Abstract
NF-protocadherin (NFPC)-mediated cell–cell adhesion plays a critical role in vertebrate neural tube formation. NFPC is also expressed during the period of axon tract formation, but little is known about its function in axonogenesis. Here we have tested the role of NFPC and its cytosolic cofactor template-activating factor 1 (TAF1) in the emergence of the Xenopus retinotectal projection. NFPC is expressed in the developing retina and optic pathway and is abundant in growing retinal axons. Inhibition of NFPC function in developing retinal ganglion cells (RGCs) severely reduces axon initiation and elongation and suppresses dendrite genesis. Furthermore, an identical phenotype occurs when TAF1 function is blocked. These data provide evidence that NFPC regulates axon initiation and elongation and indicate a conserved role for TAF1, a transcriptional regulator, as a downstream cytosolic effector of NFPC in RGCs.
Keywords: retinal growth cone, NFPC, TAF1, axon, protocadherin, cell adhesion molecule
Introduction
Nervous system development requires the coordinated regulation of cellular interactions, which are mediated through cell adhesion molecules, including the cadherins. The classical cadherins have been implicated in multiple aspects of neural development, including neurite outgrowth, axon pathfinding, and synaptogenesis (Hirano et al., 2003). More recently, new subgroups of the cadherin superfamily have been identified, including desmosomal, Fat, and seven-pass transmembrane (7-TM) cadherins. These proteins differ from the classical cadherins in that they possess varying numbers of extracellular cadherin (EC) repeats and have divergent cytoplasmic domains. Many of these molecules are critical for neural development. For instance, photoreceptor target selection in Drosophila requires the 7-TM cadherin Flamingo, (Lee et al., 2003) and Celsr3, a mammalian Flamingo ortholog, regulates the formation of axon tracts, including the anterior commissure (Tissir et al., 2005).
The protocadherins comprise another phylogenetically distinct group of the cadherin superfamily and are often expressed within spatially restricted domains in the central nervous system (Hirano et al., 2003). For instance, protocadherin 7 (pcdh7) is expressed in the Purkinje cells and vomeronasal organ of the developing mouse brain (Redies et al., 2005) and in the cerebral cortex of the adult (Yoshida et al., 1999). One function that protocadherins may regulate during neural development is axonogenesis, as a recent report has described aberrant axon tract formation in the ventral telencephalon of OL-protocadherin-deficient mice (Uemura et al., 2007). However, the mechanisms underlying protocadherin-mediated axonal extension, in particular the identity of cytosolic cofactors for neuronal protocadherins, remain poorly defined.
NF-protocadherin (NFPC), the Xenopus homolog of pcdh7, regulates ectodermal cell sorting (Bradley et al., 1998) and neural tube closure (Rashid et al., 2006) during embryogenesis. We demonstrate that NFPC is highly expressed in the developing retina and is particularly abundant in retinal ganglion cell (RGC) axons and growth cones. Dominant inhibition of NFPC function in single neurons in vivo elicited defects in RGC axon and dendrite initiation and elongation. In addition, template-activating factor 1 (TAF1) is also highly expressed in RGCs, consistent with its known role as a cytosolic cofactor for NFPC (Heggem and Bradley, 2003). Furthermore, a dominant-negative TAF1 protein produced a phenotype that mimicked NFPC inhibition, indicating that RGC axon and dendrite outgrowth in vivo requires TAF1-mediated NFPC function.
Materials and Methods
Retinal cultures.
Xenopus laevis embryos, obtained by in vitro fertilization, were raised in 0.1× modified Barth's saline (14–20°C). Retinal explant culture was performed as described previously (Piper et al., 2006).
In situ hybridization.
Whole-mount (see Fig. 1B,C) and section (see Fig. 1A,G) in situ hybridization was performed using NFPC- and TAF1-specific probes (Rashid et al., 2006). Sense probes displayed no specific staining. Horseradish peroxidase (HRP) labeling of RGC axons was performed as described previously (Piper et al., 2006).
