google Scholar]
- Loerch PM, Lu T, Dakin KA, Vann JM, Isaacs A, Geula C, Wang J, Pan Y, Gabuzda DH, Li C, Prolla TA, Yankner BA. Evolution of the aging brain transcriptome and synaptic regulation. PLoS ONE. 2008;3:e3329. doi: 10.1371/journal.pone.0003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Boado YS, Tolivia J, Lopez-Otin C. Apolipoprotein D gene induction by retinoic acid is concomitant with growth arrest and cell differentiation in human breast cancer cells. J Biol Chem. 1994;269:26871–26878. [PubMed] [Google Scholar]
- Mahadik SP, Khan MM, Evans DR, Parikh VV. Elevated plasma level of apolipoprotein D in schizophrenia and its treatment and outcome. Schizophr Res. 2002;58:55–62. doi: 10.1016/S0920-9964(01)00378-4. [DOI] [PubMed] [Google Scholar]
- Montpied P, de Bock F, Lerner-Natoli M, Bockaert J, Rondouin G. Hippocampal alterations of apolipoprotein E and D mRNA levels in vivo and in vitro following kainate excitotoxicity. Epilepsy Res. 1999;13:135–146. doi: 10.1016/S0920-1211(99)00003-0. [DOI] [PubMed] [Google Scholar]
- Moreno M, Ordóñez P, Alonso A, Díaz F, Tolivia J, González C. Chronic 17β -estradiol treatment improves skeletal muscle insulin signaling pathway components in insulin resistance associated with aging. AGE. 2010;32:1–13. doi: 10.1007/s11357-009-9095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muffat J, Walker DW, Benzer S. Human ApoD an apolipoprotein up-regulated in neurodegenerative diseases extends lifespan and increases stress resistance in Drosophila. Proc Natl Acad Sci USA. 2008;105:7088–7093. doi: 10.1073/pnas.0800896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Tolivia J, Álvarez-Uria M. The magnocellular neurosecretory system of the hamster hypothalamus: an ultrastructural and morphometric study during lifetime. Mech Ageing Dev. 1997;97:143–161. doi: 10.1016/S0047-6374(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Navarro A, Tolivia J, Alvarez-Uría M. Quantitative ultrastructural evidences suggest no age-related changes in biosynthesis and processing within parvocellular cells of the paraventricular nucleus in hamsters. Mech Ageing Dev. 1998;103:91–103. doi: 10.1016/S0047-6374(98)00036-0. [DOI] [PubMed] [Google Scholar]
- Navarro A, Tolivia J, Astudillo A, Del Valle E. Pattern of apolipoprotein D immunoreactivity in human brain. Neurosci Lett. 1998;254:17–20. doi: 10.1016/S0304-3940(98)00639-9. [DOI] [PubMed] [Google Scholar]
- Navarro A, Astudillo A, Del Valle E, González del Rey C, Tolivia J. Immunohistochemical presence of apolipoprotein D in senile plaques. J Histotechnol. 2001;24:45–49. [Google Scholar]
- Navarro A, Del Valle E, Astudillo A, Gonzalez del Rey C, Tolivia J. Immunohistochemical study of distribution of apolipoproteins E and D in human cerebral β amyloid deposits. Exp Neurol. 2003;184:697–704. doi: 10.1016/S0014-4886(03)00315-7. [DOI] [PubMed] [Google Scholar]
- Navarro A, Del Valle E, Tolivia J. Differential expression of apolipoprotein D in human astroglial and oligodendroglial cells. J Histochem Cytochem. 2004;52:1031–1036. doi: 10.1369/jhc.3A6213.2004. [DOI] [PubMed] [Google Scholar]
- Navarro A, Ordoñez C, Martínez E, Pérez C, Astudillo A, Tolivia J. Apolipoprotein D expression absence in degenerating neurons of human central nervous system. Histol Histopathol. 2008;23:995–1001. doi: 10.14670/HH-23.995. [DOI] [PubMed] [Google Scholar]
- Navarro A, Del Valle E, Juárez A, Martínez E, Ordóñez C, Astudillo A, Tolivia J. Apolipoprotein D synthesis progressively increases in frontal cortex during human lifespan. Age. 2010;32:85–96. doi: 10.1007/s11357-009-9117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NR. Glial responses to steroids as markers of brain aging. J Neurobiol. 1999;40:585–601. doi: 10.1002/(SICI)1097-4695(19990915)40:4<585::AID-NEU13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ordoñez C, Navarro A, Pérez C, Astudillo A, Martínez E, Del Valle E, Tolivia J. Apolipoprotein expression in substantia nigra of Parkinson disease. Histol Histopathol. 2006;21:361–366. doi: 10.14670/HH-21.361. [DOI] [PubMed] [Google Scholar]
- Ordoñez C, Navarro A, Pérez C, Martínez E, Astudillo A, Tolivia J (2011) Gender differences in apolipoprotein D expression during aging and in Alzheimer Disease. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2011.01.010 [DOI] [PubMed]
- Patel SC, Asotra K, Patel YC, McConathy WJ, Patel RC, Suresh S. Astrocytes synthesize and secrete the lipoprotein ligand carrier apolipoprotein D. NeuroReport. 1995;6:653–657. doi: 10.1097/00001756-199503000-00017. [DOI] [PubMed] [Google Scholar]
- Provost PR, Villeneuve L, Weech PK, Milne RW, Marcel YL, Rassart E. Localization of the major sites of rabbit apolipoprotein D gene transcription by in situ hybridization. J Lipid Res. 1991;32:1959–1970. [PubMed] [Google Scholar]
