Abstract
Soybean polyunsaturated phosphatidylcholine (PC) is thought to exert anti-inflammatory activities and has potent effects in attenuating acute renal failure and liver dysfunction. The aim of this study was to investigate the effects of PC in protecting multiple organ injury (MOI) from lipopolysaccharide (LPS). Six groups of rats (N=8) were used in this study. Three groups acted as controls and received only saline, hydrocortisone (HC, 6 mg/kg, i.v.) or PC (600 mg/kg, i.p.) without LPS (15 mg/kg, i.p.) injections. Other 3 groups, as the test groups, were administered saline, HC or PC in the presence of LPS. Six hours after the LPS injection, blood and organs (lung, liver and kidney) were collected from each group to measure inflammatory cytokines and perform histopathology and myeloperoxidase (MPO) assessment. Serum cytokines (TNF-α, IL-6 and IL-10) and MPO activities were significantly increased, and significant histopathological changes in the organs were observed by LPS challenge. These findings were significantly attenuated by PC or HC. The treatment with PC or HC resulted in a significant attenuation on the increase in serum levels of TNF-α and IL-6, pro-inflammatory cytokines, while neither PC nor HC significantly attenuated serum levels of IL-10, anti-inflammatory cytokine. In the organs, the enhanced infiltration of neutrophils and expression of ED2 positive macrophage were attenuated by PC or HC. Inductions of MPO activity were also significantly attenuated by PC or HC. From the findings, we suggest that PC may be a functional material for its use as an anti-inflammatory agent.
Keywords: Kidney, Liver, Lung, LPS, Phosphatidylcholine
INTRODUCTION
Multiple organ injury (MOI) is defined as dysfunction or failure of multiple organs or system happening simultaneously or sequentially due to various etiological factors. The well-known etiology includes infection of gram positive/negative bacteria, fungal, and virus, shock, hemorrhage, allergy, burns, trauma and severe acute pancreatitis [1-4]. Although recently, remarkable advances have been made in understanding the cellular mechanisms underlying organ dysfunction and recovery relating to these conditions, the exact mechanism is not yet fully elucidated. Current understanding of the pathophysiology of MOI focuses on the multiple cell populations and cell-signaling pathways involved in this complex condition characterized by generalized inflammation [5-8]. This overwhelming host response, systemic inflammation, is generally believed to be the cause of MOI. Treatment of patients with MOI is still largely supportive. The development of adequate treatment relies on elucidation of the mechanisms and mediators involved in its pathophysiology.
It is generally recognized that the systemic inflammatory and immune response triggered by pathogenic gram-negative bacteria largely depend on the release of lipopolysaccharide (LPS) from the bacterial cell wall [9,10]. LPS is able to induce the cellular release of NO, various proinflammatory cytokines and chemokines, as well as mediating macrophage migration and contributing to the pathogenesis of sepsis by stimulating innate immune process [11]. A high dose of LPS causes more than one organ at a time to fail, which is similar to MOI, generally resulting from serious infections, low blood pressure and serious injuries. The features of MOI induced by LPS are circulatory failure, systemic hypotension, hypo-reactivity to vasoconstrictors, subsequent problems with organ perfusion and the development of functional abnormalities [12]. The endotoxin-induced failure of lung, liver, and kidney reflect systemic inflammatory response syndrome and septic shock [13]. In addition to this overshooting proinflammatory reaction, the immune response to infection also triggers the local as well as the systemic release of anti-inflammatory mediators such as IL-10.
Soybean polyunsaturated phosphatidylcholine (PC) is a polyunsaturated fatty acid (PUFA) compound and a 94% to 96% mixture of phosphatidylcholines, about half of which is dilinoleoylphosphatidylcholine [14]. In general, phosphatidylcholine is an integral component of every cell in biology [15,16]. Extensive researches up to date suggest that phosphatidylcholine plays a pivotal role in many important areas including maintaining memory and nerve signaling, as a precursor to important neurotransmitters and attenuating acute renal failure and liver dysfunction [17-19]. A number of recent studies have demonstrated an anti-inflammatory potential for PC and its metabolites in various conditions linked to leukocyte activation, including ischemia [20], oxidative stress [21] and endotoxin-induced injuries [22,23]. PUFA, one component of PC, also has been shown to modulate the inflammatory reactions [24,25]. Moreover, in clinical practice, PC, supplemented regularly as part of our normal diets, has also been proven safe and effective over decades of study as a therapeutic agent [17,26].
Accordingly, our primary aim was to evaluate a precise characterization of PC in preventive effect for MOI using a standardized rodent model with LPS. In this model, we set out to ascertain the anti-inflammatory properties of PC by performing detailed functional and morphological analyses relative to proinflammatory cytokines.
METHODS
Chemicals
Hydrocortisone (HC, 50 mg/ml) was purchased from Il-dong pharmaceutical company (Seoul, Korea). Phosphatidylcholine (Phospholipon90G) was kindly supplied by Amipharm Co. (Seoul, Korea). Lipopolysaccharide from salmonella thyphosa and other essential chemicals were obtained from Sigma-Aldrich chemical Co. (St. Louis, MO, USA).
Animals
Male Sprague-Dawley rats (6 weeks old, 200~220 g body weight) were used in present study. These animals were supplied by Nara-biotechnology (Seoul, Korea). They were maintained at 22±2℃ in a 12 h light dark cycle, and were given a normal laboratory diet (Purina, Korea) and fresh water ad libitum. All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the protocol was approved by the Institutional Animal Care and Use Committee of Chung Ang University.