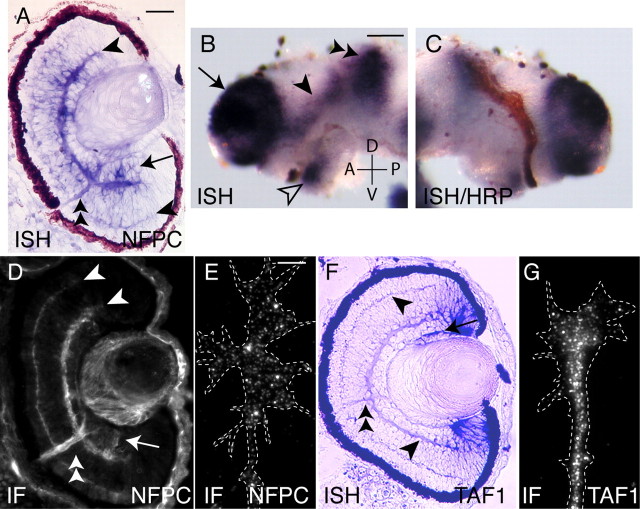
Figure 1.
Retinal expression of NFPC and TAF1. A, Transverse section of a stage 40 retina, showing NFPC mRNA expression in the plexiform layers (arrowheads), ONH (double arrowhead), and RGC layer (arrow). B, Whole-mount ISH of an isolated stage 40 brain. NFPC mRNA delineates the optic tract (arrowhead) and is seen in the forebrain (arrow), tectum (double arrowhead), and hypothalamus (open arrowhead). A, Anterior; D, dorsal; P, posterior; V, ventral. C, Reverse side of the brain shown in B, in which retinal axons have been HRP filled, so they appear brown. The HRP signal is coincident with NFPC mRNA in the optic tract. D, At stage 40, the lens, plexiform layers (arrowheads), RGCs (arrow), and ONH (double arrowhead) express NFPC protein. E, Cultured RGC neurite from a stage 24 retinal explant expressing NFPC on the neurite shaft and growth cone. F, Retinal TAF1 mRNA expression is found in the RGC layer (arrow), ONH (double arrowhead), and the plexiform layers (arrowheads) at stage 40. G, Cultured stage 24 retinal neurite expressing TAF1. IF, Immunofluorescence. Dotted lines in E and G delineate the edge of the growth cone. Scale bar: B, C, 400 μm; A, D, F, 250 μm; E, G, 5 μm.
Immunofluorescent labeling.
Polyclonal antibodies against TAF1 (Active Motif, Carlsbad, CA) or the cytoplasmic domain of NFPC, followed by incubation with a fluorescent secondary (goat anti-rabbit IgG AlexaFluor594, Invitrogen, Carlsbad, CA) were used to detect these proteins using standard protocols. To visualize cells expressing myc-tagged dominant-negative constructs, an anti-myc antibody (9E10, Sigma, St. Louis, MO) was used, followed by incubation with a fluorescent secondary antibody (goat anti-mouse IgG AlexaFluor594, Invitrogen).
Plasmids.
Membrane-targeted GFP (GAP-GFP), cloned into pCS2+ vector (Das et al., 2003), was used as the negative control. To inhibit NFPC, a deletion construct, NFΔE, lacking the 836 aa comprising the extracellular (EC) region was used (Bradley et al., 1998). The NFΔN construct lacks both the EC domain and the cytoplasmic juxtamembrane 71 aa of NFPC (Heggem and Bradley, 2003). To inhibit TAF1, a deletion construct, TAF1ΔN, lacking the first 141 aa of this protein was used (Rashid et al., 2006). Each construct was cloned into pCS2+ and was myc-tagged at the N terminus (TAF1ΔN) or C terminus (NFΔE, NFΔN).
In vivo lipofections.
For each deletion construct, colipofections were performed with GAP-GFP to enable selection of lipofection-positive embryos. DNA was mixed with DOTAP (Roche, Indianapolis, IN) at a ratio of 1 μg:3 μl before injection into the proliferating retinal neuroepithelium of stage 19 embryos. The first retinal neurons are born at stage 25/26, when expression of exogenous constructs begins (Holt et al., 1990). At stage 40, when RGC axons first enter the optic tectum, embryos were fixed (4% paraformaldehyde) and mounted in OCT (TissueTEK), and 30 μm serial cryosections cut in the transverse plane. Retinal cells expressing the dominant-negative constructs were detected using immunofluorescent detection of the myc epitope.
Analysis.
The retinotectal pathway was imaged at ×20 magnification using a Nikon Optiphot inverted microscope and a Hamamatsu digital CCD camera. Neurons were scored as RGCs if their somata were located in the ganglion cell layer of the retina. Axon and primary dendrite length of myc-positive (dominant-negative constructs) or GAP-GFP-positive (control) RGCs were traced and measured using Openlab (Improvision, Lexington, MA). Data represent pooled results from at least three independent experiments.
