Abstract
Objective: To investigate the beneficial effect of bicyclol on rat hearts subjected to ischemia-reperfusion (IR) injuries and its possible mechanism. Methods: Male Sprague-Dawley rats were intragastrically administered with bicyclol (25, 50 or 100 mg/(kg∙d)) for 3 d. Myocardial IR was produced by occlusion of the coronary artery for 1 h and reperfusion for 3 h. Left ventricular hemodynamics was continuously monitored. At the end of reperfusion, myocardial infarct was measured by 2,3,5-triphenyltetrazolium chloride (TTC) staining, and serum lactate dehydrogenase (LDH) level and myocardial superoxide dismutase (SOD) activity were determined by spectrophotometry. Isolated ventricular myocytes from adult rats were exposed to 60 min anoxia and 30 min reoxygenation to simulate IR injuries. After reperfusion, cell viability was determined with trypan blue; reactive oxygen species (ROS) and mitochondrial membrane potential of the cardiomyocytes were measured with the fluorescent probe. The mitochondrial permeability transition pore (mPTP) opening induced by Ca2+ (200 μmol/L) was measured with the absorbance at 520 nm in the isolated myocardial mitochondria. Results: Low dose of bicyclol (25 mg/(kg∙d)) had no significant improving effect on all cardiac parameters, whereas pretreatment with high bicyclol markedly reduced the myocardial infarct and improved the left ventricular contractility in the myocardium exposed to IR (P<0.05). Medium dose of bicyclol (50 mg/(kg∙d)) markedly improved the myocardial contractility, left ventricular myocyte viability, and SOD activity, as well decreased infarct size, serum LDH level, ROS production, and mitochondrial membrane potential in rat myocardium exposed to IR. The reduction of ventricular myocyte viability in IR group was inhibited by pretreatment with 50 and 100 mg/(kg∙d) bicyclol (P<0.05 vs. IR), but not by 25 mg/(kg∙d) bicyclol. The opening of mPTP evoked by Ca2+ was significantly inhibited by medium bicyclol. Conclusions: Bicyclol exerts cardioprotection against IR injury, at least, via reducing oxidative stress and its subsequent mPTP opening.
Keywords: Ischemia-reperfusion injury, Cardioprotection, Oxidative stress, Mitochondrial permeability transition pore, Bicyclol
1. Introduction
Cardiovascular disease, especially myocardial infarction, is the leading cause of death and disability all over the world (Yellon and Hausenloy, 2007). Promptly resuming the blood supply in the ischemic tissue is the most effective way to reduce the process of ischemic injury which ultimately leads to infarction. However, reperfusion itself after even brief duration of ischemia causes other irreversible myocardial damages, which is so-called myocardial ischemia-reperfusion (IR) injuries (Gross and Auchampach, 2007). The manifestation of myocardial IR injuries includes contractile dysfunction, myocardial stunning, and even cell death. Though the causes of myocardial IR injury are complicated, it is believed that overproduction of reactive oxygen species (ROS), including hydrogen peroxide (H2O2), hydroxyl radical (·OH−) and superoxide (·O2 −), and Ca2+ overload induced by reperfusion play pivotal roles in myocardial IR injuries (Venardos et al., 2007). More than 95% of ROS come from mitochondrial oxidative phosphorylation and mitochondria themselves are also very susceptible to ROS. The endogenous anti-oxidases, such as superoxide dismutase (SOD), scavenge the excessive ROS to maintain the balance of redox state. When the endogenous anti-oxidase is not enough to eliminate the overproduction of ROS during reperfusion, oxidative stress occurs and the mitochondrial permeability transition pore (mPTP) is opened (He et al., 2008). The mPTP opening leads to mitochondrial swelling, mitochondrial membrane potential collapse, and cytochrome c release, which ultimately results in cell death (Kroemer, 1998).
Bicyclol (4,4′-dimethoxy-5,6,5′,6′-dimethylene-dioxy-2-hydroxymethyl-2′-carbonyl biphenyl), a synthetic drug widely used in clinic to treat hepatitis (Lu and Li, 2002; Sun and Liu, 2006), has been demonstrated to protect the liver against viral infection and toxicity (Liu et al., 2005; Wang and Li, 2006). The hepatoprotection by bicyclol is related to its anti-inflammatory properties, improving mitochondrial function, reducing nicotinamide adenine dinucleotide phosphate (NADPH)-dependent lipid peroxidation, and suppressing apoptosis (Hu and Liu, 2006). Recently, bicyclol has been reported to lessen IR injury in kidneys by inhibiting lipid peroxidation (Zhao et al., 2002), indicating that bicyclol may exert organ protection via reducing oxidative stress. However, whether bicyclol protects heart and myocardial mitochondria against IR injuries remains unclear. Here, we explored the beneficial effect of pretreatment with bicyclol against IR injuries and the underlying mechanism, especially its anti-oxidative action and ability to inhibit mPTP opening, in rat hearts.
2. Materials and methods
2.1. Experimental animals
Male Sprague-Dawley rats (250‒300 g) from the Experimental Animal Center of Zhejiang Academy of Medical Sciences were fed on standard pellet chow and water ad libitum ((22±2) °C, a 12-h light/dark cycle). All performances were approved by the Ethics Committee for the Use of Experimental Animals in Zhejiang University, China.