Drug treatment
Six groups of 8 rats per group were randomly divided; Saline+Saline, Saline+PC, Saline+HC, LPS+Saline, LPS+HC, and LPS+PC. Three groups acted as controls and received saline, HC (6 mg/kg) or PC (600 mg/kg) with saline instead of LPS. For the test groups, saline, HC or PC was administered with LPS (15 mg/kg, i.p.). HC was injected through lateral tail-vein. PC was suspended in distilled water (100 mg/ml), and administered intraperitoneally.
Sample collection
Six hours after LPS injection, the rats were sacrificed and then collected the tissues and blood from posterior venacava. Serum was separated by centrifugation at 4,000 rpm for 15 min, and stored at -70℃ until analyzed. Liver, lung and kidney samples were immediately removed and weighed after washing 0.9% saline. One part of the tissues from each group was stored at -70℃ until analyzed and the other remaining tissues were fixed in 4% paraformaldehyde for histopathological examination.
Myeloperoxidase (MPO) assay
MPO activity was evaluated using MPO colorimetric activity assay kit (BioVision Research Products BioVision, Mountain View, CA, USA). Briefly, the frozen tissues were minced with scissors, homogenized in 0.1 M Tris-HCl buffer (pH 7.4) and centrifuged to remove insoluble materials. Supernatants were collected, mixed with MPO assay buffer and MPO substrate, incubated at room temperature for 1 h, and then mixed with tetramethylbenzidine probe. The absorbance was read at 412 nm in an ELISA reader. MPO activity was normalized to milligrams of protein. Protein concentration was measured by Qubit® Protein Assay kit (Invitrogen, Carlsbad, CA, USA).
Cytokine assays
Serum levels of TNF-α were determined using a rat TNF-α ELISA kit (eBiosource International, Burlington, Ontario, Canada). IL-6 and IL-10 in serum were quantified by ELISA with Quantikine® mouse immunoassay kits (R&D Systems, Minneapolis, MN, USA). The ELISA was performed following the manufacturer's instruction, and the concentrations of cytokine were measured by optical densitometry at 450nm in SpectramaxPlus 384 microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Histological examination
For histopathological analysis, paraformaldehyde fixed and paraffin embedded lungs, livers, and kidneys were sectioned at 4µm. After sectioning, slides were stained with hematoxylin and eosin (H&E) using the standard staining method. Expressions of ED2 positive cell were detected with the avidin-biotinylated horseradish peroxidase complex (DAKO LSAB, Los Angeles, CA, USA) according to the kit's instruction. Primary antibodies used were anti-ED2 mouse monoclonal antibody (1:50, Serotec, Kidlington, Oxford, UK). Negative control immunostaning was performed by omission of the primary antibody and by use of non-immune serum. For the microscopic analysis, each slide was examined by a blind pathologist at a magnification of ×400 (Olympus BX51, 0.949 mm2).
Statistical analysis
Data were expressed as mean±S.E.M. Statistical analysis was performed by one-way ANOVA with Tukey's multiple comparison post-tests (IBM SPSS statistics ver. 20, SPSS Inc., Chicago, USA). All statistical differences were considered to be significant with p<0.05.
RESULTS
PC attenuates LPS-induced increase in pro-inflammatory cytokines, TNF-α and IL-6, but not in anti-inflammatory cytokine, IL-10
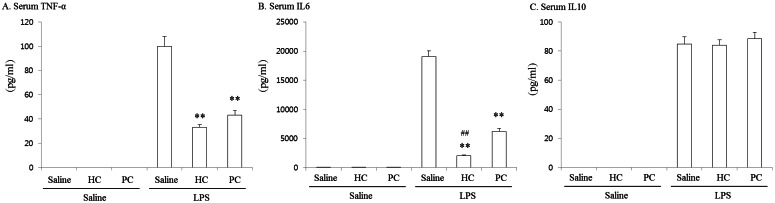
The levels of serum cytokines were measured and results are shown in Fig. 1. One-way ANOVA showed significant differences among groups in TNF-α [F(5, 42)=108.569, p<0.001], IL-6 [F(5, 42)=200.764, p<0.001] and IL-10 [F(5, 42) =223.731, p<0.001]. The serum level of cytokines was not changed by HC or PC treatment without LPS. However, the level of TNF-α, IL-6 and IL-10 were significantly increased (p<0.01) at 6h after LPS injection in comparison to Saline+Saline group. Treatment with PC resulted in protective effects on these LPS-induced increases in TNF-α and IL-6 levels. Treatment with HC also resulted in significant attenuations against LPS-induced increases in both cytokines. Although the levels of both cytokines in LPS+HC group were lower than those of LPS+PC group, a significant difference (p<0.01) between LPS+HC group and LPS+PC group was shown only in IL-6 level. On the other hand, the LPS-induced increase in serum level of IL-10 was changed by neither HC nor PC.
Fig. 1.
Effects of phosphatidylchoine (PC) on serum level of TNF-α (A), IL6 (B) and IL10 (C) in lipopolysaccharide (LPS)-exposed rats. The levels of cytokines were significantly increased at 6 h after LPS injection. Pretreatment with PC (600 mg/kg, i.p.) showed protective effects on this LPS-induced increase in TNF-α and IL-6 levels. Treatment with hydrocortisone (HC, 6 mg/kg, i.v.) also attenuated the magnitude of the increase in both cytokines. Each value is the mean±SEM of 8 mice. **p<0.01 vs. LPS+Saline, ##p<0.01 vs. LPS+PC (one-way ANOVA followed by Turkey's post-test).
