Abstract
The Drug-Resistant Pathogen Surveillance Group in Pediatric Infectious Disease conducted national surveillance for Haemophilus influenzae in 2007 (phase 3) and 2010 (phase 4), following the previous surveillance conducted from 2000 to 2001 (phase 1) and in 2004 (phase 2). We examined the antimicrobial susceptibility for H. influenzae derived from clinical specimens of pediatric patients collected nationwide from 27 institutions during phases 3 (386 strains) and 4 (484 strains). The frequency of β-lactamase-nonproducing ampicillin (ABPC)-resistant (BLNAR) strains, which rapidly increased from 11.4 % in phase 1 to 43.4 % in phase 2, has gradually decreased from 38.3 % in phase 3 to 37.8 % in phase 4. In contrast, On the other hand, the frequency of β-lactamase-producing strains, which continuously decreased from 8.3 % in phase 1 to 4.4 % in phase 3, has increased to 8.7 % in phase 4. Prevalence of β-lactamase-producing clavulanic acid/amoxicillin-resistant (BLPACR) strains, especially, has increased from 1.6 % in phase 3 to 4.8 % in phase 4. The oral antimicrobial agents with the lowest MIC90 were levofloxacin in both phases, and tosufloxacin in phase 4 (≤0.063 μg/ml), whereas for intravenous use the corresponding agent was tazobactam/piperacillin in both phases (0.125 μg/ml). There was no increase in the MIC90 of most β-lactams between phase 3 and phase 4. In relationship to sex, age, presence of siblings, attendance at a daycare center, siblings’ attendance at a daycare center, and prior administration of antimicrobial agents within 1 month, the frequency of β-lactamase-nonproducing ABPC-intermediately resistant (BLNAI) strains + BLNAR strains was high (P = 0.005) in cases with prior administration of antimicrobial agents in phase 3.
Keywords: Pediatric infectious disease, Surveillance, Haemophilus influenzae sensitivity, Drug resistance
Introduction
Haemophilus influenzae, along with Streptococcus pneumoniae, is a major pathogen in respiratory tract infection and invasive infection in children. Previously, H. influenzae developed resistance to ampicillin (ABPC) by producing β-lactamase; however, since the beginning of the 2000s, there has been a rapid increase in the prevalence of ampicillin-resistant strains that do not produce β-lactamase, that is, β-lactamase-nonproducing ABPC-resistant (BLNAR) strains [1]. With regard to the BLNAR strains, their sensitivity to cephems and carbapenems, as well as to ABPC, decreases as a result of mutations in the ftsI genes that encode penicillin-binding protein (PBP) 3 [2], causing major problems in the development of treatment strategies for pediatric infection such as meningitis. The Drug-Resistant Pathogen Surveillance Group in Pediatric Infectious Disease reported that from phase 1 (2000–2001) to phase 2 (2004) of Nationwide Surveillance there was a rapid increase in the distribution of BLNAR strains and a decrease in β-lactamase-nonproducing ABPC-sensitive (BLNAS) strains [3]. It is very important to maintain an understanding of the trends in development of drug resistance in H. influenzae to be able to choose the proper antimicrobial agent in situations where there are significant changes in the prevalence of drug-resistant strains. We therefore conducted phase 3 (2007) and phase 4 (2010) surveillance studies, following the first two phases. Here we report the results of these studies.
Materials and methods
Strains, antimicrobial susceptibility testing, and capsular typing for serotype b strains
We collected H. influenzae isolated from clinical specimens taken from pediatric patients at 27 institutions nationwide, all of which participated in the Drug-Resistant Pathogen Surveillance Group in Pediatric Infectious Disease, and used the 386 strains accumulated from January to June in 2007 for phase 3 and the 484 strains accumulated from January to June in 2010 for phase 4. The sources of the isolates were as follows in phase 3: nasopharynx, 299 strains; pharynx, 51 strains; sputum, 29 strains; blood, 3 strains; nasal discharge and pus, 1 strain for each; and unknown origin, 2 strains. Sources of isolates were as follows in phase 4: nasopharynx, 396 strains; sputum, 40 strains; pharynx, 30 strains; blood, 8 strains; cerebrospinal fluid, 4 strains; and unknown origin, 6 strains.
For antimicrobial susceptibility testing, we measured the minimum inhibitory concentration (MIC) by the broth microdilution method, complying with the Clinical and Laboratory Standards Institute (CLSI) standards [4]. Sensitivity to the following 21 drugs was tested during phase 3: ABPC, clavulanic acid/amoxicillin (CVA/AMPC), piperacillin (PIPC), tazobactam/piperacillin (TAZ/PIPC), cefaclor (CCL), cefditoren (CDTR), cefcapene (CFPN), cefpodoxime (CPDX), cefdinir (CFDN), cefotaxime (CTX), cefteram (CFTM), cefotiam (CTM), ceftriaxone (CTRX), faropenem (FRPM), panipenem (PAPM), meropenem (MEPM), azithromycin (AZM), clarithromycin (CAM), rokitamycin (RKM), telithromycin (TEL), and levofloxacin (LVFX). During phase 4, sensitivity was tested against a total of 23 drugs including those tested during phase 3 (other than TEL), with the following additional drugs: tebipenem (TBPM), doripenem (DRPM), and tosufloxacin (TFLX). β-Lactamase production was determined by the nitrocephin method.
The strains were classified according to the CLSI criteria [5]: that is, β-lactamase-nonproducing strains were classified into BLNAS strains, for which the MIC for ABPC was 1 μg/ml or less; β-lactamase-nonproducing ABPC-intermediately resistant (BLNAI) strains, with MIC for ABPC of 2 μg/ml; and BLNAR strains, with MIC for ABPC of 4 μg/ml or more. β-Lactamase-producing strains were classified into β-lactamase-producing ABPC-resistant (BLPAR) strains, with MIC for CVA/AMPC of 4 μg/ml or less; and β-lactamase-producing CVA/AMPC-resistant (BLPACR) strains, with MIC for CVA/AMPC of 8 μg/ml or more.