2.2. Chemicals
Bicyclol (C19H18O9; Beijing Union Pharmaceutical Corporation, China) was dissolved in polyethylene glycol 400 (PEG-400) for intragastric administration. The kits for measurement of SOD activity and lactate dehydrogenase (LDH) activity were purchased from Nanjing Jiancheng Bioengineering Institute, China. N-2-mercaptopropionyl glycine (MPG), 2,3,5-triphenyltetrazolium chloride (TTC), and cyclosporin A (CsA) were purchased from Sigma-Aldrich Inc. (Saint Louis, MO, USA). Tetramethylrhodamine ethyl ester (TMRE) and 2′,7′-dichlorofluorescein diacetate (DCFH-DA) were purchased from Molecular Probes (Eugene, OR, USA). DCFH-DA was dissolved in dimethyl sulfoxide (DMSO), and the final concentration of DMSO was less than 0.2%, which has been previously shown to have no effect on the heart or myocardial organelles (Hausenloy et al., 2004; Gao et al., 2007). All other chemicals were of analytical purity.
2.3. Preparation of IR heart model
Rats were anesthetized by injecting 0.04 g/ml chloral hydrate (400 mg/kg, intraperitoneally (i.p.)), and supplemented with 16 mg/kg chloral hydrate (i.p.) every 30 min to get persistent anesthesia. As in our previous study (Zhang et al., 2006), the rat was tracheotomized and ventilated with room air (2 ml/stroke tidal volume, 70 strokes/min), and the body temperature was maintained at 37 °C using a heating pad. The rat pericardium was opened through a left thoracotomy and the left anterior descending (LAD) coronary artery was ligated and loosened to simulate IR (Zhang et al., 2006). Then, the left ventricular hemodynamics, including left ventricular developed pressure (LVDP), heart rate (HR), and maximal rise/fall rate of left ventricular pressure (±dP/dt max), were continuously monitored throughout the surgery (ischemia for 1 h and reperfusion for 3 h) via a pressure transducer, which was cannulated into the left ventricle through the right carotid artery, connected to a data acquisition system (RM6240BD, Chengdu, China). After surgery, all rat hearts were carefully excised under anesthesia to be stained with TTC for infarct size measurement, or to be homogenized for SOD activity assay.
2.4. Measurement of serum LDH level
After 3 h reperfusion, the rat blood was collected after sacrifice, settled for 15 min, and centrifuged (3 000 r/min, 10 min, 4 °C). The supernatant was collected for LDH assay by the commercial kit. Briefly, according to the manual of the kit, the absorbance at 550 nm was measured using a spectrophotometer, and the LDH level in serum was reported as U/L.
2.5. Measurement of infarct size
Myocardial infarct was determined by the TTC staining method (Zhang et al., 2006). Briefly, after 3 h reperfusion, the isolated rat heart was perfused with 1% (0.01 g/ml) Evans blue on a Langendorff apparatus, and then was frozen to be cut into 2 mm. The heart slices were stained with 1% (0.01 g/ml) TTC for 10‒15 min at 37 °C. Myocardial infarct (pale) was calculated as percentage of risk (red) areas using ImageJ software from the National Institutes of Health (Bethesda, MD, USA).
2.6. Assay of SOD activity
Heart tissue samples were homogenized and centrifuged (3 000 r/min, 10 min, 4 °C) in ice-cold medium containing 10 mmol/L Tris-HCl, 0.1 mmol/L EDTA-2Na, 10 mmol/L sucrose, and 8 g/L NaCl (pH 7.4). Supernatant was collected to assay the SOD activity using the commercial kit based on the xanthine-xanthine oxidase method (Huang et al., 2007).
2.7. Preparation of isolated ventricular myocytes from adult rats
As in our previous study (Cao et al., 2003), single ventricular myocytes were prepared from the adult rats pretreated with bicyclol by enzymatic dissociation. Briefly, after the rat heart was perfused on a Langendorff apparatus with 100% oxygenated Ca2+-free modified Tyrode’s solution (composed of (mmol/L): 100.0 NaCl, 10.0 KCl, 1.2 KH2PO4, 5.0 MgSO4, 20.0 glucose, and 10.0 3-morpholinopropanesulfonic acid (MOPS; pH 7.2)), the perfusion solution was changed to 100% oxygenated low-Ca2+ (50 μmol/L) Tyrode’s solution containing 0.03% (0.3 g/L) collagenase and 1% (10 g/L) bovine serum albumin for 10 min. Then the ventricular myocytes were enzymatically isolated and suspended in Tyrode’s solution (Ca2+ concentration was gradually increased to 1.25 mmol/L within 40 min) (Cao et al., 2003).
2.8. IR in isolated ventricular myocytes
As in our previous study (Gao et al., 2007), the single ventricular myocyte preparation (0.5 ml) was centrifuged for 1 min to pellet. After removal of excess supernatant, about 0.2 ml mineral oil was layered on top of the myocyte pellet to prevent oxygen diffusion and simulate anoxia (ischemia) for 60 min at 37 °C. The myocyte was fully reoxygenated (reperfused) for 30 min by removing the mineral oil and gently pipetting with normoxic Tyrode’s solution (Gao et al., 2007).
2.9. Assay of cell viability
After incubation with 0.4% (4 g/L) trypan blue for 3 min, the isolated ventricular myocytes not permeated by trypan blue (viable cells) were counted and the cell viability was calculated as a percentage of the total cells (Hiebert and Ping, 1997).
2.10. Measurement of the mitochondrial membrane potential
Mitochondrial membrane potential was measured using the fluorescent dye TMRE based on the previous study (Katoh et al., 2002). Briefly, TMRE is positively charged and accumulates in mitochondria with auto-quenching because the potential of the mitochondrial matrix is negative. Mitochondrial depolarization causes the spread of TMRE from mitochondrial matrix to cytoplasm and enhances the whole-cell fluorescence signal (Katoh et al., 2002; Gao et al., 2006). Myocytes were loaded with TMRE (1×10−7 mol/L) for 20 min in the dark and were washed twice with Tyrode’s solution to avoid the influence of intracellular accumulation of TMRE (Gao et al., 2006). TMRE fluorescence was detected (excitation at 514 nm/emission at 590 nm) and calculated as the percentage of that in the normal control group.
