Abstract
Eukaryotic initiation factor subunit c (eIF3c) has been identified as an oncogene that is over-expressed in tumor cells and, therefore, is a potential therapeutic target for gene-based cancer treatment. This study was focused on investigating the effect of small interfering RNA (siRNA)-mediated eIF3c gene knockdown on colon cancer cell survival. The eIF3c gene was observed to be highly expressed in colon cancer cell models. The expression levels of the gene in eIF3c siRNA infected and control siRNA infected cells were compared via real-time polymerase chain reaction (PCR) and western blotting analysis. Cell proliferation levels were analyzed employing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and colony formation assays. Furthermore, the effects of eIF3c gene knockdown on the cell cycle and apoptosis were analyzed using flow cytometry. The results showed that suppression of eIF3c expression significantly (P<0.001) reduced cell proliferation and colony formation of RKO colon cancer cells. The cell cycle was arrested by decreasing the number of cells entering S phase. Further, apoptosis was induced as a result of eIF3c knockdown. Collectively, eIF3c deletion effectively reduced the survival of colon cancer cells and could be used as a therapeutic tool for colon cancer therapy.
Keywords: Eukaryotic initiation factor subunit c (eIF3c), Colon cancer, RKO cells, Small interfering RNA (siRNA)
1. Introduction
Colon cancer is the second major cause of malignancy-related death worldwide after lung cancer (Mclornan et al., 2010). Although much research has been carried out on the development of chemotherapeutics, because of inherent or acquired drug resistance, optimal chemotherapeutics for the treatment of colon cancer have not yet been found (Longley et al., 2006). Due to this resistance and the high risk factors associated with chemotherapy, other means of cancer treatment are constantly being researched. Gene expression profiling provides insights into understanding and identifying different subsets of genes with differential expression during cancer progression (van de Veer et al., 2002). This understanding has led to gene-targeted therapies to control cancer cell survival. Among those technologies, the use of interfering RNA (RNAi) to manipulate identified genes related to cancer progression has been gaining more attention in cancer therapy research. This method has been used in studies related to colon cancer on the inhibition of the β-catenin gene by small interfering RNA (siRNA). Successful silencing resulted in down-regulation of β-catenin-dependent gene expression and inhibited cellular proliferation in both in vitro and in vivo models (Verma et al., 2003). Therefore, the identification of critical genes related to colon cancer cell progression and their silencing may be a promising approach in cancer gene targeting therapy.
Translation is a fundamental process which can be divided into initiation, elongation, termination, and ribosome recycling. During the initiation phase of translation, the eukaryotic initiation factor 3 (eIF3) complex is essential as it is required for the interaction between the 40S ribosomal subunit which binds to the ternary complex (eIF2-GTP-methionine) and the 5′-end of the mRNA (Sonenberg and Hinnebusch, 2009). The expression of eIF subunits in cancers has not been well characterized. However, it has been found recently that altered expression of eIF subunits in tumors such as the over-expression of eIF4e, eIF2a, eIF3c, or eIF3h and the under-expression of eIF3e can induce cellular proliferation by initiating protein translation (Scoles et al., 2006). Scoles et al. (2006) showed that the eIF3c gene has oncogenic properties and that its over-expression induces the proliferations of gliomas, meningiomas, and ovarian carcinomas. eIF3c is the p110 subunit of eIF3. In the initiation of protein translation, eIF3c has a significant role in binding to two AUG recognition factors, eIF1 and eIF5. The eIF3c gene eIF3S8 is located on chromosome 16p11.2, which is an unstable region of the genome, and therefore duplication of the entire eIF3S8 gene is observed regularly (Loftus et al., 1999). This high duplication rate of the eIF3c gene may contribute to higher expression levels of eIF3c in various tumors (Scoles, 2008). Furthermore, it has been found that eIF3c is associated with neuro-fibromatosis 2 (NF2) tumor suppressor proteins. High expression levels of eIF3c have adverse effects on NF2 expression and thereby induce cell proliferation (Scoles et al., 2006) via the hyperactivation of translation initiation machinery (Zhang et al., 2007).
Based on these literature and clinical findings, the current study was focused on the use of eIF3c knockdown as a therapeutic tool for colon cancer therapy. Lentivirus-mediated eIF3c siRNA delivery was used as the mode of knockdown, and the effects of knockdown on colon cancer cell proliferation were observed in RKO colon cancer cell models.
2. Materials and methods
2.1. Cell culture
Colon cancer cells (RKO, HCT116, SW480, SW620, and LoVo) and human embryonic kidney cells (HEK293) were obtained from the American Type Culture Collection (ATCC). The cells were maintained in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (100 μg/ml) at 37 °C in a humidified atmosphere of 5% CO2.
