Highlights
-
•
A method to quantify tamsulosin by liquid chromatography–tandem mass spectrometry.
-
•
Simple extraction method from serum, excellent recovery, linear range 0.2–50 ng/mL.
-
•
In-house synthesis of internal standard, d9-finasteride.
-
•
Validated method with acceptable reproducibility, precision, accuracy and stability.
-
•
Useful to assess compliance and pharmacokinetics in studies of benign prostatic hyperplasia.
Abbreviation: BPH, benign prostatic hyperplasia
Keywords: Tamsulosin, Finasteride, Liquid chromatography, Mass spectrometry, Serum, Prostate
Abstract
A simple, sensitive and robust method to extract tamsulosin from human serum, and quantify by liquid chromatography–tandem mass spectrometry (LC–MS/MS) was developed and validated and is applicable as a measure of compliance in clinical research. Tamsulosin was extracted from human serum (100 μL) via liquid–liquid extraction with methyl tert-butyl ether (2 mL) following dilution with 0.1 M ammonium hydroxide (100 μL), achieving 99.9% analyte recovery. Internal standard, d9-finasteride, was synthesised in-house. Analyte and internal standard were separated on an Ascentis® Express C18 (100 mm × 3 mm, 2.7 μm) column using a gradient elution with mobile phases methanol and 2 mM aqueous ammonium acetate (5:95, v/v). Total run-time was 6 min. Tamsulosin was quantified using a triple quadrupole mass spectrometer operated in multi-reaction-monitoring (MRM) mode using positive electrospray ionisation. Mass transitions monitored for quantitation were: tamsulosin m/z 409 → 228 and d9-finasteride m/z 382 → 318, with the structural formulae of ions confirmed by Fourier transform ion cyclotron resonance mass spectrometry (within 10 ppm). The limit of quantitation was 0.2 ng/mL, and the method was validated in the linear range 0.2–50 ng/mL with acceptable inter- and intra-assay precision and accuracy and stability suitable for routine laboratory practice. The method was successfully applied to samples taken from research volunteers in a clinical study of benign prostatic hyperplasia.
1. Introduction
Benign prostatic hyperplasia (BPH) is a highly prevalent disorder in older men which causes lower urinary tract symptoms and in severe cases can lead to urinary retention and renal tract complications [1]. Tamsulosin (Fig. 1) is an α1 adrenergic antagonist, targeting uro-specific α1A and α1D receptors, and is an important therapy for many BPH patients [2]. In clinical studies of response to pharmacological intervention establishing compliance with study medication is important. Traditional methods of establishing compliance such as a ‘pill count’ at the end of a study can be complemented by measurement of drug in serum.
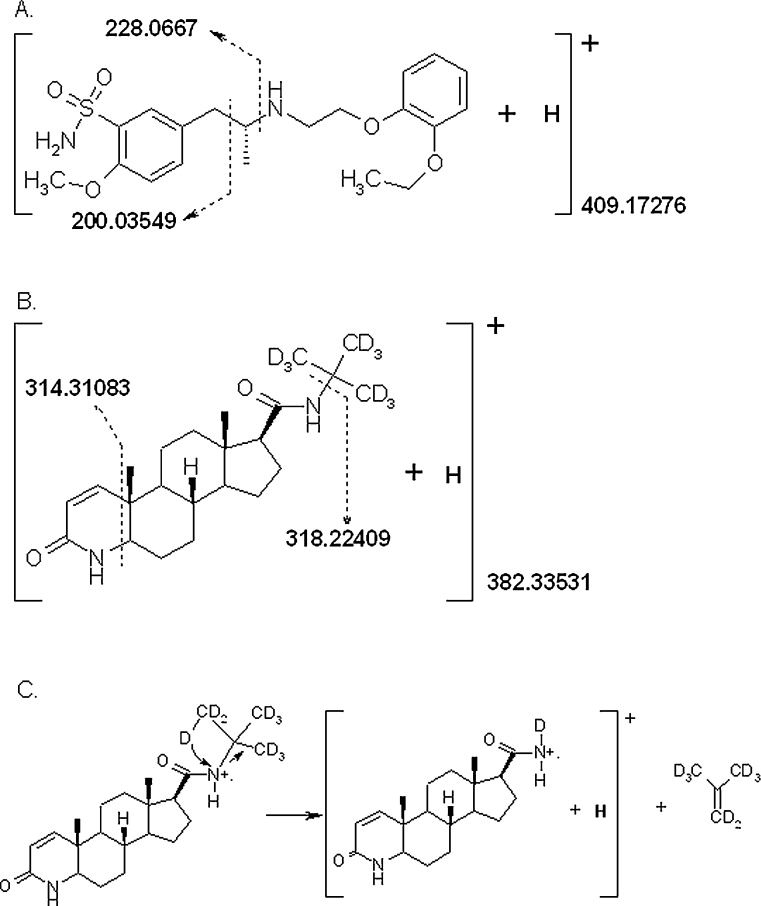
Fig. 1.
Chemical structures and proposed fragmentation patterns for analyte and internal standard. Accurate masses shown for analyte quantifier and qualifier ions were confirmed to within 10 ppm of their theoretical monoisotopic mass. (A) Structure and proposed fragmentation pattern for tamsulosin. (B) Structure and proposed fragmentation for d9-finasteride. (C) Proposed mechanism for fragmentation of d9-finasteride into quantifier ion. From the charged radical parent ion a single deuterium shifts in a concerted transfer of 2 electrons from the deuterated tert-butyl amine to the amide nitrogen with loss of neutral d8-2-methylpropene (shown). A single electron radical transfer is also possible.