Additionally, all the strains were tested for H. influenzae serotype b (Hib) by the polymerase chain reaction (PCR) method [6]. Fourteen strains (3.6 %) in phase 3 and 23 strains (4.8 %) in phase 4 were detected as Hib. The sources of Hib strains in phase 3 were as follows: nasopharynx, 9 strains; blood, 3 strains; and pharynx and pus, 1 strain for each. Sources of Hib strains in phase 4: nasopharynx, 9 strains; blood, 8 strains; cerebrospinal fluid, 4 strains; and sputum and pharynx, 1 strain each.
Background factors and statistical analysis
To examine the relationship between background factors and the development of drug resistance, the frequency of isolation was compared between drug-resistant strains and BLNAS strains in relationship to six background factors, including sex, age, presence or absence of siblings, attendance or nonattendance at a daycare center, siblings’ attendance or nonattendance at a daycare center, and prior administration of antimicrobial agents within 1 month. The standard for drug-resistant strains was the same as that used in phase 1 and phase 2 and was defined as BLNAI + BLNAR. The χ2 test was used to identify whether a significant difference exists, using two-sided testing at a 5 % level of significance. Fisher’s exact test was used when an expected value was less than 5.
Results
Figure 1 shows the number of strains by degrees of resistance. In phase 3, there were 133 strains of BLNAS (34.5 %), 88 strains of BLNAI (22.8 %), 148 strains of BLNAR (38.3 %), 11 strains of BLPAR (2.8 %), and 6 strains of BLPACR (1.6 %). In phase 4, there were 161 strains of BLNAS (33.3 %), 98 strains of BLNAI (20.2 %), 183 strains of BLNAR (37.8 %), 19 strains of BLPAR (3.9 %), and 23 strains of BLPACR (4.8 %).
Fig. 1.
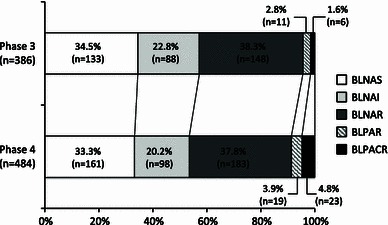
Distribution of Haemophilus influenzae strains classified by ampicillin (ABPC) or clavulanic acid/amoxicillin (CVA/AMPC) resistance in phases 3 and 4. BLNAS, β-lactamase-nonproducing ABPC-sensitive strain; BLNAI, β-lactamase-nonproducing ABPC-intermediately resistant strain; BLNAR, β-lactamase-nonproducing ABPC-resistant strain; BLPAR, β-lactamase-producing ABPC-resistant strain; BLPACR, β-lactamase-producing CVA/AMPC-resistant strain
Figure 2 shows the number of Hib strains by degrees of resistance. In phase 3, there were 9 strains of BLNAS (64.3 %), 3 strains of BLNAI (21.4 %), 1 strain of BLNAR (7.1 %), 1 strain of BLPAR (7.1 %), and no BLPACR strain. In phase 4, there were 13 strains of BLNAS (56.5 %), 4 strains of BLNAI (17.4 %), 6 strains of BLPAR (26.1 %), and no BLNAR or BLPACR strains.
Fig. 2.
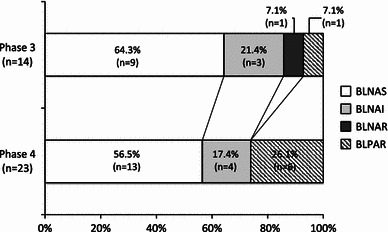
Distribution of Haemophilus influenzae serotype b strains classified by ampicillin (ABPC) or clavulanic acid/amoxicillin (CVA/AMPC) resistance in phases 3 and 4. BLNAS, β-lactamase-nonproducing ABPC-sensitive strain; BLNAI, β-lactamase-nonproducing ABPC-intermediately resistant strain; BLNAR, β-lactamase-nonproducing ABPC-resistant strain; BLPAR, β-lactamase-producing ABPC-resistant strain; BLPACR, β-lactamase-producing CVA/AMPC-resistant strain
Table 1 shows the MIC50, MIC90, and MIC range of antimicrobial agents for H. influenzae. In phase 3 with a total of 386 strains, the oral antimicrobial agent with the lowest MIC90 was LVFX (≤0.063 μg/ml), followed by CDTR (0.25 μg/ml). The intravenous antimicrobial agent with the lowest MIC90 was TAZ/PIPC (0.125 μg/ml), followed by PIPC, CTRX, and MEPM (0.25 μg/ml). In phase 4 with a total of 484 strains, the oral antimicrobial agent with the lowest MIC90 was LVFX and TFLX (≤0.063 μg/ml), followed by CDTR (0.25 μg/ml). The intravenous antimicrobial agent with the lowest MIC90 was TAZ/PIPC (0.125 μg/ml), followed by PIPC and CTRX (0.25 μg/ml). Between phase 3 and phase 4, there was a twofold increase in the MIC90 values of PAPM (2–4 μg/ml) and MEPM (0.25–0.5 μg/ml), but there were no changes in those of other drugs.
Table 1.
