Abstract
With improving survival rates of preterm newborns, adverse cognitive outcomes are increasingly recognized. Adverse cognitive outcomes are associated with decreased cerebellar volumes, and modifiable risk factors for these adverse outcomes should be identified. Animal models demonstrate reduced preterm cerebellar growth after exposure to glucocorticoids. Preterm neonates were prospectively studied with serial MRI examinations near birth and again near term-equivalent age. Adjusting for associated clinical factors, antenatal bethamethasone was not associated with changes in cerebellar volume. Postnatal exposure to clinically routine doses of hydrocortisone or dexamethasone were associated with impaired cerebellar, but not cerebral, growth. Modifying postnatal risk factors for impaired cerebellar development, and particularly glucocorticoid exposure, may help to decrease risk for adverse neurological outcome after preterm birth.
INTRODUCTION
Preterm birth accounts for 13% of all live births in the United States and is the leading cause of death or long-term motor and cognitive deficits. With improving rates of survival, continued risk for adverse neurodevelopmental outcomes(1) – in particular, increasing rates of cognitive impairment(2) – points to an urgent need to identify modifiable risk factors to improve outcomes. In addition to well-known risks for intraventricular hemorrhage (IVH) and white matter injury (WMI) in the cerebrum, preterm neonates are also at risk for postnatal cerebellar hypoplasia, which is associated with impaired motor and cognitive function.(3, 4) Risk factors for abnormal cerebellar development appear distinct from those factors impacting other brain structures. Specifically, smaller cerebellar volumes have been associated with supratentorial IVH,(5, 6) but other associated clinical factors have not been identified in human studies.
Antenatal betamethasone is routinely used for preterm lung maturation, while postnatal hydrocortisone and dexamethasone are used for persistent hypotension presumed to be a result of adrenal insufficiency and prolonged dependence on intubation due to subglottic stenosis or bronchopulmonary dysplasia. Hydrocortisone was first reported to delay growth of the neonatal rat cerebellum in 1978.(7) Since then, negative effects of glucocorticoids on cerebellar development have been confirmed in rats and other species.(8–10) Studies in human preterm newborns reveal adverse effects of postnatal dexamethasone therapy on brain development, including decreased cerebral and cerebellar tissue volumes.(11) By school age, exposed children performed worse on motor and cognitive testing.(12) In agreement with these concerns, the American Academy of Pediatrics recommends against the use of high-dose dexamethasone.(13) However, such findings have not been clearly corroborated with hydrocortisone exposure. While one study raised concerns of motor and cognitive deficits in children with over a week of hydrocortisone therapy,(14) other studies have not reported abnormalities of brain development with hydrocortisone therapy in preterm newborns, resulting in their continued clinical use.(15, 16)
The cerebellum undergoes dramatic growth during the period of prematurity (from 24 to 37 weeks gestational age) and the early post-term period, not only in regards to volume but also surface area.(17, 18) This period of dramatic growth makes the cerebellum particularly vulnerable to factors that may hinder its development. As the cerebellum has the highest levels of glucocorticoid receptors in the brain, the developmental effects of glucocorticoids on the cerebellum are an important area of study.(19)
Glucocorticoid exposure is thus a potential modifiable risk factor for adverse motor and cognitive outcomes in preterm newborns, and therefore, clarifying the effects of glucocorticoid administration on cerebellar development is important. We measured brain volumes on sequential MRI studies of preterm neonates (the first scan as soon as the neonate was stable and the second immediately before discharge as close as possible to term equivalent age) during the early postnatal period at the University of California San Francisco (UCSF) and the University of British Columbia (UBC) to assess effects of exposure to glucocorticoids both antenatally and postnatally in doses currently used in neonatal intensive care.
RESULTS
Study subjects
At UCSF, 57 subjects had a total of 92 MRI scans. At UBC, 115 subjects had a total of 206 MRI scans. Not all subjects were scanned twice due to various clinical reasons, including clinical instability for transport to scanner or transfer to other hospitals prior to second scan. Subject characteristics at the two study sites, including frequency of glucocorticoid exposure, are summarized in Table 1. Of particular note, there were higher rates of administration of antenatal betamethasone at UBC and dexamethasone was not used at UCSF in this cohort. The magnitude and duration of glucocorticoid exposure in the study subjects are summarized in Table 2.