Tamsulosin levels in those treated with the 0.4 mg modified release formulation are reported to be between 11.8 ng/mL [3] and 16.1 ng/mL [4] after a single dose, and 10 ng/mL after 21 days dosing [5]. Measurement of tamsulosin levels has been reported from plasma by HPLC [6,7], LC–MS [8] and LC–MS/MS [3,9–12], with key features of these methods described in Table 1. HPLC alone precludes additional specificity and sensitivity afforded by its use in conjunction with mass spectrometry, and in all assays described requires undesirably large (1–1.5 mL) sample volumes. While LC–MS methods have been described, it is now increasingly recognised that tandem mass spectrometry (such as LC–MS/MS) with the monitoring of 2 mass transitions is the gold standard of analyte measurement. Electrospray ionisation has been used more commonly, but atmospheric pressure ionisation (APCI) was used successfully, by Qi et al. [9]. The combination of an LC–MS/MS approach, minimal sample volume, simple extraction method and excellent analyte recovery was not achieved in any published method (Table 1). Therefore, we sought to develop an assay to measure tamsulosin from human serum with a simple extraction method, excellent analyte recovery and sufficient sensitivity to allow use of small sample volumes.
Table 1.
Summary of key characteristics of published assays to quantify tamsulosin from plasma or serum.
| Internal standard | LLE: pH modifier; solvent | Sample volume | Analytical method | LC run time (min) | Linear range (ng/mL) | Recovery (%) | LLOQ | |
|---|---|---|---|---|---|---|---|---|
| Assay reported | d9-Finasteride | NH4OH; MTBE | 100 μL | LC–MS/MS | 6 | 0.2–50 | 107.4% | 2 pg |
| [7] | ±-5-[2-[[2-(o-ethoxyphenoxy)propyl]amino]propyl]-2-methoxybenzenesulphonamide | >1 step incl. S.NaHCO3; EA | 1.5 mL | HPLC | NR (tamsulosin RT 5.9 min) | 0.5–15 | 70% | 525 pg |
| [10] | (6)-(R)-5-[3-[[2-(o-ethoxyphenoxy)ethyl] amino]butyl] – 2 – methoxybenzenesulfonamide hydrochloride (AB289) (Yamanouchi) | S. NaHCO3; H:EA | 200 μL | LC–MS/MS | 3 | 0.5–50 | >80% | 25 pg |
| [4]a | AB289 (as above) | Details NR | NR | HPLCa | NR | 0.5–50 | NR | NR |
| [8] | 1-(2,6-Dimethyl-3-hydroxylphenoxy)-2- (3,4-methoxyphenylethylamino)-propane hydrochloride | S. NaHCO3; EA | 1 mL | LC–MS | 8 | 0.2–30 | 84.2–94.5% | 40 pg |
| [6] | Analogue of tamsulosin: (R)-5-[2-[(3-(2-ethoxyphenoxy)propyl)amino]-2-methylethyl]-2-methoxybenzensulfonamide (Léčiva) | Na2CO3; BA | 1 mL | HPLC | 3.5 | 0.4–40 | NR | 360 pg |
| [3] | Mosapride | NaOH; DEE:DCM | 100 μL | LC–MS/MS | 2 | 0.1–50 | 59.3% | NR |
| [11] | Labetalol | S. NaHCO3; EA | 1 mL | LC–MS/MS | 5 | 0.1–19.3 | 66–77% | 17.5 pg |
| [12] | Diphenhydramine hydrochloride | MTBE | 500 μL | LC–MS/MS | 2 | 0.01–20 | 78% | 5 pg |
| [9] | Diphenhydramine | NaCO3, H, EA | 200 μL | LC–MS/MS | NR (tamsulosin RT 2.2 min) | 0.1–30 | 73% | 2 pg |
LLE, liquid–liquid extraction; LC, liquid chromatography; MTBE, methyl tert-butyl ether; LC–MS/MS, liquid chromatography tandem mass spectrometry; S., saturated; EA, ethyl acetate; HPLC, high performance liquid chromatography; NR, not reported; H, hexane; BA, butyl acetate; DEE, diethyl ether; DCM, dichloromethane; RT = retention time; LLOQ = lower limit of quantitation (on-column).
This method principally describes a radioreceptor assay, details of which are not included in this table. The analytical method developed, validated and presented here is summarised in the first row for comparison.
2. Experimental
2.1. Reagents and standards
All solvents were HPLC grade and chemicals were from Sigma–Aldrich (Dorset, UK) unless otherwise stated. The internal standard, d9-finasteride was synthesised in house (see Section 2.3). Sources of other chemicals were as follows: tamsulosin hydrochloride (AK Scientific, Mountain View, USA), water and ammonium hydroxide (35%, v/v) solution (Fisher Scientific, Loughborough, UK), methanol (VWR, Lutterworth, Leicestershire, UK), 4-aza-5α-androstan-1-en-one-16β-carboxylic acid (APAC pharmaceutical, LLC, Columbia, USA), and 2-amino-2methyl-d3-propane-1,1,1,3,3,3-d6 (CDN isotopes Inc., Quebec, Canada).
2.2. Biological samples
Pooled male human serum (collected from healthy men aged 17–45 years on no medications) was purchased (TCS Biosciences, Buckingham, UK) for use as blank matrix in method development, validation and standard curves (referred to as “drug-free serum”). For method application, serum was collected from 3 male subjects who had received at least 3 months of treatment with tamsulosin MR 0.4 mg daily (Synthon Hispania, Sant Boi de Llobregat, Spain). Serum was also analysed from 3 male subjects not receiving tamsulosin. All biological samples were collected with informed consent with local regulatory and ethical approval and stored at −80 °C until analysis.