2.11. Measurement of ROS
Isolated ventricular myocytes were loaded with DCFH-DA (1×10−5 mol/L) for 30 min in the dark. Dichlorofluorescein (DCF) fluorescence was detected (excitation at 488 nm/emission at 530 nm), and the intracellular ROS level was indicated by the DCF fluorescence intensity calculated as the percentage of that in the normal control group (Swift and Sarvazyan, 2000).
2.12. Isolation of myocardial mitochondria
As in our previous study (Zhang et al., 2006), mitochondria were isolated from the rat heart by differential centrifugation.
2.13. Measurement of mPTP opening
The myocardial mitochondria were incubated in the swelling buffer (120 mmol/L KCl, 10 mmol/L Tris-HCl, 20 mmol/L MOPS, 5 mmol/L KH2PO4, pH 7.4), and CaCl2 (2×10−4 mol/L) was added to produce mPTP opening (Baines et al., 2003). The mPTP opening was measured as the reduction of absorption at 520 nm (A 520) for 15 min by a spectrophotometer. Mitochondria, pre-incubated with mPTP inhibitor CsA (1 μmol/L) for 2 min before subjected to high CaCl2, were considered as a positive control group.
2.14. Experimental protocols
In the first series of experiments, rats were randomly divided into three groups: (1) sham group: age-matched healthy rats were only tracheotomized without coronary artery ligation; (2) IR group: age-matched healthy rats were only intragastrically administered with an equal volume of PEG-400 (vehicle for bicyclol) for 3 d, and then exposed to 1 h ischemia followed by 3 h reperfusion in vivo; (3) bicyclol group: rats were intragastrically administered with 25, 50, or 100 mg/(kg∙d) of bicyclol for 3 d (Wang and Li, 2006), and then subjected to IR procedure as the IR group.
The second series of experiments was to detect the effect of bicyclol pretreatment on the ventricular myocytes subjected to anoxia/reoxygenation, which was induced by 60 min anoxia followed by 30 min reoxygenation as described above. Six groups were studied in the anoxia-reoxygenation model: (1) control group: myocyte suspension isolated from normal rats not subjected to IR procedure; (2) IR group: normal myocyte suspension exposed to 60 min anoxia and 30 min reoxygenation to simulate IR procedure; (3) MPG group: normal myocyte suspension treated with MPG (5×10−7 mol/L), an ROS scavenger, for 5 min before IR procedure; (4‒6) bicyclol+IR groups: myocyte suspension isolated from rats pretreated with bicyclol (25, 50, or 100 mg/(kg∙d)) for 3 d before IR procedure.
2.15. Statistical analysis
All data are shown as mean±standard deviation (SD). Statistical comparisons were made using one-way analysis of variance (ANOVA) followed by the Newman-Keuls test with GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). The P values <0.05 were considered to be statistically significant.
3. Results
3.1. Effect of bicyclol on myocardial contractility during IR
As shown in Table 1, LAD ligation for 1 h significantly decreased LVDP in all groups compared with their own baselines (P<0.01). LVDP was increased during reperfusion period, but still markedly lowed in IR and bicyclol (25 mg/(kg∙d)) groups compared with their own baselines. Pretreatment with bicyclol at both 50 and 100 mg/(kg∙d) markedly increased the LVDP at 3 h reperfusion compared with IR group (P<0.01, P<0.05). There were no differences in heart rate among all groups during the whole IR period. The ±dP/dt max was significantly diminished by ischemia and reperfusion in all groups compared with their own baselines, which was markedly attenuated by bicyclol (50 and 100 mg/(kg∙d)) at 2 h and 3 h reperfusion, respectively (P<0.05 vs. IR).
Table 1.
Effect of pretreatment with bicyclol on left ventricular hemodynamics in rat hearts exposed to ischemia-reperfusion (IR)
| Group | Treatment | LVDP (% of baseline) | Heart rate (beats/min) | +dP/dt max (% of baseline) | −dP/dt max (% of baseline) |