Susceptibilities for Haemophilus influenzae in phase 3 and phase 4
| Phase 3 | Phase 4 | |||||
|---|---|---|---|---|---|---|
| Number of strains | 386 | 484 | ||||
| MIC | MIC50 | MIC90 | MIC range | MIC50 | MIC90 | MIC range |
| ABPC | 2 | 8 | 0.12–>128 | 2 | 8 | ≤0.063–>128 |
| CVA/AMPC | 4 | 8 | 0.25–32 | 4 | 8 | 0.125–16 |
| PIPC | ≤0.063 | 0.25 | ≤0.063–>128 | ≤0.063 | 0.25 | ≤0.063–>128 |
| TAZ/PIPC | ≤0.063 | 0.125 | ≤0.063–1 | ≤0.063 | 0.125 | ≤0.063–0.25 |
| CCL | 16 | 64 | 0.25–>128 | 16 | 64 | 0.25–128 |
| CDTR | 0.125 | 0.25 | ≤0.063–0.5 | 0.125 | 0.25 | ≤0.063–1 |
| CFPN | 1 | 2 | ≤0.063–4 | 1 | 2 | ≤0.063–8 |
| CPDX | 2 | 4 | ≤0.063–8 | 2 | 4 | ≤0.063–8 |
| CFDN | 2 | 8 | ≤0.063–16 | 2 | 8 | ≤0.063–16 |
| CFTM | 0.5 | 1 | ≤0.063–2 | 0.5 | 1 | ≤0.063–2 |
| CTM | 8 | 64 | ≤0.063–128 | 8 | 64 | 0.125–128 |
| CTRX | 0.125 | 0.25 | ≤0.063–0.5 | 0.125 | 0.25 | ≤0.063–0.5 |
| CTX | 0.5 | 1 | ≤0.063–4 | 0.5 | 1 | ≤0.063–4 |
| AZM | 1 | 2 | 0.125–64 | 1 | 2 | ≤0.063–4 |
| CAM | 4 | 8 | 1–>64 | 4 | 8 | 0.5–32 |
| RKM | 8 | 16 | 1–32 | 8 | 16 | ≤0.063–32 |
| TEL | 1 | 2 | 0.25–8 | – | – | – |
| FRPM | 2 | 4 | ≤0.063–4 | 2 | 4 | ≤0.063–8 |
| TBPM | – | – | – | 0.25 | 1 | ≤0.063–2 |
| PAPM | 1 | 2 | ≤0.063–4 | 1 | 4 | ≤0.063–8 |
| MEPM | 0.125 | 0.25 | ≤0.063–1 | 0.125 | 0.5 | ≤0.063–1 |
| DRPM | – | – | – | 0.5 | 2 | ≤0.063–4 |
| LVFX | ≤0.063 | ≤0.063 | ≤0.063–0.5 | ≤0.063 | ≤0.063 | ≤0.063–0.5 |
| TFLX | – | – | – | ≤0.063 | ≤0.063 | ≤0.063–2 |
ABPC ampicillin, CVA/AMPC clavulanic acid/amoxicillin, PIPC piperacillin, TAZ/PIPC tazobactam/piperacillin, CCL cefaclor, CDTR cefditoren, CFPN cefcapene, CPDX cefpodoxime, CFDN cefdinir, CFTM cefteram, CTM cefotiam, CTRX ceftriaxone, CTX cefotaxime, AZM azithromycin, CAM clarithromycin, RKM rokitamycin, TEL telithromycin, FRPM faropenem, TBPM tebipenem, PAPM panipenem, MEPM meropenem, DRPM doripenem, LVFX levofloxacin, TFLX tosufloxacin
Table 2 shows the MIC50, MIC90, and MIC range of antimicrobial agents for H. influenzae divided into the following five groups by degrees of resistance, the BLNAS, BLNAI, BLNAR, BLPAR, and BLPACR groups, respectively. In the BLNAR group, the MIC50 values of the β-lactams excluding PIPC and TAZ/PIPC were 4- to 64 fold higher, and the MIC90 values of all the agents were 2- to 4 fold higher than the values in the BLNAS group in phase 3. In phase 4, in the BLNAR group, the MIC50 values of the β-lactams excluding PIPC and TAZ/PIPC were 4- to 32 fold higher, and the MIC90 values of all the agents were 2- to 8 fold higher than the values in the BLNAS group. In both phase 3 and phase 4, the MIC50 values of PIPC and TAZ/PIPC were the same in the BLNAS and BLNAR groups (≤0.063 μg/ml).
Table 2.
Suscepsibilities for Haemophilus influenzae (divided into five groups) in phase 3 and phase 4
| Class | BLNAS | BLNAI | BLNAR | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase | Phase 3 | Phase 4 | Phase 3 | Phase 4 | Phase 3 | Phase 4 | ||||||||||||
| No. of strains (%) | 133 (34.5) | 161(33.3) | 88 (22.8) | 98 (20.2) | 148 (38.3) | 183 (37.8) | ||||||||||||
| MIC | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | MIC Range |
| ABPC | 0.25 | 1 | 0.125–1 | 0.5 | 1 | ≤0.063–1 | 2 | 2 | 2–2 | 2 | 2 | 2–2 | 4 | 8 | 4–16 | 4 | 8 | 4–8 |
| CVA/AMPC | 0.5 | 2 | 0.25–4 | 0.5 | 2 | 0.125–4 | 4 | 8 | 1–8 | 4 | 4 | 1–8 | 8 | 8 | 2–32 | 4 | 8 | 2–16 |
| PIPC | ≤0.063 | 0.125 | ≤0.063–0.5 | ≤0.063 | 0.125 | ≤0.063–0.5 | ≤0.063 | 0.25 | ≤0.063–0.5 | ≤0.063 | 0.125 | ≤0.063–0.25 | ≤0.063 | 0.25 | ≤0.063–1 | ≤0.063 | 0.25 | ≤0.063–0.5 |
