Abstract
Previous studies have demonstrated that nitric oxide (NO) synthase inhibitors are as efficacious as tricyclic antidepressants in preclinical antidepressant screening procedures and in attenuating behavioural deficits associated with animal models of depression. The N-methyl-𝒟-aspartate receptor (NMDA-R) complex gates Ca2+, which interacts with calmodulin to subsequently activate NO synthase. We hypothesised that uncoupling neuronal nitric oxide synthase (nNOS) from the NMDA-R through the scaffolding protein postsynaptic density protein 95 (PSD-95) would produce behavioural antidepressant effects similar to NO synthase inhibitors. Small-molecule inhibitors of the PSD-95/nNOS interaction, IC87201 (0.01–2 mg/kg) and ZL006 (10 mg/kg) were tested for antidepressant properties in tests of antidepressant activity namely the tail suspension and forced swim tests in mice. We now report that IC87201 and ZL006 produce antidepressant-like responses in the forced swimming test (FST) and tail suspension test (TST) following a single administration in mice. By contrast to the tricyclic antidepressant imipramine (25 mg/kg), the effects are not observed 1 h following drug administration but are apparent 24 and 72 h later. Furthermore prior exposure to the TST or FST is required in order to observe the antidepressant-related activity. Similar delayed and sustained antidepressant-like effects were observed following TRIM (50 mg/kg) and ketamine (30 mg/kg) in the TST. The antidepressant-like effects of ZL006 also generalise to IC87201 in the TST. IC87201 was devoid of effects on locomotor activity and step-through latency in the passive avoidance cognition test. These data support the hypothesis that targeting the PSD-95/nNOS interaction downstream of NMDA-R produces antidepressant effects and may represent a novel class of therapeutics for major depressive disorders.
Keywords: nitric oxide synthase, N-methyl-𝒟-aspartate receptor, IC87201, antidepressant, tail suspension test, mouse
INTRODUCTION
Consistent with a role for glutamate in major depressive illness, anti-glutamatergic agents have demonstrated antidepressant efficacy both clinically and in several animal models of antidepressant action (Sanacora et al, 2012; Skolnick et al, 2009). Most notable are the clinical studies that have reported a rapid improvement in depressive symptoms in treatment resistant depressed patients after intravenous administration of ketamine, a non-competitive N-methyl-𝒟-aspartic acid receptor (NMDA-R) antagonist (reviewed by Murrough, 2012). Although beneficial effects have been observed for up to 7 days, the use of ketamine in the clinic is hampered due to the risk for adverse effects, including psychosis and psychomotor stimulation.
The NMDA-R is an ionotropic-glutamate receptor subtype that activates nitric oxide synthase (NOS) and production of nitric oxide (NO). NO synthases comprise neuronal (nNOS), endothelial (eNOS), inducible (iNOS) and mitochondrial (mtNOS) isoforms where nNOS is colocalised and functionally coupled to the NMDA-R (for review, see Guix et al, 2005). As NMDA-R antagonists possess antidepressant properties, targets downstream of the receptor, such as nNOS may represent targets for antidepressant activity. In this regard, L-arginine-derived inhibitors of NOS, produce antidepressant activity in the forced swimming test (FST), a preclinical behavioural screening procedure sensitive to antidepressant activity (Gigliucci et al, 2010; Harkin et al, 2003; Harkin et al, 1999; Dhir and Kulkarni, 2011; Wegener and Volke, 2010). Genetic deletion of nNOS or treatment with NOS inhibitors (7-nitroindazole (7-NI) or 1-(2-trifluoro-methyl-phenyl) imidazole (TRIM)) prevents chronic mild stressor (CMS)-induced depression-related behavioural and cellular changes in mice, further confirming the antidepressant potential of NOS inhibition (Mutlu et al, 2009; Zhou et al, 2007).
Despite these promising leads, efforts in synthesising highly selective nNOS inhibitors have been difficult to bring to fruition. The selective targeting of nNOS, but not eNOS, would avoid eNOS-mediated changes in arterial blood pressure and circumvent adverse cardiovascular effects. In this regard, the two aforementioned inhibitors 7-NI and TRIM, which show preferential inhibition of nNOS have been reported to possess antidepressant-like properties in preclinical tests (Dhir and Kulkarni, 2011; Zhou et al, 2007). Despite the additional selectivity of these two drugs for nNOS (Alderton et al, 2001; Moore and Handy, 1997), selectivity is not exclusive. NOS inhibitors that are more selective for the neuronal isoform are not devoid of adverse effects and may decrease locomotion and/or motor coordination (Dzoljic et al, 1997; Harkin et al, 2003; Mutlu et al, 2011; Ulak et al, 2010; Volke et al, 2003). Moreover 7-NI seems to have a less favourable side-effect profile than TRIM in several paradigms that investigated learning and memory (Holscher et al, 1996; Mutlu et al, 2011; Yildiz Akar et al, 2009; Yildiz Akar et al, 2007; Zou et al, 1998).
The postsynaptic density protein 95 kDa (PSD-95) is a scaffolding protein that links nNOS to NMDA-R. Disruption of the PSD-95/nNOS interaction has been achieved with both peptide fragments and small-molecule inhibitors (for review see Doucet et al, 2012). As other functions of the NMDA receptor remain intact, this selective approach may be adopted to further test for a novel glutamatergic NO-based treatment for depression. Two compounds that disrupt the PSD-95/nNOS interaction have been identified. 2-((1H-benzo [d] [1,2,3] triazol-5-ylamino) methyl)-4,6-dichlorophenol (IC87201) disrupts the PSD-95/nNOS interaction with an IC50 of 31 μM. In mice, this drug was an effective anti-nociceptive after intraperitoneal (i.p.) injection, with an EC50 of 0.1 mg/kg. In rats, IC87201 abolished mechanical allodynia when administered intrathecally (at 50 and 100 nmol doses) or i.p. (2 mg/kg) (Florio et al, 2009). 4-(3,5-Dichloro-2-hydroxy-benzylamino)-2-hydroxybenzoic acid (ZL006) is a molecule structurally related to IC87201, which inhibits NMDA-R-dependent NO synthesis in cortical neurons with an IC50 of 82 nM and crosses the blood brain barrier after systemic administration (Zhou et al, 2010). In vivo, ZL006 produced a reduction in infarct size and attenuated neurological deficits in a middle cerebral artery occlusion model of stroke (Zhou et al, 2010). Both IC87201 and ZL006 have attractive properties to investigate the PSD-95/nNOS interface as a drug target for the treatment of depression.