| Sham | Baseline | 100 [(94.19±4.82) mmHg] | 337.4±10.56 | 100 [(4 356.81±463.26) mmHg/s] | 100 [(3 471±468.29) mmHg/s] |
| Ischemia | 90.44±9.48 | 354.3±14.03 | 91.25±6.43 | 90.35±9.23 | |
| Reperfusion 1 h | 94.28±9.48 | 352.0±23.10 | 95.38±14.95 | 93.46±2.57 | |
| Reperfusion 2 h | 95.18±7.39 | 379.4±14.80 | 94.57±12.46 | 96.35±4.31 | |
| Reperfusion 3 h | 93.43±6.51 | 369.7±24.79 | 95.44±9.51 | 95.26±7.04 | |
| IR | Baseline | 100 [(104.14±11.27) mmHg] | 387.8±68.8 | 100 [(5 078.03±1 035.58) mmHg/s] | 100 [(4 356.84±1 074.34) mmHg/s] |
| Ischemia | 69.38±14.54** ## | 376.0±61.7 | 67.36±13.22** ## | 66.58±10.60** ## | |
| Reperfusion 1 h | 76.47±10.84** # | 398.0±44.4 | 78.04±16.97* ## | 74.89±19.21* | |
| Reperfusion 2 h | 78.59±11.90** # | 387.4±53.9 | 69.42±15.26** ## | 69.83±14.09** ## | |
| Reperfusion 3 h | 76.65±13.33** # | 392.2±33.2 | 68.26±19.61** ## | 72.67±12.92** # | |
| 25 mg/(kg∙d) bicyclol+IR | Baseline | 100 [(87.44±12.11) mmHg] | 319.0±16.99 | 100 [(3 751.76±578.69) mmHg/s] | 100 [(3 425.89±361.71) mmHg/s] |
| Ischemia | 68.85±16.36** ## | 317.0±54.32 | 68.75±14.42** # | 68.79±15.95** # | |
| Reperfusion 1 h | 87.21±5.92 | 333.2±40.87 | 82.47±9.86 | 89.79±15.55 | |
| Reperfusion 2 h | 84.89±13.58* | 322.2±52.45 | 77.26±12.55** | 83.87±20.99 | |
| Reperfusion 3 h | 77.48±4.43** # | 323.6±34.67 | 79.39±22.52* | 78.06±17.49* | |
| 50 mg/(kg∙d) bicyclol+IR | Baseline | 100 [(91.47±5.55) mmHg] | 326.6±19.4 | 100 [(3 343.48±733.26) mmHg/s] | 100 [(3 306.59±300.88) mmHg/s] |
| Ischemia | 69.78±10.84** ## | 326.4±40.8 | 70.45±7.72** # | 64.11±13.35** ## | |
| Reperfusion 1 h | 80.28±7.01** | 380.2±29.9 | 76.97±6.10** | 76.38±11.58* | |
| Reperfusion 2 h | 86.86±9.80 | 392.5±11.6 | 82.52±7.88 | 83.16±9.75 | |
| Reperfusion 3 h | 94.91±6.25++ | 417.4±23.4 | 94.64±3.31++ | 96.07±13.41+ | |
| 100 mg/(kg∙d) bicyclol+IR | Baseline | 100 [(100.05±11.96) mmHg] | 383.4±62.8 | 100 [(4 697.07±948.00) mmHg/s] | 100 [(4 014.40±1 018.35) mmHg/s] |
| Ischemia | 73.94±11.59** # | 370.8±59.7 | 67.35±11.76** ## | 70.45±5.94* # | |
| Reperfusion 1 h | 90.03±10.63+ | 409.0±70.7 | 89.61±19.27 | 91.68±21.01 | |
| Reperfusion 2 h | 91.12±6.02 | 388.6±44.9 | 88.24±8.80 | 91.89±7.99+ | |
| Reperfusion 3 h | 91.58±5.82+ | 397.8±49.8 | 85.57±10.56 | 90.21±8.43 |
LVDP: left ventricular developed pressure; ±dP/dt max: maximal rise/fall rate of left ventricular pressure. Data are shown as mean±SD, n=5 rat hearts in each group
P<0.05 vs. baseline
P<0.01 vs. baseline
P<0.05 vs. sham group
P<0.01 vs. sham group
P<0.05 vs. IR group
P<0.01 vs. IR group
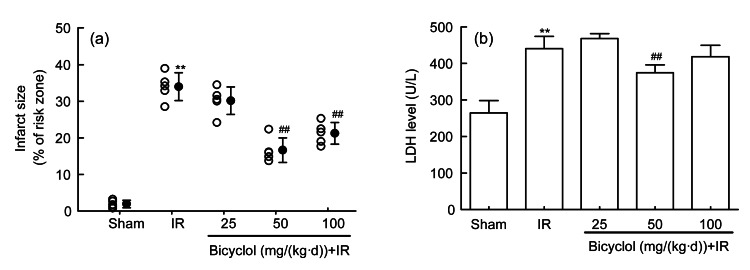
3.2. Effect of bicyclol on myocardial infarct and LDH release after IR
Compared with the sham group, myocardial infarct and LDH release were both significantly increased in IR group (Fig. 1). The increment of infarct size induced by IR was markedly diminished by pretreatment with 50 and 100 mg/(kg∙d) bicyclol (P<0.01), but not by 25 mg/(kg∙d) bicyclol (Fig. 1a). Similarly, pretreatment with 50 mg/(kg∙d) bicyclol inhibited the IR-induced myocardial LDH release (P<0.01), though 25 and 100 mg/(kg∙d) bicyclol failed to reduce the LDH release in the rat heart subjected to IR (Fig. 1b).
Fig. 1.
Effect of bicyclol on myocardial infarct (a) and LDH release (b) in rats subjected to ischemia-reperfusion (IR)
Data are shown as mean±SD, n=5 rat hearts in each group. ** P<0.01 vs. sham group; ## P<0.01 vs. IR group
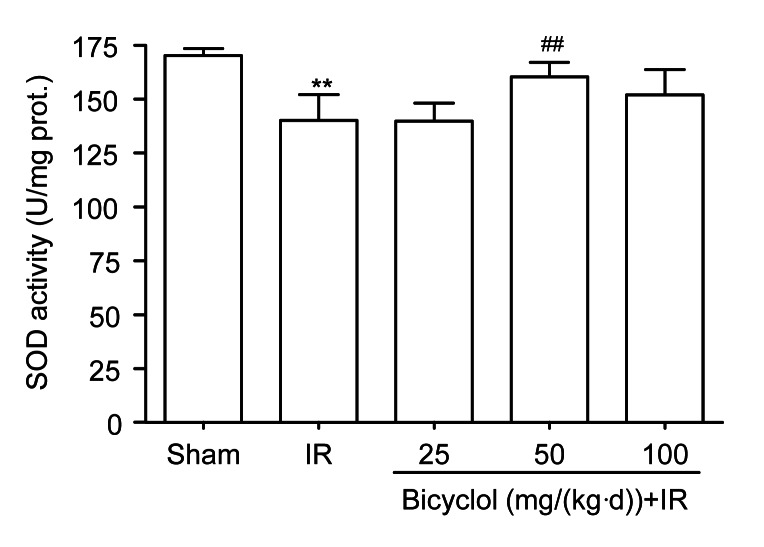
3.3. Effect of bicyclol on myocardial SOD activity after IR
Compared with the sham group, the SOD activity in rat hearts was markedly reduced in the IR group (P<0.01), which was inhibited by pretreatment with 50 mg/(kg∙d) bicyclol (P<0.01 vs. IR), but not by 25 or 100 mg/(kg∙d) bicyclol (Fig. 2).