PC attenuates LPS-induced increase in MPO activity of liver, lung and kidney
To clarify the effect of PC on LPS-induced MOI, we determined MPO activity in liver, lung and kidney. The increased activity of MPO is an evident marker of inflammation. The MPO activities of tissues are shown in Table 1. One-way ANOVA showed significant differences among groups in MPO activity of liver [F(5, 42)=59.507, p<0.001], lung [F(5, 42)=534.129, p<0.001] and kidney [F(5, 42)= 367.902, p<0.001]. MPO activity was not detected at the tissues of organs in Saline+Saline group, although low levels of MPO activities were induced by HC and PC. Post hoc comparisons using the Tukey's test indicated that LPS produces a significant increase (p<0.01) in MPO activity of the organs. These changes were significantly (p<0.01) attenuated by either PC or HC.
Table 1.
Effect of PC on LPS-induced changes in MPO activity
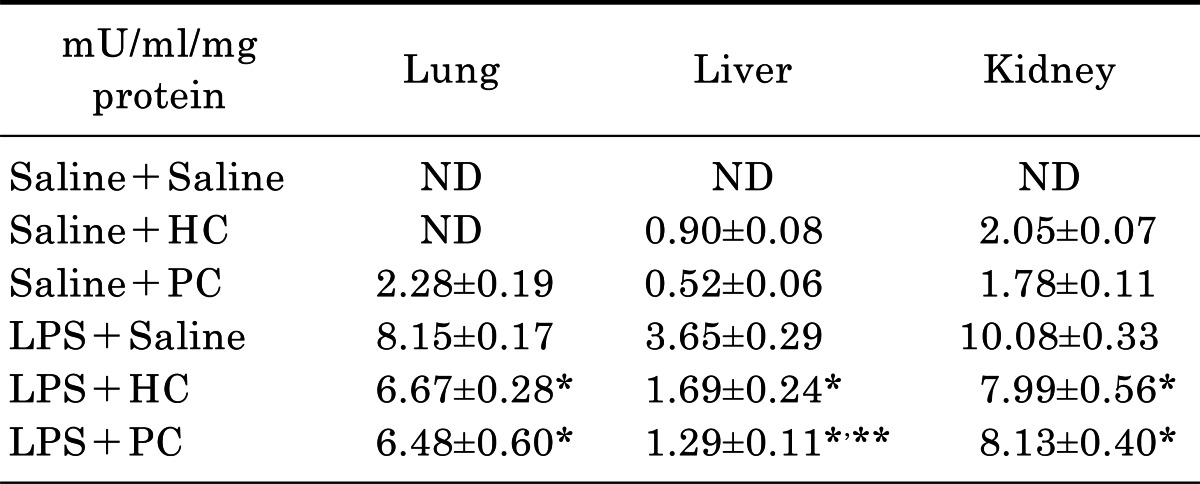
Each value is mean±SEM of 8 mice. ND, not detected, *p<0.01 vs. LPS+Saline, **p<0.05 vs. LPS+HC (one-way ANOVA followed by Turkey's post-test).
PC attenuates LPS-induced morphological changes and PMN infiltration in the organs such as lung and liver
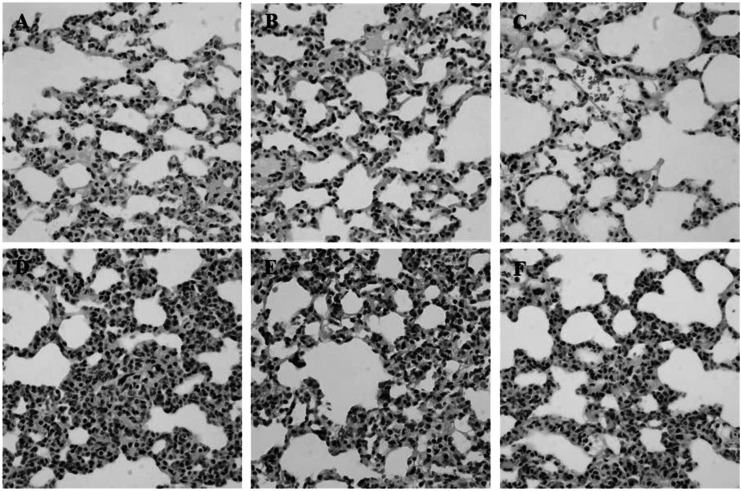
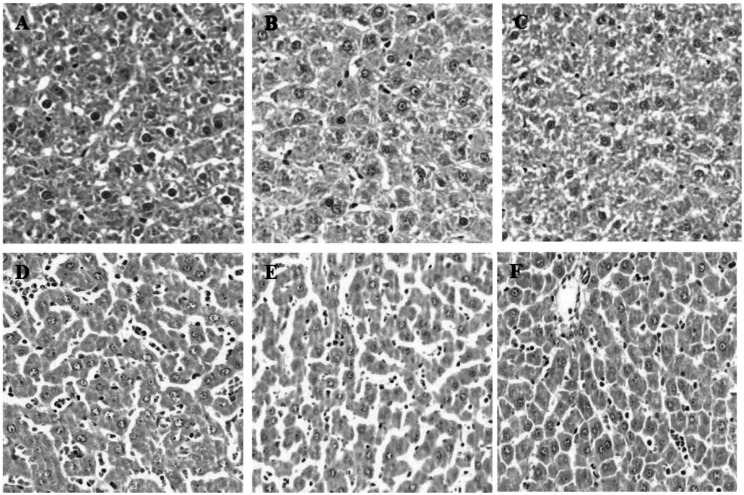
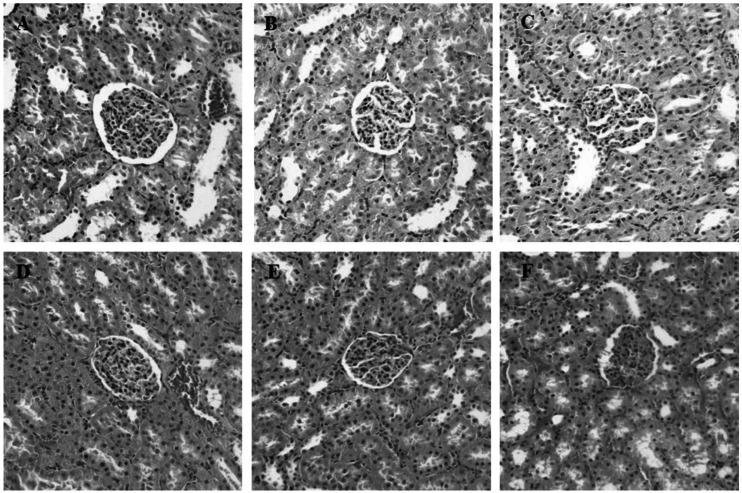