Statistics.
Statistical analyses were performed using ANOVA (Kruskal–Wallis with posttest) or χ2 tests. Error bars represent SEM.
Results
NFPC is expressed in RGCs and along the retinotectal pathway
NFPC is expressed in the developing eye (Bradley et al., 1998), but its pattern of expression in specific retinal cell types and its distribution within the entire optic pathway is uncharacterized. To address this we performed in situ hybridization (ISH) with an NFPC-specific probe. ISH on transverse sections of the eye show NFPC expression in the differentiating central portion of the retina at stage 40, including the RGC and inner nuclear cell layers, and is particularly high in the inner plexiform layer (IPL) and the optic nerve head (ONH), where the dendrites and axons of RGCs reside, respectively (Fig. 1A). This suggests that NFPC message is trafficked into RGC dendrites and axons. Whole-mount ISH on stage 40 Xenopus brains demonstrated that NFPC mRNA is also expressed in the optic tract and optic tectum (Fig. 1B). Moreover, anterograde HRP labeling of retinal axons in these brains verified that NFPC mRNA colocalizes with RGC axons in the optic tract (Fig. 1C). To determine whether the ISH labeling in the optic tract region reflected transcripts localized exclusively to retinal axons and/or the underlying neuroepithelial substrate, embryonic eyes were removed before RGC axonogenesis (stage 26). ISH performed on the brains from these eyeless embryos at stage 40 showed persistent, although slightly reduced, expression of NFPC in the optic pathway (data not shown), suggesting that NFPC is expressed in the neuroepithelial substrate in addition to the RGC axons.
To examine protein distribution in the retina, immunocytochemistry was performed on sectioned material. NFPC immunolabeling closely matched the pattern of ISH staining (Fig. 1D). To test whether NFPC protein is expressed in the growth cones of retinal axons, immunocytochemistry was performed on retinal explants cultured from embryos of ages varying from stage 24 to stage 42 (Fig. 1E; data not shown). Growth cones showed strong punctate staining, indicating that NFPC protein is present in RGC growth cones during the period of retinal axonogenesis.
Dominant-negative NFPC inhibits development of RGC processes
To determine the role of NFPC in RGC development, we sought to block its function in vivo by expressing a dominant-negative form of NFPC. We used a construct lacking the EC domain of NFPC (NFΔE), which was shown previously to inhibit endogenous function (Bradley et al., 1998). Lipofection of this myc-tagged NFPC mutant into eye primordia at stage 19 gave rise to multiple myc-positive neurons in the retina at stage 40. We first assessed whether expression of the mutant protein altered retinal patterning. Both neuronal differentiation and laminar migration appeared normal in NFΔE-expressing cells, as examples of all retinal cell types expressing the dominant-negative protein were observed and were located in their correct laminar position (Fig. 2). Staining with the nuclear marker DAPI demonstrated no evidence of increased apoptosis, characterized by pyknotic nuclei, suggesting that NFΔE-expressing cells are viable at this stage. Similar results were observed with the NFΔN (lacks both EC and cytoplasmic juxtamembrane domains; see Materials and Methods) and TAF1ΔN constructs (data not shown; see Materials and Methods). In summary, inhibition of NFPC function does not appear to affect laminar migration or fate determination.
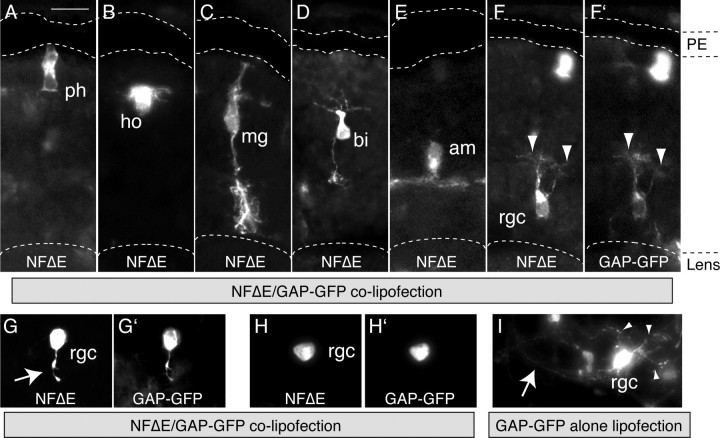
Figure 2.