Table 1.
Subject demographics
| Site | UCSF | UBC | P-value |
|---|---|---|---|
|
| |||
| Male | 27/57 (47%) | 55/115 (48%) | 0.90 |
|
| |||
| Gestational age at birth (weeks, mean ± SD) | 28.1 ± 2.2 | 27.8 ± 2.1 | 0.39 |
|
| |||
| Chorioamnionitis | 13/57 (23%) | 36/115 (31%) | 0.27 |
|
| |||
| Antenatal betamethasone | 42/57 (74%) | 104/115 (90%) | 0.006 |
|
| |||
| Persistent hypotension | 32/57 (56%) | 45/115 (39%) | 0.03 |
|
| |||
| Patent ductus arteriosus | 30/57 (53%) | 55/115 (48%) | 0.54 |
|
| |||
| Postnatal sepsis | 28/57 (29%) | 38/115 (33%) | 0.60 |
|
| |||
| Chronic lung disease | 15/57 (26%) | 47/115 (41%) | 0.05 |
|
| |||
| Intubation (days, mean ± SD) | 8.6 ± 13.2 | 16.3 ± 24.8 | 0.03 |
|
| |||
| Postmenstrual age at MRI (weeks, mean ± SD) | |||
| First scan | 31.7 ± 1.6 | 32.3 ± 3.3 | 0.21 |
| Second scan | 35.7 ± 2.2 | 40.5 ± 2.9 | <0.001 |
|
| |||
| Postnatal hydrocortisone | |||
| First scan | 10/53 (19%) | 18/113 (16%) | 0.63 |
| Second scan | 8/39 (21%) | 17/93 (18%) | 0.69 |
|
| |||
| Postnatal dexamethasone | |||
| First scan | 0/53 (0%) | 16/113 (14%) | 0.004 |
| Second scan | 0/39 (0%) | 17/93 (18%) | 0.005 |
|
| |||
| No postnatal glucocorticoids | 46/57 (81%) | 90/115 (78%) | 0.65 |
Note that 4 subjects at the University of California San Francisco (UCSF) and 2 subjects at the University of British Columbia (UBC) had only second and not first MRI scans. Means were compared using the t-test. Proportions were tested using the Fisher exact test.
Glucocorticoids and cerebellar volume
Adjusting for gestational age at time of birth, postmenstrual age at time of MRI, severity of IVH, cerebellar hemorrhage, duration of intubation, presence of hypotension requiring intervention, and study site, cerebellar volume was not associated with antenatal betamethasone exposure (0.24cm3, 95%CI −0.64 to 1.11, P=0.60). Significant interaction was found between glucocorticoid exposure and postmenstrual age in the analysis of cerebellar volume (P=0.005 for hydrocortisone, P=0.003 for dexamethasone). Considering this interaction in the regression analysis, decrease in growth of the cerebellum is associated with glucocorticoid exposure, resulting in a 1.88cm3 smaller cerebellum by 40 weeks postmenstrual age associated with hydrocortisone (95% CI −2.91 to −0.86, P<0.001) and a 2.31cm3 smaller cerebellum associated with dexamethasone (95% CI −3.52 to −1.10, P<0.001) when compared to neonates without glucocorticoid exposure. This represents 8% and 10% smaller cerebellar volumes respectively (See Figure 1).
Figure 1.