As NMDA receptors couple to nNOS via the postsynaptic protein PSD-95, inhibition of PSD-95/nNOS may elicit an antidepressant response with a better side-effect profile (Doucet et al, 2012). To further explore this hypothesis, the present investigation assessed compounds targeting the PSD-95/nNOS interface, IC87201 and ZL006 for antidepressant effects in the mouse tail suspension test (TST) and FST. The reference tricyclic antidepressant imipramine, ketamine and TRIM were also included for comparative purposes.
MATERIALS AND METHODS
Animals
Male CD-1 mice aged 6–8 weeks old (Charles River, Margate, UK or Harlan, Bicester, UK) were housed five per cage, kept on a 12 h/12 h light/dark cycle (lights on from 0800 to 2000 hours) and were given access to food and water ad libitum. Mice were habituated to animal facilities for 2 weeks before behavioural testing. All testing was conducted between 1000 and 1700 hours. All animal procedures conformed to the European Council Directive 1986 (86/609/EEC) and were approved by the Bioresources Ethics Committee of the University of Dublin, Trinity College.
Drugs
All drugs were injected i.p. in an injection volume of 10 ml/kg. Concentrations were as follows: ketamine 30 mg/kg (Vetalar; Pfizer, Dublin, Ireland), imipramine 25 mg/kg (Sigma-Aldrich, Arklow, Ireland), 1-[2-(trifluoromethyl)phenyl] imidazole (TRIM) 50 mg/kg (Alfa Aesar, Heysham, UK) and 4-(3,5-Dichloro-2-hydroxy-benzylamino)-2-hydroxybenzoic acid (ZL006) 10 mg/kg (Topchem Pharmaceuticals, Sligo, Ireland) in 0.9% saline. 2-((1H-benzo[d] [1,2,3]triazol-5-ylamino) methyl)-4,6-dichlorophenol (IC87201) 0.01–2 mg/kg (Topchem Pharmaceuticals, Sligo, Ireland) was prepared in 5% DMSO. Saline or 5% DMSO were used as vehicle control where appropriate. All drugs were prepared freshly on the day of testing and were administered before test as indicated.
TST
The TST was performed as originally described (Steru et al, 1985). Mice were suspended by their tail from a metal rod using adhesive tape attached ∼0.5–1 cm from the base of their tail. The rod was fixed 35 cm from the surface of a table. Mice were considered immobile only when they hung passively and completely motionless. The total duration of immobility during a 6 min test was calculated. A decrease in immobility time in this test indicated an antidepressant-like response.
FST
The FST was performed as previously described (Porsolt et al, 1977). Mice were placed for 6 min in a 2l Pyrex glass beaker filled with 12 cm of water at 23±1 °C. Immobility was scored during the last 4 min (with single exposure) or for the full duration of a 6 min trial (with re-exposure) and was defined as the absence of active, escape-oriented behaviours such as swimming, jumping or rearing. The water in the chamber was changed between animals. The FST is sensitive to conventional antidepressant treatment (Porsolt et al, 1977) as well as to non-monoaminergic antidepressants (Autry et al, 2011; Maeng et al, 2008). A decrease in immobility time indicated an antidepressant-like response.
Locomotor Activity
Mice were placed into activity monitor cages (32 cm × 20 cm × 18 cm; length × width × height) and locomotor activity was recorded for 60 min by infrared beams linked to computer acquisition software (AM1051 data logger; Benwick Electronics, UK) under dim light conditions. Each activity monitor was equipped with a set of horizontal infrared beams, positioned 3 cm above the base of the cage to record the activity. The set of beams consisted of a 12 × 7 beam matrix, forming a grid of 66 × 2.54 cm2 cells within the cage (Gigliucci et al, 2010). Activity was recorded as the number of times a beam changed from unbroken to broken.
Passive Avoidance Test
Mice were tested on a step-through inhibitory avoidance apparatus (UgoBasile, Comerio, Italy), in which two compartments similar in size (40 cm × 20 cm × 22 cm; length × width × height) were divided by a partition with a guillotine door. The protocol was adapted from previous studies (Maeng et al, 2008; Mutlu et al, 2011). Briefly, in the training trial, the mouse was placed in the white ‘start' compartment (10 𝒲 light) and the guillotine door was opened 60 s later. After the mouse crossed over into the dark chamber, the latency was recorded, the door was closed and a mild foot shock (0.5 mA, 3 s) was delivered. The mouse remained in the shock compartment for an additional 30 s to associate spatial cues with the treatment and was then returned to its home cage. For retention trials, mice were placed in the light compartment 24 h and 72 h post-training. Each mouse was placed in the ‘start' compartment, as in the training trial and the door was opened after a 30 s acclimatisation period. The latency to enter the dark compartment (previously associated with the foot shock) was measured as an index of inhibitory avoidance. Cutoff latency was 5 min. In the retention test session, the foot shock was omitted. The arena was cleaned with water and 70% alcohol between trials in order to remove olfactory cues.
Data Analysis
Data are expressed as group mean with SEM and were analysed using unpaired Student's t-tests or analyses of variance (ANOVA). If any statistically significant change was found following one or two factor ANOVA, post-hoc comparisons were performed using a Dunnett's or Student-Newman-Keuls test, respectively. Data were deemed significant when, P<0.05.
Experimental Design
Experiment 1. Dose-related effects of IC87201 in the TST
Mice received IC87201 (0.01, 0.1 and 1 mg/kg, i.p.) or vehicle (5% DMSO, i.p.) and were exposed to the TST 60 min later and re-tested 24 h following drug administration. For comparison, the effects of imipramine (25 mg/kg, i.p.) were determined compared with vehicle-treated (saline) controls under identical conditions. An independent group of mice received IC87201 (1 mg/kg, i.p.) or vehicle (5% DMSO, i.p.) and were exposed to the TST once 24 h following drug administration. The selection of doses was determined from a published report indicating that IC87201 was effective in treating NMDA-induced thermal hyperalgesia in mice with an EC50 of 0.1 mg/kg, i.p. (Florio et al, 2009).
Experiment 2. Effects of IC87201 on locomotor activity
Mice were habituated to the monitor cages for 60 min and subsequently received IC87201 (0.01, 0.1 and 1 mg/kg, i.p.) or vehicle (5% DMSO, i.p.). Locomotor activity was subsequently recorded for 60 min.