2.2. Construction of eIF3c short hairpin RNA (shRNA) lentivirus
The sequences of the siRNA for eIF3c and control siRNA were synthesized as 5′-GAC CAT CCG TAA TGC CAT GAA-3′ and 5′-TTC TCC GAA CGT GTC ACG T-3′, respectively. These nucleotide sequences were inserted into the plasmid using the shRNA expressing vector pFUGW (Hollybio, Shanghai, China) and lentiviral packing vectors pVSVG-1 and pCMVΔR8.92 (Hollybio, Shanghai, China). The identities of the generated lentiviral based shRNA expressing vectors were confirmed by DNA sequencing. For the transfection, HEK293T cells (1×107) were seeded in 10-cm dishes and cultured for 24 h to reach 70%–80% confluence. Two hours before transfection, the medium was replaced with serum-free DMEM and the three plasmids, including 20 μg of silencing sequence or control sequence, 15 μg of packaging vector pCMVΔR8.92, and 10 μg of VSVG-1 plasmid, were added to 200 μl of opti-MEM and 15 μl of Lipofectamine 2000. The mixture was added to the cells and incubated for 8 h prior to replacement with 10 ml of DMEM medium containing 10% FBS. The supernatant was collected after 48 h of transfection and lentiviral particles were harvested by ultra-centrifugation (4000×g) at 4 °C for 10 min (Soneoka et al., 1995).
2.3. Infection of RKO cells with eIF3c shRNA lentivirus or control lentiviru
For the infection of RKO cells with eIF3c shRNA or control shRNA, cells were seeded onto 96-well plates (50 000 cells/well) and after 24 h of incubation, the culture medium was replaced with opti-MEM medium containing the lentivirus. After 24 h, virus-containing incubation medium was replaced with fresh medium and incubated for another 72 h. The success of infection was examined by counting the green fluorescence emitted by the green florescence protein (GFP) within the lentivirus particles under fluorescence microscopy 96 h after infection.
2.4. RNA extraction and real-time polymerase chain reaction (PCR) analysis
RNA was extracted from RKO cells infected with the lentivirus for 5 d. Cells were lysed with Trizol reagent (Invitrogen, USA) and the total RNA was extracted from the lysate using standard procedures. The extracted RNA was used to synthesize the cDNA using the Promega M-MLV cDNA synthesis kit according to the manufacturer’s instructions. For real-time PCR analysis, glyceraldehydes-3-phosphate dehydrogenase (GAPDH) was used as a reference. The forward and reverse primers used were: for eIF3c, forward primer 5′-AGA TGA GGA TGA GGA TGA GGA C-3′ and reverse primer 5′-GGA ATC GGA AGA TGT GGA ACC-3′, for GAPDH, forward primer 5′-TGA CTT CAA CAG CGA CAC CCA-3′ and reverse primer 5′-CAC CCT GTT GCT GTA GCC AAA-3′. Relative gene expression level of the eIF3c gene in the presence or absence of eIF3c siRNA compared to that of GAPDH was calculated using the 2−ΔΔCT analysis method.
2.5. Reverse transcriptase PCR
RNA extraction and cDNA synthesis were carried out as described above. GAPDH was used as an internal control. The primers used for reverse transcriptase PCR were as follows: eIF3c, forward primer 5′-AGA TGA GGA TGA GGA TGA GGA C-3′ and reverse primer 5′-GGA ATC GGA AGA TGT GGA ACC-3′; GAPDH, forward primer 5′-TGA CTT CAA CAG CGA CAC CCA-3′ and reverse primer 5′-CAC CCT GTT GCT GTA GCC AAA-3′. The experiment was performed according to the users’ manual and 1 μg cDNA was used as the template. The PCR products were loaded onto an agarose gel containing ethidium bromide (EB) for electrophoresis.
2.6. Western blotting analysis
Western blotting was carried out to evaluate eIF3c gene expression levels in siRNA-infected colon cancer cells (5 d after infection) compared to those of control siRNA-infected cells. To isolate the cellular protein, the cells were washed with cold phosphate buffered saline (PBS) and lysed with radio-immune precipitation assay (RIPA) buffer [50 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, 1% nonidet P (NP)-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS)] containing phenylmethyl sulfonylfluoride (PMSF) (1 mmol/L) and protease inhibitors (2 μg/ml; Protease Inhibitor Cocktail Set III, Calbiochem) on ice for 30 min. The protein content was measured by the Lowry method and the protein concentration of each sample was adjusted to 2 μg/μl. Then, 20 μl of collected protein was loaded onto a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and electrophoresed at 60 V for 4 h. The gel was transferred to polyvinylidene difluoride membrane and the proteins were detected by respective antibodies using electrochemiluminescence (ECL) kit (Amersham, USA) and exposed to X-ray film. GAPDH was used as control and detected by an anti-GAPDH antibody (Santa Cruz Biotechnology). The bands on X-ray films were quantified with an ImageQuant densitometric scanner (Molecular Dynamics, USA).