NFΔE-expressing cells differentiate into multiple retinal cell types. A–H, Transverse sections of stage 40 retinas immunolabeled with an anti-myc antibody to identify NFΔE-expressing cells. NFΔE-expressing cells can differentiate into photoreceptors (A, ph), horizontal cells (B, ho), Müller glia (C, mg), bipolar cells (D, bi), amacrine cells (E, am), and RGCs (F–H). Some RGCs expressing NFΔE possess only dendrites (F, arrowheads) or only an axon (G, arrow), whereas some lack all processes (H). Embryos were cotransfected with GAP-GFP, and the distribution of NFΔE (F–H) and GAP-GFP (F′–H′) in RGCs was very similar, illustrating NFΔE is transported through the axodendritic field. I, RGC transfected with GAP-GFP alone, with multiple dendrites (arrowheads) and an axon extending to the ONH (arrow). PE, Pigment epithelium. Scale bar, 25 μm.
However, closer examination indicated that axon and dendrite extension were impaired in NFΔE-expressing RGCs (Fig. 2F–H). NFΔE-expressing RGCs often possessed dendrites but lacked an axon (Fig. 2F), exhibited only a short axon (Fig. 2G) or, in some cases, possessed no processes at all (Fig. 2H), a phenotype rarely observed in controls. In contrast, RGCs expressing GAP-GFP alone exhibited normal phenotypes with multiple dendrites and an axon that exited the eye via the ONH (Fig. 2I). One possible explanation for the apparent lack of axons/dendrites is that the myc-tagged truncated NFPC protein used for immunodetection is not trafficked efficiently into axons and dendrites. Thus, processes might be present but escape detection with myc immunocytochemistry. To address this, we colipofected the dominant-negative constructs with GAP-GFP. The GAP-GFP fusion protein is targeted to the membrane by the GAP-tag and so is detectable throughout the soma and the processes of transfected cells (Das et al., 2003). As can be seen in Figure 2F–H, expression of NFΔE and GAP-GFP in colipofected RGCs was almost identical, suggesting that the mutant protein is distributed to the distal processes of the RGC as efficiently as the GFP marker, validating our analysis of RGC processes through immunodetection of the mutant protein alone.
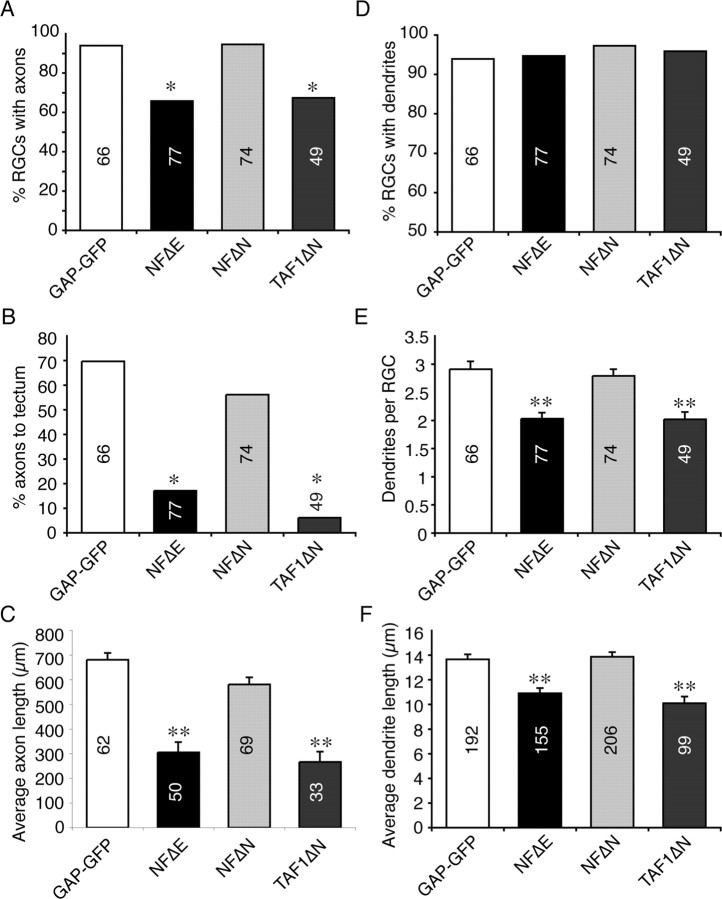
We next quantified the presence and length of both axons and dendrites in RGCs expressing NFΔE or GAP-GFP controls by tracing lengths of these processes in serial sections (Fig. 3). Axon initiation was strongly inhibited in NFΔE-expressing RGCs, with 34.2% of RGCs expressing this protein failing to extend an axon, a phenotype only observed in 6.1% of GAP-GFP controls. Of those RGCs expressing NFΔE that did extend an axon, growth through the optic pathway was severely retarded, with only 17.1% reaching the tectum by stage 40, as opposed to 69.7% in the control. Indeed, the average length of NFΔE-expressing axons was significantly shorter (305.4 ± 42 μm) than GAP-GFP-expressing axons (681.0 ± 28 μm). We also analyzed embryos at stage 43 to determine whether this phenotype was attributable to an overall delay in RGC maturation. The axon length of stage 43 RGCs expressing the NFΔE construct was not significantly increased compared with those at stage 40 (data not shown), suggesting that the impairment of axon extension is not caused by delayed RGC development.