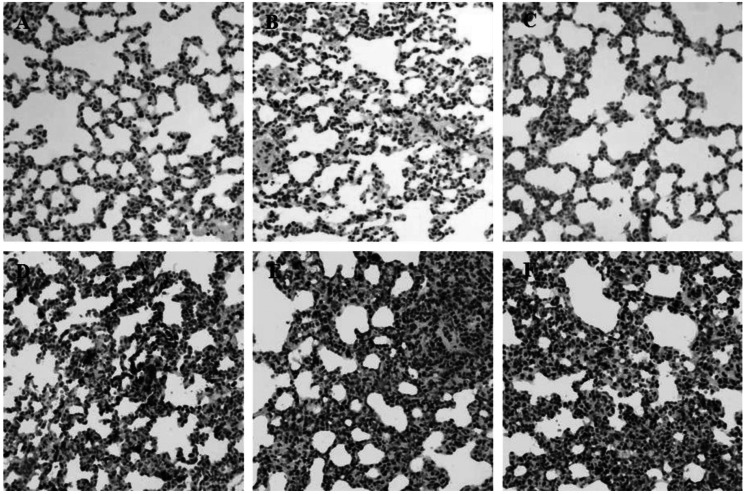
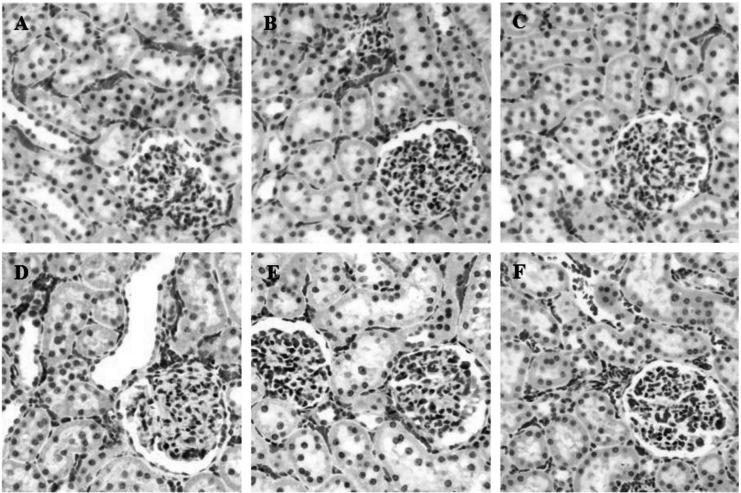
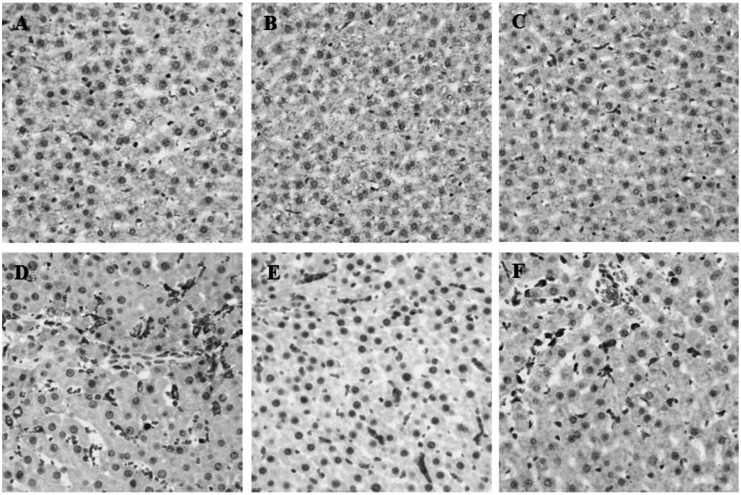
During inflammation, polymorphonuclear leukocytes (PMN) infiltration into tissues plays an important role immune response and is a major cause of tissue damage. To determine whether treatment with PC can attenuate PMN induction and morphological change, the sectioned tissues were stained with H&E. An apparent increase in PMN infiltration was observed in lung and liver after LPS challenge (Fig. 2D and 3D). Thickening of alveolar septa was observed in lung tissue after LPS administration. These morphological changes were ameliorated by either HC (Fig. 2E) or PC (Fig. 2F). However, no significant effects of PC were observed in kidney tissue (Fig. 4) as compared to effects on lung and liver.
Fig. 2.
Effects of PC on LPS-induced morphological changes and PMN infiltrations in lung. Representative micrographs are illustrated. An increase in PMN infiltration and thickening of alveolar septa were observed in lung tissue after LPS administration. These morphological changes were ameliorated by either HC or PC. (A) Saline+Saline (B) Saline+HC, (C) Saline+PC, (D) LPS+Saline, (E) LPS+HC and (F) LPS+PC (Hematoxylin and eosin staining; magnification, 400×).
Fig. 3.