2.7. Analysis of the effect of eIF3c knockdown on cell proliferation
The level of RKO cell proliferation after eIF3c siRNA infection was analyzed by counting the number of viable cells with a Cellomics ArrayScan® VTI HCS reader (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Infected cells were seeded into a 96-well plate at a concentration of 2 000 cells/well and 10 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/ml) was added into each well once daily for 5 d. The Cellomics machine detects viable cells by their green fluorescence emission. The number of viable cells was calculated for each of five days after plating. All experiments were performed in triplicates.
2.8. Colony forming assay
RKO cells transfected with eIF3c siRNA or control siRNA for five days were collected and seeded in six-well plates at a density of 500 cells/well. The medium was changed every three days. After two weeks of culture, the cells were washed and fixed in 4% paraformaldehyde. The fixed cells were stained by adding freshly prepared diluted Giemsa stain for 20 min. The cells were rinsed with distilled water and colonies with more than 50 cells were counted using a fluorescence microscope.
2.9. Cell cycle analysis
The cell cycle distribution was analyzed by flow cytometry assay following propidium iodide (PI) staining. The infected cells were seeded onto a six-well plate at a density of 1×106 cells/well. After 24-h incubation, the cells were collected and washed with ice cold PBS. The collected cells were fixed in 70% ethanol and incubated for 30 min at 4 °C. The ethanol was discarded by centrifugation and the cell pellet was suspended in 100 μg/ml of DNase-free RNase for 30 min at 37 °C. Then PI solution (100 μg/ml) was added to the cell suspension which, after filtering through a 50-μm nylon mesh, was then analyzed using a flow cytometer (FACS Cali-bur, BD Biosciences).
2.10. Detection of apoptosis
Apoptotic cells were detected using the Annexin V-APC Apoptosis Detection Kit (BioVision, USA). eIF3c siRNA- and control siRNA-infected cells were seeded onto a six-well plate at a density of 5×105 cells/well. After five days of incubation the cells were collected and washed with ice cold PBS. Cells were resuspended in 100 μl of binding buffer followed by the addition of 5 μl of Annexin V solution and 5 μl of PI solution. The mixture was incubated for 5 min in the dark before being analyzed using a flow cytometer.
