Abstract
Stress in adolescence has been widely demonstrated to have a lasting impact in humans and animal models. Developmental risk and protective factors play an important role in the responses to stress in adulthood. Mild-to-moderate stress in adolescence may resist the negative impacts of adverse events in adulthood. However, little research on resilience has been conducted. In this study, we used a predictable chronic mild stress (PCMS) procedure (5 min of daily restraint stress for 28 days) in adolescent rats (postnatal days (PNDs) 28–55) to test the resilience effect of PCMS on depressive-like behavior in the sucrose preference test and forced swim test and anxiety-like behavior in the novelty-suppressed feeding test and elevated plus maze in adulthood. We also investigated the role of mammalian target of rapamycin (mTOR) signaling in the brain during the PCMS procedure in adolescence. Moreover, we investigated the effect of PCMS in adolescence on subsequent responses to chronic unpredictable stress (CUS; PNDs 63–83) in adulthood. The results demonstrated that PCMS during adolescence produced antidepressant- and anxiolytic-like effects and increased mTOR signaling activity in the prefrontal cortex in early adulthood. Either systemic administration or intra-PFC infusion of the mTOR inhibitor rapamycin completely blocked the behavioral effects produced by PCMS in adolescence. PCMS during adolescence resisted depressive- and anxiety-like behavior caused by CUS in adulthood. These findings indicate that PCMS in adolescence can contribute to resilience against depression and anxiety caused by stress in adulthood.
Keywords: adolescence, depression, mTOR, predictable chronic mild stress, resilience
INTRODUCTION
Adolescence is a critical period of development during the transition from childhood to adulthood, and it is a key time point for the maturation of social and cognitive behavior in preparation for adulthood. It is defined by characteristic adolescent behaviors, including high levels of risk-taking, high exploration, novelty- and sensation-seeking, social interaction, high activity, and play behaviors that likely promote the acquisition of the necessary skills for maturation and independence (Spear, 2000). In rodents, the ages associated with adolescence are commonly considered to be postnatal days (PNDs) 21–59 (Tirelli et al, 2003).
Compared with adulthood, adolescence is a critical time window for stress susceptibility (Heim and Nemeroff, 2001). Stress during adolescence may have enduring consequences on mental health later in life. However, not all individuals who encounter stress or adverse events develop mental illnesses, and responses to stress vary widely (Southwick and Charney, 2012). Stress experienced during adolescence often leads to increased risk for the development of mental health problems in adulthood. Animal models indicated that stress during adolescence increased depressive- and anxiety-like behavior in adulthood (McCormick et al, 2008; Pohl et al, 2007). Conflicting studies have reported that adverse experiences during development promote resilience to adverse or stressful environments in adulthood. For example, intermittent predatory stress in adolescent rats was reported to induce greater resilience in adulthood (Kendig et al, 2011). Toth et al's (2008) research found that chronic mild stress at a young age was less or even not harmful to brain plasticity and the associated risk of depressive behavior.
The consequences of stress may also be related to its predictable or unpredictable nature (Anisman and Matheson, 2005). Unpredictable chronic mild stress always leads to depressive- and anxiety-like behavior (Zhu et al, 2011), but predictable chronic mild stress (PCMS) enhanced mood, learning, and memory in adult rats by stimulating neural stem cells and increasing neurogenesis in the hippocampus (Parihar et al, 2011). PCMS is characterized by predictability, mild intensity, and an appropriate duration of stress exposure (eg, predictable 5 min restraint stress daily for 28 days in Parihar's research). Although PCMS decreased depressive- and anxiety-like behavior for prolonged periods (Parihar et al, 2011), no study of which we are aware has reported the effects of adolescent exposure to PCMS on subsequent depressive- and anxiety-like behavior in adulthood.
Many signaling pathways have been shown to be engaged in depressive-like behavior, including the mammalian target of rapamycin (mTOR) pathway. mTOR is a conserved serine/threonine protein kinase involved in cellular translation, the control of neuronal cell size (Kwon et al, 2003), local protein synthesis in dendrites (Takei et al, 2004), and synaptic plasticity (Cammalleri et al, 2003). mTOR phosphorylates p70-kDa ribosomal protein S6 kinase and subsequently phosphorylates the S6 ribosomal protein (rpS6; Chandran et al, 2012). Recent research provided evidence that the mTOR pathway have an important role in depression. Li et al (2010, 2011) reported that the rapid antidepressant effects of ketamine were mediated by activation of the mTOR signaling pathway in the prefrontal cortex (PFC), and blockade of mTOR signaling by rapamycin completely blocked ketamine-induced molecular and behavioral alterations.
In this study, we hypothesized that (1) exposure to PCMS during adolescence provides resilience to depressive- and anxiety-like behavior in adulthood, (2) the central mTOR signaling pathway is involved in the resistance effect of PCMS during adolescence on the development of depression and anxiety in adulthood, and (3) exposure to PCMS during adolescence promotes resilience to depressive- and anxiety-like behavior induced by chronic unpredictable stress (CUS) in adulthood.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats, aged 21 days, weighed 45–55 g upon arrival to the laboratory and were housed in groups of four per cage. The animals were allowed 1 week of acclimatization after arrival before commencing stress exposure. The experiment began on PND 28. All of the animals were housed in temperature-controlled (23±2 °C) and humidity-controlled (50±5%) rooms and maintained on a 12 h/12 h light/dark cycle (lights on 0800 hours) with food and water available ad libitum. All of the animal procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the procedures were approved by the Local Animal Use Committee.
Predictable Chronic Mild Stress
The PCMS protocol was adapted from a previous report (Parihar et al, 2011). A rat restrainer was constructed of stainless steel with a grid of holes on the head side for ventilation and a sliding door on the tail side. The size of the restrainer was adjusted to the growth of the animals. The rats were restrained for 5 min each day with no mobility and then were returned to their home cage. This stress paradigm continued for 28 days, during which all of the rats were restrained at the same time every day (1500–1700 hours) to maintain the predictability of the timing of stress.
Chronic Unpredictable Stress
The CUS protocol was adapted from previous reports with minor modifications (Li et al, 2011; Willner et al, 1987; Zhu et al, 2011). The animals were exposed to a variable sequence of mild and unpredictable stressors for 21 days. A total of 10 different stressors were used, with two stressors per day. The stressors included cold for 1 h at 4 °C, water deprivation for 24 h, vibration for 1 h, tilted cages (45°) for 24 h, forced cold swim for 5 min, crowding for 24 h, soiled bedding for 24 h, light/dark cycle reversal for 36 h, food deprivation for 24 h, and tail clamp for 1 min.
Sucrose Preference Test
The sucrose preference is considered an index of anhedonia (Shi et al, 2012; Warner-Schmidt and Duman, 2008). The rats were trained to adapt to a 1% sucrose solution (w/v) for 48 h at the beginning of the experiment, in which two bottles of 1% sucrose solution were placed in each cage. After adaptation, the rats were deprived of water for 4 h, followed by the sucrose preference test (SPT), in which rats were housed in individual cages for 1 h exposure to two identical bottles, one filled with 1% sucrose solution and the other filled with water. At the end of the 1 h test, sucrose and water consumption (in ml) was measured. Sucrose preference (%) was calculated as sucrose consumption/(sucrose consumption+water consumption). All of the behavioral tests and drug administrations were performed during the dark phase.
Locomotor Activity Test
Locomotor activity was measured with an automated video tracking system (DigBehv-LM4; Shanghai Jiliang Software Technology, Shanghai, China) that contained eight identical clear Plexiglas chambers (40 cm × 40 cm × 65 cm). The video files were analyzed using DigBehv analysis software. Locomotor activity is expressed as the total distance traveled in centimeters during a predetermined period of time (in 5 min).
