Summary
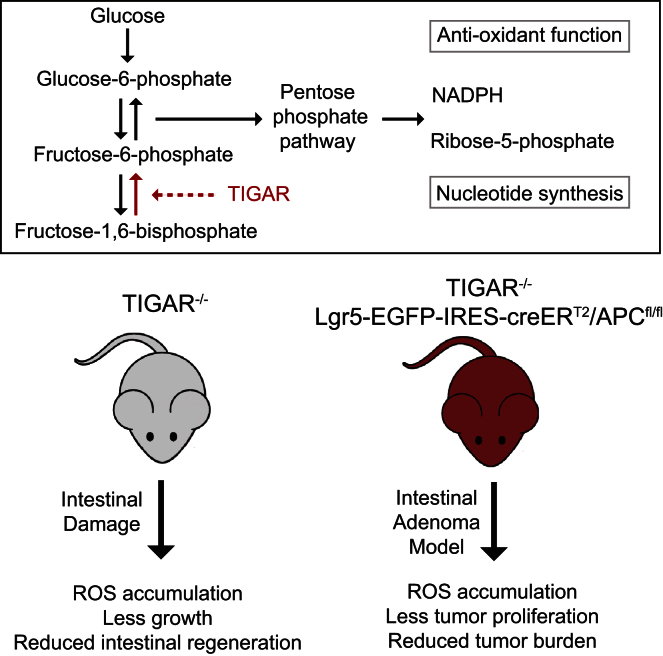
Regulation of metabolic pathways plays an important role in controlling cell growth, proliferation, and survival. TIGAR acts as a fructose-2,6-bisphosphatase, potentially promoting the pentose phosphate pathway to produce NADPH for antioxidant function and ribose-5-phosphate for nucleotide synthesis. The functions of TIGAR were dispensable for normal growth and development in mice but played a key role in allowing intestinal regeneration in vivo and in ex vivo cultures, where growth defects due to lack of TIGAR were rescued by ROS scavengers and nucleosides. In a mouse intestinal adenoma model, TIGAR deficiency decreased tumor burden and increased survival, while elevated expression of TIGAR in human colon tumors suggested that deregulated TIGAR supports cancer progression. Our study demonstrates the importance of TIGAR in regulating metabolism for regeneration and cancer development and identifies TIGAR as a potential therapeutic target in diseases such as ulcerative colitis and intestinal cancer.
Graphical Abstract
Highlights
-
•
TIGAR is expressed in most adult tissue but is dispensable for embryonic development
-
•
Intestinal damage induces TIGAR, which is important for gut regeneration
-
•
TIGAR provides reducing power and nucleotides for rapid intestinal proliferation
-
•
TIGAR contributes to the development of intestinal tumors in mice and humans
TIGAR enhances NADPH and nucleotide precursor generation for antioxidant and DNA production. Cheung et al. show that TIGAR is important for intestinal tissue regeneration despite being dispensable for development. TIGAR deficiency also impeded intestinal tumor development. As human colon cancers overexpress TIGAR, its inhibition may selectively target tumor growth.
Introduction
The ability to regulate metabolic pathways is key to maintaining normal health and homeostasis, and defects in these responses contribute to major diseases such as diabetes and cancer. Modulation of metabolic pathways plays an important role in tumorigenesis, where metabolic transformation underpins the abnormal growth, proliferation, and survival of cancer cells. The expression of the M2 isoform of pyruvate kinase, for example, allows the diversion of glycolytic intermediates into various anabolic pathways (including the pentose phosphate pathway [PPP]) to support cancer cell growth (Anastasiou et al., 2011; Ye et al., 2012). The use of glutamine for anaplerosis (Wise and Thompson, 2010) and the expression of mutant forms of isocitrate dehydrogenase (Borodovsky et al., 2012) are further examples where altered metabolism plays a role in promoting oncogenic transformation. Components of many metabolic pathways are modified by both tumor suppressors and oncogenes, and drugs that target metabolic enzymes are being developed as cancer therapies (Cairns et al., 2011; Dang, 2012). However, the contribution of these enzymes to tumor development and the balance between their roles in allowing normal cell growth and supporting cancer progression are not well explored in vivo, possibly due to the fact that modulation of many of these enzymes is deleterious to embryonic development (Piruat et al., 2004; Pollard et al., 2007; Yang et al., 2010b).
TIGAR functions as a fructose-2,6-bisphosphatase (Fru-2,6-BPase), decreasing the intracellular levels of fructose-2,6-bisphosphate (Fru-2,6-BP) and thereby lowering the activity of PFK1 and flux through the main glycolytic pathway. As a consequence, glucose metabolism may be diverted into the PPP, and TIGAR has been shown to support increased production of NADPH and antioxidant activity through the generation of reduced glutathione (Bensaad et al., 2006; Bensaad and Vousden, 2007; Li and Jogl, 2009; Wanka et al., 2012). Several studies have shown the importance of TIGAR in controlling reactive oxygen species (ROS) in tissue culture systems (Bensaad et al., 2006, 2009; Yin et al., 2012) and elevated expression of TIGAR has been reported in some cancer models (Wanka et al., 2012; Won et al., 2012).
The potential importance of TIGAR in tumor development is illustrated by a study showing that the depletion of PFKFB4 (an enzyme with fructose-2,6-bisphosphatase activity like TIGAR) and the subsequent decrease in ROS scavengers can inhibit tumor growth in a xenograph model (Ros et al., 2012). An RNA interference screen in glioma stem-like cells also identified PFKFB4 as an essential gene for tumor survival (Goidts et al., 2012). Furthermore, additional consequences of promotion of the PPP, including the support of other anabolic reactions that require NADPH, such as fatty acid synthesis, and the generation of ribose phosphate for DNA synthesis and repair (Deberardinis et al., 2008; Kroemer and Pouyssegur, 2008; Tong et al., 2009; Vander Heiden et al., 2009) may play a role in tumor development. Taken together, it is possible that expression of TIGAR and subsequent changes in metabolic pathway utilization could help to promote tumor development. However, the overall contribution of TIGAR to normal growth and development has not been determined.
TIGAR was initially identified as a transcriptional target of p53 (Bensaad et al., 2006). p53 is a critical tumor suppressor protein that functions to prevent the growth or survival of cells that are undergoing malignant progression by inducing apoptosis or senescence in response to oncogenic stress. However, more recent studies have shown that p53 can also function to control metabolism (Vousden and Ryan, 2009), and TIGAR contributes to this activity of p53. The activation of TIGAR and other genes by p53 promotes an antioxidant response (Bensaad et al., 2006; Budanov et al., 2004; Cosentino et al., 2011), which helps cells to survive transient or low levels of stress, and has recently also been suggested to play a key role in preventing malignant progression (Li et al., 2012). However, TIGAR overexpression has also been described in a few tumor types (Wanka et al., 2012; Won et al., 2012), raising the possibility that deregulated expression of TIGAR may play a role in supporting, rather than inhibiting, cancer development.
In order to determine the role of TIGAR in normal development and its contribution to proliferation under conditions of tissue repair or malignant growth, we generated TIGAR-deficient mice. While TIGAR does not appear to be essential for normal development, animals lacking TIGAR show a clear defect in their ability to mount a proliferative response in adult tissue, either in response to tissue damage or tumor development. TIGAR levels are elevated in mouse and human tumors and tumor cell lines, regardless of the presence or absence of wild-type (WT) p53, and a reduction of TIGAR increased survival in a mouse model of intestinal adenoma. Taken together, our data show that TIGAR contributes to both tissue regeneration and tumor development.
Results
Generation of TIGAR-Deficient Mice
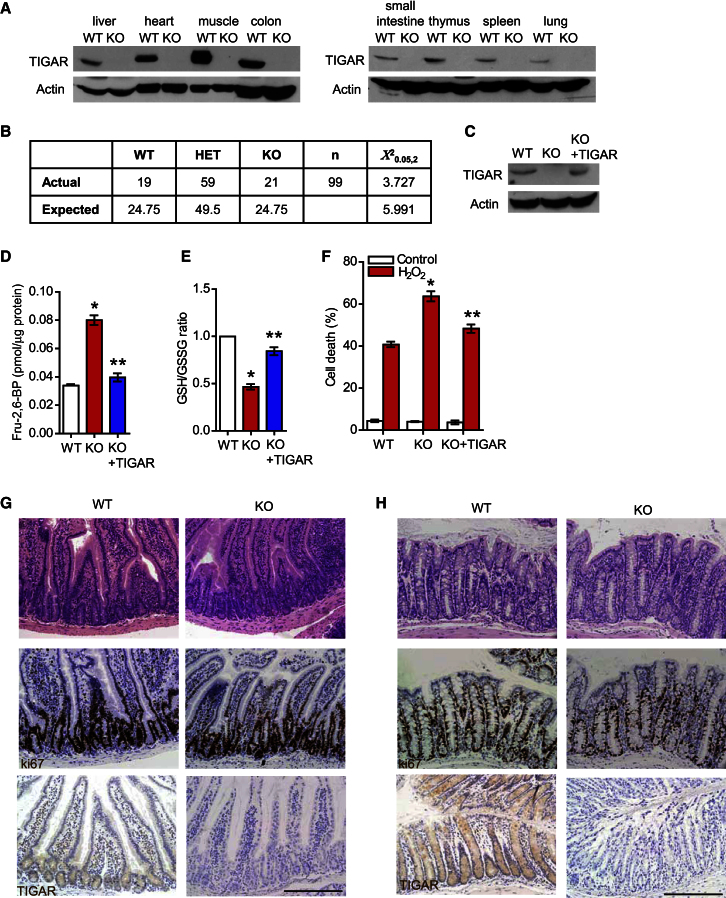
Two independent approaches were taken to generate TIGAR-deficient mice. A constitutive deletion was generated using a genetrap construct from EUCOMM, in which the targeting vector was inserted between exons 2 and 3 of the TIGAR gene (Figure S1A available online). Lack of TIGAR protein expression was confirmed by western blotting lysates from various tissues derived from these mice (Figure 1A). Deletion of TIGAR did not lead to obvious developmental problems, and TIGAR−/− mice were born at the expected Mendelian ratio (Figure 1B). In parallel, we also generated a conditional TIGARfl/fl mouse by targeting exon 3, the deletion of which resulted in a frame shift and complete loss of expression of TIGAR protein in the presence of the germline deleter cre (Figures S1B and S1C).