2.11. Statistical analysis
All data are expressed as mean±standard deviation (SD) of three independent experiments and the error bars represent the SD. Data were statistically analyzed using SPSS 16.0 software by a paired t-test analysis method. P<0.01 was considered as statistically significant.
3. Results
3.1. eIF3c siRNA infection down-regulated eIF3c expression in RKO cells
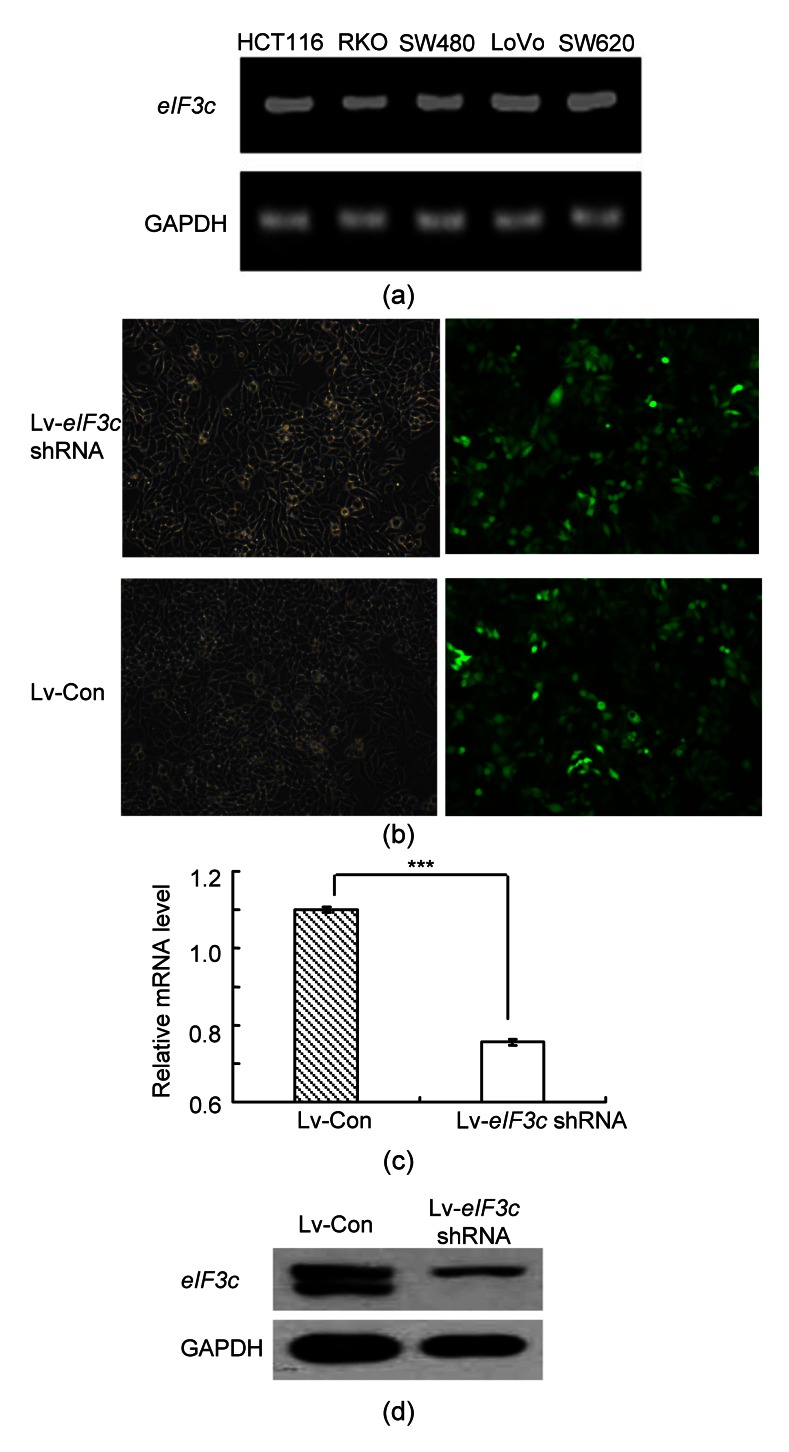
The expression levels of the eIF3c gene in different colon cancer cell models were examined and the results indicated that eIF3c was expressed in all models (Fig. 1a). RKO cells with higher eIF3c expression were selected for the further analysis. The successful infection of eIF3c siRNA or control siRNA into RKO cells was confirmed via a microscopic detection of green fluorescence. eIF3c siRNA infected cells (Lv-eIF3c shRNA) showed more than 80% successful infection compared to the control group (Lv-Con) (Fig. 1b). The protein expression levels of eIF3c in eIF3c siRNA-infected and in control sequence-infected cells were compared by western blotting. The results obtained (Figs. 1c and 1d) clearly indicated that siRNA treatment had down-regulated the protein expression level of eIF3c by 70% compared to that of the control group. Therefore, these results demonstrate that the higher expression level of eIF3c in RKO cells was suppressed by siRNA infection.
Fig. 1.
Effect of knockdown of eIF3c on the eIF3c mRNA and protein expression levels in RKO cells
(a) mRNA expression levels of eIF3c in different colon cancer cells; (b) Light microscopic (left) and fluorescent microscopic (right) pictures of RKO cells; (c) Quantitative real-time PCR data of siRNA mRNA levels following the knockdown compared with the control group; (d) eIF3c protein expression levels in eIF3c siRNA-infected and control siRNA-infected lung cancer cells. *** P<0.001 in comparison with the control
3.2. eIF3c siRNA infection suppressed the cell proliferation of RKO cells
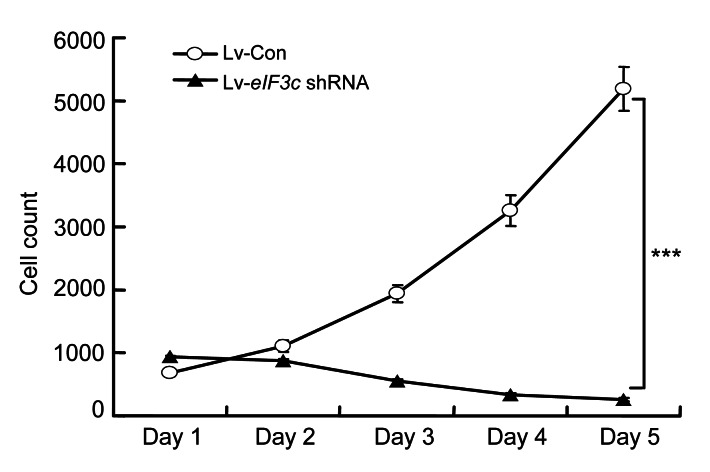
The effect of the knockdown eIF3c gene on cell proliferation was analyzed by counting the number of viable cells using a Cellomics Array Scan® VTI HCS reader. The results indicated that one and two days after infection cell proliferation was very similar in the eIF3c siRNA-infected group and the control group (Fig. 2).