Novelty-Suppressed Feeding Test
The novelty-suppressed feeding test (NSFT) was adapted from previous protocols (Bodnoff et al, 1988; Zhu et al, 2011). The rats were deprived of food for 24 h before the test in their home cages. On the test day, the rats were individually placed in an open field arena (75 cm × 75 cm × 40 cm) with several small pellets of food placed on a piece of white paper (10 cm × 10 cm) in the center. Each rat was first placed in a corner of the cage. The latency to approach the food and begin eating was recorded (in s) as the main test parameter (maximum time, 10 min). Immediately after each rat was taken back to its home cage, food consumption during the first 5 min (mg/kg bodyweight) was quantified to exclude the possibility that stress affected normal appetite and feeding. A more ‘anxious' animal will take more time to begin eating in a novel environment (Bodnoff et al, 1988).
Elevated Plus Maze
The elevated plus maze (EPM) is based on a rat's natural fear of open, unprotected, and elevated spaces (Parihar et al, 2011; Pellow et al, 1985). The EPM consisted of four crossed narrow arms elevated 70 cm from the floor (50 cm long and 10 cm wide), with two open arms and two closed arms with 40 cm high walls. Illumination was 3 lx in the closed arms and 8 lx in the open arms. Each rat was first placed in the central zone of the EPM with its head facing an open arm. The rat was allowed to freely explore the maze for 5 min. The number of entries into and time (in s) spent in the open arms were recorded by two independent observers who were blind to the group assignments and sat quietly at a distance of 2.5 m from the maze.
Forced Swim Test
The forced swim test (FST) is a well-established behavioral model of despair (Porsolt et al, 1978; Zhu et al, 2011). Each rat was first individually placed into a 25 cm diameter × 65 cm height plastic cylinder filled with water that was maintained at 23–25 °C to a depth of 45 cm for 15 min. The rats were removed from the water at the end of 15 min, gently dried, and returned to their home cages. They were placed again in the cylinders 24 h later, and the 5 min FST was conducted. Immobility (in s) was recorded by two independent observers who were blind to the animal groups. Floating was defined as the minimum movement necessary to keep the rat's head above the water.
Drugs Administration and Surgical Procedure
Rapamycin (Sigma, St Louis, MO) was dissolved in 100% dimethyl sulfoxide (DMSO; Sigma) for systemic administration of 10 mg/kg (intraperitoneally). The vehicle for the control treatment was 100% DMSO (Neasta et al, 2010). For experiments that involved central administration of the inhibitor, surgical procedures began 5 days after the adolescent PCMS paradigm described above. When the rats' body weights reached 280–310 g, they were implanted with guide cannulae (22 gauge) into the medial PFC (coordinates from bregma: anterior/posterior, +3.2 mm; medial/lateral, ±1.0 mm; dorsal/ventral, −3.0 mm from dura; Li et al, 2010). The rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally; Shi et al, 2012). After a 7-day recovery period, rapamycin (0.01 nmol in 1 μl) or vehicle (DMSO) was delivered 3 h before each behavioral test at a rate of 0.25 μl/min, with the injection cannula (26 gauge) protruding 1 mm beyond the guide cannula. The dose was chosen based on Li et al (2010). The injection cannula stayed in the guide cannula for 1 min after the infusions.
Tissue Sample Preparation
The brains were extracted, and bilateral tissue punches (13 gauge) of the PFC, hippocampus, and amygdala were obtained based on our previous study (Lu et al, 2005; Shi et al, 2012). The tissue punches were homogenized (10–15 s × 3, 5 s interval) with an electrical disperser (Wiggenhauser, Sdn Bhd) after being lysed with RIPA lysis buffer with protease inhibitor and a phosphatase inhibitor mixture (Applygen Technology, Beijing, China) for 30 min. Afterwards, the homogenate was subjected to 10 000 g centrifugation at 4 °C for 20 min. All of the above procedures were performed at low temperature (0–4°C). The protein concentrations of all of the samples were determined using the BCA assay kit (Applygen Technology). The protein concentration was normalized by diluting the samples with RIPA lysis buffer.
Western Blot Assays
The samples were treated according to our previous studies (Wang et al, 2010). Briefly, equal amounts of protein (40 μg) for each sample were loaded into 8% or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The primary antibodies used were as follows: anti-phospho-mTOR (1 : 1000, Ser2448; Cell Signaling Technology), anti-mTOR (1 : 1000; Cell Signaling Technology), anti-phospho-rpS6 (1 : 500, Ser235/236; Cell Signaling Technology), anti-rpS6 (1 : 1000, 5G10; Cell Signaling Technology), anti-β-actin antibody (1 : 2000, A5316; Sigma), and horseradish peroxidase-conjugated secondary antibody (1 : 5000, goat anti-rabbit or mouse IgG; Santa Cruz Biotechnology and Vector Laboratories, respectively). The levels of all of the molecules were normalized to the level of β-actin. Band intensities were quantified by two observers who were blind to the experimental groups using the Quantity One 4.0.3 software (Bio-Rad, Hercules, CA).
Data Analysis
The data are expressed as mean±SEM. The statistical analysis of the behavioral and molecular data in the two groups was performed using Student's t-test. For the other experiments, the data were analyzed using two-way analysis of variance (ANOVA), followed by the least significant difference post hoc test (for details, see the Results section; Shi et al, 2012). Values of p<0.05 were considered statistically significant.
RESULTS
PCMS in Adolescence Produced Antidepressant- and Anxiolytic-Like Effects and Increased mTOR Activity in the PFC in Early Adulthood
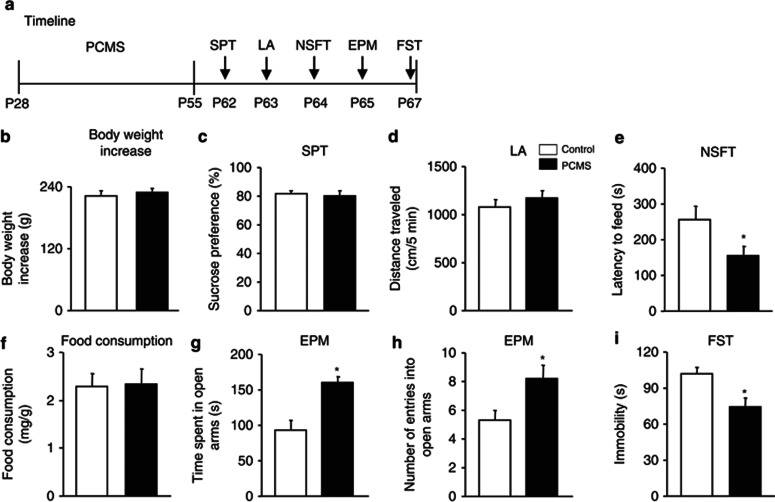
The rats were exposed to PCMS within the adolescent period (ie, PNDs 28–55), which continued for 28 days. On PND 28, the rats were randomly assigned to two groups (n=9–10 per group). One group of rats underwent the PCMS procedure for 28 days. The other rats were assigned to the control group and underwent similar handling every day for 28 days, with the exception of restraint stress. Behavioral tests began on PND 62, and each rat was subjected to the SPT, locomotor activity assessment, the NSFT, the EPM, and the FST to assess depressive- and anxiety-like behavior. The tests were conducted sequentially, one per day, to minimize the impact of immediate behavioral testing on subsequent tests. The timeline is shown in Figure 1a. PCMS during adolescence had no effects on body weight gain (p>0.05; Figure 1b), sucrose preference (p>0.05; Figure 1c), or locomotor activity (p>0.05; Figure 1d). PCMS produced anxiolytic-like effects, reflected by an decreased latency to feed (t17=2.33, p<0.05; Figure 1e), without altering home cage food consumption in the NSFT (p>0.05; Figure 1f). PCMS significantly increased the time spent in the open arms (t17=−4.19, p<0.05; Figure 1g) and the number of entries into the open arms of the EPM (t17=−2.53, p<0.05; Figure1h). PCMS produced antidepressant-like effects, reflected by a significant decrease in immobility in the FST (t17=3.08, p<0.05; Figure 1i).
Figure 1.