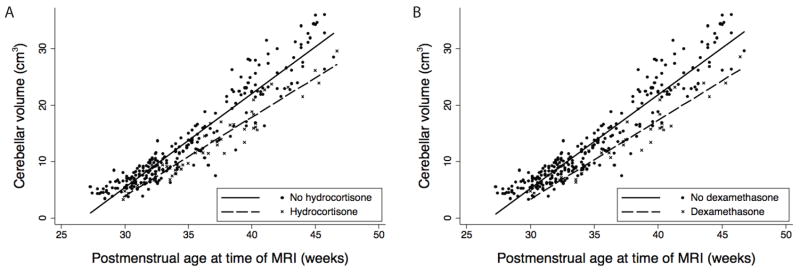
Cerebellar growth is decreased associated with A. postnatal hydrocortisone and B. postnatal dexamethasone. Results are from a generalized estimated equation for repeated measures, adjusting for gestational age at birth, postmenstrual age at time of MRI, severity of intraventricular hemorrhage, cerebellar hemorrhage, duration of intubation, presence of hypotension requiring intervention, and study site.
Accounting for the degree of exposure to antenatal betamethasone, neither exposure to a partial course of betamethasone (0.069cm3, −1.16 to 1.02, P=0.90) nor exposure to a complete course (two doses of 12mg each) of betamethasone (0.19cm3, −0.69 to 1.08, P=0.67) was associated with changes in cerebellar volume. Total exposure to glucocorticoids postnatally prior to each scan was grouped into categories of less than or greater than the median exposure to each glucocorticoid (11.58mg for hydrocortisone and 0.89mg for dexamethasone). Exposure to less than 11.58mg hydrocortisone was associated with a 1.87cm3 smaller cerebellum (95% CI −3.00 to −0.74, P=0.001), while exposure to greater than 11.58mg was associated with a 2.11cm3 smaller cerebellum (95% CI −3.32 to −0.90, P=0.001) by 40 weeks postmenstrual age. Exposure to less than 0.89mg dexamethasone was associated with a 3.34cm3 smaller cerebellum (95% CI −4.67 to −2.02, P<0.001), while exposure to greater than 0.89mg was associated with a 1.85cm3 smaller cerebellum (95% CI −3.04 to −0.66, P=0.002) by 40 weeks postmenstrual age. Thus, in the range of doses administered under the clinical treatment protocols at the study sites, a dose-dependent effect of glucocorticoids on cerebellar growth could not be determined.
Finally, to clarify if the volume changes may be a result of immediate, reversible effects of glucocorticoids on brain volume, we considered the number of days between the last exposure to glucocorticoids and MRI scanning. Using the same regression models, cerebellar volume was not associated with the number of days since the last hydrocortisone (0.020cm3/day, 95% CI −0.0001 to 0.039, P=0.052) or dexamethasone (−0.0012cm3/day, 95% CI −0.026 to 0.023, P=0.92) administration. Thus, the volume changes observed do not appear to be effects that are immediately reversible.
Glucocorticoids and the cerebrum
Given the impact of glucocorticoids on the cerebellum by term-equivalent age, the term-equivalent MRI scans were analyzed further for cerebral volumes, resulting in analysis of 123 MRI scans. Adjusting for gestational age at birth, postmenstrual age at time of MRI, severity of WMI, severity of IVH, duration of intubation, and presence of hypotension requiring medical intervention, cerebral volume at term was not associated with exposure to antenatal betamethasone (7.98cm3, −17.90 to 33.87, P=0.55), postnatal hydrocortisone (−15.06cm3, −37.51 to 7.39, P=0.19), or postnatal dexamethasone (−6.44cm3, −41.41 to 28.52, P=0.72).