Fig. 2.
Effect of bicyclol on myocardial superoxide dismutase (SOD) activity in rats subjected to ischemia-reperfusion (IR)
Data are shown as mean±SD, n=5 rat hearts in each group. ** P<0.01 vs. sham group; ## P<0.01 vs. IR group
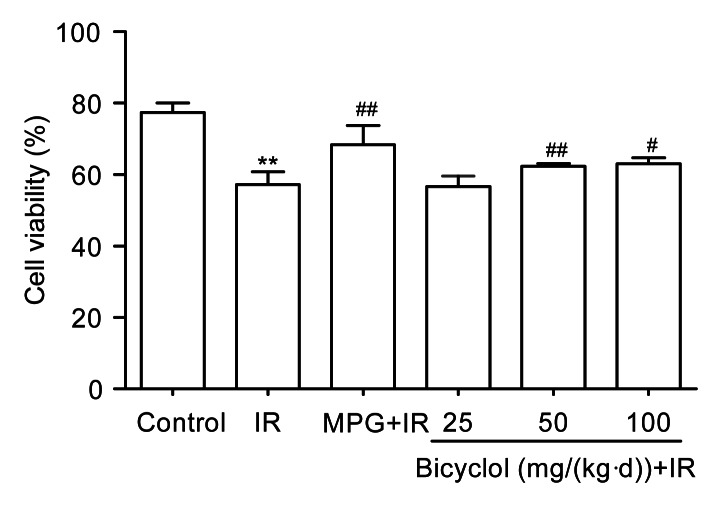
3.4. Effect of bicyclol on ventricular myocyte viability after IR
Ventricular myocyte viability was significantly reduced in IR group (P<0.01 vs. control), which was markedly inhibited by pretreatment with 50 and 100 mg/(kg∙d) bicyclol (P<0.01, P<0.05 vs. IR), but not by 25 mg/(kg∙d) bicyclol (Fig. 3). Pretreatment with MPG (5×10−7 mol/L), an ROS scavenger, for 5 min also markedly inhibited the decrease of myocyte viability induced by IR (P<0.01; Fig. 3).
Fig. 3.
Effect of bicyclol on cell viability in isolated adult rat ventricular myocytes exposed to ischemia-reperfusion (IR)
Data are shown as mean±SD, n=8 ventricular myocyte samples from 8 rat hearts in each group. ** P<0.01 vs. control group; # P<0.05, ## P<0.01 vs. IR group
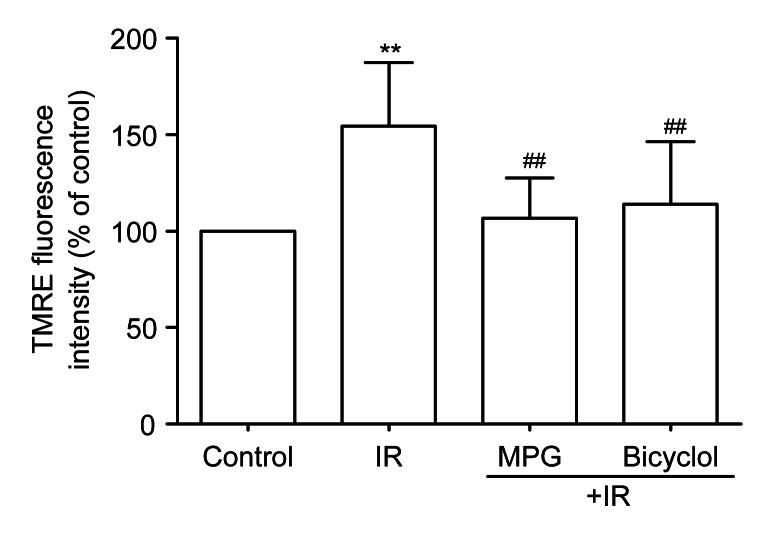
3.5. Effect of bicyclol on myocardial mitochondrial membrane potential after IR
Compared with the control group, TMRE fluorescence of isolated ventricular myocytes was enhanced in the IR group (P<0.01), indicating that mitochondrial membrane potential was depolarized by IR. Pretreatment with bicyclol at 50 mg/(kg∙d) markedly reduced the TMRE fluorescence in isolated ventricular myocytes subjected to IR (P<0.01 vs. IR), which was the same trend as pretreatment with MPG for 5 min before ischemia (Fig. 4).
Fig. 4.