Experiment 3. Sustained effects of IC87201 in the TST
Mice were exposed to the TST and were subsequently administered IC87201 (1 mg/kg, i.p.) or vehicle (5% DMSO, i.p.). Animals were re-tested in the TST 24 h and 72 h later. For comparison, the effects of imipramine (25 mg/kg, i.p.), ketamine (30 mg/kg, i.p.) and TRIM (50 mg/kg, i.p.) were determined compared with vehicle-treated (saline) controls under identical conditions. These doses were selected on the basis that they have been previously reported to reduce immobility time in the TST and FST in mice (Harkin et al, 2004; Hayase et al, 2006; Koike et al, 2011; Volke et al, 2003).
Experiment 4. Effects of ZL006 in the TST
Mice were exposed to the TST and subsequently treated with a single i.p. injection of ZL006 (10 mg/kg, i.p.) or vehicle (saline). Animals were subsequently re-exposed to the TST 24 h and 72 h later and immobility time was recorded. In a locomotor activity test, separate groups of mice were habituated to the monitor cages for 60 min and received ZL006 (10 mg/kg, i.p.) or vehicle (saline). Locomotor activity was subsequently recorded for 60 min. The dose of ZL006 was selected on the basis of a previous report indicating that ZL006 (1.5–3 mg/kg, i.v.) was effective in reducing infarct size and improving neurological outcome following ischaemic injury in mice and rats without overt effects on cerebral blood flow or learning and memory (Zhou et al, 2010).
Experiment 5. Dose-related effects of IC87201 in the FST and in the passive avoidance test
Mice received IC87201 (0.01, 0.1, 1 and 2 mg/kg, i.p.) or vehicle (5% DMSO, i.p.) and were exposed to the FST 60 min later and re-tested 24 h following drug administration. In an independent study, mice were exposed to a first session of the FST and subsequently treated with IC87201 (2 mg/kg, i.p.) or vehicle (5% DMSO, i.p.). Animals were re-tested 24 h later. Locomotor activity was determined as previously described where mice were habituated to the monitor cages for 60 min, subsequently treated with IC87201 (2 mg/kg, i.p.) or vehicle (5% DMSO, i.p.) and locomotor activity recorded for 60 min. For the passive avoidance test, mice received two i.p. injections of IC87201 (2 mg/kg): the first one was administered 60 min before the training session of the passive avoidance test and the second one immediately after training. Retention latencies were measured 24 h and 72 h after the training session. The dose of IC87201 was selected from a previous report indicating an attenuation of mechanical allodynia in rats subjected to chronic constriction injury (Florio et al, 2009).
RESULTS
Experiment 1. Dose Response of IC87201 in the TST
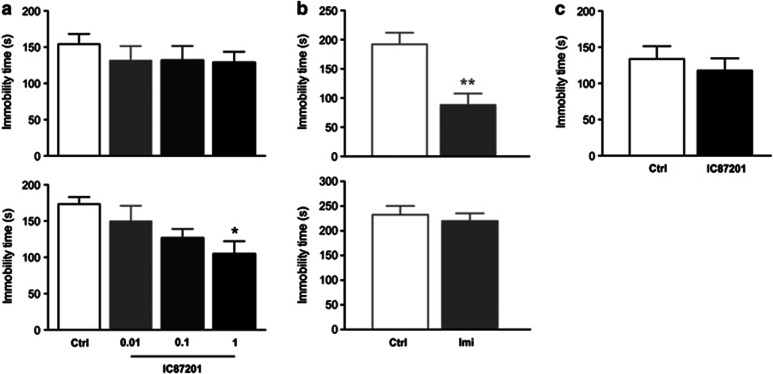
IC87201 did not reduce immobility time in the TST 1 h following drug administration. IC87201, however, dose dependently reduced immobility time when mice were re-tested 24 h later (Figure 1a). ANOVA showed a significant effect of IC87201 treatment (F3,28=3.48, P=0.029). Post-hoc comparisons revealed that IC87201 (1 mg/kg) significantly reduced immobility time when compared with vehicle-treated controls. In contrast, imipramine reduced immobility time in the TST 1 h following drug administration (Student's t-test; t=3.73, P=0.0017). Imipramine however failed to reduce immobility time when mice were re-tested 24 h later (Figure 1b). IC87201 (1 mg/kg) administered to mice exposed to a single TST session 24 h following drug administration failed to influence immobility time (Figure 1c).
Figure 1.
Dose-related effects of IC87201 in the TST. Male CD-1 mice received (a) IC87201 (0.01, 0.1 and 1 mg/kg) or (b) imipramine (25 mg/kg) and immobility time was recorded 60 min later (top panel) and following re-exposure to the test 24 h later (bottom panel). (c) Mice received IC87201 (1 mg/kg) and immobility time was examined 24 h following drug administration only. Data were collected in three independent studies and are expressed as mean±SEM. (n=8–10 per group). *P<0.05 vs control (Dunnett's), **P<0.01 vs control (Student's t-test).
Experiment 2. IC87201 does not Impair Locomotor Activity
ANOVA of activity scores showed an effect of time (F5,140=27.83, P<0.001) but there was no effect of IC87201 or interaction between IC87201 and time (Figure 2a). When expressing sum totals of activity over the course of 60 min ANOVA of the total scores did not show effects of the test compound (Figure 2b).
Figure 2.
Effects of IC87201 on locomotor activity. Male CD-1 mice were habituated to monitor cages for 60 min before receiving a single administration of IC87201 (0.01, 0.1 and 1 mg/kg). Locomotor activity was examined in (a) 10 min intervals or (b) total activity for a duration of 60 min. Data are expressed as mean±SEM (n=8 per group).