Effects of PC on LPS-induced morphological changes and PMN infiltrations in liver. Representative micrographs are illustrated. An increase in PMN infiltration was observed in liver tissue after LPS administration. Treatment with either HC or PC ameliorated the increase in PMN infiltrations. (A) Saline+Saline (B) Saline+HC, (C) Saline+PC, (D) LPS+Saline, (E) LPS+HC and (F) LPS+PC (Hematoxylin and eosin staining; magnification, 400×).
Fig. 4.
Effects of PC on LPS-induced morphological changes and PMN infiltrations in kidney. Representative micrographs are illustrated. There are no significant effects of PC or HC on kidney tissue as compared to effects on lung and liver (A) Saline+Saline (B) Saline+HC, (C) Saline+PC, (D) LPS+Saline, (E) LPS+HC and (F) LPS+PC (Hematoxylin and eosin staining; magnification, 400×).
PC attenuates LPS-induced ED2-positive cell expression in the organs such as lung, liver and kidney
Next, we performed immunohistochemistry with mouse monoclonal antibody against ED2 macrophage to examine enhanced phagocytosis after LPS exposure (Fig. 5~7). ED2 positive macrophage expression in damaged tissue reflects the production of inflammatory mediator such as TNF-α, and IL-6 [27]. PC treatment led to the attenuation of the level of ED2 positive macrophages, reaching a high peak level in LPS-challenge. The protective effects of PC were shown in all organs, not only lung and liver but also kidney. These results suggest that PC is potentially effective in the accumulation of inflammatory cells into the damaged tissue with LPS.
Fig. 5.
Effects of PC on LPS-induced ED2-positive cell expression in lung. Immunohistochemistry was performed after LPS exposure. Treatment with the PC led to the attenuation of the level of ED2 positive macrophages. Representative micrographs are illustrated. (A) Saline+Saline (B) Saline+HC, (C) Saline+PC, (D) LPS+Saline, (E) LPS+HC and (F) LPS+PC (Magnification, 400×).
Fig. 7.
Effects of PC on LPS-induced ED2-positive cell expression in kidney. Treatment with the PC led to the attenuation of the level of ED2 positive macrophages. Representative micrographs are illustrated. (A) Saline+Saline (B) Saline+HC, (C) Saline+PC, (D) LPS+Saline, (E) LPS+HC and (F) LPS+PC (Magnification, 400×).
DISCUSSION
In this study, we investigated the anti-inflammatory activity of PC in LPS-induced MOI in rats. The extent of inflammatory responses was monitored by measuring the production levels of pro-inflammatory or anti-inflammatory cytokines (TNF-α, IL-6, and IL-10) in serum. Effects of PC on the distribution of ED2 positive macrophages and infiltration of PMN were also evaluated. Assay for MPO, the most abundant protein in neutrophils, was performed in the focus of inflammatory pathologies. PC significantly inhibited LPS-induced inflammatory responses found in MOI. Results from ELISA analysis showed that the serum levels of pro-inflammatory cytokines such as TNF-α, and IL-6, were attenuated by PC, while level of anti-inflammatory cytokine IL-10 in LPS-exposed rats was not changed by PC. Although no significant effects was observed in H&E stained kidney tissue, treatment with PC resulted in significant reductions of the distribution of ED2 positive macrophages and PMN population in lung and liver. MPO was also significantly decreased by PC as compared to LPS-induced control.
A number of inflammatory mediators are released by cells in response to localized injury or trauma. These mediators elicit or enhance particular functions of the inflammatory response and may be monitored to assess an inflammatory response. In MOI, a systemic injury, inflammation is also a common problem. Further, in clinical aspect, it is a cause of death with extremely high mortality rates in patients undergoing major surgery [28]. Although glucocorticosteroids remain the most effective therapy for inflammatory disorders, the side effects limit their clinical usefulness [29]. High-dose of corticosteroids to treat sepsis has been one of controversial clinical issues [30], and administration of high-dose corticosteroids does not benefit to treat patients with early and severe sepsis [31,32].
Although low-dose of HC, as a replacement therapy, has shown anti-inflammatory effects in sepsis [33-35], more successful treatment of controlling the inflammatory state should be needed. Previous studies have shown that PC has anti-inflammatory properties in animal disease models. One study identified a protective role for PC in which the therapeutic potential may be linked to the reduction of neutrophil leukocyte-mediated microcirculatory inflammatory reactions [36]. Another investigation reported that PC acts as precursors allowing the specific effector(s) to achieve and maintain an optimal concentration at the site of action, in part, in the modulatory effects observed [37], suggesting dietary PC supplementation may be an advantageous therapeutic strategy in chronic inflammatory diseases, or as a prophylactic regime via which to decrease the risk of inflammatory complications and side effects.
In the inflammatory state, LPS stimulates the production of various endogenous inflammatory mediators and profoundly modulates immune responses and organ functions. Specially, pro-inflammatory cytokines, such as TNF-α or IL-6, and anti-inflammatory cytokines, IL-10, are produced in response to LPS [38]. In this study, PC inhibited the up-regulation of series of pro-inflammatory cytokines such as TNF-α and IL-6. TNF-α is released by monocytes and macrophages in response to various stimuli and acts as a principal mediator of the deleterious effects of LPS [39]. When TNF-α production increases to such an extent over the local infection and when it circulates in bloodstream, blood pressure drops, resulting in shock. Major organs including the kidneys, liver, lungs, and brain, stop working properly because of poor blood flow [40]. IL-6 has been implicated as mediators of LPS toxicity in vivo and in vitro [41-43]. The biological properties of IL-6 are unusually similar to those of TNF-α, and the synergistic effects are evident in several models [44,45]. One investigation [46] showed the interaction between TNF-α and IL-6. The LPS-induced IL-6 secretion was reduced in the anti-TNF-pretreated group. Parallel to the in vivo experiments, the IL-6 secretion was diminished for 25% in presence of anti-TNF antibody treatment. In this experiment, we showed that the effects of LPS challenge on the pro-inflammatory cytokine production were significantly attenuated by the treatment with PC, supporting its anti-inflammatory effect.