Figure 1.
TIGAR-Deficient Mice
(A) Western blot analysis of the indicated tissues from WT and TIGAR-deficient animals.
(B) Mendelian ratio from TIGAR+/− matings.
(C) Western blot analysis of baby mouse kidney (BMK) cultures from wild-type (WT), TIGAR−/− (KO), and KO cultures transfected with human TIGAR construct.
(D) Level of fructose-2,6-bisphosphate in WT, KO, and KO BMK cell cultures transfected with TIGAR construct (KO + TIGAR). ∗p < 0.05 compared to WT; ∗∗p < 0.05 compared to KO.
(E) Ratio of oxidized and reduced glutathione (GSH/GSSG) of WT, KO, and KO + TIGAR BMK cells. ∗p < 0.05 compared to WT; ∗∗p < 0.05 compared to KO.
(F) Cell death as measured by PI exclusion of WT, KO, and KO + TIGAR BMK cells after hydrogen peroxide treatment. ∗p < 0.05 compared to WT; ∗∗p < 0.05 compared to KO.
(G and H) Untreated small intestine (G) and colon (H) from WT and KO animals. Top: hematoxylin and eosin staining (H&E); middle: Ki67; bottom: TIGAR staining. Scale bar, 200 μm. Data are represented as mean ± SEM (n = at least 3).
See also Figure S1.
Much of the analysis of TIGAR function to date has been carried out in human cells (Bensaad et al., 2006; Li and Jogl, 2009), and to confirm a similar function of mouse TIGAR, we tested baby mouse kidney (BMK) cells derived from TIGAR−/− mice for Fru-2,6-BP levels and reduced glutathione (GSH)/oxidized glutathione (GSSG) ratios (Figures 1C–1E). Cells lacking TIGAR showed increased Fru-2,6-BP levels and lower GSH/GSSG ratios. These results are therefore consistent with the described role of human TIGAR as a Fru-2,6-BPase that increases NADPH and subsequently lowers the GSH/GSSG ratio (Bensaad et al., 2006; Li and Jogl, 2009). As expected, TIGAR-deficient cells were more sensitive to oxidative stress following treatment with H2O2 (Figure 1F). Reintroduction of human TIGAR into these cells to levels comparable with those seen in WT cells (Figure 1C) rescued these phenotypes (Figures 1D and 1F), confirming that the effects seen were due to the lack of TIGAR in these cells and that mouse TIGAR functions similarly to human TIGAR.
TIGAR Is Required for Small Intestine Proliferation after Acute Damage
While lack of TIGAR in developing embryos or unstressed adults did not result in a clear change in phenotype, we considered that TIGAR expression may play a more important role under conditions of stress in adult tissue. To address this question, we focused on the intestine as an organ in which cell proliferation plays an important role during regeneration following tissue ablation. Analysis of small intestine (Figure 1G) or colon (Figure 1H) from untreated mice showed normal intestinal crypt architecture and proliferation in TIGAR-deficient mice compared to WT animals. TIGAR protein expression in WT animals was localized mainly in the intestinal crypt (Figures 1G and 1H), where most of the proliferation occurs. Importantly, no staining was seen in the intestines or colon of TIGAR-null mice.
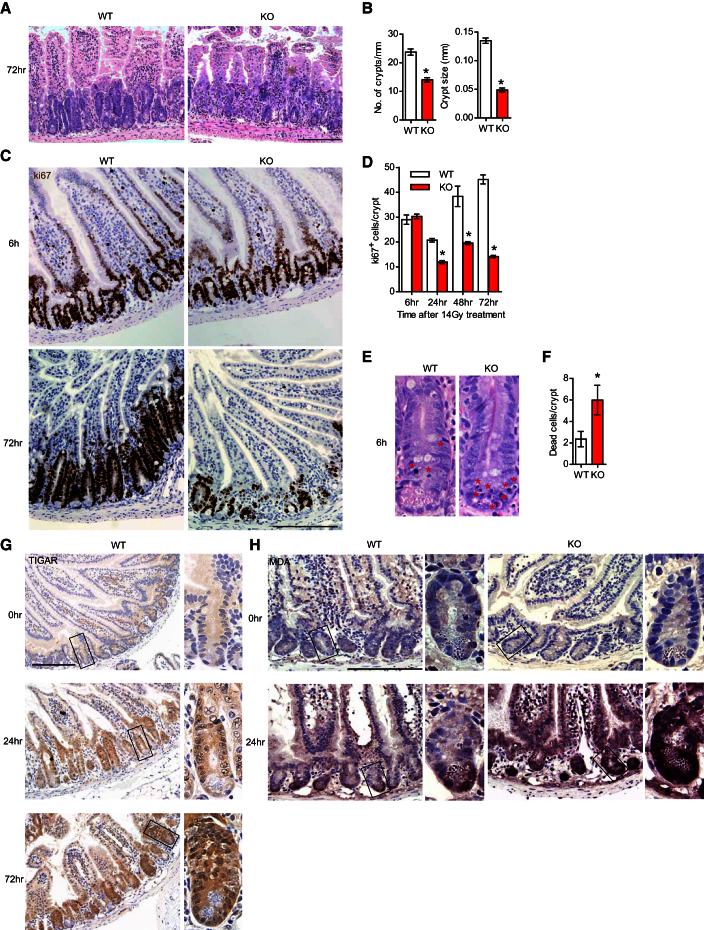
To examine the role of TIGAR in proliferation and regeneration of adult intestinal epithelium, we turned to well-defined models in which ablation of intestinal epithelium by irradiation (IR) or treatment with a genotoxic drug is followed by a period of recovery and rapid tissue regeneration. In WT mice, small intestinal regeneration is characterized by a rapid regrowth of intestinal crypts, which can be seen 72 hr following treatment with 14 Gy IR (Figures 2A and 2B). By comparison, TIGAR-deficient mice showed a significant reduction in both the size and number of regenerating crypts (Figures 2A and 2B). To determine the underlying cause of this defect, we examined both proliferation and cell death in the small intestine of mice after IR. Using the proliferation marker Ki67, it was clear that while both WT and TIGAR−/− mice showed an initial decrease in proliferation 24 hr post-IR, only the WT animals moved to a phase of rapid proliferation that was evident 48 and 72 hr post-IR and was consistent with the outgrowth of new crypt structures (Figures 2C and 2D). The TIGAR−/− mice showed a clear defect in proliferation at these time points (Figures 2C and 2D). Measurements of cell death in the intestine indicated an increase in the number of dying cells in the TIGAR−/− compared to WT intestines 6 hr post-IR (Figures 2E and 2F), although by 24 or 48 hr minimal cell death was detected in mice of either genotype. Analysis of progenitor cells using immunohistochemistry of markers such as LGR5 or Olfm4 (van der Flier et al., 2009) revealed a similar staining pattern between TIGAR−/− and WT animals (Figure S2A). The staining intensity was somewhat reduced in TIGAR-deficient animals after acute injury (Figure S2A), although the interpretation of this effect is limited by the difficulty in finding intact crypts to stain after damage. Taken together with the data showing only a transient increase in apoptosis in TIGAR-null intestines 6 hr after damage (Figures 2E and 2F), these results suggest that the defects in proliferation in the absence of TIGAR are not simply the result of massive apoptosis during the period of maximal regeneration (24–48 hr) or complete depletion of the stem cell population at an earlier time point, but also reflect a failure to proliferate.
Figure 2.
TIGAR-Deficient Mice Have Reduced Regenerative Capacity in the Intestinal Crypt after 14 Gy Whole-Body IR
(A) Small intestine from WT and TIGAR−/− (KO) animals 72 hr after 14 Gy IR. Bar = 200 μm.
(B) Number of crypts per millimeter (left) and size of crypts (right) 72 hr after 14 Gy IR. ∗p < 0.05 compared to WT.
(C) Ki67 staining of WT and KO intestines 6 and 72 hr after 14 Gy IR. Bar = 200 μm.
(D) Quantification of Ki67+ cells at the indicated times after 14 Gy IR. ∗p < 0.05 compared to WT.
(E) Apoptosis in the small intestine 6 hr after 14 Gy IR. Asterisks denote cells with apoptotic nuclear morphology.