Effect of bicyclol on mitochondrial membrane potential by 1×10−7 mol/L tetramethylrhodamine ethyl ester (TMRE) loading in isolated adult rat ventricular myocytes exposed to ischemia-reperfusion (IR)
Data are shown as mean±SD, n=8 ventricular myocyte samples from 8 rat hearts in each group. ** P<0.01 vs. control group; ## P<0.01 vs. IR group
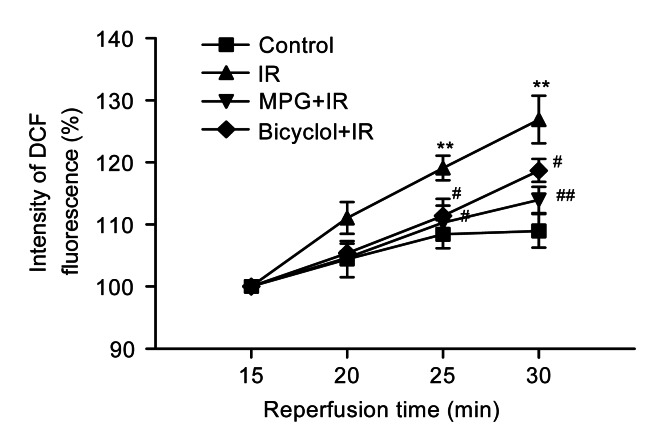
3.6. Effect of bicyclol on ventricular myocyte ROS level after IR
Compared with the control group, DCF fluorescence of myocytes at 25 and 30 min reperfusion was markedly enhanced in IR group (P<0.01), indicating an enhancement of ROS level induced by IR. Pretreatment with bicyclol at 50 mg/(kg∙d) significantly inhibited the DCF fluorescence of myocytes during the reperfusion (P<0.05 vs. IR), which was the same trend as pretreatment with MPG for 5 min before ischemia (Fig. 5).
Fig. 5.
Effect of bicyclol on reactive oxygen species (ROS) by 1×10−5 mol/L 2′,7′-dichlorofluorescein diacetate (DCFH-DA) loading in isolated adult rat ventricular myocytes exposed to ischemia-reperfusion (IR)
Data are shown as mean±SD, n=8 ventricular myocyte samples from 8 rat hearts in each group. ** P<0.01 vs. control group; # P<0.05, ## P<0.01 vs. IR group
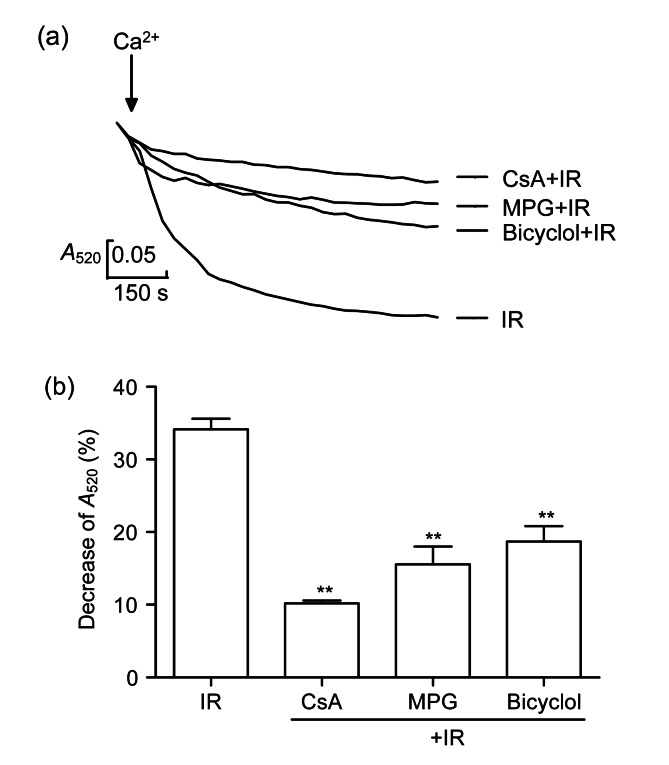
3.7. Effect of bicyclol on the opening of ventricular myocyte mPTP
The addition of CaCl2 (2×10−4 mol/L) induced a great decrease of A 520, indicating an increment of mPTP opening in the mitochondria (Fig. 6). The decrease of A 520 produced by high Ca2+ was diminished by CsA, MPG, and 50 mg/(kg∙d) bicyclol (P<0.01).
Fig. 6.
Effect of bicyclol on the mitochondrial permeability transition pore (mPTP) opening by absorbance at 520 nm (A 520) in isolated rat myocardial mitochondria exposed to 2×10−4 mol/L CaCl2
(a) Curves of the absorbance with different treatments recorded at 15 min after addition of CaCl2; (b) Decrease of absorbance at 15 min after addition of CaCl2. Data are shown as mean±SD, n=8 myocardial mitochondria samples from 8 rat hearts in each group. ** P<0.01 vs. IR group
4. Discussion
Bicyclol is a synthetic drug widely used to treat hepatic disease. A previous study demonstrated that bicyclol strongly protects the kidney from IR injury by reducing oxidative stress (Zhao et al., 2002). However, there is no evidence concerning cardioprotection of bicyclol against IR injury. Here we used a regional myocardial IR model in vivo, a ventricular myocyte model of IR, and isolated myocardial mitochondria model of IR to investigate the protection of bicyclol against myocardial IR injuries. We found that bicyclol (50 and 100 mg/(kg∙d) for 3 d) improved the cardiac function (LVDP, ±dP/dt max) and decreased myocardial infarct after IR, and pretreatment with 50 mg/(kg∙d) bicyclol decreased myocardial LDH release. Furthermore, pretreatment with 50 and 100 mg/(kg∙d) bicyclol increased isolated ventricular myocyte viability after IR, as the same as the effect of MPG, an ROS scavenger. However, the low dose of bicyclol did not affect any of the indexes measured. These results demonstrate that bicyclol has potential cardioprotective effects against IR in an appropriate dose.