PMN and macrophages are thought to contribute significantly to the pathophysiologic features of organ injury [47]. PMNs dominate the early stages of inflammation and set the stage for repair of tissue damage by macrophages. Acute inflammation is characterized by increased MPO activity increased expression and activity of cytokines, and increased oxidant stress [48]. The present study is in agreement with previous studies showing that LPS-induced organ injury accompanies changes in tissue neutrophil accumulation and neutrophil infiltration, which is relevant to an increase in MPO assay. As clearly shown in the PC-treated group, the attenuation of PMN infiltration into damaged tissue with reduction of MPO activity ensues. Macrophages also play a pivotal role in the initiation, maintenance, and resolution of inflammation. An autocrine loop in macrophages is the most probable mechanism for the regulation of an LPS-induced multiple organ injury [49]. They are activated and deactivated in the inflammatory process. Inhibition of inflammation by removal of mediators from inflammatory macrophages leads to repairing damaged tissues [47]. Activated macrophages are deactivated by anti-inflammatory cytokines (IL-10 and transforming growth factor β) mainly produced by macrophages. IL-10 is a potent regulator of the inflammatory responses [50]. Several cell types produce IL-10 and its receptor chains and these may regulate different inflammatory responses. In this study, we showed that the treatment with PC failed to significantly alter the increase in serum levels of IL-10 in LPS-challenged rats. However, the migrating macrophages, ED2 positive cell, into the tissue damage were attenuated by PC treatment. The fail of IL-10 change by PC may be correlated with the reduction of ED2-positive cells in the damaged tissue by the inhibition of migration of macrophages.
In summary, this study shows that the treatment with PC resulted in significant attenuations on serum levels of TNF-α and IL-6 in LPS-exposed rats. The cytokines is responsible for blood pressure drops, resulting in shock and MOI. MPO activity, PMNs and ED2 positive macrophages to contribute significantly to the inflammatory features of organ injury were also attenuated by PC in LPS-induced MOI. These findings give a clue that PC exerts anti-inflammatory activities in the immune challenged animals, implicating that PC may be therapeutically or prophylactically useful for the treatment of inflammatory diseases such as MOI.
Fig. 6.
Effects of PC on LPS-induced ED2-positive cell expression in liver. Immunohistochemistry was performed after LPS exposure. Treatment with the PC led to the attenuation of the level of ED2 positive macrophages. Representative micrographs are illustrated. (A) Saline+Saline (B) Saline+HC, (C) Saline+PC, (D) LPS+Saline, (E) LPS+HC and (F) LPS+PC (Magnification, 400×).
ABBREVIATIONS
- PC
phosphatidylcholine
- MOI
multiple organ injury
- LPS
lipopolysaccharide
- HC
hydrocortisone
- MPO
myeloperoxidase
References
- 1.Proulx F, Joyal JS, Mariscalco MM, Leteurtre S, Leclerc F, Lacroix J. The pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2009;10:12–22. doi: 10.1097/PCC.0b013e31819370a9. [DOI] [PubMed] [Google Scholar]
- 2.Papathanassoglou ED, Bozas E, Giannakopoulou MD. Multiple organ dysfunction syndrome pathogenesis and care: a complex systems' theory perspective. Nurs Crit Care. 2008;13:249–259. doi: 10.1111/j.1478-5153.2008.00289.x. [DOI] [PubMed] [Google Scholar]
- 3.Butt I, Shrestha BM. Two-hit hypothesis and multiple organ dysfunction syndrome. JNMA J Nepal Med Assoc. 2008;47:82–85. [PubMed] [Google Scholar]
- 4.Krau SD. Making sense of multiple organ dysfunction syndrome. Crit Care Nurs Clin North Am. 2007;19:87–97. doi: 10.1016/j.ccell.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Mizock BA. The multiple organ dysfunction syndrome. Dis Mon. 2009;55:476–526. doi: 10.1016/j.disamonth.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Lipsky M. Multiple organ dysfunction syndrome. Foreword. Dis Mon. 2009;55:475. doi: 10.1016/j.disamonth.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Johnson D, Mayers I. Multiple organ dysfunction syndrome: a narrative review. Can J Anaesth. 2001;48:502–509. doi: 10.1007/BF03028318. [DOI] [PubMed] [Google Scholar]
- 8.Wadhwa J, Sood R. Multiple organ dysfunction syndrome. Natl Med J India. 1997;10:277–282. [PubMed] [Google Scholar]
- 9.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henderson B, Wilson M. Modulins: a new class of cytokine-inducing, pro-inflammatory bacterial virulence factor. Inflamm Res. 1995;44:187–197. doi: 10.1007/BF01782257. [DOI] [PubMed] [Google Scholar]
- 11.Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13:110–116. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 12.Bone RC. Sepsis, the sepsis syndrome, multi-organ failure: a plea for comparable definitions. Ann Intern Med. 1991;114:332–333. doi: 10.7326/0003-4819-114-4-332. [DOI] [PubMed] [Google Scholar]