Clinical factors and cerebellar volume
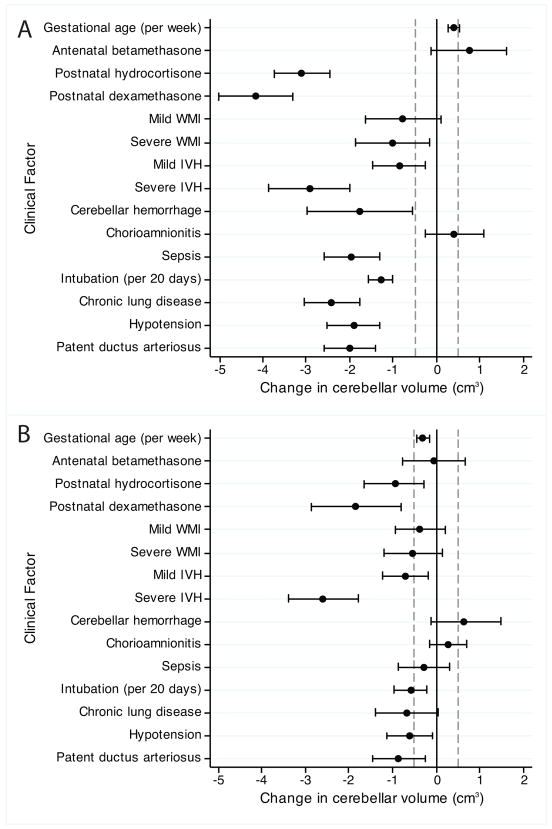
To assess whether or not the observed association between glucocorticoids and cerebellar volume can be explained by other clinical factors, a multivariate regression model was used to test for significant clinical variables that may influence cerebellar volume. The clinical variables included in the model, as well as results, are summarized in Figure 2. In the multivariate model, cardiorespiratory factors including duration of intubation, hypotension requiring medical intervention, and patent ductus arteriosus were significantly associated with decreased cerebellar volume. The factors found to be associated with the largest decreases in cerebellar volume, however, included severe IVH, postnatal hydrocortisone, and postnatal dexamethasone.
Figure 2.
Change in cerebellar volume associated with various clinical factors. A. The univariate models adjusting for postmenstrual age at time of MRI scan and study site. B. The multivariate model considering all variables. Statistical significance for this analysis was defined as a mean magnitude of change in cerebellar volume >0.5cm3 (dotted lines) and P<0.1 (whiskers not crossing zero).
DISCUSSION
This study using serial neuroimaging in preterm newborns shows strong associations between the use of postnatal glucocorticoids – either hydrocortisone or dexamethasone – and impaired cerebellar growth. Conversely, associations were not found between antenatal betamethasone use and cerebellar growth. Surprisingly, there were no associations found between the use of glucocorticoids and supratentorial brain volume. Glucocorticoid exposure is common in preterm newborns, with 85% of all patients exposed antenatally and 21% exposed postnatally in our cohort.
Hydrocortisone and dexamethasone result in decreased proliferation and increased apoptosis of the granule cells of the external granular layer of murine models during the developmental period corresponding with the preterm period in humans.(7, 10) All glucocorticoids tested in animal models, including hydrocortisone, dexamethasone, and corticosterone, result in similar findings of granule cell death in rats, mice, and chicken.(8–10) In fact, the neonatal cerebellum has the highest levels of glucocorticoid receptors in the brain,(19) localized in the external granular layer.(10) These results suggest that all of these glucocorticoids impact development of the cerebellum.
Human studies, however, suggest varying effects of different glucocorticoids upon the preterm brain. In a study comparing 11 newborns exposed to postnatal dexamethasone to 30 unexposed newborns, those exposed had smaller overall brain volumes at term-equivalent age, including cerebellar volume.(11) In a randomized controlled trial of early postnatal dexamethasone therapy, those exposed to dexamethasone were found to have decreased height and head circumference, with worse outcomes on motor and cognitive testing by 8 years of age.(12) As a result, the American Academy of Pediatrics issued specific recommendations in 2010 against the use of high-dose dexamethasone.(13)
As opposed to dexamethasone, conflicting results have been reported after hydrocortisone therapy. Benders et al. compared 19 infants treated with hydrocortisone for chronic lung disease to 19 controls matched for gestational age at birth, showing no difference between whole brain volumes at term-equivalent age, with mean decrease in cerebellar volume of 1cm3 (95%CI −4 to 2cm3).(15) Follow-up of 60 preterm newborns with chronic lung disease, including 25 treated with hydrocortisone, showed no differences in brain development and neurological outcome at 8 years, but the cerebellum was not assessed in that analysis.(20) Follow-up of 226 preterm infants to 8 years showed no difference in motor or cognitive function after adjustment for gestational age, body weight, sex, mechanical ventilation, and small for gestational age.(16) However, when considering duration of therapy in a different cohort by 2 years, longer treatment with hydrocortisone (more than 7 days) was associated with worse developmental outcome.(21)
Proposed differences for the lesser effects of hydrocortisone include its greater mineralocorticoid-mediated, as opposed to glucocorticoid-mediated, activity and shorter half-life in the brain due to specific inactivation by 11-β-hydroxysteroid dehydrogenase type 2, which has high expression in the fetal brain.(15) However, although 11-β-hydroxysteroid dehydrogenase type 2 is capable of degrading hydrocortisone but not dexamethasone, both glucocorticoids result in injury of the external granular layer in wild-type animals. This is suggested by rodent models that show similar effects of corticosterone (a known substrate of 11-β-hydroxysteroid dehydrogenase type 2) and dexamethasone, both on granule cell apoptosis with acute glucocorticoid exposure and on inhibition of cell proliferation with chronic exposure.(22)