2.3. Selection of internal standard and synthesis of d9-finasteride
In the absence of a commercially available stable isotope labelled internal standard, compounds tested as potential internal standards were trichlormethiazide, ketoconazole, phthalylsulfathiazole (QMX Laboratories, Essex, UK) and d9-finasteride.
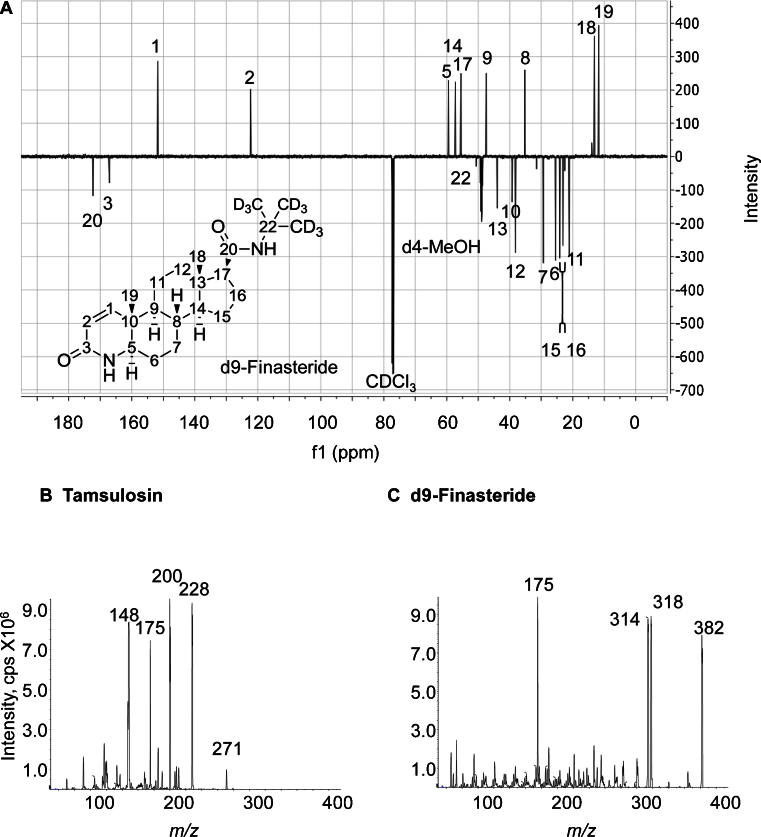
Synthesis of d9-finasteride was adapted from the general amide formation coupling reported by Rasmusson et al. [13]. 4-Aza-5α-androstan-1-en-one-16β-carboxylic acid (97% pure, 200 mg, 0.63 mmol) and 2-amino-2methyl-d3-propane-1,1,1,3,3,3-d6 (342 μL,3. 24 mmol) were used as starting materials to obtain 191 mg of crude material (80% yield) after work-up. 50 mg were purified by flash chromatography on an Isolera Biotage system (5% isopropanol-95% DCM, SNAP silica cartridge (25 mg), 254 nm) to yield 31 mg of pure d9-finasteride. The final compound was characterised by 13C NMR (d4-MeOH, Bruker AV400 NMR spectrometer). 13C NMR signals matched most of those of a finasteride standard (Fig. 2C). Characteristically the 28.7 ppm signal for the 3 primary (CD3)3CN carbons was absent and the 51.1 ppm signal for the quaternary (CD3)3CN carbon was less intense than in the finasteride reference material.
Fig. 2.
(A) d9-Finasteride DQ135 13C NMR (126 MHz, CDCl3) δ = C-20: 172.29, C-3: 167.09, C-1: 151.71, C-2: 122.30, C-5: 59.56, C-14: 57.29, C-17: 55.48, C-22: 50.63, C-9: 47.47, C-13: 43.97, C-10: 39.27, C-12: 38.16, C-8: 35.22, C-7: 29.33, C-6: 25.55, C-15: 24.15, C-16: 23.12, C-11: 21.13, C-18: 13.13, C-19: 11.76. Assignation was carried out based on previously published data [16]. A reference sample of finasteride scanned on the same instrument gave the same signals, apart from the presence of the intense tert-butyl CH3 signal at 28.7 ppm. cps, counts per second. (B) Product ion spectra for protonated tamsulosin in electrospray ionisation mode, with m/z 228 and 200 selected as quantifier and qualifier ions respectively. (C) Product ion spectra for protonated d9-finasteride in electrospray ionisation mode, with m/z 318 and 314 selected as quantifier and qualifier ions respectively. Product ion spectra for both tamsulosin and d9-finasteride were collected under the following conditions: declustering potential 119 V, collision energy 35 V, cell exit potential 16 V.
2.4. Instrumentation
Chromatographic separation was performed on a Waters Acquity™ UPLC system (Manchester, UK), and detection for quantitative analysis was performed on an ABSciex QTRAP® 5500 mass spectrometer (Warrington, UK), with nitrogen as the source and collision gas. The system was operated using Analyst® Software version 1.5.1. For confirmation of structural formulae of proposed fragment ions, electrospray-Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) was performed using a 12 T SolariX dual source (ESI/MALDI) system (Bruker Daltonics, MA, USA), operated with SolariX control version 1.5.0 (build 42.8) software.
2.4.1. Analytical, chromatographic and mass spectrometric conditions
Chromatographic separation was achieved on an Ascentis® Express C18 column (100 mm × 3 mm, 2.7 μm, Sigma–Aldrich, Dorset, UK), protected by a BDS Hypersil C18 guard cartridge (10 mm × 3 mm, 3 μm; Thermo Electron, Hemel Hempstead, UK). Column and autosampler temperatures were maintained at 40 °C and 10 °C, respectively. Elution was achieved at a flow rate of 0.35 mL/min (initial backpressure of approximately 145 bar), using a gradient from 5:95 (methanol:aqueous ammonium acetate (2 mM), pH 7.38), with an initial hold of 0.5 min followed by a linear increase in organic mobile phase to 95:5 at 2.5 min, which was sustained for a further minute (until 3.5 min) before re-equilibration, with a total run-time of 6 min. Retention times for analyte and internal standard were approximately 3.5 and 4 min respectively.