- 13.Bohlinger I, Leist M, Gantner F, Angermüller S, Tiegs G, Wendel A. DNA fragmentation in mouse organs during endotoxic shock. Am J Pathol. 1996;149:1381–1393. [PMC free article] [PubMed] [Google Scholar]
- 14.Mourelle M, Guarner F, Malagelada JR. Polyunsaturated phosphatidylcholine prevents stricture formation in a rat model of colitis. Gastroenterology. 1996;110:1093–1097. doi: 10.1053/gast.1996.v110.pm8612998. [DOI] [PubMed] [Google Scholar]
- 15.Fallbrook A, Turenne SD, Mamalias N, Kish SJ, Ross BM. Phosphatidylcholine and phosphatidylethanolamine metabolites may regulate brain phospholipid catabolism via inhibition of lysophospholipase activity. Brain Res. 1999;834:207–210. doi: 10.1016/s0006-8993(99)01570-x. [DOI] [PubMed] [Google Scholar]
- 16.Blusztajn JK, Zeisel SH, Wurtman RJ. Synthesis of lecithin (phosphatidylcholine) from phosphatidylethanolamine in bovine brain. Brain Res. 1979;179:319–327. doi: 10.1016/0006-8993(79)90447-5. [DOI] [PubMed] [Google Scholar]
- 17.Cheatham CL, Goldman BD, Fischer LM, da Costa KA, Reznick JS, Zeisel SH. Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2012;96:1465–1472. doi: 10.3945/ajcn.112.037184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung SY, Moriyama T, Uezu E, Uezu K, Hirata R, Yohena N, Masuda Y, Kokubu T, Yamamoto S. Administration of phosphatidylcholine increases brain acetylcholine concentration and improves memory in mice with dementia. J Nutr. 1995;125:1484–1489. doi: 10.1093/jn/125.6.1484. [DOI] [PubMed] [Google Scholar]
- 19.Rey JW, Schreiner O, Barreiros AP, Heise M, Krupp M, Schuchmann M, Otto G, Galle PR, Teufel A. Acute renal failure and liver dysfunction after subcutaneous injection of 3-snphosphatidylcholine (Lipostabil®)-case report. Z Gastroenterol. 2011;49:340–343. doi: 10.1055/s-0029-1245614. [DOI] [PubMed] [Google Scholar]
- 20.Ghyczy M, Torday C, Kaszaki J, Szabó A, Czóbel M, Boros M. Oral phosphatidylcholine pretreatment decreases ischemia-reperfusion-induced methane generation and the inflammatory response in the small intestine. Shock. 2008;30:596–602. doi: 10.1097/SHK.0b013e31816f204a. [DOI] [PubMed] [Google Scholar]
- 21.Al-Orf SM. Effect of oxidized phosphatidylcholine on biomarkers of oxidative stress in rats. Indian J Clin Biochem. 2011;26:154–160. doi: 10.1007/s12291-010-0064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokés T, Eros G, Bebes A, Hartmann P, Várszegi S, Varga G, Kaszaki J, Gulya K, Ghyczy M, Boros M. Protective effects of a phosphatidylcholine-enriched diet in lipopolysaccharide-induced experimental neuroinflammation in the rat. Shock. 2011;36:458–465. doi: 10.1097/SHK.0b013e31822f36b0. [DOI] [PubMed] [Google Scholar]
- 23.Dial EJ, Zayat M, Lopez-Storey M, Tran D, Lichtenberger L. Oral phosphatidylcholine preserves the gastrointestinal mucosal barrier during LPS-induced inflammation. Shock. 2008;30:729–733. doi: 10.1097/SHK.0b013e318173e8d4. [DOI] [PubMed] [Google Scholar]
- 24.Orr SK, Trépanier MO, Bazinet RP. n-3 Polyunsaturated fatty acids in animal models with neuroinflammation. Prostaglandins Leukot Essent Fatty Acids. 2013;88:97–103. doi: 10.1016/j.plefa.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Das UN. Infection, inflammation, and polyunsaturated fatty acids. Nutrition. 2011;27:1080–1084. doi: 10.1016/j.nut.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Tandy S, Chung RW, Kamili A, Wat E, Weir JM, Meikle PJ, Cohn JS. Hydrogenated phosphatidylcholine supplementation reduces hepatic lipid levels in mice fed a high-fat diet. Atherosclerosis. 2010;213:142–147. doi: 10.1016/j.atherosclerosis.2010.07.050. [DOI] [PubMed] [Google Scholar]
- 27.Polfliet MM, Fabriek BO, Daniëls WP, Dijkstra CD, van den Berg TK. The rat macrophage scavenger receptor CD163: expression, regulation and role in inflammatory mediator production. Immunobiology. 2006;211:419–425. doi: 10.1016/j.imbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Englert JA, Fink MP. The multiple organ dysfunction syndrome and late-phase mortality in sepsis. Curr Infect Dis Rep. 2005;7:335–341. doi: 10.1007/s11908-005-0006-0. [DOI] [PubMed] [Google Scholar]
- 29.Alves C, Robazzi TC, Mendonça M. Withdrawal from glucocorticosteroid therapy: clinical practice recommendations. J Pediatr (Rio J) 2008;84:192–202. doi: 10.2223/JPED.1773. [DOI] [PubMed] [Google Scholar]
- 30.Annane D. Corticosteroids for septic shock. Crit Care Med. 2001;29(7 Suppl):S117–S120. doi: 10.1097/00003246-200107001-00036. [DOI] [PubMed] [Google Scholar]
- 31.Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995;23:1294–1303. doi: 10.1097/00003246-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Cronin L, Cook DJ, Carlet J, Heyland DK, King D, Lansang MA, Fisher CJ., Jr Corticosteroid treatment for sepsis: a critical appraisal and meta-analysis of the literature. Crit Care Med. 1995;23:1430–1439. doi: 10.1097/00003246-199508000-00019. [DOI] [PubMed] [Google Scholar]