Figure 3.
NFΔE and TAF1ΔN impair axon and dendrite initiation and outgrowth from RGCs. Quantitative analysis of GAP-GFP-, NFΔE-, NFΔN-, and TAF1ΔN-expressing RGCs at stage 40. A, The percentage of RGCs with axons was significantly reduced in cells expressing NFΔE or TAF1ΔN compared with RGCs expressing GAP-GFP. B, C, Although many axons from GAP-GFP- or NFΔN-expressing RGCs reached the tectum, the percentage of axons reaching the tectum was significantly smaller in NFΔE- or TAF1ΔN-expressing cells (B), and these RGCs had on average significantly shorter axons (C). D–F, The number of RGCs with dendrites was similar between all sample groups (D), but RGCs expressing NFΔE or TAF1ΔN had significantly fewer (E) and shorter (F) dendrites per cell. Numbers in bars indicate RGCs analyzed for each group. *p < 0.0005, χ2 test; **p < 0.001, Kruskal–Wallis test.
The effect of NFΔE expression on RGC dendritogenesis was less severe than on axonogenesis. Although the percentage of NFΔE-expressing RGCs with dendrites versus the control was very similar (94.7% vs 93.9%), those cells expressing NFΔE exhibited significantly fewer dendrites (2.03 ± 0.11) than the control (2.91 ± 0.14) and, moreover, the average length of dendrites in cells expressing NFΔE (10.90 ± 0.43 μm) was significantly shorter than the control (13.65 ± 0.42 μm). These data demonstrate that NFPC function is required for axon and dendrite initiation and elongation in vivo.
Dominant-negative blockade of TAF1 phenocopies NFPC inhibition
The cytoplasmic juxtamembrane region of NFPC interacts with TAF1, and TAF1 is required for NFPC-mediated cell adhesion in the embryonic ectoderm (Heggem and Bradley, 2003). Therefore, we extended our analysis to investigate the pattern of TAF1 expression in the optic pathway and found that it is highly enriched in the IPL and ONH of the developing retina (Fig. 1F). Furthermore, immunocytochemistry on cultured retinal explants showed that TAF1 protein is also expressed in retinal growth cones (Fig. 1G). Importantly, the data indicate that both NFPC and TAF1 are expressed in RGCs, prompting us to assess the functional role of TAF1 in their development. We used a deletion construct, TAF1ΔN, previously shown to inhibit TAF1 function in vivo (Rashid et al., 2006). The results observed were similar to that obtained with NFΔE. Axon initiation from TAF1ΔN-expressing RGCs was severely impaired, with only 67.3% possessing an axon (Fig. 3). Of these, only a small percentage had reached the tectum by stage 40 (6.1%), with the average axon length (267.3 ± 41 μm) being significantly shorter than the GAP-GFP controls. Similarly, although most of the RGCs expressing the mutant protein possessed dendrites, the average number of dendrites per RGC (2.02 ± 0.13) was reduced compared with the control, as was the average dendrite length (10.10 ± 0.55 μm). This phenocopy of NFPC perturbation suggests that TAF1 could play an important role by acting downstream of NFPC during the development of RGC processes in vivo.