The mass spectrometer was operated in positive electrospray ionisation (ESI) mode, with curtain gas 25 psi, collision gas medium, spray voltage of 5 kV, source temperature 550 °C, and source gases both set to 55 psi. Multiple reaction monitoring of the analyte transitions (collision energy, cell exit potential, declustering potential (all V)) were m/z 409 → 228 (33, 20,141 V; quantifier), m/z 409 → 200 (45, 16, 141 V; qualifier) and internal standard transitions m/z 382 → 318 (31, 12, 96 V; quantifier) and m/z 382 → 314 (39, 14, 96 V; qualifier) was performed. The quantifier and qualifier transitions were selected as those with the greatest signal-to-noise ratios.
2.4.2. Fourier transform ion cyclotron resonance mass spectrometry (FTICRMS)
Analyte and internal standard were directly infused separately (20 ng/μL) in acetonitrile: 0.1% trifluoroacetic acid in water (60:40, v/v). Ions were detected between m/z 250 and 1500, yielding a 1 Mword time-domain transient. Ions of interest were isolated for 20 s prior to collision-induced dissociation (CID) experiments. CID was carried out using 35 eV as the collision energy.
2.5. Extraction method
As tamsulosin is not present endogenously, it was appropriate to optimise and perform this assay in the biological matrix, serum. Extraction efficiency was compared between different extraction methods attempted and the most effective was selected. Repetitions (n = 6) were performed to ensure reproducibility.
Tamsulosin (1 mg) and d9-finasteride (1 mg) were dissolved separately in methanol (1 mL) and stored at −20 °C. Stock solutions (10 μg/mL in methanol) of tamsulosin and d9-finasteride (internal standard) were prepared and stored at −20 °C. Standards of lower concentration were prepared on the day of analysis by serial dilution of the stock solutions.
Serum (100 μL) was dispensed into a glass tube, and d9-finasteride (1 ng) added (as 10 μL of 100 ng/mL solution). NH4OH (0.1 M, 100 μL) was added and samples mixed (5 min, 100 rpm). Analyte and internal standard (IS) were extracted via a liquid–liquid extraction with methyl tert-butyl ether (MTBE; 2 mL). Following mixing (5 min, 100 rpm), and centrifugation (1791 g, 4 °C, 10 min), the organic layer was transferred to another glass tube. Extracts were reduced to dryness under oxygen free nitrogen (40 °C) and the residue reconstituted in mobile phase (100 μL, methanol:2 mM ammonium acetate, 5:95). Injection volume was 10 μL.
2.6. Assay validation
2.6.1. Recovery
Recovery was calculated by expressing the mean of the integrated peak areas from extracted standards, as a percentage of the mean integrated peak area from post-spiked standards. This was performed in 6 replicate samples of drug-free serum enriched with analyte (1 ng) and internal standard (1 ng).
2.6.2. Assessment of ion suppression
Effect of the biological matrix (human serum) on ionisation efficiency was assessed in replicates of 6 by post-spiking extracts of drug-free serum with tamsulosin (1 ng), and the response compared to standards (1 ng) dissolved directly in mobile phase.
2.6.3. Specificity
Analyte specificity was ensured to avoid potential interferences by other endogenous components in serum. Extracted drug-free serum analysed using the described method was checked for interferences at or close to the expected retention times for tamsulosin and d9-finasteride. Additional analyte and internal standard specificity was ensured with measurement of quantifier and qualifier ions. Acceptable quantifier:qualifier ratios in biological samples were those within 20% of the mean ratio seen in standards.
2.6.4. Limit of detection (LOD)
Limits of detection (LOD) were determined by analysing solutions prepared by serial dilution of analyte and internal standard stock solutions (0.1–0.3 ng/mL), with the LOD corresponding to the quantity of analyte generating a peak with signal-to-noise ratio (SNR) of approximately 3.
2.6.5. Lower limit of quantitation (LLOQ)
Lower limit of quantitation (LLOQ) following extraction was determined by extracting analyte and internal standard from serum at amounts approximating LOD (0.02 ng), 2× LOD (0.04 ng), and 4× LOD (0.08 ng), corresponding to concentrations of 0.2 ng/mL, 0.4 ng/mL and 0.8 ng/mL respectively. The LLOQ was defined as the amount where the relative standard deviation of the mean (RSD), in replicates of 6, was ≤20%, where RSD (%) = standard deviation/mean × 100.
2.6.6. Linearity
A standard curve was generated by adding d9-finasteride (1 ng) to drug-free serum and increasing amounts (0.02, 0.1, 0.2, 0.5, 1, 2, 3, 4, 5 ng) of tamsulosin (corresponding to a concentration range of 0–50 ng/mL). Peak areas of quantifier ions of tamsulosin and d9-finasteride were integrated and a calibration curve constructed (peak area ratio of tamsulosin:d9-finasteride versus amount of tamsulosin). Regression lines of best fit were constructed and deemed acceptable if the regression coefficient, r, was >0.99. Weightings compared were none, 1/x and 1/x2 to improve accuracy and precision at the lowest concentrations and to afford intercepts as close to zero as possible.