Our study found similar results in premature newborns, showing decreased cerebellar volumes by term-equivalent age associated with either postnatal hydrocortisone or dexamethasone. In contrast to previous studies, no dose-dependent effects could be found in the clinically relevant range of exposures seen in this cohort. One reason for the lack of dose-dependent effects may be the narrow clinical range of exposures in this cohort, and lower dosage range for use of dexamethasone. Another possible reason is the small number of subjects exposed to dexamethasone, limiting the power of the study to detect a clinical effect.
Of particular interest, the mean decrease in cerebellar volume detected in this current study is comparable to that observed by Benders et al.(15). In addition, with this larger sample size, statistical significance is now evident. In agreement with Benders et al., no changes were seen in supratentorial brain structures. Considering the high frequency of use of antenatal betamethasone in mothers in preterm labor, it is reassuring that cerebellar growth reduction is not associated with this therapy. Potential reasons for the lack of effect of antenatal betamethasone may include low dose and short exposure to the drug, earlier exposure in the antenatal period, or protective effects of placental 11-β-hydroxysteroid dehydrogenase type 2 which decreases infant exposure to the drug.(23)
Many clinical factors were associated with cerebellar volume, the most important being postnatal glucocorticoid exposure, IVH, cardiorespiratory compromise as indicated by prolonged intubation, hypotension requiring medical intervention, and patent ductus arteriosus. Indeed, the continued strong association between cerebellar volume and postnatal glucocorticoid exposure after multivariate analysis supports the strength of this finding. Associations between IVH and cerebellar volume have been reported previously.(6) The finding that several postnatal factors are associated with decreased cerebellar volume suggests a possibility that measures to correct these potentially modifiable risk factors may improve the late growth of the cerebellum. Of particular note, frank cerebellar hemorrhage was associated with increased cerebellar volumes in the perinatal period (areas of hemorrhage were not included in the cerebellar volume determinations). Since it is known that cerebellar hemorrhages result in atrophy in the long-term, these increased volumes may suggest effects of local parenchymal edema at the time of the MRIs.
The incorporation of two independent prospective cohorts and a large sample size for volumetric analysis provide strength in this study on the association between glucocorticoid exposure and cerebellar growth. However, in this observational study, no specific dosing regimen for corticosteroids was defined. Although causality cannot be concluded based on association studies, animal models are convincing for a causal pathway between glucocorticoid exposure and external granular cell apoptosis. In combination with the animal models in multiple species, this large prospective observational study suggests similar effects of glucocorticoids in human preterm newborns.
This study reports on outcomes of cerebellar volumes in the neonatal period, showing decreased growth velocity over the early postnatal period translating to a 10% decreased volume by term-equivalent age. Recent research has shown that altered brain development after preterm birth continues through childhood.(24) As previously reported, smaller cerebellar volumes seen by 14 years of age are associated with significantly worse cognitive testing using the Wechsler Intelligence Scale for Children-Revised, the Kaufman Assessment Battery for Children, and the Schonnel reading age.(3) Since other studies have shown no associated motor and cognitive deficits by 2 years associated with glucocorticoid exposure,(16) follow-up to school age may be required to detect the long-term motor and cognitive consequences of impaired cerebellar development as observed by Allin et al. at 14 years.(3) If long-term sequelae are found by school age, decreased cerebellar volumes could be useful as an early surrogate biomarker for adverse long-term outcome after preterm birth. The use of MRI at term-equivalent age as a biomarker for long-term outcome would provide a convenient measure for rapid identification of important modifiable clinical factors such as glucocorticoid exposure, as well as a potentially rapid assessment of the efficacy of clinical management improvements on future neurological outcome.