Predictable chronic mild stress (PCMS) during adolescence produced antidepressant- and anxiolytic-like effects in early adulthood. (a) Schematic of the timeline for PCMS (5 min daily restraint stress) exposure and the behavioral tests. All of the behavioral tests were separated by 1 day and conducted sequentially after stress exposure. We assessed the effects of PCMS during adolescence on (b) body weight gain, (c) depressive-like behavior in the sucrose preference test (SPT), (d) locomotor activity, (e, f) anxiety-like behavior in the novelty-suppressed feeding test (NSFT), reflected by the latency to feed and food consumption during 5 min in the home cage, (g, h) anxiety-like behavior in the elevated plus maze (EPM), and (i) depressive-like behavior in the forced swim test (FST). The data are expressed as mean±SEM (n=9–10 per group). *p<0.05, compared with the control group. LA, locomotor activity.
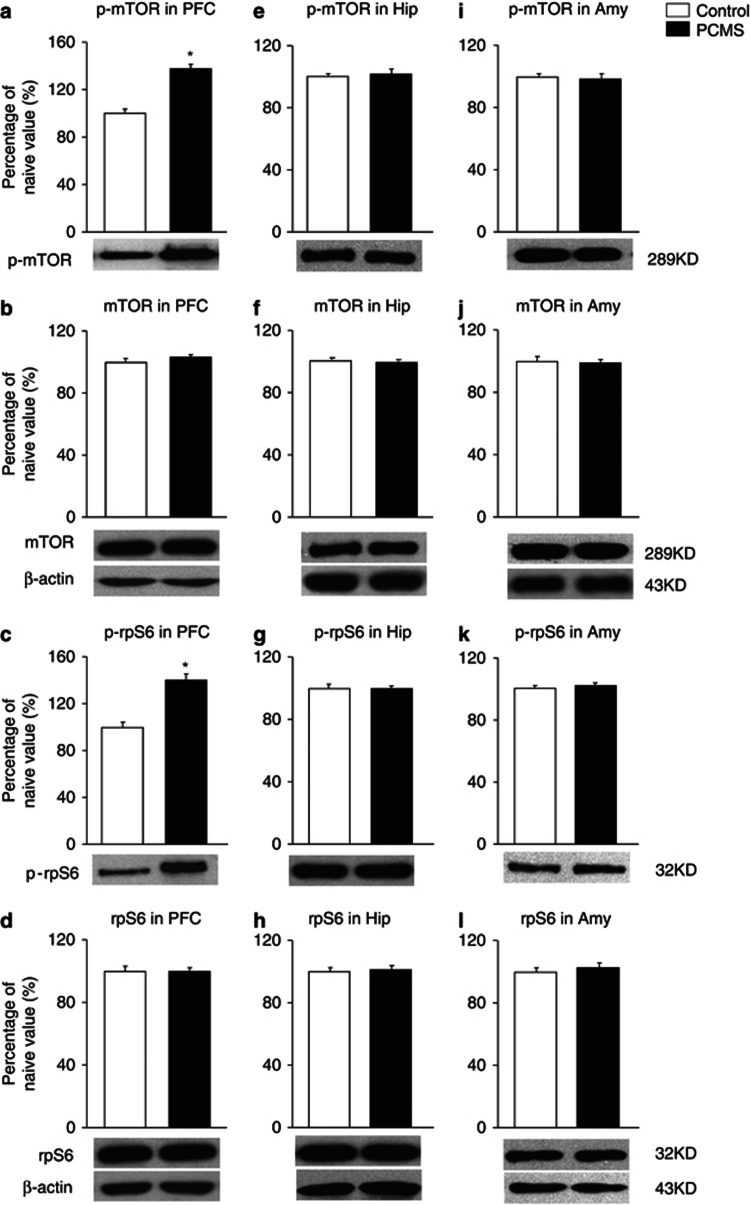
One day after all of the behavioral tests ended, the brains were removed for subsequent measurement of mTOR signaling activity. Six samples were randomly selected for the assessment of phospho-mTOR (Ser2448) and phospho-rpS6 (Ser235/236) levels using western blot (n=6 per group; Wang et al, 2010). In rats that were subjected to PCMS, the levels of phospho-mTOR (t10=−7, p<0.05; Figure 2a) and phospho-rpS6 (t10=−5.84, p<0.05; Figure 2c) were significantly elevated in the PFC but not in the hippocampus (Figure 2e and g) or amygdala (Figure 2i and k) compared with the control groups. However, total mTOR and rpS6 levels were not significantly changed in these brain regions (Figure 2b), reflected by increased mTOR signaling activity in the PFC. Altogether, these results suggest that PCMS during adolescence effectively resisted depressive- and anxiety-like behavior in early adulthood and elevated mTOR signaling activity in the PFC.
Figure 2.
Predictable chronic mild stress (PCMS) during adolescence increased mammalian target of rapamycin (mTOR) signaling activity in the prefrontal cortex (PFC). After the rats were exposed to PCMS and the behavioral tests, the levels of total and phosphorylated (p)-mTOR and S6 ribosomal protein (rpS6) were determined in the PFC, hippocampus (Hip), and amygdala (Amy) by western blot. The levels of p-mTOR and p-rpS6 (a, c) were increased in the PFC, suggesting that PCMS during adolescence increased mTOR signaling activity in the PFC. The levels of p-mTOR and p-rpS6 were not altered in the Hip (e, g) or Amy (i, k). Total mTOR and rpS6 levels were not changed in these brain regions (b, d, f, h, j and l). The data are expressed as mean±SEM. Representative band intensities of the western blot are shown on the bottom (n=6 per group). *p<0.05, compared with the control group.
Systemic Inhibition of mTOR Signaling Activity Reversed the Antidepressant- and Anxiolytic-Like Behavior Produced by PCMS During Adolescence
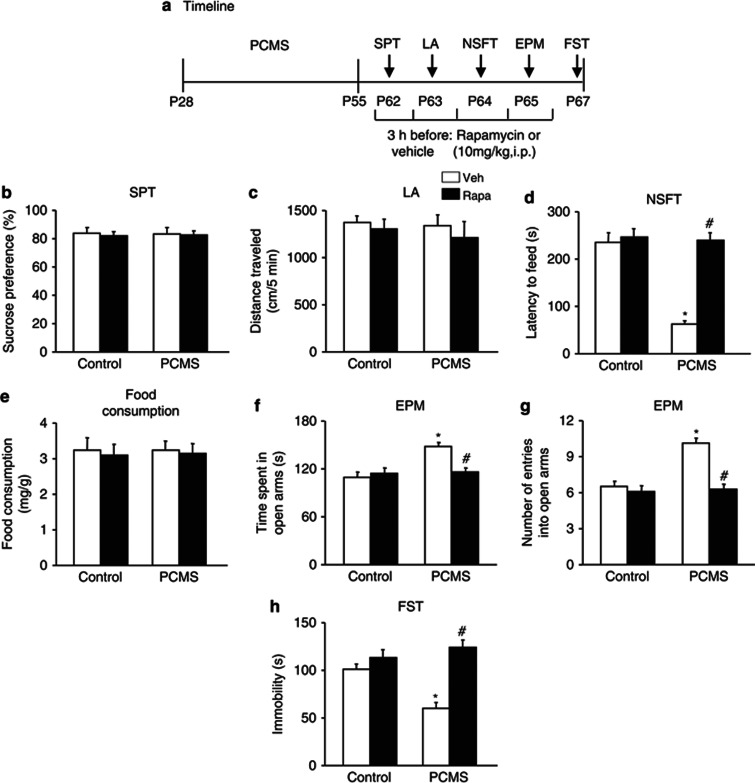
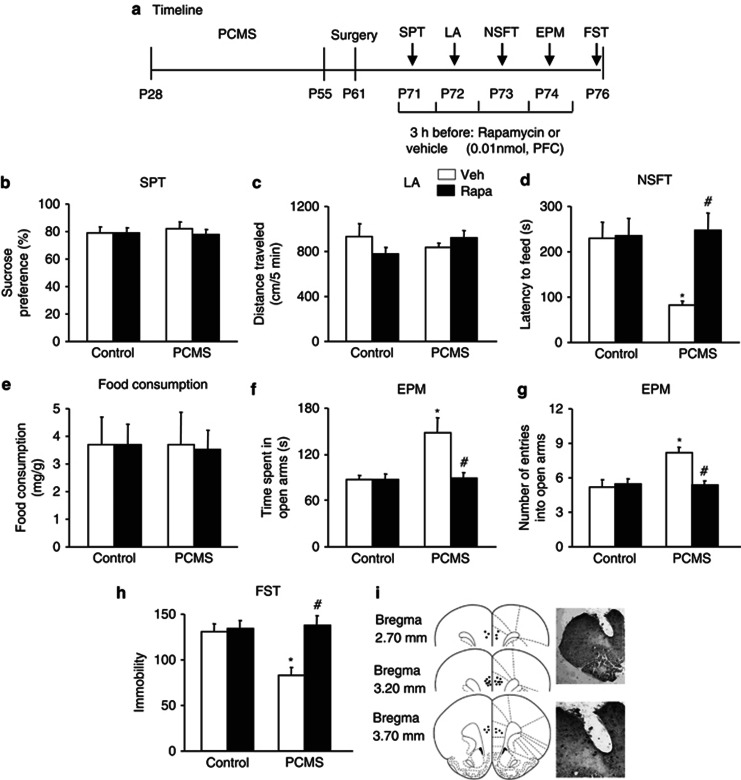
To determine the relationship between mTOR signaling and the antidepressant- and anxiolytic-like effects of PCMS exposure during adolescence, four groups of rats (n=10–11 per group) were assigned to PCMS or non-PCMS conditions during adolescence and then received vehicle (DMSO) or rapamycin (10 mg/kg, intraperitoneally) during early adulthood. Rapamycin was administered 3 h before each behavioral test. All of the behavioral tests were separated by 1 day and were conducted sequentially after stress exposure. The timeline of this experiment is shown in Figure 3a. Rapamycin did not influence sucrose preference (p>0.05; Figure 3b) or locomotor activity (p>0.05; Figure 3c). Rapamycin completely