(F) Number of apoptotic cells in the crypts 6 hr after 14 Gy IR. ∗p < 0.05 compared to WT.
(G) TIGAR staining of WT animals before IR and 24 and 72 hr after 14 Gy IR. Scale bar, 200 μm. Panels show details of crypt structures indicated in the box.
(H) Malondialdehyde (MDA) staining of WT and KO animals before and 24 hr after 14 Gy IR. Scale bar, 200 μm. Data are represented as mean ± SEM (n = at least 3).
See also Figure S2.
Consistent with the role of TIGAR in supporting intestinal proliferation after damage, TIGAR immunostaining in WT tissues revealed a marked and sustained increase in TIGAR expression in intestinal crypts 24 and 72 hr after IR (Figure 2G). Previous studies have demonstrated a role of TIGAR in lowering ROS (Bensaad et al., 2006; Li and Jogl, 2009; Wanka et al., 2012), and we have shown that TIGAR-deficient BMK cells have less GSH and are more sensitive to H2O2 (Figures 1E and 1F). Analysis of intestinal tissue for markers of ROS accumulation also showed an increase in MDA (malondialdehyde, an indicator for lipid peroxidation) staining after IR in TIGAR−/− mice compared to WT animals (Figure 2H), indicating that TIGAR is also important to limit ROS levels in vivo. The importance of TIGAR in response to IR damage is underscored by similar effects observed following damage induced by cisplatin (Figure S2). After cisplatin treatment, TIGAR−/− mice showed a reduced ability to regenerate crypts (Figures S2B and S2C), which was accompanied by a decrease in proliferation (Figures S2D and S2E) and an increase in apoptosis at early time point (Figures S2F and S2G).
TIGAR Is Required for Colon Regeneration in a Model of Ulcerative Colitis
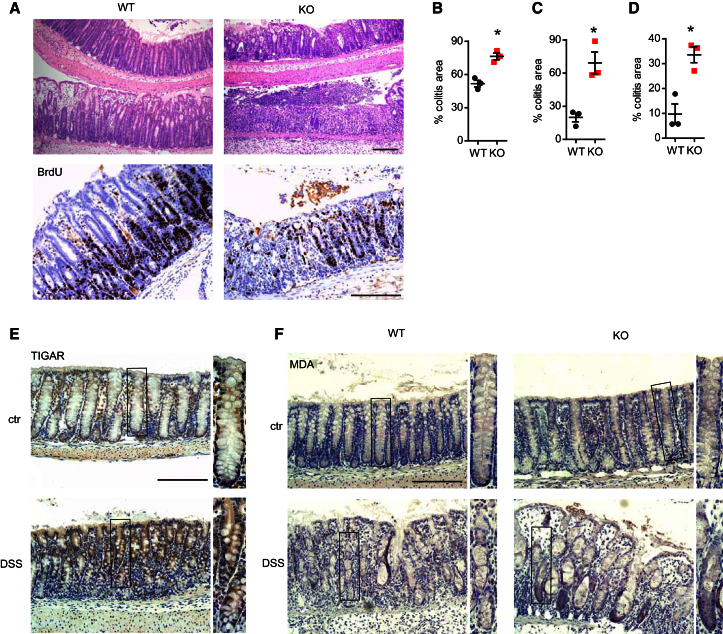
The results described above revealed an interesting role for TIGAR in supporting tissue growth and survival under conditions of rapid regeneration in adult mice, consistent with the proposed metabolic function of TIGAR. To determine whether TIGAR can also play a role in recovery following damage in a different setting, we investigated a model of ulcerative colitis in the colon. Treatment of mice with dextran sulfate sodium (DSS) over several days resulted in an ablation of colon epithelium, followed by inflammation and hyperproliferation of the crypts to allow for repair (Cooper et al., 1993). While the resting colons of WT and TIGAR-deficient mice were indistinguishable (Figure 1H), the extensive formation of new crypts seen in WT colons following DSS treatment and recovery was much less apparent in TIGAR−/− animals (Figure 3A). Furthermore, compared to WT mice, there is a defect in proliferation in TIGAR-deficient animals after DSS treatment, as indicated by bromodeoxyuridine (BrdU) incorporation (Figure 3A). Consistently, TIGAR−/− mice subjected to DSS treatment showed poorer recovery and succumbed approximately 1 day earlier than WT animals. The difference between WT and TIGAR−/− mice was more obvious following exposure to milder damage (2% DSS) compared to a more damaging treatment (3.5% DSS) (Figures 3B and 3C), and the difference was sustained days after treatment, although at this time point some recovery in TIGAR−/− mice was also seen (Figures 3C and 3D; note difference in scale of y axis). TIGAR expression can therefore limit but not completely prevent damage, and even in the absence of TIGAR some recovery is possible.
Figure 3.
TIGAR-Deficient Mice Are More Sensitive to DSS-Induced Colitis
(A) Colon from WT and TIGAR−/− (KO) animals 2 days after 2% DSS treatment. Top: H&E; bottom: BrdU staining. Bar = 200 μm.
(B) Percentage of colitis area one day after 3.5% DSS in WT and KO animals. ∗p < 0.05 compared to WT.
(C) Percentage of colitis area one day after 2% DSS in WT and KO animals. ∗p < 0.05 compared to WT.
(D) Percentage of colitis area 2 days after 2% DSS in WT and KO animals. ∗p < 0.05 compared to WT.
(E) TIGAR staining in WT animals, untreated (ctr) or after 3.5% DSS treatment. Scale bar, 200 μm. Panels show details of crypt structures indicated in the box.
(F) MDA staining of WT and KO animals before and after 2% DSS treatment. Scale bar, 200 μm. Data are represented as mean ± SEM (n = at least 3).
See also Figure S3.
Analysis of TIGAR protein levels in WT mice showed increased expression following DSS treatment (Figure 3E), similar to that seen in small intestine after IR damage (Figure 2G). TIGAR-deficient animals accumulated elevated ROS following DSS treatment, as indicated by an increase in MDA staining (Figure 3F), suggesting an important role of TIGAR in limiting ROS damage after DSS-induced colitis. These results indicated that, as seen in the regenerating small intestine after IR or treatment with cisplatin, efficient repair of damage to the colon was dependent on TIGAR expression.
The requirement for TIGAR in intestinal regeneration was confirmed using animals with conditional TIGAR allele (TIGARfl/fl) crossed with deleter cre TIGARfl/fl;cre/+ animals (Figures S1B and S1C). Loss of TIGAR expression in these animals also resulted in increased sensitivity to DSS-induced colitis in the colon (Figure S3), recapitulating the phenotype seen with the TIGAR−/− mouse. Taken together, these results show that lack of TIGAR compromises the ability of cells to limit ROS and undergo proliferation necessary to regenerate intestinal epithelium after ablation.
TIGAR Provides Antioxidants and Nucleosides for Growth
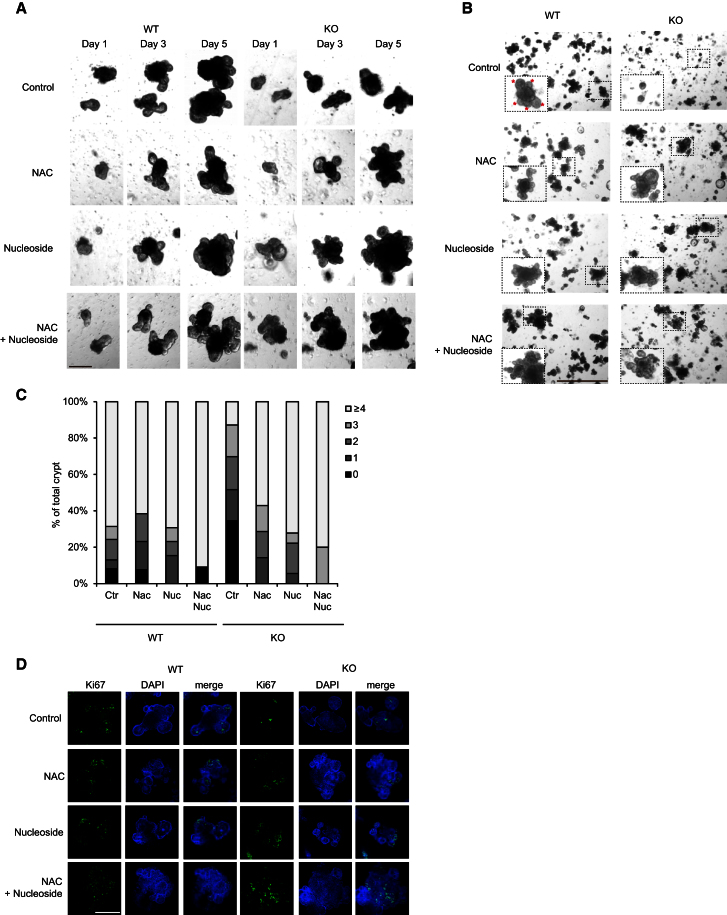
Our analysis of the recovering small intestine suggested that TIGAR might be important in both lowering apoptosis and allowing cell growth and proliferation. Previous work has indicated that TIGAR could contribute to these responses by limiting oxidative damage and may promote the generation of metabolic intermediates, such as nucleotides, that are necessary for tissue expansion. To examine directly the defect that underlies the failure of TIGAR−/− intestinal crypts to regenerate properly, we turned to an in vitro intestinal crypt culture model (Sato et al., 2009). Compared to cells derived from WT mice, intestinal crypts from TIGAR−/− mice were defective in their ability to proliferate in this three-dimensional tissue culture model (Figures 4A and 4B). TIGAR−/− cells were less able to form crypt structures, and the culture of these cells produced fewer new crypt outgrowths over time (Figures 4A and 4B). Quantification of 100 individual crypt structures of each genotype clearly showed the defect in crypt formation in cultures from TIGAR−/− cells (Figure 4C). Direct staining of the crypt structures with Ki67 also showed less proliferation in the TIGAR−/− cultures, even when comparing crypt structures of similar size (Figure 4D), which is consistent with the regeneration failure seen in vivo in these mice. This defect could be rescued by the addition of N-acetyl L-cysteine (NAC; to control ROS) and nucleosides (to compensate for any lack of ribose phosphate production). Indeed, compared to the WT crypts, essentially complete rescue was observed following treatment with nucleosides or NAC alone (Figures 4A–4D). The effect of NAC indicates an importance of antioxidant function to the rescue, and interestingly we showed that treatment of cells with nucleosides also resulted in an increase in the GSH/GSSG ratio (Figure S4A), showing that nucleosides can also provide antioxidant function. To explore the contribution of the nonoxidative branch of the PPP, we treated cultures with oxythiamine (which inhibits transketolase in the nonoxidative PPP). Levels of oxythiamine that did not affect the growth of WT crypts exacerbated the growth defect of TIGAR−/− crypts (Figure S4B), showing that loss of TIGAR makes cells more sensitive to inhibition of this branch of the PPP. Interestingly, oxythiamine also somewhat impeded, but did not completely inhibit, the ability of NAC to rescue the growth of TIGAR-null cells, again suggesting that the main defect in these cells is in antioxidant function.