Experiment 3. IC87201, Ketamine and TRIM Produce Sustained Antidepressant-like Effects in the TST
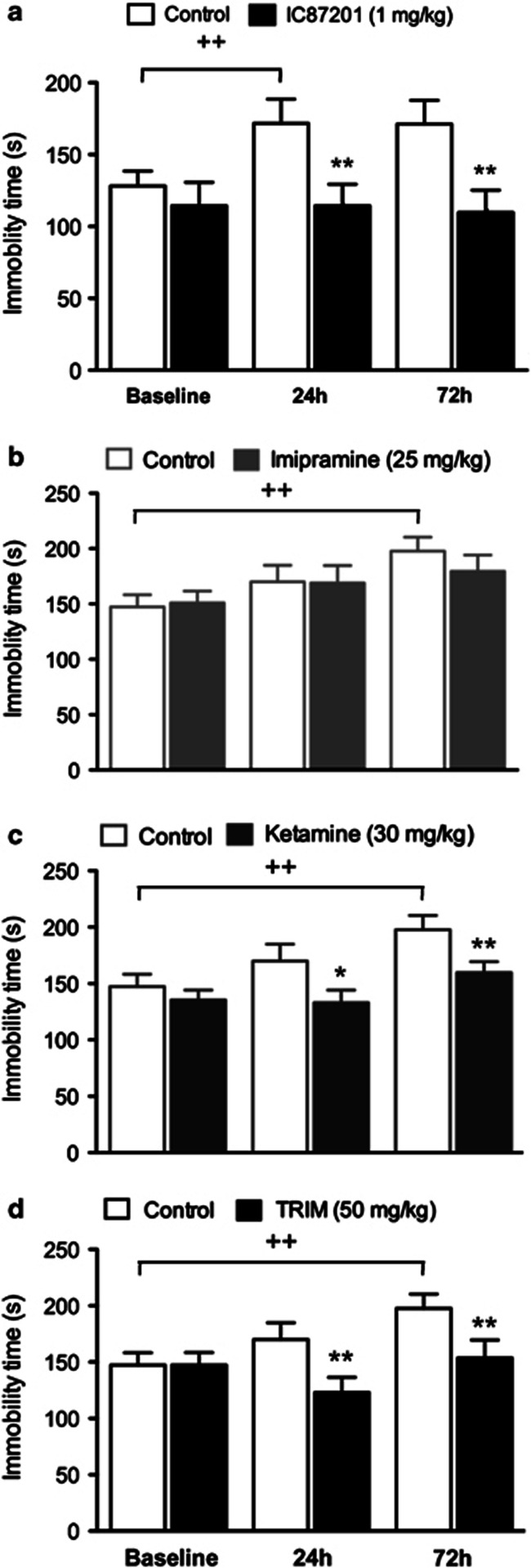
ANOVA of immobility time showed an effect of IC87201 (F1,36=5.81, P=0.027) and a time × IC87201 interaction (F2,36=3.72, P=0.034). Post-hoc analysis revealed an increase in immobility time in control group upon re-exposure to the test compared with the initial trial. IC87201 treatment prevented this increase in immobility time at 24 and 72 h when compared with vehicle-treated controls (Figure 3a).
Figure 3.
Sustained effects of IC87201 in the TST. (a) Male CD-1 mice received IC87201 (1 mg/kg) immediately after a first exposure to the TST (baseline). Immobility time was then re-assessed at 24 and 72 h (n=10 per group). In an independent experiment, male CD-1 mice received (b) imipramine (25 mg/kg), (c) ketamine (30 mg/kg) or (d) TRIM (50 mg/kg) immediately after a first exposure to the TST (baseline). Immobility time was then re-assessed 24 and 72 h following drug administration (n=17–19 per group). Data are expressed as mean±SEM. ++P<0.01 vs baseline control, *P<0.05, **P<0.01 vs respective 24 h and 72 h control (Student-Newman-Keuls).
ANOVA of immobility times following imipramine treatment showed an effect of time (F2,68=9.93, P<0.001) but failed to demonstrate effects of imipramine or an imipramine × time interaction. Post-hoc analysis revealed an increase in immobility time in control group upon re-exposure to the test compared with the initial trial (Figure 3b).
ANOVA of immobility times following ketamine treatment showed an effect of time (F2,68=12.14, P<0.001) and ketamine (F1,68=4.34, P=0.045). Post-hoc analysis revealed an increase in immobility time in control group upon re-exposure to the test compared with the initial trial. Ketamine treatment prevented this increase when animals were re-exposed to the test 24 and 72 h following drug administration when compared with vehicle-treated controls (Figure 3c).
ANOVA of immobility times following TRIM administration showed an effect of time (F2,72=7.68, P<0.001) and a TRIM × time interaction (F2,72=4.87, P=0.0103). Post-hoc analysis revealed an increase in immobility time in control group upon re-exposure to the test compared with the initial trial. Treatment with TRIM prevented this increase when animals were re-exposed to the test 24 and 72 h following drug administration when compared with vehicle-treated controls (Figure 3d).
Experiment 4. ZL006 Produces Sustained Antidepressant-like Effects in the TST
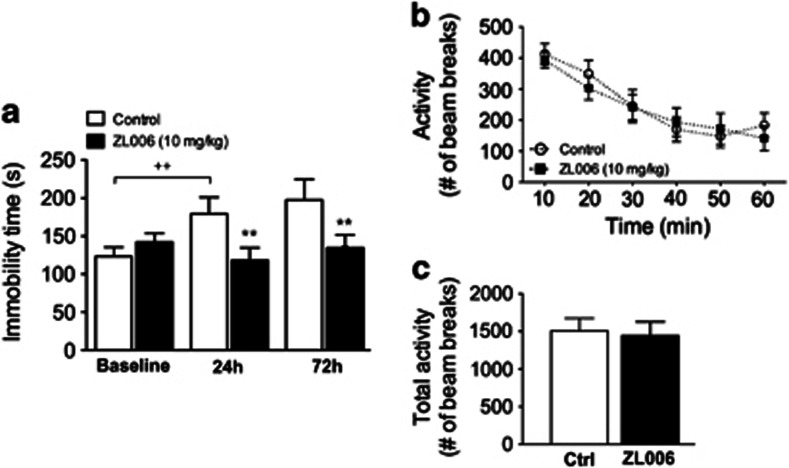
ANOVA of immobility times showed an effect of time (F2,36=5.53, P=0.0081) and a ZL006 × time interaction (F2,36=11.08, P<0.001). Post-hoc comparisons revealed an increase in immobility time in control group upon re-exposure to the test compared with the initial trial. Treatment with ZL006 prevented this increase at 24 and 72 h following drug administration when compared with vehicle-treated controls (Figure 4a).
Figure 4.
Sustained effects of ZL006 in the TST. (a) Male CD-1 mice received ZL006 (10 mg/kg) immediately after a first exposure to the TST (baseline). Immobility time was then re-assessed 24 and 72 h following drug administration. In a companion experiment, locomotor activity was examined in (b) 10 min intervals or (c) total activity for a total duration of 60 min. Data are expressed as mean±SEM (n=10 per group).++P<0.01 vs baseline control, **P<0.01 vs respective 24 h and 72 h control (Student-Newman-Keuls).