The traditional focus of brain injury in preterm newborns has been on supratentorial injury, including IVH and WMI. This study highlights that specific modifiable clinical factors with major impacts on long-term neurological outcome, such as glucocorticoid exposure, may have specific detrimental effects on the cerebellum equivalent to, or greater than, the more traditionally studied supratentorial brain structures. Clinicians caring for preterm newborns may need to weigh the benefits of using glucocorticoids for hypotension and prolonged intubation with these findings of associated impaired cerebellar growth. With improvements in neonatal care and focus on supratentorial brain injury in the past decade, we have failed to improve neurodevelopmental outcomes.(1, 2) By turning our focus to modifying factors that impair cerebellar development, we may reach our critical target of improving long term outcomes after preterm birth.
SUBJECTS AND METHODS
Study subjects
All preterm newborns (<33 weeks gestational age at birth) born between July 2006 and February 2009 admitted to the intensive care nurseries at UCSF and UBC who consented for study were included in this prospective cohort.(6) The inclusion and exclusion criteria are the same for both centers. Exclusion criteria included (1) clinical evidence of a congenital malformation or syndrome, (2) congenital infection, and (3) newborns too clinically unstable for transport to the MRI scanner. Parental consent was obtained for all cases following a protocol approved by both institutional committees on human research.
Clinical data was collected prospectively from patient charts. Antenatal betamethasone was used for preterm lung maturation when preterm birth was expected. Postnatal hydrocortisone and dexamethasone were used for persistent hypotension or prolonged dependency on intubation based on clinical decision by the neonatologist. Drug administration records from the hospital pharmacist were used to verify dates and dosages of hydrocortisone and dexamethasone administered to each subject. Standard dosing at UCSF and UBC for hydrocortisone was 1–3 mg/kg/day followed by tapering daily doses and for dexamethasone was 0.15mg/kg/day followed by tapering daily doses for bronchopulmonary dysplasia or 0.15mg/kg/dose for up to 3 doses prior to extubation for airway edema.
Neuroimaging studies
Study subjects underwent up to two MRI scans – one as soon after birth as clinically stable and again near term-equivalent age. Custom MR-compatible incubators with specialized neonatal head coils were used to provide a quiet, well-monitored environment for the neonate, minimizing movement and improving signal-to-noise ratio.(25) MRI scans at both sites were performed on 1.5-T scanners with nearly identical acquisition methods as previously described.(6)
MRI scans were reviewed by a single pediatric neuroradiologist at each study site (AJB, KJP) blinded to patient history and using scores with high intra and inter-rater reliability.(26, 27) IVH was graded using the grading system of Papile.(28) WMI was graded using a clinically predictive scoring system previously shown to be associated with neurodevelopmental outcomes at 12–18 months of age.(26) Cerebellar hemorrhage was defined as visible blood of any age in the cerebellum on ultrasound or MRI.
Head ultrasounds were performed by clinical protocol, with one scan at 7 days of life and another at 4 weeks of life, or earlier if clinically indicated. The severity of IVH was graded as the highest grade of hemorrhage seen on any ultrasound or MRI scan.