Figure 4.
Reduction of Proliferation in TIGAR-Deficient Intestinal Crypt Can Be Rescued by the Addition of ROS Scavengers and Nucleoside
(A) Crypt cultures from WT and TIGAR−/− (KO) small intestines after the indicated treatment. The same crypts were followed on days 1, 3, and 5. Scale bar, 100 μm.
(B) Crypt cultures from WT and KO small intestines 5 days after the indicated treatment. Asterisks indicate growing buds from the crypt. Scale bar, 300 μm.
(C) Quantification of the number of buds (0 to ≥4) from the crypts from WT and KO small intestinal crypt cultures.
(D) Ki67 staining of the crypt cultures from WT and KO small intestine treated with the indicated drugs for 5 days. Scale bar, 100 μm.
See also Figure S4.
TIGAR Is Critical for Tumor Development in an Intestinal Adenoma Model
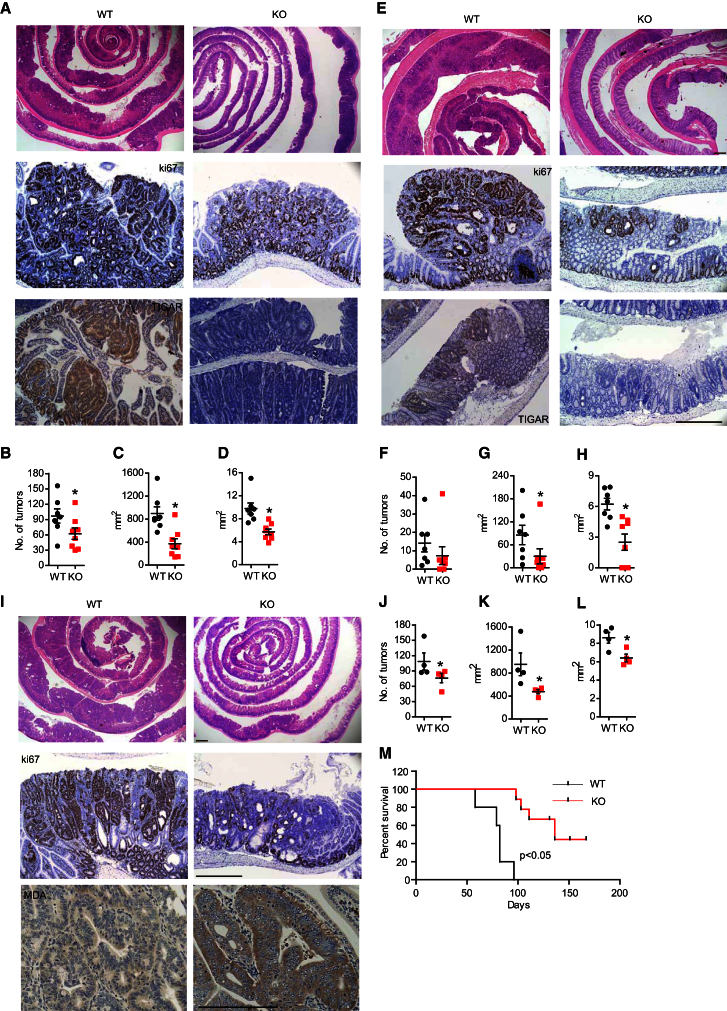
Our results show that TIGAR plays an important role in allowing growth and proliferation required for the repair of damaged intestinal epithelium in adult mice. The modulation of metabolic pathways to allow anabolism and ROS limitation has been proposed to play an important role in tumorigenesis. We were therefore interested to determine whether TIGAR also contributes to the abnormal proliferation of tumor cells in the intestine. To address this question, we used a model in which the tumor suppressor APC is deleted in LGR5+ intestinal stem cells, leading to the development of intestinal adenoma (Barker et al., 2007, 2009). Using a protocol involving multiple doses of tamoxifen to maximize the incidence of adenoma development, TIGAR-deficient mice (knockout [KO]: TIGAR−/− Lgr5-EGFP-IRES-creERT2/APCfl/fl) showed a reduced burden of abnormally proliferating adenoma in the small intestine compared to mice with WT TIGAR (WT: TIGAR+/+ Lgr5-EGFP-IRES-creERT2/APCfl/fl) (Figures 5A–5D). Specifically, TIGAR-deficient mice showed a significant reduction in total tumor burden and average tumor size compared to WT mice (Figures 5B–5D). Staining of TIGAR in WT tissues show a higher expression in the adenoma compared with surrounding normal tissue (Figure 5A), which is consistent with the role of TIGAR in supporting proliferation in tumor tissues. Similarly, the TIGAR-deficient mice showed a reduction in size of colon adenomas compared to WT mice (Figures 5E–5H). Interestingly, when examining the colon, there were no significant differences in the number of tumors in the colon (Figure 5F), indicating that TIGAR may be more important for tumor growth than for tumor initiation in this tissue. These results indicated that the absence of TIGAR limited tumor development, and so to determine the effect of TIGAR depletion on the survival of mice, we turned to a protocol of a single tamoxifen induction, which allows a slower onset of adenoma. As seen previously, fewer and smaller tumors developed in the small intestine of the TIGAR-deficient mice (Figures 5I–5L). In addition, there was less proliferation (less Ki67 staining) and more ROS damage (more MDA staining) in TIGAR-null compared to WT animals (Figure 5I), indicating a contribution of TIGAR to proliferation and ROS limitation that is similar to that seen following acute intestinal damage. Staining of GFP as a marker of LGR5-positive stem cells in these models (Barker et al., 2007, 2009) revealed that the absence of TIGAR does not deplete the tumors of stem cells (Figure S5). Overall, there appeared to be a slight decrease in the number of crypts with positive GFP staining, although as with the damage model this was difficult to interpret in view of the defects in proliferation in TIGAR-null animals. Importantly, the reduction in tumor burden correlated with an increased survival of mice lacking TIGAR (Figure 5M).
Figure 5.
TIGAR Deficiency Reduced the Tumor Burden in a Mouse Model of Intestinal Adenoma
(A) H&E staining (top), Ki67 staining (middle), and TIGAR staining (bottom) of the small intestines from TIGAR+/+Lgr5-EGFP-IRES-creERT2/APCfl/fl (WT) mice and TIGAR−/−Lgr5-EGFP-IRES-creERT2/APCfl/fl (KO) mice after multiple rounds of tamoxifen induction.
(B) Number of tumors from WT and KO mice. ∗p < 0.05 compared to WT.
(C) Total tumor burden in the small intestine of WT and KO mice. ∗p < 0.05 compared to WT.
(D) Average size of tumor from WT and KO mice. ∗p < 0.05 compared to WT.
(E) H&E staining (top), Ki67 staining (middle), and TIGAR staining (bottom) of the colon from WT and KO mice after multiple dosage of tamoxifen induction. Scale bar, 200 μm.
(F) Number of tumors from the colon of WT and KO mice.
(G) Total colon tumor burden of WT and KO mice. ∗p < 0.05 compared to WT.
(H) Average colon tumor size of WT and KO mice. ∗p < 0.05 compared to WT.
(I) H&E staining (top), Ki67 staining (middle), and MDA staining (bottom) of the small intestines from TIGAR+/+Lgr5-EGFP-IRES-creERT2/APCfl/fl (WT) and TIGAR−/−Lgr5-EGFP-IRES-creERT2/APCfl/fl (KO) after a single dosage of tamoxifen induction. Scale bar, 200 μm.