blocked the resilience effects of PCMS during adolescence, reflected by no changes in the latency to feed (p>0.05; Figure 3d), without altering home cage food consumption in the NSFT (p>0.05; Figure 3e), the time spent in the open arms or number of entries into the open arms in the EPM (p>0.05; Figure 3f), and immobility in the FST (p>0.05; Figure 3h), compared with the control groups. A two-way ANOVA, with inhibitor treatment (vehicle and rapamycin) and stress (non-PCMS and PCMS) as the between-subjects factors, was used to analyze the effects of mTOR inhibition on the behavioral measures. The analysis revealed no significant main effects or interactions (all p>0.05) for sucrose preference, locomotor activity, or home cage food consumption in the NSFT. Significant main effects of treatment (F1,37=32.48, p<0.05) and stress (F1,37=29.34, p<0.05) and a significant treatment × stress interaction (F1,37=25.18, p<0.05) were found for the latency to feed in the NSFT. Significant main effects of treatment (F1,37=5.67, p<0.05; F1,37=21.02, p<0.05) and stress (F1,37=10.83, p<0.05; F1,37=16.74, p<0.05) and a significant treatment × stress interaction (F1,37=9.90, p<0.05; F1,37=13.81, p<0.05) were found for the time spent in the open arms and number of entries into the open arms in the EPM, respectively. Significant main effects of treatment (F1,37=27.48, p<0.05) and stress (F1,37=4.48, p<0.05) and a significant treatment × stress interaction (F1,37=12.61, p<0.05) were found for immobility in the FST (Figure 3b–h).
Figure 3.
Systemic rapamycin (Rapa) reversed predictable chronic mild stress (PCMS)-induced antidepressant- and anxiolytic-like effects. (a) Schematic of the timeline for PCMS exposure, drug administration, and the behavioral tests. Vehicle (Veh) or Rapa (10 mg/kg, intraperitoneally) was injected 3 h before each behavioral test. All of the behavioral tests were separated by 1 day and conducted sequentially after stress exposure. We assessed the effects of Rapa on (b) depressive-like behavior in the sucrose preference test (SPT), (c) locomotor activity, (d, e) anxiety-like behavior in the novelty-suppressed feeding test (NSFT), reflected by the latency to feed and food consumption during 5 min in the home cages, (f, g) anxiety-like behavior in the elevated plus maze (EPM), and (h) depressive-like behavior in the forced swim test (FST). The data are expressed as mean±SEM (n=10–11 per group). *p<0.05, compared with the control group; #p<0.05, PCMS-Rapa group compared with PCMS-Veh group. LA, locomotor activity.
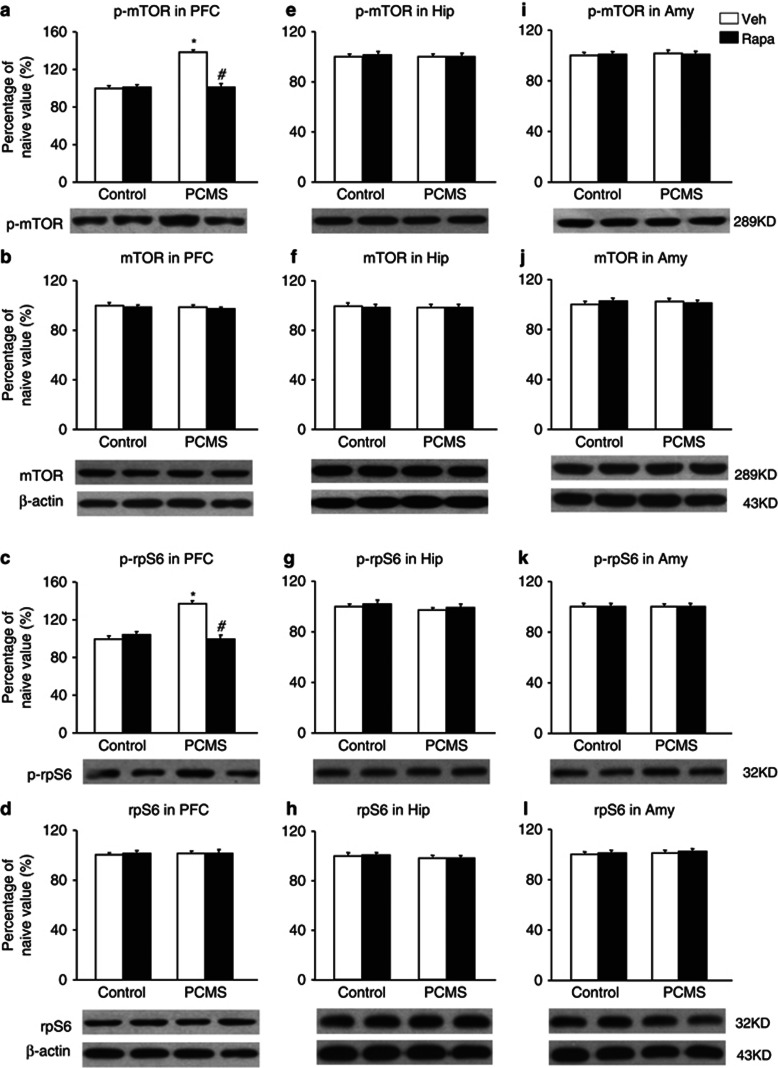
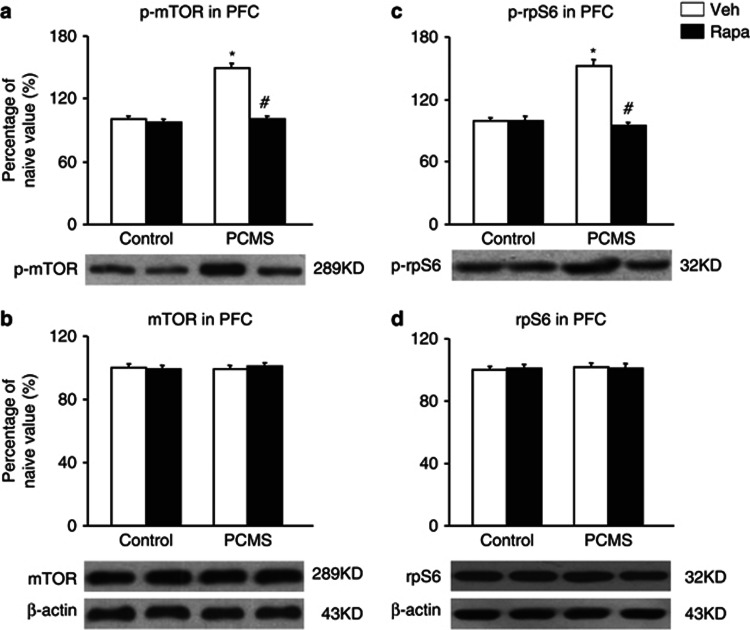
One day after the behavioral tests, the brains were removed, and six samples were randomly selected for subsequent western blot (n=6 per group; Wang et al, 2010). The data analysis showed that rapamycin completely blocked the effect of PCMS on the elevations of phospho-mTOR and phospho-rpS6 levels in the PFC compared with the control groups and did not change phospho-mTOR or phospho-rpS6 levels in the PFC in the control groups (p>0.05; Figure 4a and c). The analysis revealed main effects of treatment (F1,20=30.67, p<0.05; F1,20=48.99, p<0.05) and stress (F1,20=27.43, p<0.05; F1,20=59.44, p<0.05) and a treatment × stress interaction (F1,20=45.76, p<0.05; F1,20=57.83, p<0.05) for phospho-mTOR and phospho-rpS6 levels in the PFC, respectively (Figure 4a and c). The levels of phospho-mTOR and phospho-rpS6 in the hippocampus (Figure 4e and g) and amygdala (Figure 4i and k) were not significantly changed in the four groups, and total mTOR and rpS6 levels were not significantly changed in these three brain regions in the four groups (p>0.05; Figure 4b).