Fig. 2.
Effect of eIF3c knockdown on the proliferation of RKO cells
*** P<0.001 in comparison with the control
However, cell proliferation significantly (P<0.001) decreased from the third day onwards, such that after five days, the number of cells in the control group was 60 times higher than that in the eIF3c siRNA-infected group. These results indicated that deletion of eIF3c had a significant influence on the survival of RKO colon cancer cells.
3.3. eIF3c siRNA infection suppressed colony formation in RKO cells
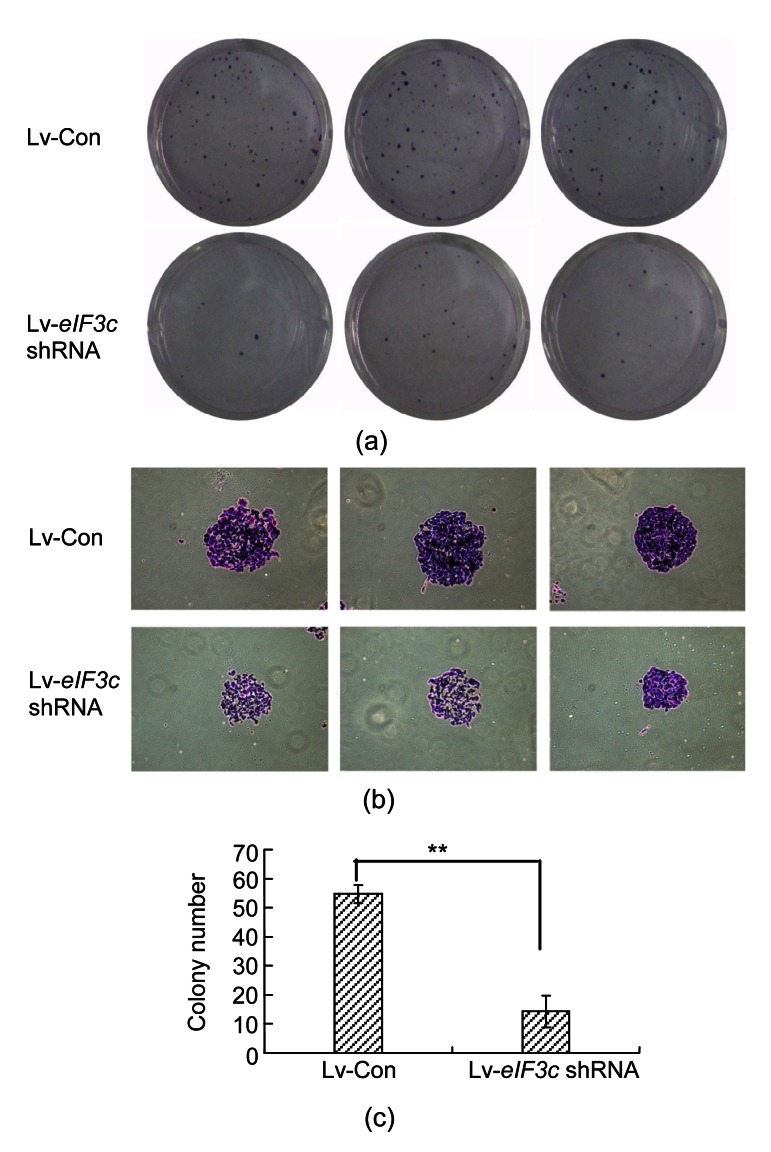
RKO colon cancer cells tend to grow in colonies under normal conditions. Therefore, the effect of eIF3c deletion on colony formation was determined by performing a colony formation assay. The number and size of colonies were observed in eIF3c siRNA infected-cells and control siRNA-infected cells. The number of the colonies decreased significantly (P<0.01) with the eIF3c deletion, showing a 3.5-fold decrease compared to the control group (Figs. 3a and 3c). The size of the colonies in eIF3c siRNA-infected cells was smaller than that of those in the control group (Fig. 3b). It appears that because of the decrease in cell proliferation in the absence of the eIF3c gene, the number of colonies decreased.