| TAZ/PIPC | ≤0.063 | ≤0.063 | ≤0.063–0.5 | ≤0.063 | ≤0.063 | ≤0.063–0.25 | ≤0.063 | 0.125 | ≤0.063–0.25 | ≤0.063 | 0.125 | ≤0.063–0.25 | ≤0.063 | 0.25 | ≤0.063–1 | ≤0.063 | 0.125 | ≤0.063–0.25 |
| CCL | 4 | 16 | 0.25–64 | 4 | 16 | 0.25–64 | 32 | 64 | 4–>128 | 16 | 64 | 2–128 | 64 | 128 | 4–to 128 | 32 | 64 | 2–128 |
| CDTR | ≤0.063 | ≤0.063 | ≤0.063–0.5 | ≤0.063 | 0.125 | ≤0.063–0.5 | 0.125 | 0.25 | ≤0.063–0.5 | 0.25 | 0.25 | ≤0.063–0.5 | 0.25 | 0.25 | ≤0.063–0.5 | 0.25 | 0.5 | ≤0.063–1 |
| CFPN | ≤0.063 | 0.5 | ≤0.063–2 | ≤0.063 | 0.5 | ≤0.063–1 | 1 | 2 | ≤0.063–4 | 1 | 2 | ≤0.063–4 | 2 | 2 | 0.25–4 | 2 | 4 | ≤0.063–8 |
| CPDX | ≤0.063 | 1 | ≤0.063–8 | ≤0.063 | 1 | ≤0.063–4 | 2 | 8 | 0.25–8 | 2 | 4 | 0.125–8 | 4 | 4 | 1–8 | 2 | 4 | 0.25–8 |
| CFDN | 0.25 | 2 | ≤0.063–8 | 0.5 | 2 | ≤0.063–4 | 4 | 8 | 0.5–16 | 2 | 4 | 0.5–8 | 4 | 8 | 0.5–16 | 4 | 8 | 0.5–16 |
| CFTM | ≤0.063 | 0.5 | ≤0.063–1 | ≤0.063 | 0.5 | ≤0.063–1 | 0.5 | 1 | ≤0.063–1 | 0.5 | 1 | ≤0.063–2 | 0.5 | 1 | 0.125–2 | 1 | 1 | ≤0.063–1 |
| CTM | 2 | 8 | ≤0.063–64 | 2 | 8 | 0.125–32 | 16 | 64 | 2–64 | 8 | 32 | 1–64 | 32 | 64 | 2–128 | 32 | 64 | 1–128 |
| CTRX | ≤0.063 | 0.125 | ≤0.063–0.25 | ≤0.063 | 0.125 | ≤0.063–0.25 | 0.125 | 0.25 | ≤0.063–0.5 | 0.125 | 0.25 | ≤0.063–0.5 | 0.25 | 0.25 | ≤0.063–0.5 | 0.25 | 0.25 | ≤0.063–0.5 |
| CTX | ≤0.063 | 0.5 | ≤0.063–1 | ≤0.063 | 0.5 | ≤0.063–0.5 | 0.5 | 1 | ≤0.063–2 | 0.5 | 1 | ≤0.063–2 | 0.5 | 2 | 0.125–4 | 1 | 2 | ≤0.063–4 |
| AZM | 1 | 2 | 0.125–4 | 1 | 1 | ≤0.063–4 | 1 | 2 | 0.25–4 | 1 | 2 | 0.125–4 | 1 | 2 | 0.25–4 | 1 | 2 | 0.125–2 |
| CAM | 4 | 8 | 1–16 | 4 | 8 | 0.5–32 | 4 | 8 | 1–16 | 4 | 16 | 1–16 | 8 | 8 | 1–16 | 8 | 8 | 2–16 |
| RKM | 8 | 16 | 1–32 | 4 | 16 | ≤0.063–32 | 8 | 16 | 2–16 | 8 | 16 | 1–32 | 8 | 16 | 2–16 | 8 | 16 | 0.25–16 |
| TEL | 1 | 2 | 0.25–8 | – | – | – | 1 | 2 | 0.5–8 | – | – | – | 2 | 2 | 0.5–4 | – | – | – |
| FRPM | 0.5 | 1 | ≤0.063–2 | 0.5 | 2 | ≤0.063–4 | 2 | 2 | 0.25–4 | 1 | 2 | 0.25–4 | 2 | 4 | 0.25–4 | 2 | 4 | 0.5–8 |
| TBPM | – | – | – | 0.125 | 0.25 | ≤0.063–1 | – | – | – | 0.25 | 1 | ≤0.063–1 | – | – | – | 0.5 | 1 | ≤0.063–2 |
| PAPM | 0.5 | 1 | ≤0.063–2 | 0.5 | 2 | ≤0.063–4 | 1 | 2 | 0.125–4 | 1 | 4 | ≤0.063–8 | 2 | 2 | 0.125–4 | 2 | 4 | 0.25–8 |
| MEPM | ≤0.063 | 0.125 | ≤0.063–0.125 | ≤0.063 | 0.125 | ≤0.063–0.5 | 0.125 | 0.25 | ≤0.063–0.5 | 0.25 | 0.5 | ≤0.063–0.5 | 0.25 | 0.5 | ≤0.063–1 | 0.25 | 0.5 | ≤0.063–1 |
| DRPM | – | – | – | 0.125 | 0.5 | ≤0.063–1 | – | – | – | 0.5 | 1 | ≤0.063–2 | – | – | – | 1 | 2 | ≤0.063–4 |
| LVFX | ≤0.063 | ≤0.063 | ≤0.063–0.125 | ≤0.063 | ≤0.063 | ≤0.063–0.5 | ≤0.063 | ≤0.063 | ≤0.063–0.125 | ≤0.063 | ≤0.063 | ≤0.063–0.5 | ≤0.063 | ≤0.063 | ≤0.063–0.5 | ≤0.063 | ≤0.063 | ≤0.063–0.125 |
| TFLX | – | – | – | ≤0.063 | ≤0.063 | ≤0.063–2 | – | – | – | ≤0.063 | ≤0.063 | ≤0.063–≤0.063 | – | – | – | ≤0.063 | ≤0.063 | ≤0.063–0.125 |
| Class | BLPAR | BLPACR | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase | Phase 3 | Phase 4 | Phase 3 | Phase 4 | ||||||||
| No. of strains (%) | 11 (2.8) | 19 (3.9) | 6 (1.6) | 23 (4.8) | ||||||||
| MIC | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | MIC Range | MIC50 | MIC90 | MIC Range |
| ABPC | 128 | >128 | 2 to >128 | 32 | >128 | 16 to >128 | >128 | >128 | >128 to >128 | >128 | >128 | 64 to >128 |
| CVA/AMPC | 1 | 4 | 0.25–4 | 2 | 4 | 0.5–4 | 8 | 16 | 8–16 | 8 | 16 | 8–16 |
| PIPC | 128 | >128 | 0.25 to >128 | 16 | >128 | 2 to >128 | >128 | >128 | >128 to >128 | 64 | >128 | 8 to >128 |
| TAZ/PIPC | ≤0.063 | ≤0.063 | ≤0.063–≤0.063 | ≤0.063 | 0.125 | ≤0.063–0.125 | ≤0.063 | 0.125 | ≤0.063–0.125 | ≤0.063 | ≤0.063 | ≤0.063–0.125 |