2.6.7. Accuracy and precision
The intra-assay accuracy and precision were determined in a standard curve with 4 points of the standard curve (LLOQ, low, mid, high points) prepared in replicates of 6 (standard concentration: 0.2, 1, 20, 50 ng/mL). The inter-assay accuracy and precision were determined from 6 standard curves prepared on 6 independent occasions. Replicate peak area ratios were interpolated onto the matched calibration line to yield calculated amounts. The precision was calculated as the % RSD of the calculated values, and % accuracy was calculated as the measured value/theoretical value × 100. Injector precision was assessed by injecting the same standards as above 6 times on the same day. Precision and injector variability were also assessed in a volunteer sample.
2.6.8. Stability
Stability was assessed by reinjection of a calibration curve and patient sample after 24 h in auto-sampler (10 °C), and then again following 28 day storage (−20 °C). Storage conditions giving no greater that 10% change in response were accepted. Samples (n = 5) were subject to one freeze–thaw cycle and concentrations quantified before and after this process.
3. Results and discussion
3.1. Method development
3.1.1. Optimisation of mass spectrometric conditions
The mass of ions formed was determined by direct infusion into the ion source and the most abundant ions undergoing transitions to abundant product ions were selected for use in subsequent analysis as quantifier ions (following confirmation that they were also associated with the best signal-to-noise ratios in biological extracts). To provide additional specificity, the second most abundant product ion was used as the qualifier ion. Tamsulosin ionised efficiently under positive electrospray ionisation conditions, yielding the mono-protonated molecular ions with m/z 409.1. Mass spectrometric source conditions (curtain gas, collision gas, spray voltage, source temperature, source gases) and MS/MS parameters (mass transition, collision energy, cell exit potential, declustering potential) were then optimised for tamsulosin. The MS/MS spectrum is presented in Fig. 2A and is similar to those reported previously using both electrospray and APCI [9,11,12]. The identity of the fragment ions of tamsulosin were confirmed by FT-ICR-MS, with mass accuracy of ±10 ppm from theoretical monoisotopic masses (Fig. 1), corroborating the proposal by Matushima et al. [10]; loss of the 2-(o-ethoxyphenoxy)-ethyl amine moiety, yielding m/z 228 and m/z 200 corresponding to the 2-methoxy-5-methyl benzene-sulfonamide moiety.
3.1.2. Selection of internal standard
In the absence of a commercially available stable-isotope labelled tamsulosin, trichlormethiazide (reported by [13]) and further structurally similar compounds (ketoconazole and phthalylsulfathiazole) were explored as potential internal standards. However, while extraction efficiency of trichlormethiazide was reproducible, extraction of ketoconazole and phthalylsulfathiazole required pre-extraction pH modification that differed from the conditions optimised for tamsulosin. In addition, these compounds exhibited very poor ionisation efficiency in positive ESI mode and indeed were better suited to ionisation in negative mode; however analysis of tamsulosin in negative ion mode was not sufficiently sensitive for clinical samples. While switching between positive and negative ionisation modes is possible, this approach was not pursued.
While testing methods of analysis of drugs used to treat benign prostatic hyperplasia, it was noted that finasteride was well suited as an internal standard. While lacking in structural similarities to tamsulosin, it exhibited very similar behaviour throughout the analytical process, including extraction efficiency, ionisation and detection. The internal standard ultimately selected was d9-finasteride (Fig. 2C), rather than finasteride, due to the possibility of patients being co-prescribed this drug along with tamsulosin. The transitions monitored for analysis of d9-finasteride were m/z 382–318 and m/z 382–314 and the fragmentation pattern confirmed by FT-ICR-MS (Figs. 1 and 2C). Analyte and internal standard were well resolved chromatographically (Fig. 3). In the future, however, stable-isotope labelled tamsulosin would be preferred if available.
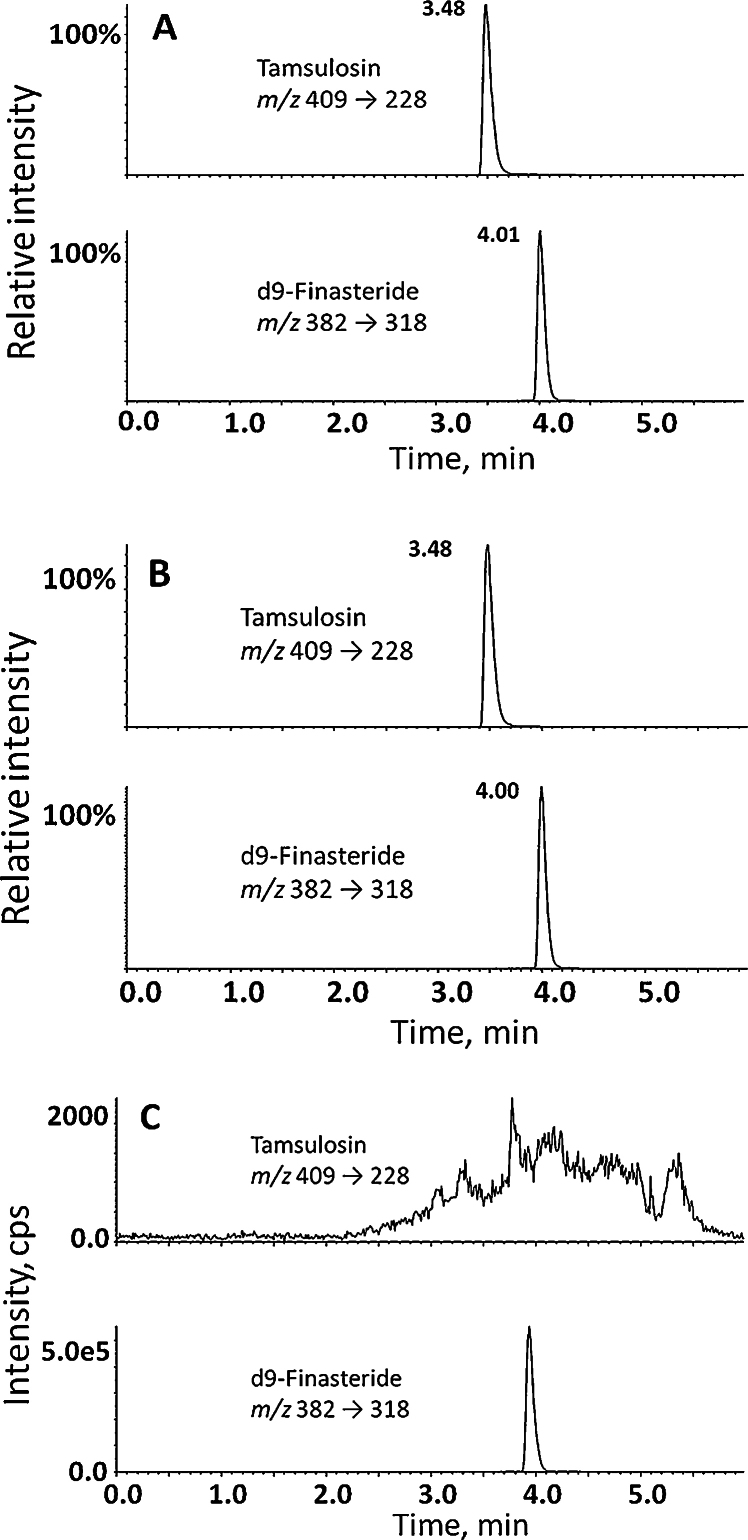
Fig. 3.