(J) Number of tumors from WT and KO animals. ∗p < 0.05 compared to WT.
(K) Total tumor burden in the small intestine of WT and KO animals. ∗p < 0.05 compared to WT.
(L) Average size of tumor from WT and KO animals. ∗p < 0.05 compared to WT.
(M) Kaplan-Meier survival curves showing adenoma-free survival of WT (n = 5) and KO (n = 7) mice. Data are represented as mean ± SEM (n = at least 3).
See also Figure S5.
TIGAR Provides Nucleosides, NADPH, and Antioxidants for the Development of Intestinal Adenomas
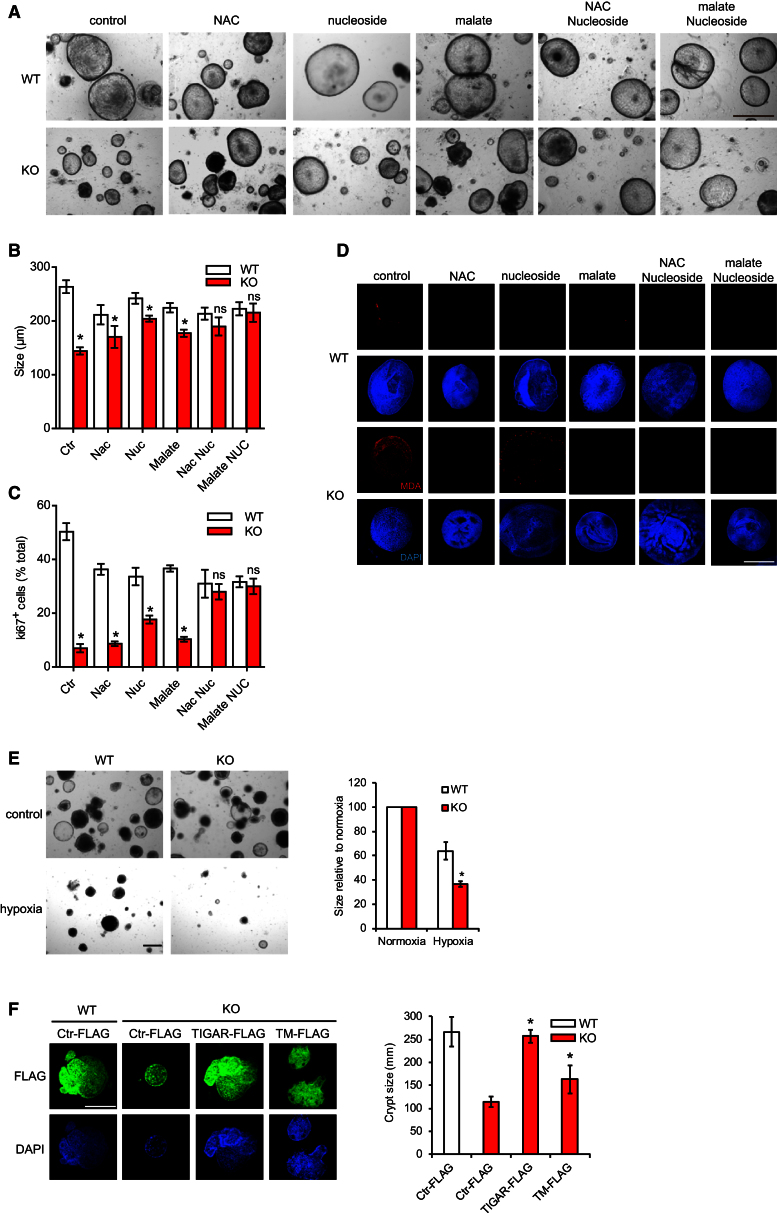
The reduction of tumor progression in TIGAR-null mice was also reflected in an in vitro tumor crypt culture model (Sato et al., 2011). These cultures grow as large cystic organoids, which do not show the differentiation or structural organization of similar cultures derived from normal intestine (compare Figures 4A and 6A). The tumor crypt cultures from TIGAR-deficient mice showed a significant impairment of growth, as revealed by the reduction of the crypt size (Figures 6A and 6B) and decreased proliferation (Figures 6C and S6A) compared to those from WT animals. To determine the underlying cause of the failure of TIGAR-null tumor cells to grow, we examined the effect of exogenous addition of either methyl-malate, which can provide additional NADPH by a non-PPP mechanism via malic enzyme (Frenkel, 1975; Schafer et al., 2009; Yang et al., 2010a), nucleosides, or NAC to decrease ROS damage. Each of these treatments partially rescued the defect in growth (Figures 6A and 6B) and proliferation (Figures 6C and S6A), while the addition of NAC and malate also prevented the accumulation of ROS, as measured by MDA staining (Figure 6D). Interestingly, treatment of these tumor crypts with nucleosides also partially inhibited ROS damage (Figure 6D), consistent with our earlier observation that nucleosides can help maintain GSH/GSSG ratios (Figure S4A). However, in this system, complete rescue of crypt size (Figures 6A and 6B) and proliferation (Figures 6C and S6A) was only achieved when both malate and nucleosides or NAC and nucleosides were provided. These results therefore support an importance of TIGAR in generating both NADPH and ribose phosphate to allow the growth of these highly proliferative tumor cells. Although there are several mechanisms for NADPH production, the PPP has been shown to be critical for redox balance in hypoxia (Anastasiou et al., 2011). Consistently, we found that TIGAR-deficient crypts are more sensitive to hypoxia than WT crypts (Figure 6E).
Figure 6.
Reduction of Proliferation in TIGAR-Deficient Tumor Crypt Can Be Rescued by the Addition of Malate and Nucleoside
(A) Tumor cystic organoid cultures from TIGAR+/+Lgr5-EGFP-IRES-creERT2/APCfl/fl (WT) and TIGAR−/−Lgr5-EGFP-IRES-creERT2/APCfl/fl (KO) small intestines after the indicated treatment for 5 days. Bar = 100 μm.
(B) Quantification (average diameter of the organoids) of (A). ∗p < 0.05 compared to WT. ns, no significant difference.
(C) Quantification of the percentage of Ki67-positive cells in the tumor crypt cultures from WT and KO animals treated with the indicated treatments for 5 days. ∗p < 0.05 compared to WT. ns, no significant difference.
(D) MDA staining of the tumor crypt cultures from WT and KO animals with the indicated treatments for 5 days. Scale bar, 100 μm.
(E) Size of TIGAR-deficient and control crypts following exposure to 1% oxygen (hypoxia) for 5 days. ∗p < 0.05 compared to WT.
(F) TIGAR-deficient and control crypts were electroporated with the indicated constructs and then cultured for 5 days. Crypts expressing comparable levels of each protein, as visualized by FLAG staining, were measured for size. ∗p < 0.05 compared to KO with Ctr-FLAG. Data are represented as mean ± SEM (n = 3).
See also Figure S6.
To confirm that the defects seen in these crypts were the result of loss of TIGAR, we re-expressed human TIGAR in these cells (Figure 6F). Interestingly, while WT TIGAR completely rescued crypt growth, a partial rescue was also seen following expression of a catalytic inactive TIGAR construct (TIGAR-TM) (Bensaad et al., 2006) (Figure 6F). These results suggest that a catalytically independent role of TIGAR, such as a recently described ability to enhance HK2 activity (Cheung et al., 2012), can contribute to the maintenance of crypt growth.
Taken together, our results indicate that the defect in growth shown by TIGAR-deficient tumor crypts reflects increased ROS and decreased nucleotide synthesis. Intriguingly, the TIGAR−/− crypts also showed an increased sensitivity to chemotherapeutic drugs compared to WT tumor crypts (Figure S6B).
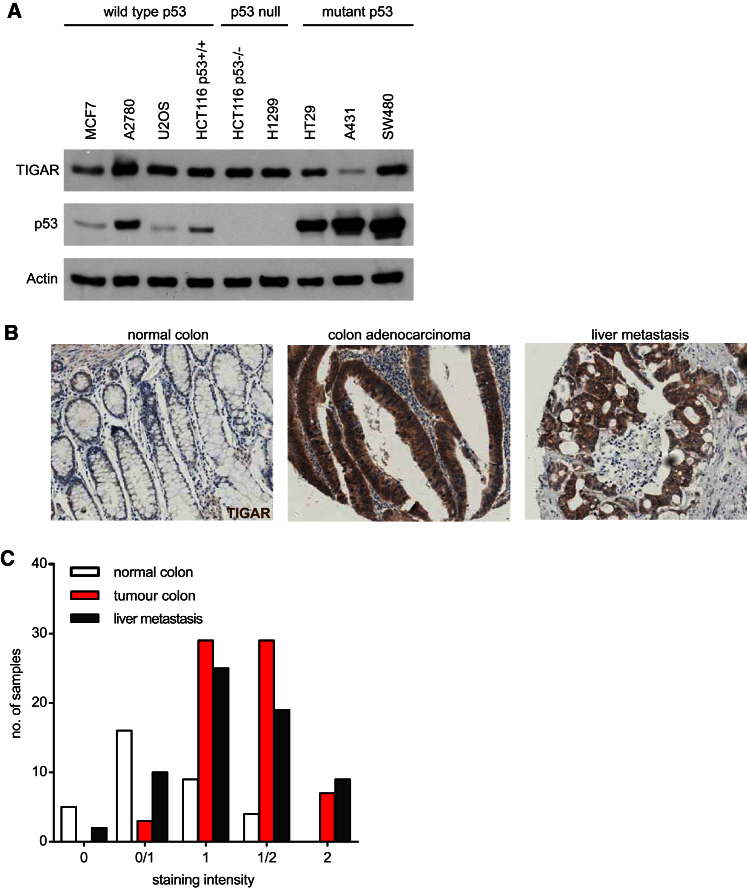
The human TIGAR gene is p53 responsive, and cells containing WT p53 show elevation of TIGAR levels in response to various forms of stress (Bensaad et al., 2006). The function of TIGAR under these conditions contributes to cell survival and is likely to allow for repair or adaptation of normal tissue to mild, transient, or nongenotoxic damage. However, our results in mice suggest that TIGAR expression may also be advantageous to tumor cells, and so we examined tumor cell lines to determine whether the expression of TIGAR was strictly dependent on the presence of WT p53. A survey of a number of p53−/− or mutant-p53-expressing tumor cell lines showed that TIGAR expression was not dependent on the retention of WT p53 (Figure 7A). Therefore, the expression of TIGAR can become decoupled from p53 and there may be a selection for expression of TIGAR during malignant progression.