| CCL | 8 | 32 | 2–64 | 8 | 32 | 2–32 | 64 | 128 | 4–128 | 32 | 64 | 16–128 |
| CDTR | ≤0.063 | 0.125 | ≤0.063–0.25 | ≤0.063 | 0.25 | ≤0.063–0.5 | 0.125 | 0.25 | 0.125–0.25 | 0.25 | 0.25 | ≤0.063–0.25 |
| CFPN | ≤0.063 | 1 | ≤0.063–2 | 0.5 | 1 | ≤0.063–1 | 2 | 2 | 0.5–2 | 2 | 2 | 0.5–2 |
| CPDX | ≤0.063 | 2 | ≤0.063–4 | 1 | 2 | ≤0.063–2 | 4 | 4 | 2–4 | 2 | 4 | 2–4 |
| CFDN | 0.25 | 2 | 0.125–4 | 1 | 4 | 0.25–4 | 4 | 8 | 2–8 | 4 | 8 | 2–8 |
| CFTM | ≤0.063 | 0.5 | ≤0.063–0.5 | 0.5 | 0.5 | ≤0.063–0.5 | 0.5 | 1 | 0.5–1 | 0.5 | 1 | 0.25–1 |
| CTM | 2 | 32 | 0.5–64 | 4 | 16 | 0.5–16 | 64 | 128 | 8–128 | 32 | 64 | 8–128 |
| CTRX | ≤0.063 | 0.25 | ≤0.063–0.25 | ≤0.063 | 0.25 | ≤0.063–0.25 | 0.25 | 0.25 | 0.125–0.25 | 0.25 | 0.25 | ≤0.063–0.5 |
| CTX | ≤0.063 | 0.5 | ≤0.063–1 | 0.25 | 0.5 | ≤0.063–1 | 1 | 1 | 0.5–1 | 1 | 1 | 0.25–1 |
| AZM | 1 | 2 | 0.5–64 | 0.5 | 1 | 0.25–1 | 1 | 64 | 0.5–64 | 1 | 2 | 0.5–4 |
| CAM | 8 | 8 | 2 to >64 | 4 | 8 | 2–8 | 4 | >64 | 4 to >64 | 8 | 16 | 4–16 |
| RKM | 8 | 16 | 4–16 | 4 | 8 | 1–8 | 2 | 8 | 2–8 | 8 | 8 | 2–16 |
| TEL | 2 | 4 | 1–4 | – | – | – | 1 | 8 | 1–8 | – | – | – |
| FRPM | 0.5 | 2 | 0.125–2 | 0.5 | 2 | ≤0.063–4 | 2 | 2 | 2–2 | 2 | 4 | 1–4 |
| TBPM | – | – | – | ≤0.063 | 0.5 | ≤0.063–0.5 | – | – | – | 0.5 | 1 | ≤0.063–2 |
| PAPM | 0.5 | 2 | ≤0.063–2 | 0.25 | 4 | ≤0.063–4 | 1 | 4 | 0.5–4 | 2 | 8 | 0.25–8 |
| MEPM | ≤0.063 | 0.25 | ≤0.063–0.5 | ≤0.063 | 0.25 | ≤0.063–0.25 | 0.125 | 0.25 | ≤0.063–0.25 | 0.25 | 0.5 | ≤0.063–1 |
| DRPM | – | – | – | 0.125 | 1 | ≤0.063–1 | – | – | – | 1 | 2 | ≤0.063–2 |
| LVFX | ≤0.063 | ≤0.063 | ≤0.063–≤0.063 | ≤0.063 | ≤0.063 | ≤0.063–≤0.063 | ≤0.063 | 0.25 | ≤0.063–0.25 | ≤0.063 | ≤0.063 | ≤0.063–≤0.063 |
| TFLX | – | – | – | ≤0.063 | ≤0.063 | ≤0.063–≤0.063 | – | – | – | ≤0.063 | ≤0.063 | ≤0.063–≤0.063 |
ABPC ampicillin, CVA/AMPC clavulanic acid/amoxicillin, PIPC piperacillin, TAZ/PIPC tazobactam/piperacillin, CCL cefaclor, CDTR cefditoren, CFPN cefcapene, CPDX cefpodoxime, CFDN cefdinir, CFTM cefteram, CTM cefotiam, CTRX ceftriaxone, CTX cefotaxime, AZM azithromycin, CAM clarithromycin, RKM rokitamycin, TEL telithromycin, FRPM faropenem, TBPM tebipenem, PAPM panipenem, MEPM meropenem, DRPM doripenem, LVFX levofloxacin, TFLX tosufloxacin
The MIC90 values of antimicrobial agents for H. influenzae, categorized by degrees of resistance, are as follows: in the BLNAS group, the oral antimicrobial agents with the lowest MIC90 were CDTR and LVFX (≤0.063 μg/ml) in phase 3, and LVFX and TFLX (≤0.063 μg/ml) in phase 4, whereas the intravenous agent with the lowest MIC90 was TAZ/PIPC (≤0.063 μg/ml) in both phase 3 and phase 4. In the BLNAI, BLNAR, and BLPAR groups, the oral antimicrobial agent with the lowest MIC90 was LVFX (≤0.063 μg/ml) in phase 3, and LVFX and TFLX (≤0.063 μg/ml) in phase 4. In the BLNAI group, the intravenous antimicrobial agent with the lowest MIC90 was TAZ/PIPC (0.125 μg/ml) in phase 3, and PIPC and TAZ/PIPC (0.125 μg/ml) in phase 4. In the BLNAR group, the intravenous antimicrobial agents with the lowest MIC90 were PIPC, TAZ/PIPC, and CTRX (0.25 μg/ml) in phase 3, and TAZ/PIPC (0.125 μg/ml) in phase 4. In the BLPAR group, the intravenous antimicrobial agent with the lowest MIC90 was TAZ/PIPC in both phase 3 and phase 4 (phase 3, ≤0.063 μg/ml; phase 4, 0.125 μg/ml). In the BLPACR group, the oral antimicrobial agent with the lowest MIC90 was CDTR and LVFX (0.25 μg/ml) in phase 3, and LVFX and TFLX (≤0.063 μg/ml) in phase 4, whereas the intravenous antimicrobial agent with the lowest MIC90 was TAZ/PIPC in both phase 3 and phase 4 (phase 3, 0.125 μg/ml; phase 4, ≤0.063 μg/ml).