The cytoplasmic juxtamembrane region of NFΔE is essential for inhibition of NFPC
NFΔE is thought to function by competing with endogenous NFPC for binding to cytosolic cofactors. Because previous research demonstrated that TAF1 binds to the juxtamembrane region of NFPC's cytoplasmic domain (Heggem and Bradley, 2003), we sought to verify that this region is important for the dominant-negative effect of NFΔE in developing RGCs. To address this, we transfected RGCs with a NFPC construct lacking both the EC and cytoplasmic juxtamembrane domains (NFΔN). This construct, when expressed in the embryonic ectoderm, does not disrupt NFPC function, because it is unable to compete for the binding of endogenous TAF1 (Heggem and Bradley, 2003). As shown in Figure 3, analysis of RGCs expressing NFΔN showed that neither axon nor dendrite initiation differed significantly from the GAP-GFP control, nor did the proportion of NFΔN-expressing axons reaching the tectum. Furthermore, the length of NFΔN-expressing axons (581.4 ± 29 μm) and dendrites (13.87 ± 0.38 μm) was comparable with those expressing GAP-GFP. These data demonstrate that the cytoplasmic juxtamembrane domain is essential for the dominant-negative effect of NFΔE and, collectively, these data point to TAF1 as the cytoplasmic binding partner important for axonal and dendritic behavior regulated by NFPC.
Inhibition of axon growth throughout the optic pathway
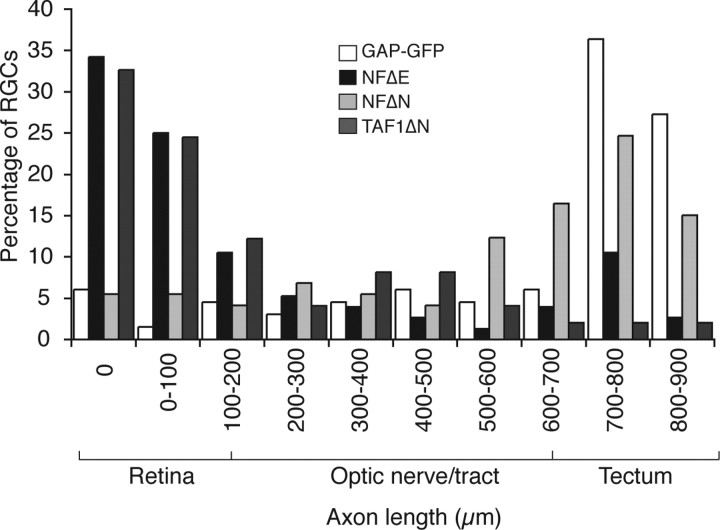
The localized expression of NFPC within retinal axons and along the optic pathway is indicative of a role during RGC axon elongation. To clearly visualize where dominantly inhibited axons were located within the optic pathway, we next did a distribution analysis of axon lengths from each sample group by placing them into 100 μm intervals and plotted the resulting spread of data onto a histogram. From this, it is clear that axons from RGCs expressing either GAP-GFP or NFΔN were most frequently found in the distal optic tract and tectum, with the majority having lengths >500 μm (Fig. 4). In contrast, most RGCs expressing NFΔE or TAF1ΔN have either no axons or shorter axons (100–200 μm), with the subsequent intervals generally having decreasing representation. Because we did not observe a specific point within the retinotectal pathway in which axons from NFΔE- or TAF1ΔN-expressing RGCs were accumulating, we infer that inhibition of NFPC or TAF1 causes a general impairment of retinal axon growth during development.
Figure 4.
Distribution of axon lengths from transfected RGCs. Axon lengths from all samples were pooled into 100 μm bins. NFΔE- or TAF1ΔN-expressing RGCs do not accumulate at a specific point in the optic pathway, suggesting a general impairment in axonogenesis.
Discussion
Our results show that NFPC and TAF1 are expressed in RGCs and that inhibition of either NFPC or TAF1 function in vivo severely impairs axon and dendrite extension. These findings, together with recent reports showing that γ-protocadherins regulate synaptic development in spinal cord neurons (Weiner et al., 2005), and OL-protocadherin controls striatal axon elongation in the ventral telencephalon (Uemura et al., 2007), implicate protocadherins as general players in axonogenesis and dendritogenesis.