Figure 7.
Increased TIGAR Expression in Primary Human Colon Cancer and Associated Metastases
(A) Expression of TIGAR in various human cancer cell lines with different p53 status.
(B) Example of TIGAR staining in matched samples of human normal colon, colon adenocarcinoma, and liver metastasis from the same patient.
(C) Quantification of the staining intensity in normal human colon, primary adenocarcinoma (tumor), and liver metastases.
To establish whether TIGAR expression is also associated with the development of human cancers, we examined a series of colon tissue microarrays (TMAs) comparing normal, tumor, and metastatic tissue from the same patient. Staining for TIGAR expression by IHC showed an increase in TIGAR protein in both the primary colon cancer and associated metastases compared to normal tissue (Figures 7B and 7C). These results are consistent with the suggestion that TIGAR expression can help to support malignant development, potentially by allowing rapid proliferation and expansion of the abnormal lesion.
Discussion
We have generated TIGAR-deficient mice to investigate the contribution of TIGAR to normal development and in rapidly proliferating cells after acute tissue damage and during tumor growth. TIGAR−/− mice did not show a clear developmental defect, suggesting either that TIGAR function is not required during embryogenesis or that there is compensatory adaptation to the constitutive lack of TIGAR during these stages of development. However, we found a clear need for TIGAR in supporting rapid proliferation of adult tissue, in particular the ability of the adult intestinal epithelium to regenerate following damage. The requirement for TIGAR under these conditions is also highlighted by the increase in TIGAR expression seen in crypts during the recovery of proliferation in WT mice and by the corresponding increase in oxidative damage in the TIGAR-deficient animals. Whether there is a similar requirement for TIGAR in other proliferative tissues remains to be determined, and a role for TIGAR in recovery from partial hepatectomy or in mounting an immune response will be extremely interesting to examine. Our studies show that TIGAR plays an important role in tissue regeneration and that lack of TIGAR results in a failure to repair damaged intestinal epithelium, with an associated increased morbidity in a model of ulcerative colitis. In these systems, therefore, TIGAR expression is necessary to maintain normal healthy tissues following stress or damage. The ability of TIGAR to contribute to the growth and survival of cells in rapidly proliferating tissue also suggested a possible contribution of TIGAR to the development or progression of malignancies. In a mouse model of intestinal tumor induced by deletion of APC in the intestinal stem cells, TIGAR expression was increased and we detected a reduction in tumor development and enhanced survival in TIGAR−/− mice.
There has been much interest recently in the revival of the suggestion that altered metabolism can contribute to, as well as respond to, oncogenic transformation. Several elegant studies have illustrated the importance of metabolic transformation in cancer development (Freed-Pastor et al., 2012; Locasale et al., 2011; Schafer et al., 2009; Ying et al., 2012), although there is limited information about how these metabolic changes may impact on tumorigenicity in vivo. The regulation of glucose metabolism by TIGAR may have several important consequences; while the contribution of TIGAR to antioxidant activity has been shown in several cell systems (Bensaad et al., 2006; Li and Jogl, 2009; Wanka et al., 2012), the potential promotion of the pentose phosphate pathway would also help to support anabolic pathways for the production of lipids and nucleic acids, and so contribute directly to cell growth (Tong et al., 2009). Although an inability to properly regulate ROS may account for enhanced apoptosis and so an indirect failure in cell proliferation, analysis of the regenerating epithelium suggested that a transient increase in apoptosis in the TIGAR−/− mice (although this did not result in complete loss of stem cells) was accompanied by a failure to recover proliferation. The importance of TIGAR in limiting ROS was supported by the analysis of normal crypt cultures in vitro, where the defect in TIGAR−/− crypts was efficiently rescued by treatment of the cells with antioxidant or nucleosides, with the observation that nucleoside treatment also provided antioxidant function. However, in the tumor crypt cultures, a full rescue of growth defects in TIGAR-null cells required the addition of both antioxidants (or NADPH generating capacity) and nucleosides, suggesting that in these cells TIGAR is important in supporting both antioxidant activity and nucleotide synthesis.
ROS has been suggested to both limit and promote tumor progression, reflecting the observation that different species of ROS have different functions. While elevated ROS can both induce potentially oncogenic mutations and function as a signaling molecule to drive proliferation, excess mitochondrial ROS can induce cell death and activation of pathways that block proliferation (Hamanaka and Chandel, 2010). These different ROS species can be differentially controlled; for example, the glutathione pool regulates oxidative stress while the peroxiredoxin pool regulates ROS signaling (Murphy, 2012). Therefore, it is possible that TIGAR mainly lowers oxidative stress via managing the glutathione level and has less of an impact on the regulation of redox signaling (which would drive proliferation).
In humans, TIGAR is p53 inducible and has been suggested to play a role under conditions of mild or transient stress to help promote the repair and survival of damaged cells (Bensaad et al., 2006; Li and Jogl, 2009; Wanka et al., 2012). These metabolic functions of p53 can play a role in tumor suppression (Li et al., 2012), and the activation of the pentose phosphate pathway by ATM has been shown to enhance NADPH production and increase nucleotide synthesis, so controlling ROS and contributing to DNA repair. These responses were shown to be important in allowing ATM to limit cancer development (Cosentino et al., 2011). However, there is also a clear potential that TIGAR activity, by limiting ROS and providing precursors for nucleotide synthesis, could be advantageous to tumor cells (Trachootham et al., 2006). Neoplastic cells generally produce more ROS than normal cells (Szatrowski and Nathan, 1991); therefore, they are more vulnerable to oxidative stress because they function with a higher basal level of ROS. On one hand, these higher levels of ROS could promote tumor progression by stimulating cell growth and proliferation (Hu et al., 2005), genetic instability (Radisky et al., 2005), and anchorage-independent growth (Weinberg et al., 2010). However, increased ROS also render tumor cells more vulnerable to cell death and it is therefore essential for the tumor cells to dynamically regulate ROS level to avoid prolonged oxidative damage. For example, oncogenes such as K-Ras, B-Raf, and Myc all increase the activity of NRF2, a transcriptional factor that increases ROS detoxification and tumorigenesis at the early stages of tumor development (DeNicola et al., 2011). Deletion of NRF2 in vivo reduces the ability of K-Ras to induce proliferation and tumorigenesis (DeNicola et al., 2011), supporting the importance of ROS limitation in tumor outgrowth. One response to increased ROS is a decrease in the activity of PKM2 through oxidation at cysteine in PKM2. This diverts glycolytic intermediates into the PPP and, as we describe for TIGAR, generates reducing potential for ROS detoxification. Importantly, this activity has been shown to be essential for tumor formation in a xenograft model (Anastasiou et al., 2011; Tong et al., 2009).
Taken together, therefore, it would appear that TIGAR can help to support tumorigenesis, both by limiting ROS and by providing precursors for nucleotide synthesis. Both of these activities can be provided by promotion of the PPP, although we also provide evidence that the recently described catalytically independent function of TIGAR in ROS regulation under hypoxia (Cheung et al., 2012) also contributes to its overall ability to support tumor cell growth.
It was therefore very interesting to determine that TIGAR was highly expressed in many human colon cancers, consistent with a contribution to the tumorigenic phenotype. Increased expression of TIGAR has recently also been described in other tumor types, including glioblastomas and breast cancers (Wanka et al., 2012; Won et al., 2012). Importantly, p53 is mutated in the majority of colon cancers, suggesting that, as seen in the cancer cell lines, selection for TIGAR overexpression is uncoupled from the activity of WT p53. This potential oncogenic activity of a p53 target gene has also recently been described for carnitine palmitoyltransferase 1c (Cpt1c), a p53-inducible gene that contributes to fatty acid oxidation. Cpt1c is upregulated in many cancer cell types and can protect against metabolic stress and contribute to growth of the tumor (Zaugg et al., 2011). Our observations indicate that the inappropriate or sustained activation of proteins like TIGAR, uncoupled from the dependence on p53, may help to support the growth and survival of cancer cells.