The frequency of isolation was compared between drug-resistant strains and BLNAS strains in relationship to six background factors: sex, age, presence or absence of siblings, attendance or nonattendance at a daycare center, siblings’ attendance or nonattendance at a daycare center, and prior administration of antimicrobial agents within 1 month (Table 3). In phase 3, the isolation rate of drug-resistant strains was higher (P = 0.005) in cases with prior administration of antimicrobial agents.
Table 3.
Number of cases of β-lactamase-nonproducing ABPC-sensitive strain (BLNAS) or β-lactamase-nonproducing ABPC-intermediately resistant strain (BLNAI) + β-lactamase-nonproducing ABPC-resistant strain (BLNAR) according to background factor
| Background factor | Phase 3 | Phase 4 | ||||
|---|---|---|---|---|---|---|
| Number of cases | Statistics | Number of cases | Statistics | |||
| BLNAS | BLNAI + BLNAR | BLNAS | BLNAI + BLNAR | |||
| Sex | ||||||
| Boy | 78 | 123 | χ2 | 91 | 152 | χ2 |
| Girl | 46 | 111 | P = 0.0607 | 70 | 128 | P = 0.6495 |
| Age category | ||||||
| Infant | 26 | 40 | χ2 | 28 | 74 | χ2 |
| Toddler | 91 | 177 | P = 0.4703 | 122 | 196 | P = 0.0546 |
| Schoolchild | 13 | 17 | 11 | 11 | ||
| Sibling/siblings | ||||||
| Yes | 80 | 144 | χ2 | 94 | 179 | χ2 |
| No | 51 | 91 | P = 0.9688 | 67 | 102 | P = 0.2684 |
| Group daycare | ||||||
| Yes | 78 | 141 | χ2 | 88 | 147 | χ2 |
| No | 48 | 83 | P = 0.8467 | 70 | 108 | P = 0.6972 |
| Group daycare (siblings) | ||||||
| Yes | 62 | 114 | χ2 | 70 | 122 | χ2 |
| No | 8 | 16 | P = 0.8552 | 21 | 37 | P = 0.9722 |
| Previous use of antimicrobial agents | ||||||
| Yes | 58 | 139 | χ2 | 87 | 176 | χ2 |
| No | 75 | 97 | P = 0.0047 | 74 | 105 | P = 0.0765 |
| Penicillins | 8 | 29 | 20 | 46 | ||
| Cephems | 29 | 85 | 36 | 93 | ||
| Macrolides | 21 | 55 | 40 | 74 | ||
| β-Lactam | 36 | 103 | χ2 | 53 | 123 | χ2 |
| Macrolides | 21 | 55 | P = 0.7832 | 40 | 74 | P = 0.3754 |
ABPC ampicillin
Discussion
The Drug-Resistant Pathogen Surveillance Group in Pediatric Infectious Disease has continued to conduct national surveillance for the antimicrobial susceptibility of H. influenzae since 2000. The previous study reported that the frequency of BLNAR strains dramatically increased from 28.8 % in phase 1 (2000–2001) to 59.3 % in phase 2 (2004) [3]. An ABPC MIC of 2 μg/ml or more was used as a criterion for the identification of BLNAR strains in phase 1 and phase 2. Had an ABPC MIC of 4 μg/ml or more been used as in the current study, the frequency of BLNAR strains would have decreased to 11.4 % and 43.4 % in phases 1 and 2, respectively. Nonetheless, the distribution of BLNAR strains still increased dramatically between phase 1 and phase 2.
In this study, the frequency of BLNAR strains was 38.3 % in phase 3 and 37.8 % in phase 4, indicating a downward tendency from its peak in phase 2. According to a report on the national surveillance conducted in Spain, the frequency of BLNAR strains gradually decreased from 13.5 % in 1996–1997 to 0.7 % in 2006–2007 [7]. Possible reasons for the decline in the number of BLNAR strains are changes in the number of prescriptions and clonal spread of sensitive strains. A positive correlation between the dosage of antimicrobial agents and the development of drug resistance has been observed with the use of population genetic methods [8]. Owing to the widespread use of guidelines in the pediatric field [9, 10], the proper use of antimicrobial agents may have been promoted in Japan.