Fig. 3.
Effect of eIF3c knockdown on the colony forming ability of RKO cells
(a) Giemsa staining of the cancer cell colonies under light microscope; (b) Images showing the reduction in the number of colonies following Giemsa staining; (c) Numerical representation of the number of colonies in the eIF3c-suppressed group and the control group. ** P<0.01 in comparison with the control
3.4. eIF3c gene deletion arrested the cell cycle of RKO cells
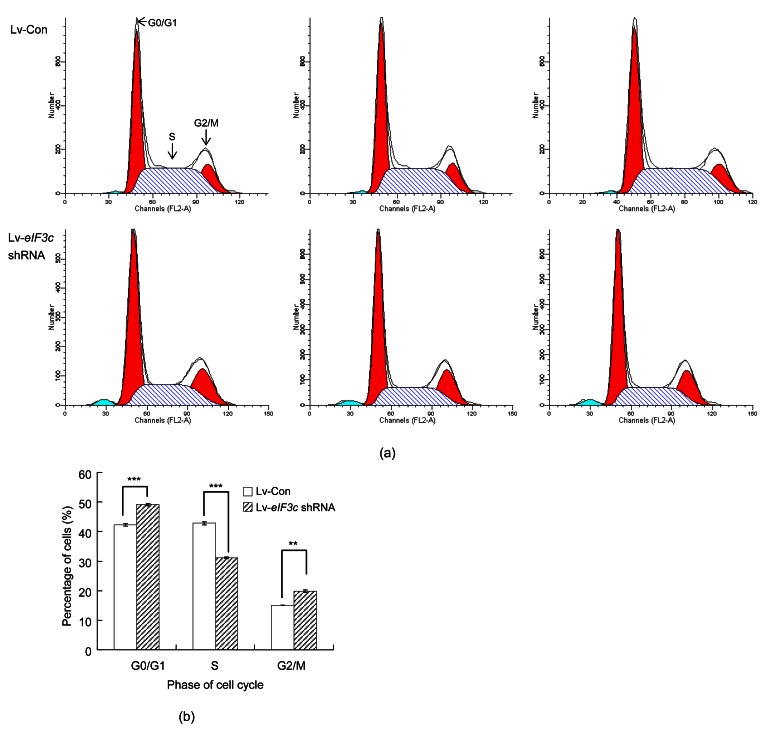
As the eIF3c gene was found to be essential for cell proliferation, the effect of eIF3c deletion on the cell cycle distribution of colon cancer cells was analyzed using a flow cytometer. The cell cycle distribution of eIF3c siRNA-infected cells was different from that of control cells (Fig. 4a). The eIF3c-deleted group showed an 8% increase in cells in G0/G1 phases (P<0.001) and a 5% increase in cells in the G2/M phases (Fig. 4b). However, cell numbers in the S phase decreased by 12% (P<0.001) with the eIF3c deletion (Fig. 4b). Collectively, these results indicate that the eIF3c gene and the RKO cell cycle distribution were significantly correlated.
Fig. 4.
Effect of eIF3c knockdown on the cell cycle distribution of RKO cells
(a) The histograms obtained from flow cytometric analysis of eIF3c deleted and non-deleted cells from three independent trials; (b) Percentages of RKO cells at different phases of the cell cycle following eIF3c siRNA or control siRNA infection. *** P<0.001, ** P<0.01 in comparison to the control
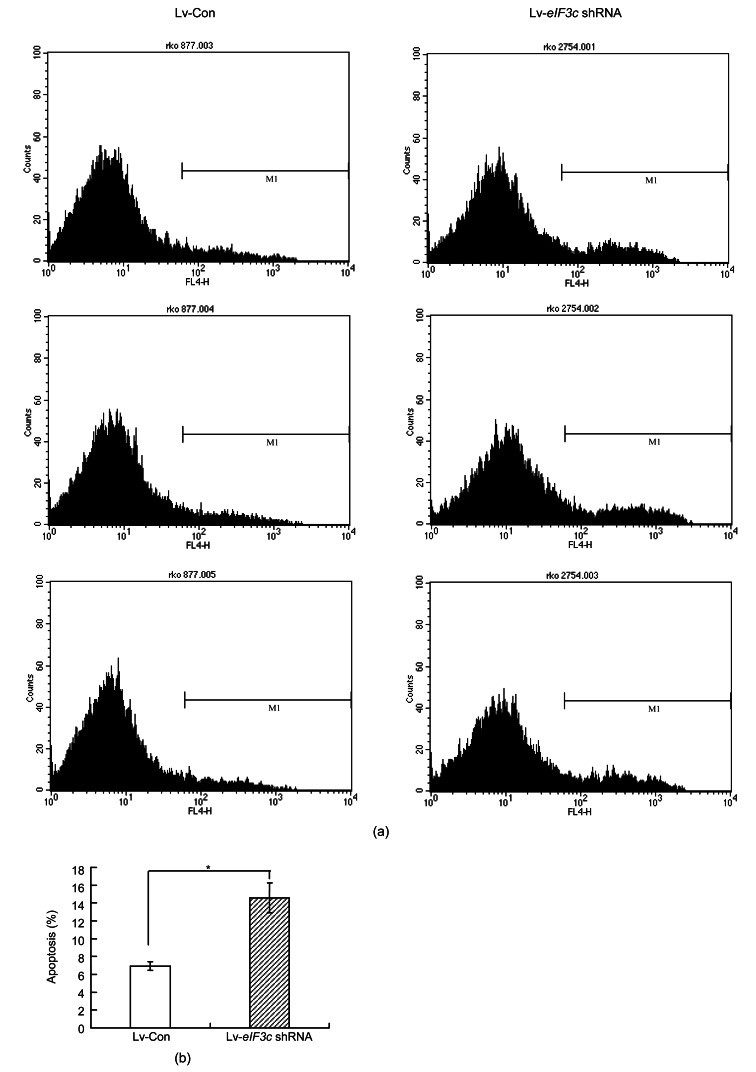
3.5. eIF3c gene knockdown induced apoptosis in RKO cells
The effect of eIF3c gene deletion on apoptosis was analyzed by comparing the apoptosis levels in eIF3c knockdown and control cells. eIF3c gene suppression induced apoptosis in RKO cells (P<0.05) (Fig. 5).
Fig. 5.
Effect of eIF3c knockdown on apoptosis in RKO cells
(a) The histograms obtained from PI-Annexin V analysis of eIF3c knockdown and control cells from three independent trials; (b) Percentage of apoptotic cells in control group and eIF3c knockdown group. * P<0.05 in comparison with control