(A) Representative mass chromatograms of quantifier mass transitions for analyte, tamsulosin, 20 ng/mL (upper panel) and internal standard, d9-finasteride, 10 ng/mL (lower panel) from spiked extracted serum. (B) Representative mass chromatograms of tamsulosin (quantified as 17.1 ng/mL; upper panel) extracted from a patient sample enriched with internal standard, d9-finasteride (10 ng/mL; lower panel). The patient had received tamsulosin (0.4 mg daily) for 90 days. (C) To assess specificity, the method was applied to serum from patients not receiving tamsulosin (n = 3) with representative chromatograms shown (mass transition of tamsulosin (upper panel), d9-finasteride lower panel)). cps, counts per second.
3.1.3. Chromatographic conditions
The addition of ammonium acetate to the aqueous mobile phase, in contrast to acidic modifiers, consistently gave increased peak areas for tamsulosin. Mobile phases containing methanol yielded better peak shape than acetonitrile. Chromatographic conditions, including flow rate and temperature optimised initially for tamsulosin were suitable for elution and detection of d9-finasteride. Representative chromatograms show tamsulosin and d9-finasteride from standard solutions (Fig. 3A) and from a patient sample spiked with internal standard (Fig. 3B).
One of the challenges faced during development of the assay was achieving symmetrical chromatographic peaks, a common problem with basic analytes such as tamsulosin. Tamsulosin has a secondary amine group with a pKa of 8.4 and problems with peak tailing have been encountered by other researchers [8], typically requiring modification of the pH or buffering of the mobile phase. The addition of formic acid as a mobile phase modifier has been reported to be helpful [3], however on our chromatographic system, similarly to Choi et al. [12] who used ammonium formate, buffering rather than acidic modifiers yielded larger peak areas for tamsulosin.
Using the Ascentis column, trials of several aqueous mobile phases demonstrated the superiority of buffering with ammonium acetate for peak shape and area of both analyte and internal standard. This achieved a pH of 7.38 at which approximately 90% of tamsulosin would be ionised, enhancing the intensity of mass spectrometric response. Varying concentrations of ammonium acetate showed 2 mM (pH 7.38) to be the optimum in terms of analyte peak area, while still retaining consistency in chromatographic response. Higher concentrations of ammonium acetate were associated with poorer peak areas, which could be attributed to the formation of ammonium adducts and associated ion suppression. Inconsistency in retention time was seen with lower (1 mM, 0.5 mM) concentrations of ammonium acetate, suggesting insufficient buffering capacity to overcome the silanol interactions. The addition of the stronger base triethylamine (TEA) has been reported to reduce peak tailing [8], however an improvement was not seen in our hands.
Under the final conditions selected, a total run-time of 6 min allowed high sample throughput. While shorter run-times of 2–3 min are described with isocratic methods [3,9,10,12], when attempted, these isocratic methods resulted in broad and tailing peaks, with decreased selectivity [14]. A shorter run time with gradient elution was not possible due to incomplete column re-equilibration. This could be addressed using UPLC columns in future.
3.1.4. Extraction
While a proportion of the analyte added was recovered following extraction by most methods tested (based on literature summarised in Table 1), the key developmental difficulties were maximising recoveries, while ensuring reproducibility. Several extraction methods were compared during method development including liquid–liquid, supported liquid, and solid phase extraction. Upon initial testing and as with others, liquid–liquid extraction (LLE) methods were taken forward as efficient recovery was achieved and the other techniques did not offer sufficient advantage, either in increased recovery or less ion suppression, to justify the additional expense. As in other publications, a notable feature was the need for pre-extraction pH modification with basic modifiers such as NaHCO3 [8,10], Na2CO3 [6,9] or NaOH [3]. Without modifying the pH of samples prior to extraction there was unacceptably high variability and poor recovery with all types of extractions in replicate sample, probably a reflection of tamsulosin being partially ionised at neutral pH. The strongest base tested, ammonium hydroxide with a pH of 10.9, provided the best response in terms of peak area and consistency with acceptable RSDs between replicate samples for both analyte and internal standard.