In contrast to the decline in the number of BLNAR strains, the number of β-lactamase-producing strains, which had continued to decrease, from 8.3 % in phase 1 to 6.4 % in phase 2, and to 4.4 % in phase 3, increased to 8.7 % in phase 4. This difference was greatly influenced by the BLPACR strains, whose number increased from 1.6 % in phase 3 to 4.8 % in phase 4. The BLPACR strains, which have two mechanisms of antimicrobial resistance that comprise β-lactamase production and mutations in ftsI genes, show resistance to many β-lactams. High detection rates of BLPACR strains have been reported in France (13.9 %) from 1999 to 2000 [11] and in Spain (22.4 %) from 2005 to 2007 [12], and in some cases clonal dissemination of the BLPACR strains has been observed [12, 13]. A similar phenomenon may have happened in Japanese children; analysis of clonality using multilocus sequence typing or pulsed-field gel electrophoresis method will thus be required to explain the increase of BLPACR strains.
The BLNAR strains, which have mutations in the ftsI genes that make PBP3 with poor affinity for antimicrobial agents, showed reduced susceptibility to various types of β-lactams including cephems [2]. When the number of BLNAR strains rapidly increased between phase 1 and phase 2, the MIC90 values of most β-lactams showed a corresponding 2- to 4 fold increase [3]. On the other hand, the BLNAR strains showed a trend toward improved susceptibility after phase 2, when the number of BLNAR strains decreased and the MIC90 value of each β-lactam antimicrobial agent decreased from one half to one fourth between phase 2 and phase 3. There were no increases in MIC90 values of β-lactams between phase 3 and phase 4 with the exception of CDTR, CFPN, and PAPM, which showed a 2-fold increase. However, the MIC90 values of β-lactams in BLNAR strains were 2- to 4 fold higher in phase 3 and 2- to 8 fold higher in phase 4, when compared with BLNAS strains. As BLNAR strains show lower susceptibility to most oral β-lactams, it is considered that limited treatment options will continue to be a particular problem.
In this study, the antimicrobial agents with the lowest MIC90 were LVFX in phase 3 and LVFX and TFLX in phase 4. Fluoroquinolones, which are not affected by mutations in ftsI genes, showed lower MIC50 and MIC90 values of ≤0.063 μg/ml for the BLNAR strains. Although the MIC of LVFX was continuously measured in phases 3 and 4, no reduced susceptibility was observed. However, the emergence of fluoroquinolone-resistant H. influenzae strains has been detected in adult patients who use fluoroquinolones frequently [14]. Treatment with TFLX in pediatric patients with otitis media or pneumonia has been covered by insurance since 2009; the use of fluoroquinolones in pediatric patients can thus be expected to increase steadily. The proper use of fluoroquinolones is extremely important if we are to prevent fluoroquinolone-resistant strains from spreading in pediatric patients.
In Japan, Hib vaccine was introduced as a voluntary vaccination in December 2008, between phases 3 and 4. Two years after its introduction, at the end of 2010, publicly subsidized vaccines became available, and since then the incidence of invasive Hib infection has decreased because of an improvement in the vaccination rate [15]. In our study, 14 (3.6 %) and 23 Hib strains (4.8 %) were isolated in phase 3 and phase 4, respectively, indicating no decrease in the number of Hib cases after the introduction of the vaccine. However, the strains in phase 4 were acquired between January and June 2010, before the initiation of the publicly subsidized vaccine program; it is likely that the Hib vaccine was not then widely available. We will have to wait for the next phase of surveillance to confirm the effect of vaccination on Hib detection rates.
During phase 1 and phase 2 surveillance, the relationship between three background factors (age, prior administration of antimicrobial agents, and attendance at a daycare center) and the isolation rate of drug-resistant strains was examined, and there were significant differences in age and prior administration of antimicrobial agents in phase 2 [3]. In this study, in relationship to a total of six background factors, including the three factors from the previous study and three additional factors (sex, siblings, and siblings’ attendance at a daycare center), there were significant differences as regards prior administration of antimicrobial agents in phase 3.
In phase 3, the isolation rate of drug-resistant strains was high in those cases with prior administration of antimicrobial agents. In phase 2, there were differences related to the types of antimicrobial agents; the frequency of drug-resistant strains in the cases treated with β-lactams was higher than that in the cases treated with macrolides, while no differences were observed in phase 3. Although the mutations in PBP3 in the BLNAR strains are considered to be easily induced by the abuse of the oral cephems [2], no significant difference was found among penicillins, cephems, and macrolides. It is likely that each type of antimicrobial agent was prescribed in a balanced manner.
In relationship to other background factors, no significant difference was found in the frequency of drug-resistant strains. In phase 2, the isolation rate of drug-resistant strains was significantly higher in children under 3 years of age, while no such difference was found in phases 3 and 4 (data not shown). As there was also an apparent decrease in the number of the BLNAR strains in infants, similar future transitions deserve attention.
It has been 10 years since the establishment of this group. We would like to continue conducting surveillance so that we can provide useful information on the drug resistance of H. influenzae for use in clinical practice.
Acknowledgments
The authors express their sincere appreciation to all the physicians who cooperated in the survey (listed in alphabetical order without honorifics): Haruhi Ando (Aichi Prefectural Colony Central Hospital), Hironobu Akita (St. Marianna University School of Medicine, Yokohama City Seibu Hospital), Hiroshi Sakata (Asahikawa Kosei Hospital), Hiroyuki Shiro (Yokohama Rosai Hospital), Kazunobu Ouchi (Kawasaki Medical School Hospital), Keita Matsubara (National Hospital Organization Tokyo Medical Center), Kenji Okada (National Hospital Organization Fukuoka Hospital), Masahiro Bamba (Yokosuka Kyosai Hospital), Masato Nonoyama (Ebina General Hospital), Miki Tanaka (Fukuoka University Hospital), Naohisa Kawamura (Osaka Rosai Hospital), Naoichi Iwai (Meitetsu Hospital), Reiko Takayanagi (Tohoku Rosai Hospital), Satoru Kojika (Fujiyoshida Municipal Medical Center), Satoshi Iwata (National Hospital Organization Tokyo Medical Center), Takashige Okada (Kagawa National Children’s Hospital), Takuma Kato (Ashikaga Red Cross Hospital), Tomoaki Sano (Yamanashi Red Cross Hospital), Tomohiro Oishi (Niigata Prefectural Shibata Hospital), Tomonobu Aoki (Fukuoka Children’s Hospital & Medical Center for Infectious Diseases), Tsunehiro Shimizu (Kyoto City Hospital), Tsunekazu Haruta (Kobe City Medical Center General Hospital), Yoichi Taneda (Inazawa City Hospital), Yoshio Yamaguchi (Tochigi National Hospital), Yoshiro Morikawa (Yodogawa Christian Hospital), Takeshi Tajima (Hakujikai Memorial Hospital) and Yoshiyuki Otomo (Juntendo University Nerima Hospital).