Our studies show that the regulation of metabolism by TIGAR is not required for normal tissue growth and development but becomes important in supporting rapid proliferation in adult intestinal epithelium. This specific requirement under conditions of tissue regeneration has also been described for FAK (Ashton et al., 2010), which is not needed in normal adult tissue but is required for intestinal regeneration and tumorigenesis. Therefore, while loss of TIGAR is deleterious to the recovery from intestinal damage, lack of TIGAR becomes advantageous under conditions where enhanced proliferation occurs in the context of tumor development. These data clearly predict that inhibition of TIGAR expression may carry some therapeutic advantage, although TIGAR inhibition would also be predicted to enhance the deleterious effects of genotoxic cancer therapies that damage normal gut and other proliferating cells. Indeed, an increase in the TIGAR level in the intestine may provide protection from acute intestinal damage such as ulcerative colitis and intestinal radiosensitivity. We are presently generating tumor models with our conditional TIGAR allele to allow us to determine the effect of targeted deletion of TIGAR in premalignant or malignant lesions.
Experimental Procedures
Transgenic Mouse Models
Details of the creation of TIGAR−/− and TIGARfl/fl mice are provided in the Supplemental Experimental Procedures. All animal work was carried out in line with the Animals (Scientific Procedures) Act 1986 and the EU Directive 2010 and sanctioned by local ethical review process (University of Glasgow).
Creation of the Intestinal Adenoma Model with TIGAR−/−
To investigate the role of TIGAR in the development of intestinal adenoma due to deletion of Apc in LGR5+ intestinal crypt cells (Barker et al., 2007, 2009), TIGAR−/− mice were interbred with Lgr5-EGFP-IRES-creERT2/APCfl/fl mice to generate TIGAR+/+ Lgr5-EGFP-IRES-creERT2/APCfl/fl (WT) and TIGAR−/− Lgr5-EGFP-IRES-creERT2/APCfl/fl (KO). To induce recombination of APC in LGR5-expressing cells, mice aged 6–8 weeks were injected intraperitoneally with single dosage of tamoxifen (3 mg tamoxifen) or multiple dosages of tamoxifen (3 mg of tamoxifen for 1 day followed by 2 mg of tamoxifen for 3 days). Mice were monitored until showing signs of intestinal illness, and tissues were collected for histology, immunohistochemistry, and intestinal crypt culture.
Cell Culture and Protein Analysis
BMK cells were cultured and used as previously described (Mathew et al., 2008). Western blot analysis, transfection of the BMK cells with TIGAR constructs, measurement of Fru-2,6-BP, and GSH/GSSG were performed as previously described (Bensaad et al., 2006).
IR, Cisplatin Treatment, and DSS-Induced Colitis
Gamma IR-induced intestinal damage was performed as previously described (Ashton et al., 2010). For cisplatin-induced intestinal damage, cisplatin (10 mg/kg) was intraperitoneally injected into 8- to 10-week-old mice. Saline was used as control. For DSS-induced colitis, mice received 2% or 3.5% of DSS in drinking water for 5 days. Then DSS was omitted and mice received tap water for 2 days. Tap water was used as control. Intestinal tissue was isolated and processed as described previously (Jamieson et al., 2012).
Histology and Immunohistochemistry
Histology and immunohistochemistry was performed as described previously (Sansom et al., 2004). Primary antibodies used were anti-Ki67 (Lab Vision), anti-TIGAR (Millipore), anti-MDA (Abcam), anti-BrdU (Sigma), anti-Olfm4 (Abcam), anti-LGR5/GPR49 (Abcam), anti-GFP (Abcam), and anti-FLAG (Sigma).
Small Intestinal Crypt Culture and Immunofluorescence
Small intestinal crypt culture and tumor crypt culture were performed as described previously (Sato et al., 2009, 2011). Briefly, small intestine was washed in cold PBS and villi were scraped using a glass coverslip. Then the small intestine was cut into small pieces and the pieces were further washed with cold PBS. The pieces were transferred into PBS with 2 mM EDTA and incubated for 30 min, after which crypts were collected by pipetting mechanically and supernatant enriched with crypts were collected. The crypts were then pelleted by centrifugation (1,200 rpm, 5 min), suspended in ADF media (advanced DMEM F12 [Invitrogen], 1% glutamine, 1% penicillin/streptomycin, 0.1% BSA, 10 mM HEPES), and passed through 70 μm cell strainer. The fraction was then centrifuged at lower speed (600 rpm, 2 min) to avoid single cells being spun down. The final pellet with crypt was resuspended with growth factor reduced Matrigel (356231, BD) in ADF that contained 0.05 μg/ml EGF (Peprotech), 0.1 μg/ml NOGGIN (Peprotech), and 0.5 μg/ml mR-Spondin (R&D Systems). For tumor crypts, adenomas were cut into small pieces, washed, and then dissociated with trypsin for 30 min in 37°C. After several washes the cells were pelleted and were suspended with Matrigel in ADF that contained EGF and NOGGIN. The cultures were passaged every 7–10 days. For the rescue experiments, 200 μM NAC (Sigma), 1× nucleoside (Millipore), 2.5 mM methyl-malate (Sigma), and 10 μM oxythiamine (Sigma) were added to the culture on the first day. Hypoxia was performed as described previously (Cheung et al., 2012). For Amaxa nucleofection into cancer crypt organoids, 1 μg (per well) control, WT TIGAR, or catalytically TIGAR mutant (TIGAR-TM) (Bensaad et al., 2006) constructs was transfected into cancer crypt organoids using solution T and program X-001 (Lonza). The rate of growth of the intestinal crypt was measured by the number of buds present in at least 100 crypt structures as previously described (Mustata et al., 2011). The rate of growth of the tumor crypt was measured by the average size (diameter) of at least 150 crypts in each treatment from tumors from three individual animals (WT and KO). For nucleofected cancer crypt measurements, the average size (diameter) of at least 100 crypts that were stained positive with FLAG was measured from cultures of three individual animals (WT and KO).
Immunofluorescence of Crypt Cultures
Crypts were fixed with 2% paraformaldehyde (PFA) in PBS for 20 min at room temperature and then permeabilized with 0.5% Triton X-100 in PBS for 10 min at 4°C. After three 10 min washes of 1× PBS-glycine (7.5 mg/ml) to quench PFA, crypts were incubated with 10% BSA primary block in immunofluorescence wash (0.13 M NaCl, 13 mM Na2HPO4, 3.5 mM NaH2PO4, 0.2% Triton X-100, 0.04% × Tween-20) for 1 hr, followed by incubation with secondary block (10% BSA and 1:100 Fab immunoglobulin G [Jackson Immunoresearch] in immunofluorescence wash) for 30 min. The primary antibody (prepared in secondary block) was incubated overnight at 4°C and washed three times in immunofluorescence wash before incubation with the appropriate fluorescent dye coupled secondary antibody (Alexa Fluor, Molecular Probes) for 1.5 hr. DAPI (1 μg/ml) in 1× PBS was used to visualize nuclei.
Human TMA analysis
TMA of human cancer patients was obtained from AccuMax Array (A203(II)-colon cancer tissues liver metastasis) and was stained with anti-human TIGAR antibody (ProSci). The scoring of the TMAs was carried out by two independent scorers.
Quantifications and Statistical Analysis
For crypt number counting in the small intestine, the intestinal length corresponding to a minimum of 100 crypts per animal per treatment was measured and was expressed as number of crypts per millimeter of intestinal length. For crypt size measurements in the intestine, a minimum of 50 crypts were measured for the height of the crypt. For cell death measurement in the crypts, the number of cells exhibiting apoptotic morphology (Li et al., 1992; Merritt et al., 1997) from at least 50 crypts per animal per treatment was scored and was expressed as the average number per crypt. The number of Ki67-positive cells was measured in at least 50 crypts per animal per treatment. The survival data were analyzed by log-rank test using GraphPad Prism 5 software. The data represent mean values ± SEM from at least three independent experiments (n = 3) unless otherwise noted. All p values were obtained using two-way ANOVA and Fisher’s post hoc tests.
Acknowledgments
Work in the K.H.V., K.B., D.S., and O.J.S. laboratories is funded by CR-UK. E.C.C. is supported by a fellowship from the Canadian Institutes of Health Research, and P.L. is in receipt of an MRC studentship. The authors thank Eyal Gottlieb for advice and critical reading of the manuscript and the Biological Services at the Beatson Institute for Cancer Research for technical assistance.
Published: May 30, 2013
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and six figures and can be found with this article online at http://dx.doi.org/10.1016/j.devcel.2013.05.001.