Several extraction solvents were compared including ethyl acetate and hexane which gave poor and inconsistent recovery, diethyl ether and dichloromethane which gave consistent but very poor recovery. Following method optimisation (solvent type, proportions, multiples of extraction), excellent recovery and consistent responses were achieved with a liquid–liquid extraction with 20 volumes MTBE, to 1 volume sample, following mixing with 0.1 M ammonium hydroxide solution. Using this approach, analyte recovery was 107.4% (RSD 11.6%) and internal standard recovery was 93.3% (RSD 6.3%), an improvement on all previous reported methods.
3.2. Assay validation
Results from assay validation of LLE are summarised in Table 2.
Table 2.
Summary table of precision and accuracy data, demonstrating acceptable intra-assay precision and accuracy to limits of 0.2 ng/mL. Inter-assay precision became acceptable at low point (1 ng/mL) as defined U.S. FDA guidance [17]. RSD, relative standard deviation.
| Intra-assay (n = 6) |
Inter-assay (n = 6) |
|||||
|---|---|---|---|---|---|---|
| Concentration found (mean ± SD, ng/mL) | Precision (% RSD) | Accuracy (%) | Concentration found (mean ± SD, ng/mL) | Precision (% RSD) | Accuracy (%) | |
| LOQ (0.2 ng/mL) | 0.18 ± 0.02 | 11.1 | 89.4 | 0.21 ± 0.07 | 36.1 | 103.3 |
| Low (1 ng/mL) | 1.1 ± 0.09 | 7.9 | 111.4 | 1.1 ± 0.08 | 7.4 | 100.5 |
| Mid (20 ng/mL) | 22.8 ± 2.9 | 12.9 | 114.1 | 23.2 ± 2.7 | 11.8 | 104.3 |
| High (50 ng/mL) | 45.7 ± 3.8 | 8.3 | 91.5 | 49.0 ± 1.8 | 3.8 | 96.8 |
| Patient sample | 17.8 ± 0.97 | 5.5 | 18.1 ± 1.0 | 5.6 | ||
3.2.1. Ion suppression
The presence of matrix did not significantly affect the intensity of response of tamsulosin (94.7% response, RSD 7.8%).
3.2.2. Specificity
Analyte specificity was ensured through monitoring of both quantifier and qualifier mass transitions by LC–MS/MS. Metabolites of tamsulosin [10,15] would be anticipated to generate different precursor ions and mass transitions from their parent drug. Extracted drug-free serum had no interfering peaks at, or close to, the retention times of tamsulosin or d9-finasteride (Fig. 3C).
3.2.3. Limits of detection (LOD)
Corresponding to a signal:noise ratio of 3, the LOD of tamsulosin was 1.28 pg on column (0.13 ng/mL) and that of d9-finasteride was 1.46 pg on column (0.15 ng/mL).
3.2.4. Lower limit of quantitation (LLOQ)
The intra-assay LLOQ following extraction for tamsulosin was 2 pg on column (0.2 ng/mL, RSD 11.1%).
3.2.5. Linearity
The standard curve was linear in the range 0.2–50 ng/mL with an average r value of 0.9952 (n = 6) with 1/x weighting applied, and average intercept of 0.001 (n = 6). The mean equation of the regression line derived from 6 replicates was y = 0.01690x − 1.2 × 10−3 with regression coefficients in the range 0.991–0.999.
3.2.6. Precision and accuracy
Intra- and inter-assay accuracy and intra-assay precision were acceptable (<20% RSD for precision and 80–120% accuracy) at the LLOQ, and inter-assay precision was acceptable from the 1 ng/mL (RSD 7.9%). Above these values, variability relating to precision (RSD) and accuracy was <15% and were therefore acceptable. Results are summarised in Table 2.
3.2.7. Stability
Acceptable autosampler and extract storage stability were demonstrated (Table 3). Data collated from standard curve and patient samples reinjected after 24 h in the autosampler (10 °C) were unchanged, with a relative response (stored/original) of 100.3%. Extracts reinjected following storage at −20 °C for 28 days had 93.9% response compared to the original run. Concentrations of tamsulosin following one freeze–thaw cycle were not different from those measured at the outset, on average 104% (relative mean error 7.9%) of the original value.
Table 3.
Calculated concentration of tamsulosin in patient sample, demonstrating acceptable stability at 10 °C for 24 h (in the autosampler) and at −20 °C for 28 days.
| Initial run | After 24 h in autosampler | After 28 days stored at −20 °C | Relative response |
|---|---|---|---|
| 17.1 ng/mL | 17.2 ng/mL | 100.3% | |
| 17.9 ng/mL | 16.8 ng/mL | 93.9% |
3.2.8. Injector reproducibility
Acceptable reproducibility upon repeat (n = 6) injections of standards and sample was demonstrated with RSDs of: LOQ (0.2 ng/mL) 6%, low (1 ng/mL) 3%, mid (20 ng/mL) 2%, high (50 ng/mL) 3%, patient sample 1%.
4. Method application
The method was applied to samples from men who had received treatment with tamsulosin MR 0.4 mg daily (n = 3), where concentrations of 16.7–36.1 ng/mL were quantified using 100 μL serum. These were within the quantitation limits of the assay. As 0.4 mg daily is the maximum dose of tamsulosin prescribed in routine clinical practice, this assay is applicable to BPH studies where tamsulosin levels would be measured. In serum obtained from men who had not received tamsulosin treatment (n = 3), analyte was not detected (Fig. 3C).