Conflict of interest
Dr. Sunakawa has received lecture fees from Taisho Toyama, Meiji Seika Pharma. Dr. Toyonaga has received remuneration from Taisho Toyama, Astellas. Dr. Sato has received lecture fees from Taisho Toyama, Abbott Japan, and remuneration from Abbott Japan, Dainippon Sumitomo, Glaxo Smith Kline, Meiji Seika Pharma, MSD. All other authors report no conflicts of interests.
References
- 1.Hasegawa K, Chiba N, Kobayashi R, Murayama SY, Iwata S, Sunakawa K, et al. Rapidly increasing prevalence of β-lactamase-nonproducing, ampicillin-resistant Haemophilus influenzae type b in patients with meningitis. Antimicrob Agents Chemother. 2004;48:1509–1514. doi: 10.1128/AAC.48.5.1509-1514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasegawa K, Kobayashi R, Takada E, Ono A, Chiba N, Morozumi M, et al. High prevalence of type b β-lactamase-nonproducing ampicillin-resistant Haemophilus influenzae in meningitis: the situation in Japan where Hib vaccine has not been introduced. J Antimicrob Chemother 2006;57:1077–1082. [DOI] [PubMed]
- 3.Sakata H, Toyonaga Y, Sato Y, Hanaki H, Nonoyama M, Oishi T, et al. Nationwide survey of the development of drug-resistance in the pediatric field: drug sensitivity of Haemophilus influenzae in Japan. J Infect Chemother. 2009;15:402–409. doi: 10.1007/s10156-009-0729-1. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute . Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. Seventh edition M7–A7. Wayne: Clinical and Laboratory Standards Institute; 2006. [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 17th informational supplement. M100-S17. Wayne, PA: Clinical and Laboratory Standards Institute; 2007.
- 6.Falla TJ, Crook DW, Brophy LN, Maskell D, Kroll JS, Moxon ER. PCR for capsular typing of Haemophilus influenzae. J Clin Microbiol. 1994;32:2382–2386. doi: 10.1128/jcm.32.10.2382-2386.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Trallero E, Martín-Herrero JE, Mazón A, García-Delafuente C, Robles P, Iriarte V, et al. Antimicrobial resistance among respiratory pathogens in Spain; latest data and change over 11 years (1996–1997 to 2006–2007) Antimicrob Agents Chemother. 2010;54:2953–2959. doi: 10.1128/AAC.01548-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austin DJ, Kristinsson KG, Anderson RM. The relationship between volume of antimicrobial consumption in human communities and the frequency of resistance. Proc Natl Acad Sci USA. 1999;96:1152–1156. doi: 10.1073/pnas.96.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Committee for Guidelines for the Management of Respiratory Infectious Diseases in Children in Japan (2011) Guidelines for the management of respiratory infectious diseases in children in Japan (in Japanese). Japanese Society of Pediatric Pulmonology, Japanese Society for Pediatric Infectious Diseases, Tokyo
- 10.The Subcommittee on Clinical Practice Guidelines for the Diagnosis and Management of Acute Otitis Media in Children (2009) Clinical practice guideline for diagnosis and management of acute otitis media (AOM) in children in Japan (in Japanese). Japan Otological Society, Japan Society for Pediatric Otorhinolaryngology, Japan Society for Infectious Diseases in Otolaryngology, Tokyo
- 11.Dabernat H, Delmas C, Seguy M, et al. Diversity of β-lactam resistance-conferring amino acid substitutions in penicillin-binding protein 3 of Haemophilus influenzae. Antimicrob Agents Chemother. 2002;46:2208–2218. doi: 10.1128/AAC.46.7.2208-2218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sevillano D, Giménez MJ, Cercenado E, Cafini F, Gené A, Alou L, et al. Genotypic versus phenotypic characterization, with respect to β-lactam susceptibility, of Haemophilus influenzae isolates exhibiting decreased susceptibility to β-lactam resistance markers. Antimicrob Agents Chemother. 2009;53:267–270. doi: 10.1128/AAC.00402-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbosa AR, Giufré M, Cerquetti M, Bajanca-Lavado MP. Polymorphism in ftsI gene and β-lactam susceptibility in Portuguese Haemophilus influenzae strains: clonal dissemination of β-lactamase-positive isolates with decreased susceptibility to amoxicillin/clavulanic acid. J Antimicrob Chemother. 2011;66:788–796. doi: 10.1093/jac/dkq533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokota S, Ohkoshi Y, Sato K, Fujii N. Emergence of fluoroquinolone-resistant Haemophilus influenzae strains among elderly patients but not among children. J Clin Microbiol. 2008;46:361–365. doi: 10.1128/JCM.01561-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ubukata K, Chiba N, Morozumi M, Iwata S, Sunakawa K. Longitudinal surveillance of Haemophilus influenzae isolates from pediatric patients with meningitis throughout Japan, 2000–2011. J Infect Chemother. doi: 10.1007/s10156-012-0448-x. [DOI] [PubMed]