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information
References
- Anastasiou D., Poulogiannis G., Asara J.M., Boxer M.B., Jiang J.K., Shen M., Bellinger G., Sasaki A.T., Locasale J.W., Auld D.S. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton G.H., Morton J.P., Myant K., Phesse T.J., Ridgway R.A., Marsh V., Wilkins J.A., Athineos D., Muncan V., Kemp R. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev. Cell. 2010;19:259–269. doi: 10.1016/j.devcel.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barker N., Ridgway R.A., van Es J.H., van de Wetering M., Begthel H., van den Born M., Danenberg E., Clarke A.R., Sansom O.J., Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Bensaad K., Vousden K.H. p53: new roles in metabolism. Trends Cell Biol. 2007;17:286–291. doi: 10.1016/j.tcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Bensaad K., Tsuruta A., Selak M.A., Vidal M.N., Nakano K., Bartrons R., Gottlieb E., Vousden K.H. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Bensaad K., Cheung E.C., Vousden K.H. Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J. 2009;28:3015–3026. doi: 10.1038/emboj.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodovsky A., Seltzer M.J., Riggins G.J. Altered cancer cell metabolism in gliomas with mutant IDH1 or IDH2. Curr. Opin. Oncol. 2012;24:83–89. doi: 10.1097/CCO.0b013e32834d816a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov A.V., Sablina A.A., Feinstein E., Koonin E.V., Chumakov P.M. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- Cairns R.A., Harris I.S., Mak T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Cheung E.C., Ludwig R.L., Vousden K.H. Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc. Natl. Acad. Sci. USA. 2012;109:20491–20496. doi: 10.1073/pnas.1206530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper H.S., Murthy S.N., Shah R.S., Sedergran D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- Cosentino C., Grieco D., Costanzo V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011;30:546–555. doi: 10.1038/emboj.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C.V. Links between metabolism and cancer. Genes Dev. 2012;26:877–890. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deberardinis R.J., Sayed N., Ditsworth D., Thompson C.B. Brick by brick: metabolism and tumor cell growth. Curr. Opin. Genet. Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Yu K.H., Yeo C.J., Calhoun E.S. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed-Pastor W.A., Mizuno H., Zhao X., Langerød A., Moon S.H., Rodriguez-Barrueco R., Barsotti A., Chicas A., Li W., Polotskaia A. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell. 2012;148:244–258. doi: 10.1016/j.cell.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel R. Regulation and physiological functions of malic enzymes. Curr. Top. Cell. Regul. 1975;9:157–181. doi: 10.1016/b978-0-12-152809-6.50012-3. [DOI] [PubMed] [Google Scholar]
- Goidts V., Bageritz J., Puccio L., Nakata S., Zapatka M., Barbus S., Toedt G., Campos B., Korshunov A., Momma S. RNAi screening in glioma stem-like cells identifies PFKFB4 as a key molecule important for cancer cell survival. Oncogene. 2012;31:3235–3243. doi: 10.1038/onc.2011.490. [DOI] [PubMed] [Google Scholar]
- Hamanaka R.B., Chandel N.S. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Rosen D.G., Zhou Y., Feng L., Yang G., Liu J., Huang P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: role in cell proliferation and response to oxidative stress. J. Biol. Chem. 2005;280:39485–39492. doi: 10.1074/jbc.M503296200. [DOI] [PubMed] [Google Scholar]
- Jamieson T., Clarke M., Steele C.W., Samuel M.S., Neumann J., Jung A., Huels D., Olson M.F., Das S., Nibbs R.J., Sansom O.J. Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J. Clin. Invest. 2012;122:3127–3144. doi: 10.1172/JCI61067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Li H., Jogl G. Structural and biochemical studies of TIGAR (TP53-induced glycolysis and apoptosis regulator) J. Biol. Chem. 2009;284:1748–1754. doi: 10.1074/jbc.M807821200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.Q., Fan C.Y., O’Connor P.J., Winton D.J., Potten C.S. Target cells for the cytotoxic effects of carcinogens in the murine small bowel. Carcinogenesis. 1992;13:361–368. doi: 10.1093/carcin/13.3.361. [DOI] [PubMed] [Google Scholar]
- Li T., Kon N., Jiang L., Tan M., Ludwig T., Zhao Y., Baer R., Gu W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale J.W., Grassian A.R., Melman T., Lyssiotis C.A., Mattaini K.R., Bass A.J., Heffron G., Metallo C.M., Muranen T., Sharfi H. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R., Degenhardt K., Haramaty L., Karp C.M., White E. Immortalized mouse epithelial cell models to study the role of apoptosis in cancer. Methods Enzymol. 2008;446:77–106. doi: 10.1016/S0076-6879(08)01605-4. [DOI] [PubMed] [Google Scholar]
- Merritt A.J., Allen T.D., Potten C.S., Hickman J.A. Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene. 1997;14:2759–2766. doi: 10.1038/sj.onc.1201126. [DOI] [PubMed] [Google Scholar]
- Murphy M.P. Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxid. Redox Signal. 2012;16:476–495. doi: 10.1089/ars.2011.4289. [DOI] [PubMed] [Google Scholar]
- Mustata R.C., Van Loy T., Lefort A., Libert F., Strollo S., Vassart G., Garcia M.I. Lgr4 is required for Paneth cell differentiation and maintenance of intestinal stem cells ex vivo. EMBO Rep. 2011;12:558–564. doi: 10.1038/embor.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piruat J.I., Pintado C.O., Ortega-Sáenz P., Roche M., López-Barneo J. The mitochondrial SDHD gene is required for early embryogenesis, and its partial deficiency results in persistent carotid body glomus cell activation with full responsiveness to hypoxia. Mol. Cell. Biol. 2004;24:10933–10940. doi: 10.1128/MCB.24.24.10933-10940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard P.J., Spencer-Dene B., Shukla D., Howarth K., Nye E., El-Bahrawy M., Deheragoda M., Joannou M., McDonald S., Martin A. Targeted inactivation of fh1 causes proliferative renal cyst development and activation of the hypoxia pathway. Cancer Cell. 2007;11:311–319. doi: 10.1016/j.ccr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Radisky D.C., Levy D.D., Littlepage L.E., Liu H., Nelson C.M., Fata J.E., Leake D., Godden E.L., Albertson D.G., Nieto M.A. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros S., Santos C.R., Moco S., Baenke F., Kelly G., Howell M., Zamboni N., Schulze A. Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discov. 2012;2:328–343. doi: 10.1158/2159-8290.CD-11-0234. [DOI] [PubMed] [Google Scholar]
- Sansom O.J., Reed K.R., Hayes A.J., Ireland H., Brinkmann H., Newton I.P., Batlle E., Simon-Assmann P., Clevers H., Nathke I.S. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Sato T., Stange D.E., Ferrante M., Vries R.G., Van Es J.H., Van den Brink S., Van Houdt W.J., Pronk A., Van Gorp J., Siersema P.D., Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- Schafer Z.T., Grassian A.R., Song L., Jiang Z., Gerhart-Hines Z., Irie H.Y., Gao S., Puigserver P., Brugge J.S. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatrowski T.P., Nathan C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- Tong X., Zhao F., Thompson C.B. The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr. Opin. Genet. Dev. 2009;19:32–37. doi: 10.1016/j.gde.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D., Zhou Y., Zhang H., Demizu Y., Chen Z., Pelicano H., Chiao P.J., Achanta G., Arlinghaus R.B., Liu J., Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- van der Flier L.G., Haegebarth A., Stange D.E., van de Wetering M., Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden K.H., Ryan K.M. p53 and metabolism. Nat. Rev. Cancer. 2009;9:691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- Wanka C., Steinbach J.P., Rieger J. Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects glioma cells from starvation-induced cell death by up-regulating respiration and improving cellular redox homeostasis. J. Biol. Chem. 2012;287:33436–33446. doi: 10.1074/jbc.M112.384578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg F., Hamanaka R., Wheaton W.W., Weinberg S., Joseph J., Lopez M., Kalyanaraman B., Mutlu G.M., Budinger G.R., Chandel N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise D.R., Thompson C.B. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won K.Y., Lim S.J., Kim G.Y., Kim Y.W., Han S.A., Song J.Y., Lee D.K. Regulatory role of p53 in cancer metabolism via SCO2 and TIGAR in human breast cancer. Hum. Pathol. 2012;43:221–228. doi: 10.1016/j.humpath.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Yang C.S., Thomenius M.J., Gan E.C., Tang W., Freel C.D., Merritt T.J., Nutt L.K., Kornbluth S. Metabolic regulation of Drosophila apoptosis through inhibitory phosphorylation of Dronc. EMBO J. 2010;29:3196–3207. doi: 10.1038/emboj.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Brosel S., Acin-Perez R., Slavkovich V., Nishino I., Khan R., Goldberg I.J., Graziano J., Manfredi G., Schon E.A. Analysis of mouse models of cytochrome c oxidase deficiency owing to mutations in Sco2. Hum. Mol. Genet. 2010;19:170–180. doi: 10.1093/hmg/ddp477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Mancuso A., Tong X., Ward P.S., Fan J., Rabinowitz J.D., Thompson C.B. Pyruvate kinase M2 promotes de novo serine synthesis to sustain mTORC1 activity and cell proliferation. Proc. Natl. Acad. Sci. USA. 2012;109:6904–6909. doi: 10.1073/pnas.1204176109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Kosugi M., Kufe D. Inhibition of the MUC1-C oncoprotein induces multiple myeloma cell death by down-regulating TIGAR expression and depleting NADPH. Blood. 2012;119:810–816. doi: 10.1182/blood-2011-07-369686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying H., Kimmelman A.C., Lyssiotis C.A., Hua S., Chu G.C., Fletcher-Sananikone E., Locasale J.W., Son J., Zhang H., Coloff J.L. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaugg K., Yao Y., Reilly P.T., Kannan K., Kiarash R., Mason J., Huang P., Sawyer S.K., Fuerth B., Faubert B. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 2011;25:1041–1051. doi: 10.1101/gad.1987211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.