ANOVA of activity scores over 10 min intervals following ZL006 administration showed an effect of time (F5,90=20.20, P<0.001) but no IC87201 or IC87201 × time interaction (Figure 4b). There was no difference in sum totals of activity counts over the course of the 60 min test period between IC87201 and vehicle-treated mice (Figure 4c).
Experiment 5. IC87201 Produces Sustained Antidepressant-like Effects in the FST
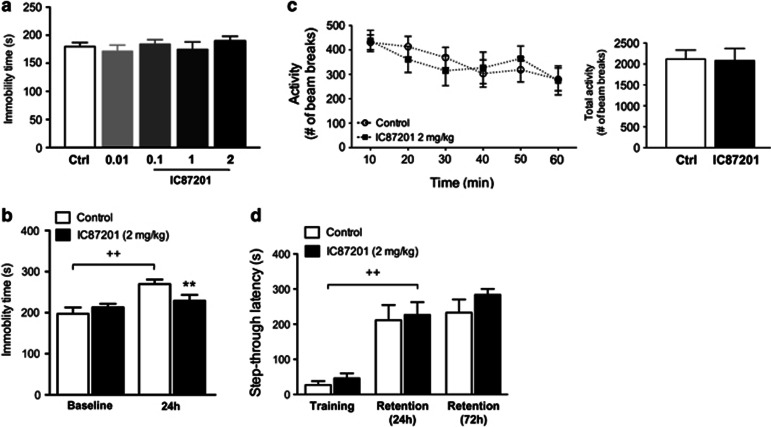
IC87201 did not influence immobility time in the FST 60 min following drug administration or following re-test 24 h later when compared with vehicle-treated controls (Figure 5a). When IC87201 (2 mg/kg) was injected following an initial exposure to the FST (baseline), ANOVA of immobility times showed an effect of time (F1,18=31.56, P<0.001) and a IC87201 × time interaction (F1,18=12.39, P=0.0024). Post-hoc comparisons revealed an increase in immobility time in the control group upon re-exposure to the test compared with the baseline trial. Treatment with IC87201 prevented this increase 24 h following drug administration when compared with vehicle-treated controls (Figure 5b). ANOVA of locomotor activity scores determined over 10 min intervals showed an effect of time (F5,70=6.53, P<0.001) but there was no IC87201 and no IC87201 × time interaction. There was no difference in sum totals of activity counts over the course of the 60 min test period between IC87201 and vehicle-treated mice (Figure 5c). ANOVA of latencies in the step-through passive avoidance trials showed an effect of time (F2,20=57.40, P<0.001) but there was no IC87201 or IC87201 × time interaction. In both control and IC87201-treated mice, post-hoc comparisons revealed that step-through latencies were significantly increased during the retention trials at 24 h and 72 h when compared with latencies during the training session (Figure 5d).
Figure 5.
Sustained effects of IC87201 in the FST. Male CD-1 mice received (a) IC87201 (0.01, 0.1, 1 and 2 mg/kg) and immobility time was determined 60 min later (top panel; Ctrl n=26, IC87201 n=8–10 per group). (b) In an independent study, mice received IC87201 (2 mg/kg) immediately after a first exposure to the TST (baseline) and immobility time was recorded 24 h later (n=10 per group). (c) Locomotor activity was examined in 10 min intervals or total activity for a duration of 60 min following IC87201 (2 mg/kg) (n=8 per group). Data are expressed as mean±SEM. ++P<0.01 vs control baseline, **P<0.01 vs 24 h control (Student-Newman-Keuls). (d) Male CD-1 mice received two doses of IC87201 (2 mg/kg): the first one was administered 60 min before the training session of the passive avoidance task and the second dose was administered immediately after the training session. Mice were tested in retention trials 24 and 72 h following training. Data are expressed as mean±SEM (n=6 per group). ++P<0.01 vs training (Student-Newman-Keuls).
DISCUSSION
The results of the present investigation demonstrate that small-molecule inhibitors of the PSD-95/nNOS interface, IC87201 and ZL006, possess antidepressant-like behavioural properties by reducing immobility time in the TST at doses that are without effect on locomotor activity. IC87201, unlike imipramine, failed to influence immobility time in the TST 1 h following drug administration. Instead, a dose-related antidepressant response was obtained 24 h later upon re-exposure to the TST. This behavioural profile is in contrast to that obtained following the administration of various classes of antidepressants, which have more immediate activity in the TST (for reviews, see Cryan et al, 2005b; Petit-Demouliere et al, 2005). Prior exposure to the TST was deemed necessary to expose the antidepressant-related activity of IC87201 as a single exposure to the test 24 h following drug administration did not influence the immobility time.
To further characterise the antidepressant-related effects of IC87201, the TST was adapted where a first exposure before drug administration was used to establish a baseline immobility score, followed by drug administration and subsequent re-testing 24 and 72 h later. Vehicle-treated control mice display an increase in immobility on re-exposure suggestive of a learned behavioural despair, which becomes apparent upon repeated exposures. Indeed the rat equivalent of the FST routinely adopts a 2 day exposure and re-exposure schedule to enhance the level of immobility observed in order to assess the antidepressant properties of test compounds (Porsolt et al, 1978). In this regard, the TST is being increasingly used in animal models of depression where increased immobility times are reported as characteristic of depression-related behaviour in response to provocative stimuli or genetically determined vulnerability. IC87201 prevents the increase in immobility obtained in the TST 24 and 72 h following drug administration. The related compound ZL006 was tested in a similar fashion and was also found to prevent the increase in immobility time associated with re-exposure to the TST 24 and 72 h following drug administration providing additional support for the hypothesis that inhibitors of the PSD-95/nNOS interface possess antidepressant-related properties. By contrast, imipramine failed to demonstrate sustained effects at the time points under investigation. Such a sustained action following a single dose represents a departure from the more traditional acute response obtained with conventional antidepressants.