The percentage of apoptotic cells doubled in the absence of the eIF3c gene compared to that of the control group (Fig. 5b). Therefore, the eIF3c gene may have a strong influence on the survival of RKO cells and in the absence of eIF3c, the survival rate decreases via induction of apoptosis.
4. Discussion
Targeted treatment methods have not yet been discovered for the treatment of colon cancer despite it being one of the most prominent causes of malignancy-related deaths worldwide (Karaayvaz et al., 2011). Therefore, this study was focused on the identification of possibilities for the manipulation of cancer-related genes as a mode of colon cancer therapy. eIF3c is over-expressed in some tumors, including seminomas and meningiomas, where it facilitates cell survival. It has been found that eIF3c can interact with the NF2 tumor suppressor merlin/schwannomin, where merlin can inhibit eIF3c-mediated cell proliferation (Zhang et al., 2007). However, in malignant conditions eIF3c expression is inversely related to merlin expression, as increased expression of eIF3c down-regulates merlin expression. This results in increased clonogenicity, increased viability, facilitated S-phase entry, and decreased apoptosis levels (Scoles, 2008). All these factors contribute to increased proliferation rates of cancer cells.
Thus, we investigated whether specifically reducing the levels of eIF3c protein in established colon cancer cell lines, in which this protein was over-expressed, might result in decreased cell proliferation levels. To achieve this objective, we used RNAi with siRNAs designed for use against eIF3c. RNAi is a conserved mechanism operating in insects, nematodes, plants, and mammalian cells for gene manipulation (Caplen et al., 2001; Elbashir et al., 2001). In this process, sequence specific post transcriptional knockdown is initiated by the introduced RNAi. The RNAi consists of double-stranded annealed sense and antisense RNAs which have sequences similar to those of the gene to be silenced (Bass, 2000). In experimental conditions lentivirus-based vectors have been used successfully to deliver siRNA efficiently with prominent gene knockdown effects (Devi, 2006).