Figure 4.
Systemic rapamycin (Rapa) resisted the elevation of mammalian target of Rapa (mTOR) signaling activity in the prefrontal cortex (PFC). After the rats were exposed to predictable chronic mild stress (PCMS), drug administration, and the behavioral tests, the levels of total and phosphorylated (p) mTOR and S6 ribosomal protein (rpS6) in the PFC, hippocampus, and amygdala were determined by western blot. Rapa blocked the elevation of p-mTOR and p-rpS6 levels in the PFC compared with the control groups (a, c) and did not change p-mTOR or p-rpS6 levels in the hippocampus (Hip) (e, g) or amygdala (Amy) (i, k). Total mTOR and rpS6 levels were not significantly changed in the PFC (b, d), Hip (f, h), or Amy (j, l) in the four groups. The data are expressed as mean±SEM. Representative band intensities of the western blot are shown on the bottom (n=6 per group). *p<0.05, compared with the control group; #p<0.05, PCMS-Rapa group compared with PCMS-vehicle (Veh) group.
Altogether, these results indicate that systemic rapamycin administration completely reversed the antidepressant- and anxiolytic-like behavior produced by PCMS during adolescence, and these effects were mediated by mTOR signaling in the PFC.
Intra-PFC Infusion of Rapamycin Reversed the Antidepressant- and Anxiolytic-Like Behavior Produced by PCMS During Adolescence
To further determine the relationship between mTOR signaling in the PFC and antidepressant- and anxiolytic-like effects of PCMS exposure during adolescence, four groups of rats (n=10 per group) were also assigned to PCMS and non-PCMS conditions during adolescence and then received vehicle (DMSO) or rapamycin (0.01 nmol, PFC) during early adulthood. Rapamycin was administered 3 h before each behavioral test. All of the behavioral tests were separated by 1 day and were conducted sequentially after stress exposure. The timeline of this experiment is shown in Figure 5a. Rapamycin did not influence sucrose preference (p>0.05; Figure 5b) or locomotor activity (p>0.05; Figure 5c) and completely blocked the resilience effects of PCMS during adolescence, reflected by no changes in the latency to feed (p>0.05; Figure 5d), without altering home cage food consumption in the NSFT (p>0.05; Figure 5e), the time spent in the open arms or number of entries into the open arms in the EPM (p>0.05; Figure 5f), and immobility time in the FST (p>0.05; Figure 5h), compared with the control groups. A two-way ANOVA, with inhibitor treatment (vehicle and rapamycin) and stress (non-PCMS and PCMS) as the between-subjects factors, was used to analyze the effects of mTOR inhibition on the behavioral measures. The analysis revealed no significant main effects or interactions (all p>0.05) for sucrose preference, locomotor activity, or home cage food consumption in the NSFT. Significant main effects of treatment (F1,36=7.01, p<0.05) and stress (F1,36=4.31, p<0.05) and a significant treatment × stress interaction (F1,36=6.02, p<0.05) were found for the latency to feed in the NSFT. Significant main effects of treatment (F1,36=6.41, p<0.05; F1,36=7.2, p<0.05) and stress (F1,36=6.96, p<0.05; F1,36=9.69, p<0.05) and a significant treatment × stress interaction (F1,36=6.41, p<0.05; F1,36=11.07, p<0.05) were found for the time spent in the open arms and number of entries into the open arms in the EPM, respectively. Significant main effects of treatment (F1,36=10.63, p<0.05) and stress (F1,36=6.56, p<0.05) and a significant treatment × stress interaction (F1,36=8.21, p<0.05) were found for immobility in the FST (Figure 5b–h).
Figure 5.
Intra-prefrontal cortex (PFC) infusion of rapamycin (Rapa) reversed predictable chronic mild stress (PCMS)-induced antidepressant- and anxiolytic-like effects. (a) Schematic of the timeline for PCMS exposure, drug administration, and the behavioral tests. Vehicle (Veh) or Rapa (0.01 nmol, PFC) was injected 3 h before each behavioral test. All of the behavioral tests were separated by 1 day and conducted sequentially after stress exposure. We assessed the effects of Rapa on (b) depressive-like behavior in the sucrose preference test (SPT), (c) locomotor activity, (d, e) anxiety-like behavior in the novelty-suppressed feeding test (NSFT), reflected by the latency to feed and food consumption during 5 min in the home cages, (f, g) anxiety-like behavior in the elevated plus maze (EPM), and (h) depressive-like behavior in the forced swim test (FST). (i) Schematic representation of the injection sites in the PFC. The data are expressed as mean±SEM (n=10 per group). *p<0.05, compared with the control group; #p<0.05, PCMS-Rapa group compared with PCMS-Veh group. LA, locomotor activity.
One day after the behavioral tests, the brains were removed, and six samples were randomly selected for subsequent western blot (n=6 per group; (Wang et al, 2010). The data analysis showed that rapamycin completely blocked the effect of PCMS on the elevations of phospho-mTOR and phospho-rpS6 levels in the PFC compared with the control groups and did not change phospho-mTOR or phospho-rpS6 levels in the PFC in the control groups (p>0.05; Figure 6a and c). The analysis revealed main effects of treatment (F1,20=51.68, p<0.05; F1,20=48.51, p<0.05) and stress (F1,20=54.52, p<0.05; F1,20=33.6, p<0.05) and a treatment × stress interaction (F1,20=43.02, p<0.05; F1,20=48.01, p<0.05) for phospho-mTOR and phospho-rpS6 levels in the PFC, respectively (Figure 6a and c). Total mTOR and rpS6 levels were not significantly changed in the PFC in the four groups (p>0.05; Figure 6b and d).
Figure 6.
Intra-prefrontal cortex (PFC) infusion of rapamycin (Rapa) resisted the elevation of mammalian target of Rapa (mTOR) signaling activity in the PFC. Rapa blocked the elevation of phosphorylated (p)-mTOR and p-S6 ribosomal protein (rpS6) levels in the PFC compared with the control groups (a, c). Total mTOR and rpS6 levels were not significantly changed in the PFC (b, d) in the four groups. The data are expressed as mean±SEM. Representative band intensities of the western blot are shown on the bottom (n=6 per group). *p<0.05, compared with the control group; #p<0.05, predictable chronic mild stress (PCMS)-Rapa group compared with PCMS-vehicle (Veh) group.