Cerebellar volumetric measurements were obtained from all studies using semiautomated tools available within the RView V9 software (http://rview.colin-studholme.net), allowing simultaneous display of multiple orthogonal slices and surface renderings. Cerebral volumes were obtained from the later (term-equivalent) MRI only. Tracings of the cerebellum were performed on the high-resolution 3D T1-weighted MRI sequences by a single pediatric neurologist (EWYT). Cerebral volumes were obtained using the same technique by a single researcher (EDYF). Cerebellar volumes were considered as the whole cerebellum ending at the cerebellar peduncles. Cerebral volumes considered all cerebral brain tissues including the midbrain tegmentum, rostral to the pons and the superior medullary velum. Cerebrospinal fluid spaces were excluded. This technique showed excellent reproducibility when testing by performing 10 tracings in a blinded manner from five different scans, with an intraclass correlation coefficient of 0.99 for both cerebellar and cerebral tracings. The average Dice coefficients of 0.90 and 0.95 for the cerebellum and cerebrum respectively indicated a high level of accuracy in the anatomical localization of the delineated volumes.(29)
Statistical analysis
Statistical analysis was performed using Stata 11 (Stata Corporation, College Station, Texas). Descriptive statistics were used to compare the subjects at the two study sites, using the Fisher exact test for comparing proportions and t-test for comparing means. Generalized estimating equations with robust standard errors were used to study the association between clinical variables and brain volume in the entire prospective cohort. For the primary analysis of the association between exposure to glucocorticoids and cerebellar volume, the predictor variables were exposure to antenatal betamethasone, postnatal hydrocortisone, and postnatal dexamethasone. To adjust for known confounders, adjustment was made for gestational age at birth, postmenstrual age at time of MRI, IVH, cerebellar hemorrhage, duration of intubation, hypotension requiring medical intervention, and study site. Categorization of subjects into lower or higher than median exposure for the cohort was done to test for effect of dosage on brain volume. Adjustment for number days since last glucocorticoid exposure was used to test for effect of immediate reversible effects of glucocorticoid exposure on brain volume. To analyze the effect of glucocorticoids on cerebral brain volumes, the same model was used, adjusting for postmenstrual age at time of MRI, IVH, WMI, duration of intubation, hypotension requiring medical intervention, and study site. Statistical significance was set at an alpha of 0.05.
To study the effect of other clinical variables on cerebellar volume, a multivariate regression model was used, with variables considered to be clinically important if they were found to have an effect magnitude of >0.5cm3 change in cerebellar volume (approximately 5% of cerebellar volume at 32 weeks postmenstrual age) and P<0.1.
Acknowledgments
Funding: Supported by the National Institutes of Health [R01 NS346432, UL1 RR024131]; and the Canadian Institutes for Health Research [CHI 151135]. EWYT is a Cerebral Palsy International Research Foundation Ethel & Jack Hausman Clinical Research Scholar. SPM is supported by a Canada Research Chair in Neonatal Neuroscience, and is a Canadian Institutes of Health Research Clinician Scientist and Michael Smith Foundation for Health Research Scholar.
Footnotes
Author contributions: EWYT had primary responsibility for designing and conducting the study, as well as writing the first draft of the manuscript. EWYT, CS and EDYK were responsible for analyzing the MRI data for brain volumes. AJB and KJP evaluated the MRI scans for brain injury. DMF, REG, and SPM also contributed to study design and data interpretation. DVG was the statistician involved with data analysis and interpretation. All authors contributed to critical review of the manuscript for publication.
Competing interests: None.