The results clearly indicated that RKO colon cancer cells over-expressed the eIF3c gene under normal conditions and siRNA-mediated knockdown significantly decreased (P<0.001) the expression level. In the absence of the eIF3c gene, the survival rate of colon cancer cells drastically decreased by 60-fold. Furthermore, the cell cycle was arrested as the number of cells entering the S phase was significantly reduced (P<0.05) compared to the control, and the induction of apoptosis was prominent in the absence of the eIF3c gene. These results indicated a strong relationship between expression of the eIF3c gene and colon cancer cell survival. According to the literature, eIF3c is strongly associated with the maintenance of cell viability and it functions in the same manner in colon cancer cells. Over-expression of the eIF3c gene due to its higher tendency to double or undergo other mutations would positively contribute to colon cancer cell survival. Negative effects on colon cancer cell survival upon knockdown of eIF3c further support this correlation. Therefore, eIF3c could be regarded as a gene that promotes tumor progression, and regulation of its expression via siRNA knockdown would be an interesting and novel therapeutic tool for the treatment of colon cancer.
5. Conclusions
Over-expression of the eIF3c gene was observed in RKO colon cancer cells and this over-expression was suppressed by lentivirus-mediated infection of eIF3c siRNA. The deletion of the eIF3c gene resulted in a significant decrease in RKO cell survival and colony formation. Furthermore, the RKO cell cycle was arrested by inhibiting entry into S phase, and apoptosis was induced in the absence of the eIF3c gene. Collectively, these results show that the eIF3c gene has a positive effect on colon cancer cell survival and progression. Therefore, siRNA-mediated knockdown of the eIF3c gene could be considered as a novel therapeutic tool for colon cancer treatment.
Footnotes
Compliance with ethics guidelines: Ning SONG, Yan WANG, Xiao-dong GU, Zong-you CHEN, and Liu-bin SHI declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Bass BL. Double-stranded RNA as a template for gene silencing. Cell. 2000;101(3):235–238. doi: 10.1016/S0092-8674(02)71133-1. [DOI] [PubMed] [Google Scholar]
- 2.Caplen NJ, Parrish S, Imani F, Fire A, Morgan RA. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. PNAS. 2001;98(17):9742–9747. doi: 10.1073/pnas.171251798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devi GR. SiRNA-based approaches in cancer therapy. Cancer Gene Ther. 2006;13(9):819–829. doi: 10.1038/sj.cgt.7700931. [DOI] [PubMed] [Google Scholar]
- 4.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 5.Karaayvaz M, Pal T, Song B, Zhang C, Georgakopoulos P, Mehmood S, Burke S, Shroyer K, Ju J. Prognostic significance of miR-215 in colon cancer. Clin Colorect Cancer. 2011;10(4):340–347. doi: 10.1016/j.clcc.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loftus BJ, Kim UJ, Sneddon VP, Kalush F, Brandon R, Fuhrmann J, Mason T, Crosby ML, Barnstead M, Cronin L. Genome duplications and other features in 12 mb of DNA sequence from human chromosome 16p and 16q. Genomics. 1999;60(3):295–308. doi: 10.1006/geno.1999.5927. [DOI] [PubMed] [Google Scholar]
- 7.Longley DB, Allen WL, Johnston PG. Drug resistance, predictive markers and pharmacogenomics in colorectal cancer. Biochim Biophys Acta Rev Cancer. 2006;1766(2):184–196. doi: 10.1016/j.bbcan.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Mclornan DP, Barrett HL, Cummins R. Prognostic significance of trail signaling molecules in stage II and III colorectal cancer. Clin Cancer Res. 2010;16(13):3442–3451. doi: 10.1158/1078-0432.CCR-10-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scoles DR. eIF3c (eukaryotic translation initiation factor 3, subunit c) Atlas Genet Cytogenet Oncol Haematol. 2008;12:428–429. [Google Scholar]
- 10.Scoles DR, Yong WH, Qin Y, Wawrowsky K, Pulst SM. Schwannomin inhibits tumorigenesis through direct interaction with the eukaryotic initiation factor subunit c (eIF3c) Human Mol Genet. 2006;15(7):1059–1070. doi: 10.1093/hmg/ddl021. [DOI] [PubMed] [Google Scholar]
- 11.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136(4):731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soneoka Y, Cannon PM, Ramsdale EE, Griffiths JC, Romano G, Kingsman SM, Kingsman AJ. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucl Acids Res. 1995;23(4):628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Veer LJ, Dai H, van de Vijver MJ. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 14.Verma UN, Surabhi RM, Schmaltieg A, Becerra C, Gaynor RB. Small interfering RNAs directed against β-catenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res. 2003;9(4):1291–1300. [PubMed] [Google Scholar]
- 15.Zhang L, Pan X, Hershey JW. Individual overexpression of five subunits of human translation initiation factor eIF3 promotes malignant transformation of immortal fibroblast cells. J Biol Chem. 2007;282(8):5790–5800. doi: 10.1074/jbc.M606284200. [DOI] [PubMed] [Google Scholar]