5. Conclusions
MS has proven the best method for analysis of tamsulosin, with several methods reported which are suitable to detect the drug in clinical samples. However the method reported here offers distinct advantages in terms of the small sample volume required (100 μL), permitted by a lower limit of detection (0.13 ng/mL). This reflects both efficient recovery and also the superior sensitivity of the QTrap 5500 instrument. Sample preparation was simple and economical, and overall this assay presents a combination not previously achieved. This may be of additional use in future studies where analysis of free and bound fraction may be desired, since tamsulosin is highly protein bound (with α1-acid glycoprotein) [10].
Expected concentration of tamsulosin fell within the linear range of the standard curve. Validation steps demonstrated the assay to be applicable to normal laboratory practice and the assay was successfully applied to samples taken from research volunteers treated with tamsulosin. The use of d9-finasteride as an internal standard suggests this method may be easily adapted to also measure finasteride. This would be particularly useful in other clinical studies in BPH where finasteride and tamsulosin are often used in different treatment groups, or administered as combination therapy.
Acknowledgements
The authors wish to thank Dr Lorna Murray (Chemistry, University of Edinburgh) for valuable assistance with 13C NMR signal assignation, the Wellcome Trust Clinical Research Facility Mass Spectrometry Core and SIRCAMS for technical support and the Chief Scientist Office and British Heart Foundation for funding this work.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Rita Upreti, Email: rupreti@staffmail.ed.ac.uk.
Natalie Z.M. Homer, Email: n.z.m.homer@ed.ac.uk.
Gregorio Naredo, Email: gnaredo@staffmail.ed.ac.uk.
Diego F. Cobice, Email: D.F.Cobice@sms.ed.ac.uk.
Katherine A. Hughes, Email: Katherine.Hughes@ed.ac.uk.
Laurence H. Stewart, Email: laurence.stewart@luht.scot.nhs.uk.
Brian R. Walker, Email: b.walker@ed.ac.uk.
Ruth Andrew, Email: ruth.andrew@ed.ac.uk.
References
- 1.Wilt T.J., N’Dow J. BMJ. 2008;336:146. doi: 10.1136/bmj.39421.685023.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lepor H. Rev. Urol. 2007;9:181. [PMC free article] [PubMed] [Google Scholar]
- 3.Ramakrishna N.V.S., Vishwottam K.N., Manoj S., Koteshwara M., Wishu S., Varma D.P. Biomed. Chromatogr. 2005;19:709. doi: 10.1002/bmc.498. [DOI] [PubMed] [Google Scholar]
- 4.Taguchi K., Schafers R.F., Michel M.C. Br. J. Clin. Pharmacol. 1998;45:49. doi: 10.1046/j.1365-2125.1998.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolzt M., Fabrizii V., Dorner G.T., Zanaschka G., Leufkens P., Krauwinkel W.J., Eichler H.G. Eur. J. Clin. Pharmacol. 1998;54:367. doi: 10.1007/s002280050477. [DOI] [PubMed] [Google Scholar]
- 6.Macek J., Klima J., Ptacek P. J. Chromatogr B – Anal. Technol. Biomed. Life Sci. 2004;809:307. doi: 10.1016/j.jchromb.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Soeishi Y., Kobori M., Kobayashi S.I., Higuchi S. J. Chromatogr. B – Anal. Technol. Biomed. Life Sci. 1990;533:291. doi: 10.1016/s0378-4347(00)82216-1. [DOI] [PubMed] [Google Scholar]
- 8.Ding L., Li L., Tao P., Yang J., Zhang Z. J. Chromatogr. B – Anal. Technol. Biomed. Life Sci. 2002;767:75. doi: 10.1016/s0378-4347(01)00546-1. [DOI] [PubMed] [Google Scholar]
- 9.Qi M.L., Wang P., Liu L.H. J. Chromatogr. B – Anal. Technol. Biomed. Life Sci. 2004;805:7. doi: 10.1016/j.jchromb.2004.01.059. [DOI] [PubMed] [Google Scholar]
- 10.Matsushima H., Takanuki K., Kamimura H., Watanabe T., Higuchi S. J. Chromatogr. B – Anal. Technol. Biomed. Life Sci. 1997;695:317. doi: 10.1016/s0378-4347(97)00200-4. [DOI] [PubMed] [Google Scholar]
- 11.Keski-Rahkonen P., Parssinen A., Leppanen E., Mauriala T., Lehtonen M., Auriola S. J. Pharm. Biomed. Anal. 2007;43:606. doi: 10.1016/j.jpba.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 12.Choi C.-I., Lee H.-I., Bae J.-W., Lee Y.-J., Byeon J.-Y., Jang C.-G., Lee S.-Y. J. Chromatogr. B – Anal. Technol. Biomed. Life Sci. 2012;909:65. doi: 10.1016/j.jchromb.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Rasmusson G.H., Reynolds G.F., Utne T., Jobson R.B., Primka R.L., Berman C., Brooks J.R. J. Med. Chem. 1984;27 doi: 10.1021/jm00378a028. [DOI] [PubMed] [Google Scholar]
- 14.Schellinger A.P., Carr P.W. J. Chromatogr. A. 2006;1109:253. doi: 10.1016/j.chroma.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 15.Taguchi K., Saitoh M., Sato S., Asano M., Michel M.C. J. Pharmacol. Exp. Ther. 1997;280:1. [PubMed] [Google Scholar]
- 16.Morzycki J.W., Wawer I., Gryszkiewicz A., Maj J., Siergiejczyk L., Zaworska A. Steroids. 2002;67:307–311. doi: 10.1016/s0039-128x(02)00012-0. [DOI] [PubMed] [Google Scholar]
- 17.USFDA, in U.S.F.a.D. Administration (Editor), www.fda.gov, 2001.