Altogether, these results indicate that the antidepressant- and anxiolytic-like behaviors produced by PCMS during adolescence were mediated by mTOR signaling in the PFC.
PCMS in Adolescence Resisted CUS-Induced Depressive- and Anxiety-Like Behavior in Adulthood
Four groups of rats were used in a 2 (non-PCMS and PCMS) × 2 (non-CUS and CUS) factorial design to test the effects of PCMS during adolescence (PNDs 28–55) on responses to CUS (PNDs 63–83) in adult rats (n=9–10 per group). Briefly, 7 days after the PCMS procedure (ie, PND 63), the rats in the PCMS group were separated into two groups. One group underwent CUS for 21 days, and the other rats were assigned to the control group. The non-PCMS control group was also separated into two groups: CUS and control. At 2 days after CUS (ie, PND 86), all of the behavioral tests were performed sequentially to measure depressive- and anxiety-like behavior. The timeline of this experiment is shown in Figure 7a.
Figure 7.
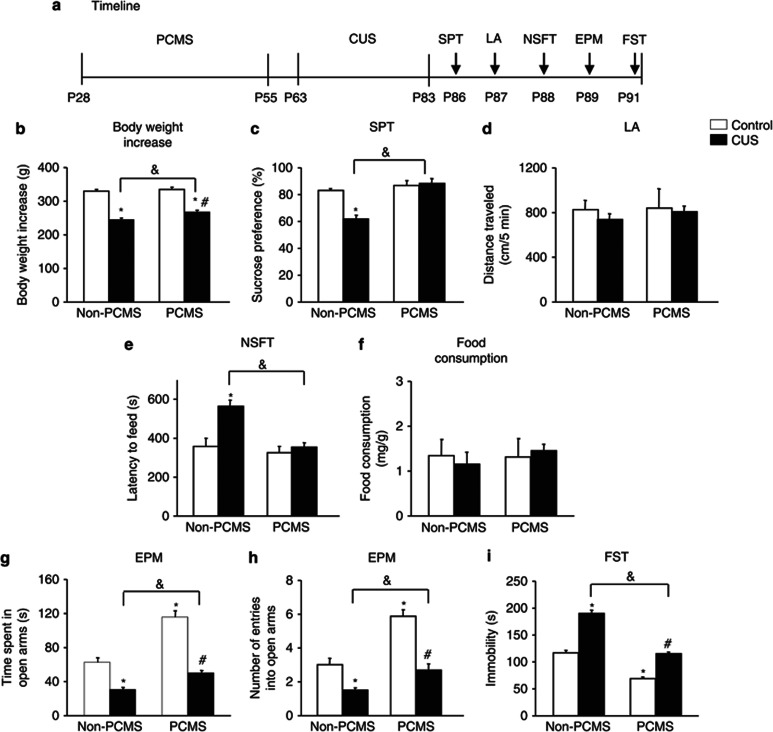
Predictable chronic mild stress (PCMS) during adolescence resisted chronic unpredictable stress (CUS)-induced depressive- and anxiety-like behavior in adulthood. (a) Schematic of the timeline for PCMS and CUS exposure and the behavioral tests. All of the behavioral tests were separated by 1 day and conducted sequentially after stress exposure. We assessed the effects of PCMS and CUS on (b) body weight gain, (c) depressive-like behavior in the sucrose preference test (SPT), (d) locomotor activity, (e, f) anxiety-like behavior in the novelty-suppressed feeding test (NSFT), reflected by the latency to feed and food consumption during 5 min in the home cages, (g, h) anxiety-like behavior in the elevated plus maze (EPM), and (i) depressive-like behavior in the forced swim test (FST). The data are expressed as mean±SEM (n=9-10 per group). *p<0.05, compared with the control group; #p<0.05, PCMS+CUS group compared with PCMS+control group; &p<0.05, PCMS+CUS group compared with the CUS group. LA, locomotor activity.
Body weight gain in the group exposed to PCMS followed by CUS was significantly less than in the control and PCMS groups (p<0.05), but significantly more than in the CUS group (p<0.05; Figure 7b). Locomotor activity was not influenced by stress in adolescence or adulthood (p>0.05; Figure 7d). However, PCMS during adolescence resisted the depressive-like behavior induced by CUS during adulthood, measured in the SPT and FST (Figure 7c and i), and anxiety-like behavior, measured in the NSFT and EPM (Figure 7e–h). A two-way ANOVA, with stress in adolescence (non-PCMS and PCMS) and stress in adulthood (non-CUS and CUS) as the between-subjects factors, revealed main effects of stress in adolescence (F1,35=12.10, p<0.05) and stress in adulthood (F1,35=291.75, p<0.05) and a stress in adolescence × stress in adulthood interaction (F1,35=4.39, p<0.05) for body weight gain (Figure 7b). Main effects of stress in adolescence (F1,35=25.36, p<0.05) and stress in adulthood (F1,35=10.44, p<0.05) and a stress in adolescence × stress in adulthood interaction (F1,35=14.23, p<0.05) were found for sucrose preference (Figure 7c). No significant main effects or interactions were found for locomotor activity (all p>0.05; Figure 7d). Main effects of stress in adolescence (F1,35=12.62, p<0.05) and stress in adulthood (F1,35=11.83, p<0.05) and a stress in adolescence × stress in adulthood interaction (F1,35=6.73, p<0.05) were found for the latency to feed in the NSFT (Figure 7e), but no significant main effects or interactions were found for home cage food consumption (all p>0.05; Figure 7f). Significant main effects of stress in adolescence (F1,35=52.14, p<0.05; F1,35=37.97, p<0.05) and stress in adulthood (F1,35=95.37, p<0.05; F1,35=49.93, p<0.05) and a stress in adolescence × stress in adulthood interaction (F1,35=10.53, p<0.05; F1,35=6.48, p<0.05) were found for the time spent in the open arms (Figure 7g) and the number of entries into the open arms (Figure 7h) in the EPM, respectively. Main effects of stress in adolescence (F1,35=163.79, p<0.05) and stress in adulthood (F1,35=155.39, p<0.05) and a stress in adolescence × stress in adulthood interaction (F1,35=7.65, p<0.05) were found for immobility in the FST (Figure 7i).
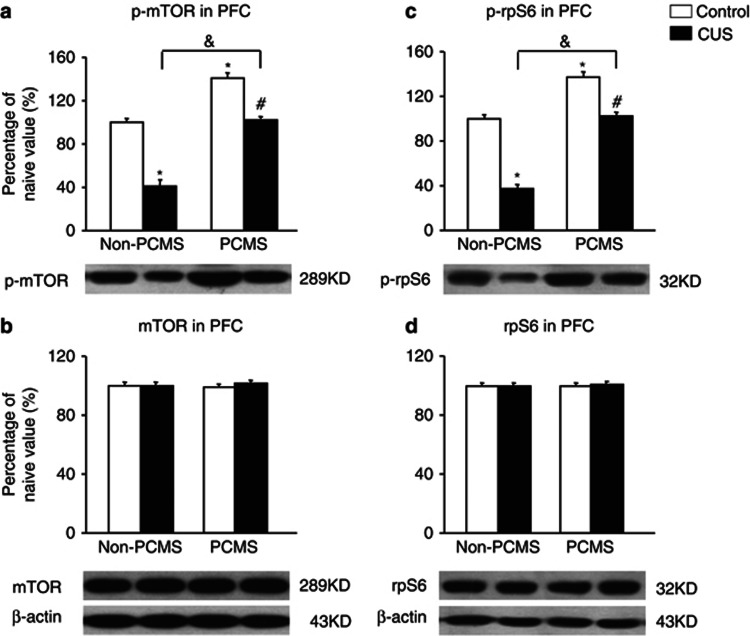
One day after the behavioral tests, the brains were removed, and six samples were randomly selected for subsequent western blot (n=6 per group; Wang et al, 2010). The data analysis showed that PCMS during adolescence increased phospho-mTOR and phospho-rpS6 levels in the PFC (p<0.05; Figure 8a and c), and CUS in adulthood decreased phospho-mTOR and phospho-rpS6 levels in the PFC (p<0.05; Figure 8a and c). PCMS during adolescence resisted the CUS-induced decrease in mTOR signaling activity in the PFC in adulthood, in which the levels of phospho-mTOR and phospho-rpS6 in the PCMS+CUS group were not significantly changed compared with the no stress group (p>0.05; Figure 8a and c). The analysis revealed main effects of stress in adolescence (F1,20=162.37, p<0.05; F1,20=161.61, p<0.05) and stress in adulthood (F1,20=159.28, p<0.05; F1,20=146.27, p<0.05) and a stress in adolescence × stress in adulthood interaction (F1,20=5.15, p<0.05; F1,20=12.35, p<0.05) for phospho-mTOR and phospho-rpS6 levels in the PFC, respectively (Figure 8a and c). Total mTOR and rpS6 levels were not significantly changed in the PFC in the four groups (p>0.05; Figure 8b and d).