- 33.van der Poll T, Barber AE, Coyle SM, Lowry SF. Hypercortisolemia increases plasma interleukin-10 concentrations during human endotoxemia--a clinical research center study. J Clin Endocrinol Metab. 1996;81:3604–3606. doi: 10.1210/jcem.81.10.8855809. [DOI] [PubMed] [Google Scholar]
- 34.Briegel J, Kellermann W, Forst H, Haller M, Bittl M, Hoffmann GE, Büchler M, Uhl W, Peter K The Phospholipase A2 Study Group. Low-dose hydrocortisone infusion attenuates the systemic inflammatory response syndrome. Clin Investig. 1994;72:782–787. doi: 10.1007/BF00180547. [DOI] [PubMed] [Google Scholar]
- 35.Osman MO, Jacobsen NO, Kristensen JU, Larsen CG, Jensen SL. Beneficial effects of hydrocortisone in a model of experimental acute pancreatitis. Dig Surg. 1999;16:214–221. doi: 10.1159/000018730. [DOI] [PubMed] [Google Scholar]
- 36.Hartmann P, Szabó A, Eros G, Gurabi D, Horváth G, Németh I, Ghyczy M, Boros M. Anti-inflammatory effects of phosphatidylcholine in neutrophil leukocyte-dependent acute arthritis in rats. Eur J Pharmacol. 2009;622:58–64. doi: 10.1016/j.ejphar.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Eros G, Varga G, Váradi R, Czóbel M, Kaszaki J, Ghyczy M, Boros M. Anti-inflammatory action of a phosphatidylcholine, phosphatidylethanolamine and N-acylphosphatidylethanolamine-enriched diet in carrageenan-induced pleurisy. Eur Surg Res. 2009;42:40–48. doi: 10.1159/000167856. [DOI] [PubMed] [Google Scholar]
- 38.Aono K, Isobe K, Kiuchi K, Fan ZH, Ito M, Takeuchi A, Miyachi M, Nakashima I, Nimura Y. In vitro and in vivo expression of inducible nitric oxide synthase during experimental endotoxemia: involvement of other cytokines. J Cell Biochem. 1997;65:349–358. [PubMed] [Google Scholar]
- 39.Riches DW, Chan ED, Winston BW. TNF-alpha-induced regulation and signalling in macrophages. Immunobiology. 1996;195:477–490. doi: 10.1016/s0171-2985(96)80017-9. [DOI] [PubMed] [Google Scholar]
- 40.Qiu P, Cui X, Barochia A, Li Y, Natanson C, Eichacker PQ. The evolving experience with therapeutic TNF inhibition in sepsis: considering the potential influence of risk of death. Expert Opin Investig Drugs. 2011;20:1555–1564. doi: 10.1517/13543784.2011.623125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song R, Kim J, Yu D, Park C, Park J. Kinetics of IL-6 and TNF-α changes in a canine model of sepsis induced by endotoxin. Vet Immunol Immunopathol. 2012;146:143–149. doi: 10.1016/j.vetimm.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Oda S, Hirasawa H, Shiga H, Nakanishi K, Matsuda K, Nakamua M. Sequential measurement of IL-6 blood levels in patients with systemic inflammatory response syndrome (SIRS)/sepsis. Cytokine. 2005;29:169–175. doi: 10.1016/j.cyto.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Leon LR, White AA, Kluger MJ. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol. 1998;275:R269–R277. doi: 10.1152/ajpregu.1998.275.1.R269. [DOI] [PubMed] [Google Scholar]
- 44.Neri M, Bello S, Bonsignore A, Centini F, Fiore C, Földes-Papp Z, Turillazzi E, Fineschi V. Myocardial expression of TNFalpha, IL-1beta, IL-6, IL-8, IL-10 and MCP-1 after a single MDMA dose administered in a rat model. Curr Pharm Biotechnol. 2010;11:413–420. doi: 10.2174/138920110791591517. [DOI] [PubMed] [Google Scholar]
- 45.Refsum SE, Halliday MI, Campbell G, McCaigue M, Rowlands BJ, Boston VE. Modulation of TNF alpha and IL-6 in a peritonitis model using pentoxifylline. J Pediatr Surg. 1996;31:928–930. doi: 10.1016/s0022-3468(96)90413-3. [DOI] [PubMed] [Google Scholar]
- 46.Engelberts I, von Asmuth EJ, van der Linden CJ, Buurman WA. The interrelation between TNF, IL-6, and PAF secretion induced by LPS in an in vivo and in vitro murine model. Lymphokine Cytokine Res. 1991;10:127–131. [PubMed] [Google Scholar]
- 47.Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train. 2006;41:457–465. [PMC free article] [PubMed] [Google Scholar]
- 48.Reumaux D, de Boer M, Meijer AB, Duthilleul P, Roos D. Expression of myeloperoxidase (MPO) by neutrophils is necessary for their activation by anti-neutrophil cytoplasm autoantibodies (ANCA) against MPO. J Leukoc Biol. 2003;73:841–849. doi: 10.1189/jlb.1102567. [DOI] [PubMed] [Google Scholar]
- 49.Pils MC, Pisano F, Fasnacht N, Heinrich JM, Groebe L, Schippers A, Rozell B, Jack RS, Müller W. Monocytes/macrophages and/or neutrophils are the target of IL-10 in the LPS endotoxemia model. Eur J Immunol. 2010;40:443–448. doi: 10.1002/eji.200939592. [DOI] [PubMed] [Google Scholar]
- 50.Zhou X, Liu Z, Jang F, Xiang C, Li Y, He Y. Autocrine Sonic hedgehog attenuates inflammation in cerulein-induced acute pancreatitis in mice via upregulation of IL-10. PLoS One. 2012;7:e44121. doi: 10.1371/journal.pone.0044121. [DOI] [PMC free article] [PubMed] [Google Scholar]