References
- 1.Doyle LW, Anderson PJ. Adult outcome of extremely preterm infants. Pediatrics. 2010;126:342–351. doi: 10.1542/peds.2010-0710. [DOI] [PubMed] [Google Scholar]
- 2.Synnes AR, et al. School Entry Age Outcomes for Infants with Birth Weight </=800 Grams. J Pediatr. 2010;157:989–994. doi: 10.1016/j.jpeds.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Allin M, et al. Cognitive and motor function and the size of the cerebellum in adolescents born very pre-term. Brain. 2001;124:60–66. doi: 10.1093/brain/124.1.60. [DOI] [PubMed] [Google Scholar]
- 4.Spittle AJ, et al. Reduced cerebellar diameter in very preterm infants with abnormal general movements. Early Hum Dev. 2010;86:1–5. doi: 10.1016/j.earlhumdev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Limperopoulos C, et al. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics. 2005;116:844–850. doi: 10.1542/peds.2004-2282. [DOI] [PubMed] [Google Scholar]
- 6.Tam EWY, et al. Differential effects of intraventricular hemorrhage and white matter injury on preterm cerebellar growth. J Pediatr. 2011;158:366–371. doi: 10.1016/j.jpeds.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohn MC, Lauder JM. The Effects of Neonatal Hydrocortisone on Rat Cerebellar Development. Dev Neurosci. 1978;1:250–266. [Google Scholar]
- 8.Jacobs CM, Trinh MD, Rootwelt T, Lomo J, Paulsen RE. Dexamethasone induces cell death which may be blocked by NMDA receptor antagonists but is insensitive to Mg2+ in cerebellar granule neurons. Brain Res. 2006;1070:116–123. doi: 10.1016/j.brainres.2005.10.093. [DOI] [PubMed] [Google Scholar]
- 9.Aden P, et al. Low-potency glucocorticoid hydrocortisone has similar neurotoxic effects as high-potency glucocorticoid dexamethasone on neurons in the immature chicken cerebellum. Brain Res. 2008;1236:39–48. doi: 10.1016/j.brainres.2008.07.095. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi KK, et al. Acute neonatal glucocorticoid exposure produces selective and rapid cerebellar neural progenitor cell apoptotic death. Cell Death Differ. 2008;15:1582–1592. doi: 10.1038/cdd.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parikh NA, et al. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics. 2007;119:265–272. doi: 10.1542/peds.2006-1354. [DOI] [PubMed] [Google Scholar]
- 12.Yeh TF, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350:1304–1313. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]
- 13.Watterberg KL. Policy statement--postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics. 2010;126:800–808. doi: 10.1542/peds.2010-1534. [DOI] [PubMed] [Google Scholar]
- 14.Needelman H, Hoskoppal A, Roberts H, Evans M, Bodensteiner JB. The effect of hydrocortisone on neurodevelopmental outcome in premature infants less than 29 weeks’ gestation. J Child Neurol. 2010;25:448–452. doi: 10.1177/0883073809348059. [DOI] [PubMed] [Google Scholar]
- 15.Benders MJ, et al. Brain development of the preterm neonate after neonatal hydrocortisone treatment for chronic lung disease. Pediatr Res. 2009;66:555–559. doi: 10.1203/PDR.0b013e3181b3aec5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rademaker KJ, et al. Neonatal hydrocortisone treatment: neurodevelopmental outcome and MRI at school age in preterm-born children. J Pediatr. 2007;150:351–357. doi: 10.1016/j.jpeds.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 17.Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limperopoulos C, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. 2005;115:688–695. doi: 10.1542/peds.2004-1169. [DOI] [PubMed] [Google Scholar]
- 19.Pavlik A, Buresova M. The neonatal cerebellum: the highest level of glucocorticoid receptors in the brain. Brain Res. 1984;314:13–20. doi: 10.1016/0165-3806(84)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Lodygensky GA, et al. Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics. 2005;116:1. doi: 10.1542/peds.2004-1275. [DOI] [PubMed] [Google Scholar]
- 21.Needelman H, Hoskappal A, Roberts H, Evans M, Bodensteiner JB. The effect of hydrocortisone on neurodevelopmental outcome in premature infants less than 29 weeks’ gestation. J Child Neurol. 2010;25:448–452. doi: 10.1177/0883073809348059. [DOI] [PubMed] [Google Scholar]
- 22.Heine VM, Rowitch DH. Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11betaHSD2-dependent mechanism. J Clin Invest. 2009;119:267–277. doi: 10.1172/JCI36376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benediktsson R, Calder AA, Edwards CR, Seckl JR. Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf) 1997;46:161–166. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- 24.Ment LR, et al. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics. 2009;123:503–511. doi: 10.1542/peds.2008-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Partridge SC, et al. Tractography-based quantitation of diffusion tensor imaging parameters in white matter tracts of preterm newborns. J Magn Reson Imaging. 2005;22:467–474. doi: 10.1002/jmri.20410. [DOI] [PubMed] [Google Scholar]
- 26.Miller SP, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147:609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 27.Block AJ, et al. Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. J Thorac Cardiovasc Surg. 2010;140:550–557. doi: 10.1016/j.jtcvs.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 29.Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302. [Google Scholar]