Figure 8.
Predictable chronic mild stress (PCMS) during adolescence resisted the chronic unpredictable stress (CUS)-induced decrease in mammalian target of rapamycin (mTOR) signaling activity in the prefrontal cortex (PFC) in adulthood. PCMS during adolescence increased phosphorylated (p)-mTOR and p-S6 ribosomal protein (rpS6) levels in the PFC (a, c), and CUS in adulthood decreased p-mTOR and p-rpS6 levels in the PFC (a, c). PCMS during adolescence resisted the CUS-induced decrease in mTOR signaling activity in the PFC in adulthood (a, c). Total mTOR and rpS6 levels were not significantly changed in the PFC (b, d) in the four groups. The data are expressed as mean±SEM. Representative band intensities of the western blot are shown on the bottom (n=6 per group). *p<0.05, compared with the control group; #p<0.05, PCMS+CUS group compared with PCMS+control group; &p<0.05, PCMS+CUS group compared with the CUS group.
Altogether, these results suggest that PCMS during adolescence resisted the depressive- and anxiety-like behavior induced by CUS in adulthood, and PCMS during adolescence resisted the CUS-induced decrease in mTOR signaling activity in the PFC in adulthood.
DISCUSSION
This study showed that PCMS during adolescence promoted antidepressant- and anxiolytic-like effects in early adulthood, and mTOR signaling in the PFC played an important role in these processes. Moreover, PCMS during adolescence enhanced the ability to resist depressive- and anxiety-like behavior induced by CUS in adulthood.
PCMS is a mild and chronic stressor and that occurs predictably. We found that PCMS in adolescence had no effect on body weight gain, contrary to previous studies that found that adolescent rodents were more sensitive to stress-induced weight loss (Chiba et al, 2012). These results suggest that PCMS in adolescence had no effect on physical development from adolescence to adulthood. Furthermore, PCMS in adolescence resisted anhedonia-, despair-, and anxiety-like behavior in the SPT, FST, NSFT, and EPM. The resilience associated with PCMS did not focus on the absence of depressive- and anxiety-like behavior, but promoted antidepressant- and anxiolytic-like effects that depend on active resilience mechanisms (Russo et al, 2012).
Several underlying mechanisms of the resilience associated with PCMS in adolescence may be proposed. One mechanism involves the characteristics of the stressor, including controllability, predictability, ambiguity, chronicity, and intermittence (Anisman and Matheson, 2005). Unpredictability refers to an event that will happen, but its occurrence is not signaled by environmental cues (Anisman and Matheson, 2005). In human and animal studies, predictable and unpredictable stressors may have different effects. Unpredictable events are generally thought to have more aversive effects than predictable events. Unpredictable events are more likely to provoke excessive brain monoamine utilization (Tsuda et al, 1989), variations in stress-related hormones (De Boer et al, 1989), disturbances in immune functioning (Mormede et al, 1988), and changes in blood pressure (Lawler et al, 1993). Consistent with previous studies, the stressor used in our study was predictable. Exposure during adolescence produced resistance to depressive- and anxiety-like behavior in adulthood. However, many other chronic restraint stress procedures have been shown to cause depressive- and anxiety-like behavior (Chiba et al, 2012; Eiland et al, 2012; Swiergiel et al, 2007). Although these stress procedures were predictable, the excessive intensity and duration of the stressor exceeded the endurance of organism, which may result in a much greater increase in corticosterone levels and aberrant adaptation, thus making the organism unable to cope with life changes, eventually leading to depressive- and anxiety-like behavior.
A second explanation for these results is that stress during adolescence determines behavioral strategies to cope with subsequent stressors in adulthood. The brain undergoes extensive morphological and functional remodeling in adolescence (Casey et al, 2008; Spear, 2000). Risk and protective factors during development greatly impact brain development and shape neural circuits that regulate future responses to stress and adversity (Southwick and Charney, 2012). Stressful events during early life were associated with later non-adaptive behaviors (Spear, 2000) and represented a significant risk factor for the development of mental disorders in later life (Heim and Nemeroff, 2001). Many studies in animal models found that juvenile stress protocols resulted in long-lasting alterations in stress responses and depressive- and anxiety-like behavior in adulthood (Tsoory et al, 2007). For example, rats exposed to chronic juvenile stress (ie, 6 h restraint daily from PNDs 20 to 41) exhibited anhedonia, reflected by sucrose preference (Eiland et al, 2012). In contrast, mild-to-moderate stressors that are controllable can have an inoculating effect (Levine, 1962) and help adolescents develop adaptive stress responses and become more resilient to the negative effects of future stressors (Southwick and Charney, 2012). Animal studies have shown that intermittent mother–offspring separation in squirrel monkeys produced fewer signs of anxiety and more exploration in a novel environment (Parker et al, 2004). Maternal deprivation (PNDs 7–14), combined with CUS (ie, elevated platform, forced swim, and food deprivation; PNDs 28–84) promoted a greater degree of resilience to stress than maternal deprivation alone. Juvenile-onset CUS (PNDs 30–90) induced more resilience than adult-onset CUS (PNDs 60–90; Ricon et al, 2012). Animals subjected to early stress during postnatal life and young adulthood exhibited enhanced hippocampal neurogenesis, decreased repressive histone methylation at brain-derived neurotrophic factor (BDNF) promoter IV, enhanced BDNF levels, and improved performance in the stress-associated Morris water maze. In contrast, middle-aged early stress animals exhibited a reduction of neurogenesis and epigenetic modifications associated with decreased BDNF IV expression (Suri et al, 2012). Thus, the characteristics, timing, duration, and severity of stressors are likely to be important factors in determining whether early experiences ultimately produce a protective or deleterious outcome.
Our results also showed that PCMS in adolescence elevated mTOR signaling activity in the PFC. Rapamycin administration completely blocked the behavioral effects produced by PCMS in adolescence, indicating that mTOR signaling is involved in the PCMS-induced resilience to adolescence depression and anxiety in adulthood. The mTOR pathway has been shown to play an important role in depression. The majority of evidence of mTOR function comes from studies that utilized rapamycin (Li et al, 2010, 2011). Rapamycin inhibits mTOR signaling (Kim et al, 2002). Recent animal studies showed that chronic treatment with the N-methyl-D-aspartate (NMDA) receptor antagonist MK-801 activated mTOR and related proteins in rat cortical tissue (Yoon et al, 2008). Li et al (2010) reported that the NMDA receptor antagonist ketamine, a fast-acting antidepressant, rapidly activated the mTOR signaling pathway, leading to increased synaptic signaling proteins and an increased number and function of new spine synapses in the PFC in rats. Li et al (2011) also found that a single dose of ketamine rapidly reversed the behavioral and synaptic deficits caused by long-term CUS exposure in an mTOR-dependent manner. Other studies found robust deficits in the mTOR signaling pathway in the PFC in subjects clinically diagnosed with major depressive disorder (Jernigan et al, 2011) and reduced the phosphorylation of mTOR signaling pathway components in the amygdala in rats exposed to CUS (Chandran et al, 2012). Therefore, activation of the mTOR pathway may be related to antidepressant actions.