A prolonged duration of ischemia followed by reperfusion damages the heart irreversibly, which is mainly mediated by the overproduction of ROS, mitochondrial dysfunction, and intracellular Ca2+ overload (Yellon and Hausenloy, 2007). Oxidative stress due to the ROS burst at the onset of reperfusion and the weakness of endogenous anti-oxidase causes irreversible cellular damage and even cell death (Solaini and Harris, 2005; Venardos et al., 2007). ROS induced by reperfusion mainly include ·O2 −, H2O2, and peroxynitrite (Watanabe et al., 2007; Burwell and Brookes, 2008), all of which damage cellular proteins, membrane lipids, and nucleic acids, leading to cell death (Venardos et al., 2007). In addition, excessive ROS mainly generated from mitochondria in IR periods accelerate the opening of the mPTP, a non-specific large pore across the inner mitochondrial membrane. The opening of mPTP allows cytoplasmic solutes freely entering the mitochondria to enlarge the mitochondrial matrix volume, collapse the mitochondrial membrane potential, release the apoptotic factor cytochrome c from the intermembrane of mitochondria into the cytoplasm (Borutaite and Brown, 2003), and augment ROS production by uncoupling oxidative phosphorylation (Wasilewski et al., 2004), all of which are thought to be the causes of reperfusion injury (Halestrap et al., 2004). Therefore, scavenging toxic ROS or preventing their formation is a crucial strategy to reduce myocardial IR. CsA, the inhibitor of mPTP, has already been proven to blunt the loss of cardiac myocytes and protect the heart from myocardial IR injury (Hausenloy et al., 2002; Nakagawa et al., 2005). In the current ventricular myocyte model of IR, we found that bicyclol (50 mg/(kg∙d)) inhibited CaCl2 (2×10−4 mol/L) induced mPTP opening just like CsA, and reduced cell death, ROS production, and the depolarization of mitochondrial membrane potential induced by IR just like MPG. Such findings suggest that the cardioprotection of bicyclol is related to its reducing ROS, inhibiting mPTP opening and maintaining the mitochondrial membrane integrity.
Oxidative stress occurs when the generation of ROS overrides the ability of the endogenous anti-oxidase system to neutralize ROS (Anand et al., 2011). The activity of endogenous antioxidative enzymes, such as SOD, is inhibited by acute myocardial ischemia, and even is washed out markedly during reperfusion. All of these events stimulate the production of more ROS, which aggravates the myocardial IR injury (Hamilton, 2007; Venardos et al., 2007). So scavenging ROS might be an effective pathway to protect heart against IR (Tao et al., 2009). Our results show that pretreatment with bicyclol (50 mg/(kg∙d)) markedly increased myocardial SOD activity after IR, consistent with the findings in the previous study of bicyclol reducing cerebral IR injury by attenuating oxidative stress in mice (Sun et al., 2009). This anti-oxidative action of bicyclol is further supported by the isolated ventricular myocyte and myocardial mitochondria model. It is suggested that pretreatment with a medium dose of bicyclol (50 mg/(kg∙d)) might enhance endogenous SOD activity, inhibit ROS production, and then diminish oxidative stress-induced mPTP opening to stabilize the mitochondrial membrane potential, all of which ultimately reduce cell death and protect myocardium against IR injury.
Altogether, our current findings indicate that bicyclol protected the myocardium against IR injury, at least, through reducing oxidative stress and its subsequent mPTP opening.
Footnotes
Project (Nos. 2011C23105 and 2012C33088) supported by the Department of Science and Technology of Zhejiang Province, China
Compliance with ethics guidelines: Jie CUI, Zhi LI, Ling-bo QIAN, Qin GAO, Jue WANG, Meng XUE, Xiao-e LOU, Iain C. BRUCE, Qiang XIA, and Hui-ping WANG declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Anand KV, Anandhi R, Pakkiyaraj M, Geraldine P. Protective effect of chrysin on carbon tetrachloride (CCl4)-induced tissue injury in male Wistar rats. Toxicol Ind Health. 2011;27(10):923–933. doi: 10.1177/0748233711399324. [DOI] [PubMed] [Google Scholar]
- 2.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, Guo Y, Bolli R, Cardwell EM, Ping P. Protein kinase Cε interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003;92(8):873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borutaite V, Brown GC. Mitochondria in apoptosis of ischemic heart. FEBS Lett. 2003;541(1-3):1–5. doi: 10.1016/S0014-5793(03)00278-3. [DOI] [PubMed] [Google Scholar]
- 4.Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10(3):579–599. doi: 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- 5.Cao CM, Xia Q, Bruce IC, Zhang X, Fu C, Chen JZ. Interleukin-2 increases activity of sarcoplasmic reticulum Ca2+-ATPase, but decreases its sensitivity to calcium in rat cardiomyocytes. J Pharmacol Exp Ther. 2003;306(2):572–580. doi: 10.1124/jpet.102.048264. [DOI] [PubMed] [Google Scholar]
- 6.Gao Q, Pan HY, Qiu S, Lu Y, Bruce IC, Luo JH, Xia Q. Atractyloside and 5-hydroxydecanoate block the protective effect of puerarin in isolated rat. Life Sci. 2006;79(3):217–224. doi: 10.1016/j.lfs.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 7.Gao Q, Yang B, Ye ZG, Wang J, Bruce IC, Xia Q. Opening the calcium-activated potassium channel participates in the cardioprotective effect of puerarin. Eur J Pharmacol. 2007;574(2-3):179–184. doi: 10.1016/j.ejphar.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Gross GJ, Auchampach JA. Reperfusion injury: does it exist? J Mol Cell Cardiol. 2007;42(1):12–18. doi: 10.1016/j.yjmcc.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion—a target for cardioprotection. Cardiovasc Res. 2004;61(3):372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton KL. Antioxidants and cardioprotection. Med Sci Sports Exerc. 2007;39(9):1544–1553. doi: 10.1249/mss.0b013e3180d099e8. [DOI] [PubMed] [Google Scholar]