NOS inhibitors have been reported to reduce immobility time acutely in the TST and FST in mice (da Silva et al, 2000; Ghasemi et al, 2008; Harkin et al, 2004; Harkin et al, 1999; Rosa et al, 2003; Volke et al, 2003). However, to our knowledge, there are no reports on delayed or sustained effects of NOS inhibitors in these paradigms following a single dose. Sustained antidepressant effects have been reported following ketamine administration in the TST and FST in mice although reports to date are equivocal (Autry et al, 2011; Bechtholt-Gompf et al, 2011; Koike et al, 2011; Maeng et al, 2008; Popik et al, 2008). Such a profile is consistent with other reports where acute administration with ketamine rapidly ameliorates anhedonic and anxiogenic behaviours in rats exposed to chronic unpredictable stress (Garcia et al, 2009; Li et al, 2011) and depression-related behaviour in a nerve injury model of neuropathic pain (Wang et al, 2011). It was therefore of interest to determine if compounds, which modulate the NMDA-R/PSD-95/nNOS signalling pathway produce sustained actions similar to those obtained with IC87201. Both ketamine and TRIM reduced immobility times in the TST 24 and 72 h following a single dose administered after a pre-test exposure. Taken together, results indicate that compounds acting on the NMDA-R/PSD-95/nNOS pathway produce sustained antidepressant-like effects in the TST following a single dose. Importantly, IC87201 treatment did not alter retention of fear memory acquired in the passive avoidance test 24 and 72 h following drug administration indicating that memory impairment does not contribute to the antidepressant-related activity of IC87201. These results are consistent with previous studies demonstrating that ZL006, TRIM and subanaesthetic doses of ketamine do not cause memory impairment in a variety of hippocampal- and amygdala-dependent tasks performed in mice (Maeng et al, 2008; Mutlu et al, 2011; Zhou et al, 2010).
The fact that IC87201 effects developed at 24 h but not acutely in the TST is not likely to be due to pharmacokinetic characteristics of the compound. Specifically, IC87201 peak plasma levels were previously reported to occur 15 min following i.p. administration of 1 or 2 mg/kg in mice and rats, respectively, and behavioural effects were delayed by 45 min, an interval thought to reflect the time needed for distribution of IC87201 to its site of action (Florio et al, 2009). Alternatively, IC87201 may produce its effects by a process of neurobiological adaptation. The pattern of response obtained with IC87201 in the TST is reminiscent of observations in a murine model of hyperalgesia where IC87201 produced a dose-dependent inhibition of NMDA-induced thermal hyperalgesia having no effect in non-hypersensitized (absence of prior NMDA challenge) mice (Florio et al, 2009). As NMDA-R-related NO production represents an important mechanism underlying the development and maintenance of neuropathic pain and IC87201 is effective following NMDA-induced hyperalgesia similar mechanisms may account for antidepressant-like activity where prior exposure to the TST may promote a NMDA-R-related sensitization, which is blocked by IC87201. In this regard, changes in neural plasticity have been proposed to account for the rapid and sustained antidepressant-related actions of ketamine. Exposure to stress has been reported to induce neural atrophy associated with a reduced density of dendritic spines and a decrease in the number and length of dendritic branches in the brain regions implicated in depression (Duman et al, 2012). Treatment with a single dose of ketamine increased the number and function of mature spines in the prefrontal cortex of rats 24 h following drug administration (Li et al, 2010) and reversed the reduction in spine density caused by chronic unpredictable stress (Li et al, 2011). Such studies have raised the possibility that increased synaptogenesis may be a relevant feature of sustained antidepressant activity of compounds modulating the NMDA-R. Further work will be required to determine if similar changes are associated with inhibitors of the PSD-95/nNOS interface.
Considering that the FST is one of the most commonly used animal models of antidepressant action (Cryan and Holmes, 2005a; Cryan and Mombereau, 2004; Petit-Demouliere et al, 2005), we then verified if the antidepressant-like effects of IC87201 generalised to the FST. In agreement with TST studies, IC87201 did not acutely reduce immobility time in the FST and this was not due to impairment in locomotion. In fact, the results of locomotor testing indicate that IC87201 does not alter ambulatory behaviour at any of the four doses tested. In contrast to our previous observations in the TST, IC87201 did not reduce immobility time in the FST upon re-exposure at 24 h, even at a dose of 2 mg/kg. This may be explained by the fact that some antidepressants differ in the pattern of their dose-response curves between the TST and FST procedures (Bai et al, 2001; Cryan et al, 2005b; Li et al, 2001) and that the TST can be more sensitive in detecting the effects of some classes of antidepressants than the FST (eg, SSRIs) (Cryan et al, 2005b).
For these reasons, a modified FST protocol based on the TST-modified paradigm was applied and the dose of IC87201 delivered was increased to 2 mg/kg in order to examine the effects of IC87201 in the FST. As seen previously with the TST-modified paradigm, a first exposure to the FST (baseline ‘stress') led to an increase in immobility time 24 h later when control animals were re-tested. This increase was evident when scoring was applied to the whole duration of a 6 min test, indicating that the first 2 min of the FST are as important as the typically scored final 4 min of the test upon re-exposure. IC87201 (2 mg/kg) attenuated the increase in immobility time observed 24 h following drug administration. These results further demonstrate the antidepressant efficacy of IC87201 in a related test of antidepressant action.
Given the antidepressant-related activity of the test compounds in the FST and TST, further tests should be carried out in other animal behaviour analogues of depressive behaviour along with some anxiety-relevant paradigms. Preliminary data shows that IC87201 (2 mg/kg, i.p.) administered concurrently with exposure to CMS prevents a deterioration in coat state of the animals (data not shown). Such a deterioration may be taken as an index of the animals inability to maintain their fur in good condition when exposed to stress and is indicative of a depression-related state (Mutlu et al, 2009). We have been encouraged by this data to investigate the effect of IC87201 on CMS-induced reductions in sucrose/saccharin preference indicative of anhedonia. Further experiments are underway to determine an optimal dosing schedule (single vs repeated administration schedules, pre-treatment, concurrent and treatment postCMS-induced anhedonia) in line with experiments described previously (Harkin et al, 2002). The effects of IC87201 (2 mg/kg, i.p.) were further assessed in the light dark box and elevated plus maze for potential anxiolytic properties 1 h and 24 h following drug administration, respectively. IC87201 did not provoke anxiety-related behaviour or produce an anxiolytic effect in either test. IC87201 did not influence the latency to cross into the dark compartment, time spent in the lit compartment or number of transitions between compartments in the light dark box. In the elevated plus maze IC87201 had no effect on the percentage of time spent in or the percentage of entries into the open arms (data not shown). The lack of anxiolytic activity implies that the compound produces a selective antidepressant-related action in keeping with the antidepressant actions of ketamine, which to date has not been reported to produce anxiolytic activity either in experimental animals or in the clinic.