In addition, our findings showed that PCMS in adolescence resisted the depressive- and anxiety-like effects of CUS in adulthood. The CUS model of depression has high validity (Willner, 1997), and numerous studies have confirmed that CUS increases depressive- and anxiety-like behavior (Zhu et al, 2011). In this study, PCMS in adolescence not only resisted acute stress in the FST but also resisted chronic stress-induced mood deficits.
The effects of PCMS in adolescence that resisted the effects of CUS in adulthood may be mediated by adaptive neurobiological changes, such as the modulation of mTOR signaling, activation of glutamatergic signaling (Adamec et al, 2012), modulation of the hypothalamic-pituitary-adrenal axis (Romeo et al, 2007), elevation of corticosterone levels at an optimal level (Feder et al, 2009), elevation of neuropeptide Y levels (Cohen et al, 2012), and enhancement of hippocampal neurogenesis (Joels, 2008; Toth et al, 2008), thus leading to adaptation and resistance and making the organism better able to cope with impending challenges (Parihar et al, 2011).
The processes by which PCMS in adolescence increases resilience in adulthood may involve alterations in the neural encoding of action in limbic circuitry. A recent study provided circuit-level insights into the causal dynamics of depression-related behavior (Tye et al, 2013). Our future studies will target specific circuits and continue to advance our understanding of the biological bases of stress and resilience.
Our findings indicate that specific adolescent stress exposure may improve the ability to deal with stressful experiences in adulthood and not impair the ability to cope with stress. The findings also suggest that mTOR signaling may be a potential target for the development of new treatments for stress-associated mental disorders.
Acknowledgments
This work was supported, in part, by the Natural Science Foundation of China (nos. 31230033, 81221002, and 91132716) and Specific Foundation for Doctoral Institute Program from the State Education Ministry of China (no. 20121323120002).
The authors declare no conflict of interest.
References
- Adamec R, Toth M, Haller J, Halasz J, Blundell J. A comparison of activation patterns of cells in selected prefrontal cortical and amygdala areas of rats which are more or less anxious in response to predator exposure or submersion stress. Physiol Behav. 2012;105:628–638. doi: 10.1016/j.physbeh.2011.09.016. [DOI] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Cammalleri M, Lutjens R, Berton F, King AR, Simpson C, Francesconi W, et al. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci USA. 2003;100:14368–14373. doi: 10.1073/pnas.2336098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran A, Iyo AH, Jernigan CS, Legutko B, Austin MC, Karolewicz B. Reduced phosphorylation of the mTOR signaling pathway components in the amygdala of rats exposed to chronic stress. Prog Neuropsychopharmacol Biol Psychiatry. 2012;40:240–245. doi: 10.1016/j.pnpbp.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:112–119. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Cohen H, Liu T, Kozlovsky N, Kaplan Z, Zohar J, Mathe AA. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology. 2012;37:350–363. doi: 10.1038/npp.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boer SF, Van der Gugten J, Slangen JL. Plasma catecholamine and corticosterone responses to predictable and unpredictable noise stress in rats. Physiol Behav. 1989;45:789–795. doi: 10.1016/0031-9384(89)90296-5. [DOI] [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joels M. Functional actions of corticosteroids in the hippocampus. Eur J Pharmacol. 2008;583:312–321. doi: 10.1016/j.ejphar.2007.11.064. [DOI] [PubMed] [Google Scholar]
- Kendig MD, Bowen MT, Kemp AH, McGregor IS. Predatory threat induces huddling in adolescent rats and residual changes in early adulthood suggestive of increased resilience. Behav Brain Res. 2011;225:405–414. doi: 10.1016/j.bbr.2011.07.058. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Baker SJ. mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci USA. 2003;100:12923–12928. doi: 10.1073/pnas.2132711100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler JE, Naylor SK, Abel MM. Predictability of foot shock differentially affects the phasic blood pressure of SHR, BHR, and WKY rats. Physiol Behav. 1993;54:369–374. doi: 10.1016/0031-9384(93)90125-y. [DOI] [PubMed] [Google Scholar]
- Levine S. Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science. 1962;135:795–796. doi: 10.1126/science.135.3506.795-a. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-𝒟-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Mormede P, Dantzer R, Michaud B, Kelley KW, Le Moal M. Influence of stressor predictability and behavioral control on lymphocyte reactivity, antibody responses and neuroendocrine activation in rats. Physiol Behav. 1988;43:577–583. doi: 10.1016/0031-9384(88)90211-9. [DOI] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci USA. 2010;107:20093–20098. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Kuruba R, Shuai B, Shetty AK. Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol Psychiatry. 2011;16:171–183. doi: 10.1038/mp.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM. Prospective investigation of stress inoculation in young monkeys. Arch Gen Psychiatry. 2004;61:933–941. doi: 10.1001/archpsyc.61.9.933. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL. Repeated exposure to stress across the childhood-adolescent period alters rats' anxiety- and depression-like behaviors in adulthood: the importance of stressor type and gender. Behav Neurosci. 2007;121:462–474. doi: 10.1037/0735-7044.121.3.462. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Ricon T, Toth E, Leshem M, Braun K, Richter-Levin G. Unpredictable chronic stress in juvenile or adult rats has opposite effects, respectively, promoting and impairing resilience. Stress. 2012;15:11–20. doi: 10.3109/10253890.2011.572207. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Karatsoreos IN, Jasnow AM, McEwen BS. Age- and stress-induced changes in corticotropin-releasing hormone mRNA expression in the paraventricular nucleus of the hypothalamus. Neuroendocrinology. 2007;85:199–206. doi: 10.1159/000102950. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HS, Zhu WL, Liu JF, Luo YX, Si JJ, Wang SJ, et al. PI3K/Akt signaling pathway in the basolateral amygdala mediates the rapid antidepressant-like effects of trefoil factor 3. Neuropsychopharmacology. 2012;37:2671–2683. doi: 10.1038/npp.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Charney DS. The science of resilience: implications for the prevention and treatment of depression. Science. 2012;338:79–82. doi: 10.1126/science.1222942. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Suri D, Veenit V, Sarkar A, Thiagarajan D, Kumar A, Nestler EJ, et al. Early stress evokes age-dependent biphasic changes in hippocampal neurogenesis, Bdnf expression, and cognition. Biol Psychiatry. 2012. [DOI] [PMC free article] [PubMed]
- Swiergiel AH, Zhou Y, Dunn AJ. Effects of chronic footshock, restraint and corticotropin-releasing factor on freezing, ultrasonic vocalization and forced swim behavior in rats. Behav Brain Res. 2007;183:178–187. doi: 10.1016/j.bbr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, et al. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, et al. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–532. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Tsoory M, Cohen H, Richter-Levin G. Juvenile stress induces a predisposition to either anxiety or depressive-like symptoms following stress in adulthood. Eur Neuropsychopharmacol. 2007;17:245–256. doi: 10.1016/j.euroneuro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Tsuda A, Ida Y, Satoh H, Tsujimaru S, Tanaka M. Stressor predictability and rat brain noradrenaline metabolism. Pharmacol Biochem Behav. 1989;32:569–572. doi: 10.1016/0091-3057(89)90198-6. [DOI] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Luo YX, He YY, Li FQ, Shi HS, Xue LF, et al. Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2010;30:12632–12641. doi: 10.1523/JNEUROSCI.1264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. VEGF as a potential target for therapeutic intervention in depression. Curr Opin Pharmacol. 2008;8:14–19. doi: 10.1016/j.coph.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Yoon SC, Seo MS, Kim SH, Jeon WJ, Ahn YM, Kang UG, et al. The effect of MK-801 on mTOR/p70S6K and translation-related proteins in rat frontal cortex. Neurosci Lett. 2008;434:23–28. doi: 10.1016/j.neulet.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Zhu WL, Shi HS, Wang SJ, Wu P, Ding ZB, Lu L. Hippocampal CA3 calcineurin activity participates in depressive-like behavior in rats. J Neurochem. 2011;117:1075–1086. doi: 10.1111/j.1471-4159.2011.07285.x. [DOI] [PubMed] [Google Scholar]