- 11.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55(3):534–543. doi: 10.1016/S0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 12.Hausenloy D, Wynne A, Duchen M, Yellon D. Transient mitochondrial permeability transition pore opening mediates preconditioning-induced protection. Circulation. 2004;109(14):1714–1717. doi: 10.1161/01.CIR.0000126294.81407.7D. [DOI] [PubMed] [Google Scholar]
- 13.He W, Zhang FJ, Wang SP, Chen G, Chen CC, Yan M. Postconditioning of sevoflurane and propofol is associated with mitochondrial permeability transition pore. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2008;9(2):100–108. doi: 10.1631/jzus.B0710586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiebert L, Ping T. Protective effect of dextran sulfate and heparin on adult rat cardiomyocytes damaged by free radicals. J Mol Cell Cardiol. 1997;29(1):229–235. doi: 10.1006/jmcc.1996.0267. [DOI] [PubMed] [Google Scholar]
- 15.Hu QW, Liu GT. Effects of bicyclol on dimethylnitrosamine-induced liver fibrosis in mice and its mechanism of action. Life Sci. 2006;79(6):606–612. doi: 10.1016/j.lfs.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Shan J, Pan XH, Wang HP, Qian LB, Xia Q. Carvedilol improved diabetic rat cardiac function depending on antioxidant ability. Diabetes Res Clin Pract. 2007;75(1):7–13. doi: 10.1016/j.diabres.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Katoh H, Nishigaki N, Hayashi H. Diazoxide opens the mitochondrial permeability transition pore and alters Ca2+ transients in rat ventricular myocytes. Circulation. 2002;105(22):2666–2671. doi: 10.1161/01.CIR.0000016831.41648.04. [DOI] [PubMed] [Google Scholar]
- 18.Kroemer G. The mitochondrion as an integrator/coordinator of cell death pathways. Cell Death Differ. 1998;5(6):547. doi: 10.1038/sj.cdd.4400387. [DOI] [PubMed] [Google Scholar]
- 19.Liu GT, Li Y, Wei HL, Lu H, Zhang H, Gao YG, Wang LZ. Toxicity of novel anti-hepatitis drug bicyclol: a preclinical study. World J Gastroenterol. 2005;11(5):665–671. doi: 10.3748/wjg.v11.i5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu H, Li Y. Effects of bicyclol on aflatoxin B1 metabolism and hepatotoxicity in rats. Acta Pharmacol Sin. 2002;23(10):942–945. [PubMed] [Google Scholar]
- 21.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Ostu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434(7033):652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 22.Solaini G, Harris DA. Biochemical dysfunction in heart mitochondria exposed to ischaemia and reperfusion. Biochem J. 2005;390(Pt2):377–394. doi: 10.1042/BJ20042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun H, Liu GT. Chemopreventive effect of bicyclol on malignant transformation of WB-F344 rat liver epithelial cells and its effect on related signal transduction in vitro. Cancer Lett. 2006;236(2):239–249. doi: 10.1016/j.canlet.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Sun LN, Shen J, Su F, Wang Q, Zhu YJ, Lou XE, Liang HW, Bruce IC, Xia Q. Bicyclol attenuates oxidative stress and neuronal damage following transient forebrain ischemia in mouse cortex and hippocampus. Neurosci Lett. 2009;459(2):84–87. doi: 10.1016/j.neulet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Swift LM, Sarvazyan N. Localization of dichlorofluorescin in cardiac myocytes: implications for assessment of oxidative stress. Am J Physiol Heart Circ Physiol. 2000;278(3):H982–H990. doi: 10.1152/ajpheart.2000.278.3.H982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao X, Lu LQ, Xu Q, Li SR, Lin MT. Cardioprotective effects of anesthetic preconditioning in rats with ischemia-reperfusion injury: propofol versus isoflurane. J Zhejiang Univ-Sci B (Biomed & Biotechno) 2009;10(10):740–747. doi: 10.1631/jzus.B0920119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venardos KM, Perkins A, Headrick J, Kaye DM. Myocardial ischemia-reperfusion injury, antioxidant enzyme systems, and selenium: a review. Curr Med Chem. 2007;14(14):1539–1549. doi: 10.2174/092986707780831078. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Li Y. Protective effect of bicyclol on acute hepatic failure induced by lipopolysaccharide and D-galactosamine in mice. Eur J Pharmacol. 2006;534(1-3):194–201. doi: 10.1016/j.ejphar.2005.12.080. [DOI] [PubMed] [Google Scholar]
- 29.Wasilewski M, Wieckowski MR, Dymkowska D, Wojtczak L. Effects of N-acylethanolamines on mitochondrial energetics and permeability transition. Biochim Biophys Acta. 2004;1657(2-3):151–163. doi: 10.1016/j.bbabio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe T, Owada S, Kobayashi HP, Kawakami H, Nagaoka S, Murakami E, Ishiuchi A, Enomoto T, Jinnouchi Y, Sakurai J, et al. Protective effects of MnM2Py4P and Mn-salen against small bowel ischemia/reperfusion injury in rats using an in vivo and an ex vivo electron paramagnetic resonance technique with a spin probe. Transplant Proc. 2007;39(10):3002–3006. doi: 10.1016/j.transproceed.2007.08.091. [DOI] [PubMed] [Google Scholar]
- 31.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 32.Zhang SZ, Wang NF, Xu J, Gao Q, Lin GH, Bruce IC, Xia Q. Kappa-opioid receptors mediate cardioprotection by remote preconditioning. Anesthesiology. 2006;105(3):550–556. doi: 10.1097/00000542-200609000-00019. [DOI] [PubMed] [Google Scholar]
- 33.Zhao DM, Sun T, Li Y. The protective effect of bicyclol on ischemia-reperfusion induced kidney injury in rats. Acta Pharm Sin. 2002;37(6):412–414. doi: 10.3321/j.issn:0513-4870.2002.06.004. (in Chinese) [DOI] [PubMed] [Google Scholar]