Reports to date have provided evidence that IC87201 had no effects on binding to a panel of 34 targets that include receptors, transporters and ion channels (Florio et al, 2009) and that the structurally related compound ZL006 did not disrupt the interaction between CAPON/nNOS, synGAP/nNOS and NR2B/PSD-95, which are all PDZ-related interactions (Zhou et al, 2010). The serotonin transporter SERT interacts with nNOS via a PDZ motif/PDZ domain interaction, an interaction that may well be of relevance to depression and antidepressant action. More specifically, the PDZ motif (-NAV) at the C-terminus of SERT interacts with the PDZ domain of nNOS (Chanrion et al, 2007). In contrast, the internal PDZ motif of nNOS (-ETTF-) interacts with the PDZ domain of PSD-95 (Christopherson et al, 1999; Tochio et al, 2000). SERT and PSD-95 therefore interact with two distinct domains of nNOS. In addition, while SERT is distributed pre-synaptically, PSD-95 is localised at the postsynaptic density. It therefore seems unlikely that the PSD-95/nNOS inhibitor IC87201 will disrupt the SERT/nNOS interaction (for a review, see Doucet et al, 2012).
The potential for regio-selectivity actions of the test compounds targeting the PSD-95/nNOS interface is an interesting prospect. The presumed mode of action of these compounds would be a somewhat region-selective disruption of the NMDA-R/PSD-95/nNOS complex in the hippocampus, while eg, NO signalling in the basal ganglia, cerebellum and amygdala should remain unchanged. Our approach to assessing this potential is to develop lentiviral tools constructed to deliver genetic material for region specific and sustained expression of a ‘nNOS decoy' peptide designed to interrupt interaction between nNOS and PSD-95 (see Doucet et al, 2012). Data generated in the laboratory thus far indicate that delivery of the decoy to the prefrontal cortex fails to produce antidepressant-related activity (forced swim test-related mobility) in rats. Further work is required to assess the impact of nNOS decoy delivery to the hippocampus, cerebellum or basal ganglia where nNOS is expressed and to assess if NO signalling in the basal ganglia, cerebellum and amygdala remains unchanged following local or systemic administration of the test compounds.
In summary, IC87201 and ZL006 both display antidepressant-like activity in the TST and FST. While most antidepressants display acute but not sustained effects, these compounds are novel in their mode of action with delayed and sustained antidepressant activity following a single dose that may promote adaptive changes in neural plasticity. Ketamine and TRIM, two compounds acting on the NMDA-R/PSD-95/nNOS pathway demonstrated similar effects in an adapted TST established to test for delayed and sustained antidepressant effects. As putative test compounds for further development, IC87201 and ZL006 present advantages over ketamine and TRIM as they are designed to uncouple NMDA-R from nNOS in a targeted fashion not possible with either receptor antagonists or non-selective NOS inhibitors. Such an approach holds promise as a strategy to harness the antidepressant potential of ketamine without incurring adverse effects typically associated with NMDA-R antagonism. Further work is required on the molecular and cellular effects of uncoupling nNOS from the NMDA-R in vivo to understand the underlying mechanisms of action and facilitate the development of the PSD-95/nNOS interface as a therapeutic target for the design of next generation antidepressant drugs.
Acknowledgments
The authors would like to thank Henri Mattes (Novartis, Basel, Switzerland) for the generous gift of IC87201 to undertake pilot investigations on the compound. This research is supported by the Health Research Board of Ireland.
The authors declare no conflict of interest.
References
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357 (Pt 3:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of ‘behavioral despair'. Pharmacol Biochem Behav. 2001;70:187–192. doi: 10.1016/s0091-3057(01)00599-8. [DOI] [PubMed] [Google Scholar]
- Bechtholt-Gompf AJ, Smith KL, John CS, Kang HH, Carlezon WA, Cohen BM, et al. CD-1 and Balb/cJ mice do not show enduring antidepressant-like effects of ketamine in tests of acute antidepressant efficacy. Psychopharmacology (Berl) 2011;215:689–695. doi: 10.1007/s00213-011-2169-8. [DOI] [PubMed] [Google Scholar]
- Chanrion B, Mannoury la Cour C, Bertaso F, Lerner-Natoli M, Freissmuth M, Millan MJ, et al. Physical interaction between the serotonin transporter and neuronal nitric oxide synthase underlies reciprocal modulation of their activity. Proc Natl Acad Sci USA. 2007;104:8119–8124. doi: 10.1073/pnas.0610964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005a;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behaviour in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005b;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- da Silva GD, Matteussi AS, dos Santos AR, Calixto JB, Rodrigues AL. Evidence for dual effects of nitric oxide in the forced swimming test and in the tail suspension test in mice. Neuroreport. 2000;11:3699–3702. doi: 10.1097/00001756-200011270-00022. [DOI] [PubMed] [Google Scholar]
- Dhir A, Kulkarni SK. Nitric oxide and major depression. Nitric Oxide. 2011;24:125–131. doi: 10.1016/j.niox.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Doucet MV, Harkin A, Dev KK. The PSD-95/nNOS complex: new drugs for depression. Pharmacol Ther. 2012;133:218–229. doi: 10.1016/j.pharmthera.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signalling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62:35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzoljic E, De Vries R, Dzoljic MR. New and potent inhibitors of nitric oxide synthase reduce motor activity in mice. Behav Brain Res. 1997;87:209–212. doi: 10.1016/s0166-4328(97)02281-x. [DOI] [PubMed] [Google Scholar]