- Rassart E, Bedirian A, Do Carmo S, Guinard O, Sirois J, Terrisse L, Milne R. Apolipoprotein D. Biochim Biophys Acta. 2000;1482:185–198. doi: 10.1016/S0167-4838(00)00162-X. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM. Effects of hormone replacement therapy on cognitive and brain aging. Ann NY Acad Sci. 2001;949:203–214. doi: 10.1111/j.1749-6632.2001.tb04023.x. [DOI] [PubMed] [Google Scholar]
- Sanchez D, Lopez-Arias B, Torroja L, Canal I, Wang X, Bastiani MJ, Ganfornina MD. Loss of glial lazarillo a homolog of apolipoprotein d reduces lifespan and stress resistance in Drosophila. Curr Biol. 2006;16:680–686. doi: 10.1016/j.cub.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Sánchez D, Ganfornina MD, Martínez S. Expression pattern of the lipocalin apolipoprotein D during mouse embryogenesis. Mech Dev. 2002;110(1–2):225–229. doi: 10.1016/S0925-4773(01)00578-0. [DOI] [PubMed] [Google Scholar]
- Saravia F, Beauquis J, Pietranera L, De Incola A. Neuroprotective effects of estradiol in hipocampal neurons and glia of middle age mice. Psychoneuroendocrinology. 2007;32:480–492. doi: 10.1016/j.psyneuen.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Seguin D, Desforges M, Rassart E. Molecular characterization and differential mRNA tissue distribution of mouse apolipoprotein D. Mol Brain Res. 1995;30:242–250. doi: 10.1016/0169-328X(95)00008-G. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women. Endocr Rev. 2003;24:133–151. doi: 10.1210/er.2001-0016. [DOI] [PubMed] [Google Scholar]
- Simard J, Veilleux R, de Launoit Y, Haagensen DE, Labrie F. Stimulation of apolipoprotein D secretion by steroids coincides with inhibition of cell proliferation in human LNCaP prostate cancer cells. Cancer Res. 1991;51:4336–4341. [PubMed] [Google Scholar]
- Simard J, de Launoit Y, Haagensen DE, Labrie F. Additive stimulatory action of glucocorticoids and androgens on basal and estrogen-repressed apolipoprotein D Messenger ribonucleic acid levels and secretion in human breast cancer cells. Endocrinology. 1992;130:1115–1121. doi: 10.1210/en.130.3.1115. [DOI] [PubMed] [Google Scholar]
- Smith KM, Lawn RM, Wilcox JN. Cellular localization of apolipoprotein D and lecithin: cholesterol acyltransferase mRNA in rhesus monkey tissues by in situ hybridization. J Lipids Res. 1990;31:995–1004. [PubMed] [Google Scholar]
- Søiland H, Søreide K, Janssen EA, Körner H, Baak JP, Søreide JA. Emerging concepts of apolipoprotein D with possible implications for breast cancer. Cell Oncol. 2007;29:195–209. doi: 10.1155/2007/487235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrisse L, Poirier J, Bertrand P, Merched A, Visvikis S, Siest G, Milne R, Rassart E. Increased levels of apolipoprotein D in cerebrospinal fluid hippocampus of Alzheimer’s patients. J Neurochem. 1998;71:1643–1650. doi: 10.1046/j.1471-4159.1998.71041643.x. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Danielson PE, Nelson PA, Pribyl TM, Hilbush BS, Hasel KW, Sutcliffe JG. Clozapine increases apolipoprotein D expression in rodent brain: towards a mechanism for neuroleptic pharmacotherapy. J Neurochem. 2001;76:789–796. doi: 10.1046/j.1471-4159.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Dean B, Pavey G, Sutcliffe JG. Increased CNS levels of apolipoprotein D in schizophrenic and bipolar subjects: implications for the pathophysiology of psychiatric disorders. Proc Natl Acad Sci USA. 2001;98:4066–4071. doi: 10.1073/pnas.071056198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EA, Laws SM, Sutcliffe JG, Harper C, Dean B, McClean C, Masters C, Lautenschlager N, Gandy SE, Martins RN. Apolipoprotein D levels are elevated in prefrontal cortex of subjects with Alzheimer’s disease: no relation to apolipoprotein E expression or genotype. Biol Psychiatry. 2003;54:136–141. doi: 10.1016/S0006-3223(02)01976-5. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Sci USA. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu VN, Uckum FM. Apolipoprotein E and Apolipoprotein D expression in a murine model of singlet oxygen-induced cerebral stroke. Biochem Biophys Res Commun. 2000;268:835–841. doi: 10.1006/bbrc.2000.2205. [DOI] [PubMed] [Google Scholar]
- Walker DW, Muffat J, Rundel C, Benzer S. Overexpression of a Drosophila homolog of apolipoprotein D leads to increased stress resistance and extended lifespan. Curr Biol. 2006;16:674–679. doi: 10.1016/j.cub.2006.01.057. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubai DB, Wilson ME, Rau SW, Bottner M, Rosewell KL. Estradiol is a protective factor in the adult and aging brain Understanding of mechanisms derived from in vivo and in vitro studies. Brain Res Rev. 2001;37:313–319. doi: 10.1016/S0165-0173(01)00136-9. [DOI] [PubMed] [Google Scholar]