- Florio SK, Loh C, Huang SM, Iwamaye AE, Kitto KF, Fowler KW, et al. Disruption of nNOS-PSD95 protein-protein interaction inhibits acute thermal hyperalgesia and chronic mechanical allodynia in rodents. Br J Pharmacol. 2009;158:494–506. doi: 10.1111/j.1476-5381.2009.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Reus GZ, Stertz L, Kapczinski F, et al. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:450–455. doi: 10.1016/j.pnpbp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Ghasemi M, Sadeghipour H, Mosleh A, Sadeghipour HR, Mani AR, Dehpour AR. Nitric oxide involvement in the antidepressant-like effects of acute lithium administration in the mouse forced swimming test. Eur Neuropsychopharmacol. 2008;18:323–332. doi: 10.1016/j.euroneuro.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Gigliucci V, Buckley KN, Nunan J, O'Shea K, Harkin A. A role for serotonin in the antidepressant activity of NG-Nitro-L-arginine, in the rat forced swimming test. Pharmacol Biochem Behav. 2010;94:524–533. doi: 10.1016/j.pbb.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Guix FX, Uribesalgo I, Coma M, Munoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76:126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Harkin A, Connor TJ, Burns MP, Kelly JP. Nitric oxide synthase inhibitors augment the effects of serotonin re-uptake inhibitors in the forced swimming test. Eur Neuropsychopharmacol. 2004;14:274–281. doi: 10.1016/j.euroneuro.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Harkin A, Connor TJ, Walsh M, John N, Kelly JP. Serotonergic mediation of the antidepressant-like effects of nitric oxide synthase inhibitors. Neuropharmacology. 2003;44:616–623. doi: 10.1016/s0028-3908(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Harkin A, Houlihan DD, Kelly JP. Reduction in preference for saccharin by repeated unpredictable stress in mice and its prevention by imipramine. J Psychopharmacol. 2002;16:115–123. doi: 10.1177/026988110201600201. [DOI] [PubMed] [Google Scholar]
- Harkin AJ, Bruce KH, Craft B, Paul IA. Nitric oxide synthase inhibitors have antidepressant-like properties in mice. 1. Acute treatments are active in the forced swim test. Eur J Pharmacol. 1999;372:207–213. doi: 10.1016/s0014-2999(99)00191-0. [DOI] [PubMed] [Google Scholar]
- Hayase T, Yamamoto Y, Yamamoto K. Behavioral effects of ketamine and toxic interactions with psychostimulants. BMC Neurosci. 2006;7:25. doi: 10.1186/1471-2202-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C, McGlinchey L, Anwyl R, Rowan MJ. 7-Nitro indazole, a selective neuronal nitric oxide synthase inhibitor in vivo, impairs spatial learning in the rat. Learn Mem. 1996;2:267–278. doi: 10.1101/lm.2.6.267. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-𝒟-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Tizzano JP, Griffey K, Clay M, Lindstrom T, Skolnick P. Antidepressant-like actions of an AMPA receptor potentiator (LY392098) Neuropharmacology. 2001;40:1028–1033. doi: 10.1016/s0028-3908(00)00194-5. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Moore PK, Handy RL. Selective inhibitors of neuronal nitric oxide synthase—is no NOS really good NOS for the nervous system. Trends Pharmacol Sci. 1997;18:204–211. doi: 10.1016/s0165-6147(97)01064-x. [DOI] [PubMed] [Google Scholar]
- Murrough JW. Ketamine as a novel antidepressant: from synapse to behaviour. Clin Pharmacol Ther. 2012;91:303–309. doi: 10.1038/clpt.2011.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu O, Ulak G, Belzung C. Effects of nitric oxide synthase inhibitors 1-(2-trifluoromethylphenyl)—imidazole (TRIM) and 7-nitroindazole (7-NI) on learning and memory in mice. Fundam Clin Pharmacol. 2011;25:368–377. doi: 10.1111/j.1472-8206.2010.00851.x. [DOI] [PubMed] [Google Scholar]
- Mutlu O, Ulak G, Laugeray A, Belzung C. Effects of neuronal and inducible NOS inhibitor 1-[2-(trifluoromethyl) phenyl] imidazole (TRIM) in unpredictable chronic mild stress procedure in mice. Pharmacol Biochem Behav. 2009;92:82–87. doi: 10.1016/j.pbb.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Popik P, Kos T, Sowa-Kucma M, Nowak G. Lack of persistent effects of ketamine in rodent models of depression. Psychopharmacology (Berl) 2008;198:421–430. doi: 10.1007/s00213-008-1158-z. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Rosa AO, Lin J, Calixto JB, Santos AR, Rodrigues AL. Involvement of NMDA receptors and L-arginine-nitric oxide pathway in the antidepressant-like effects of zinc in mice. Behav Brain Res. 2003;144:87–93. doi: 10.1016/s0166-4328(03)00069-x. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Tochio H, Mok YK, Zhang Q, Kan HM, Bredt DS, Zhang M. Formation of nNOS/PSD-95 PDZ dimer requires a preformed beta-finger structure from the nNOS PDZ domain. J Mol Biol. 2000;303:359–370. doi: 10.1006/jmbi.2000.4148. [DOI] [PubMed] [Google Scholar]
- Ulak G, Mutlu O, Tanyeri P, Komsuoglu FI, Akar FY, Erden BF. Involvement of serotonin receptor subtypes in the antidepressant-like effect of TRIM in the rat forced swimming test. Pharmacol Biochem Behav. 2010;95:308–314. doi: 10.1016/j.pbb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Volke V, Wegener G, Bourin M, Vasar E. Antidepressant- and anxiolytic-like effects of selective neuronal NOS inhibitor 1-(2-trifluoromethylphenyl)-imidazole in mice. Behav Brain Res. 2003;140:141–147. doi: 10.1016/s0166-4328(02)00312-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Goffer Y, Xu D, Tukey DS, Shamir DB, Eberle SE, et al. A single subanesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology. 2011;115:812–821. doi: 10.1097/ALN.0b013e31822f16ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener G, Volke V. Nitric oxide synthase inhibitors as antidepressants. Pharmaceuticals. 2010;3:273–299. doi: 10.3390/ph3010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz Akar F, Celikyurt IK, Ulak G, Mutlu O. Effects of L-arginine on 7-nitroindazole-induced reference and working memory performance of rats. Pharmacology. 2009;84:211–218. doi: 10.1159/000235997. [DOI] [PubMed] [Google Scholar]
- Yildiz Akar F, Ulak G, Tanyeri P, Erden F, Utkan T, Gacar N. 7-Nitroindazole, a neuronal nitric oxide synthase inhibitor, impairs passive-avoidance and elevated plus-maze memory performance in rats. Pharmacol Biochem Behav. 2007;87:434–443. doi: 10.1016/j.pbb.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Zhou L, Li F, Xu HB, Luo CX, Wu HY, Zhu MM, et al. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat Med. 2010;16:1439–1443. doi: 10.1038/nm.2245. [DOI] [PubMed] [Google Scholar]
- Zhou QG, Hu Y, Hua Y, Hu M, Luo CX, Han X, et al. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J Neurochem. 2007;103:1843–1854. doi: 10.1111/j.1471-4159.2007.04914.x. [DOI] [PubMed] [Google Scholar]
- Zou LB, Yamada K, Tanaka T, Kameyama T, Nabeshima T. Nitric oxide synthase inhibitors impair reference memory formation in a radial arm maze task in rats. Neuropharmacology. 1998;37:323–330. doi: 10.1016/s0028-3908(98)00042-2. [DOI] [PubMed] [Google Scholar]