Although it is apparent that NFPC plays an integral role in RGC axon and dendrite elongation, its precise function at the level of the growth cone is unclear. NFPC was first identified as a regulator of embryonic ectodermal formation in Xenopus. In vivo inhibition of NFPC with NFΔE resulted in a failure of the nascent ectodermal layers to juxtapose, indicating a failure of adhesion in those NFΔE-expressing regions (Bradley et al., 1998). NFPC has also been implicated in the regulation of neural tube closure (Rashid et al., 2006). Interestingly, ectodermal overexpression of NFPC induced cell sorting, with NFPC-expressing cells clustering together, a phenotype indicative of NFPC acting as a homophilic adhesion molecule (Bradley et al., 1998). Homophilic binding has also been reported for other protocadherins (Hirano et al., 2003). The expression of NFPC on RGC axons and along the optic pathway neuroepithelium (Fig. 1) is certainly consistent with a role for homophilic NFPC binding regulating axon extension through the retinotectal pathway. Similarly, homophilic binding between NFPC expressed on RGC dendrites and the processes of other retinal neurons may facilitate dendrite extension.
Alternatively, NFPC may regulate the activity of other cell adhesion molecules. Evidence supporting this hypothesis has come from an investigation of Xenopus paraxial protocadherin (PAPC). Blastomere adhesion assays and in vitro cell culture experiments indicate that PAPC, instead of mediating adhesion via homophilic binding, may function to downregulate the activity of C-cadherin (Chen and Gumbiner, 2006). NFPC could function in an analogous manner during optic pathway development, because RGCs also express N-cadherin, and, moreover, blockade of N-cadherin function causes axonal and dendritic defects similar to that caused by NFPC inhibition (Riehl et al., 1996). Our data are also consistent with this hypothesis. Further experimentation will be required to ascertain whether NFPC acts as a homophilic adhesion molecule during RGC axon and dendrite development or whether it functions to regulate N-cadherin within the growth cone.
Our data also demonstrates a novel role for TAF1 in the regulation of axon and dendrite elongation in vivo. Interestingly, TAF1 regulates histone acetylation and transcription as part of the INHAT (inhibitor of acetyltransferase) complex (Seo et al., 2001). As well as being localized to the nucleus, TAF1 is found in the cytosol and interacts with the cytoplasmic juxtamembrane region of NFPC in vitro and in vivo (Heggem and Bradley, 2003). Although we have not demonstrated a direct interaction between these molecules in RGCs, coexpression of NFPC and TAF1, as well as the very similar defects observed when either molecule is inhibited, strongly suggest that they interact during retinal development. Furthermore, the importance of the TAF1-binding domain of NFPC in mediating the function of this protocadherin is demonstrated by the fact that NFΔN does not inhibit NFPC function in ectodermal adhesion assays (Heggem and Bradley, 2003) or RGC process extension (this study). What remains to be defined are the events occurring downstream of TAF1 binding. TAF1 may play a role in downstream signaling from NFPC, because it has been shown to bind p35nck5a, the activator of cyclin-dependent kinase 5 (Cdk5), and to enhance Cdk5/p35nck5a activity (Qu et al., 2002). Cdk5 is abundant in postmitotic neurons and is localized to extending axons of chick RGCs, where it regulates growth cone elongation and stabilization (Hahn et al., 2005). Protein phosphatase 2A (PP2A), which regulates phosphorylation of key signaling pathways including the MEK/ERK cascade in neurons (Mao et al., 2005), is another candidate for signaling downstream of NFPC/TAF1, because TAF1 can interact with and regulate the activity of this enzyme (Li et al., 1996).
Aside from extension, the disruption of axon and dendrite initiation reflects a defect in early RGC development. How could NFPC and TAF1 regulate this process? Consistent with a role in early RGC development, TAF1 could itself be translocated to the nucleus to regulate programs of gene expression leading to process initiation. A second possibility is that downstream signaling from TAF1 within the cytosol may regulate axonal and dendritic specification, perhaps through Cdk5 (Qu et al., 2002). The Akt signaling pathway, a key node within the process of neuronal polarization (Yoshimura et al., 2006), is regulated by Cdk5 in rat cortical neurons (Li et al., 2003), and Rac1, another integral component for axon specification, functions with Cdk5 to control axon initiation (Ruchhoeft et al., 1999). PP2A may also be relevant for this process, because it modulates the activity of the Par complex [Par3/Par6/atypical protein kinase C (aPKC)], an important determinant for neuronal polarization and axon specification. PP2A regulates the activity and distribution of aPKC (Nunbhakdi-Craig et al., 2002) and is required for the localization of Bazooka, a component of the Par6 complex, in Drosophila photoreceptors (Nam et al., 2007). Although the mechanism is currently unclear, our data clearly point to TAF1 as an important mediator of NFPC function during the initial stages of RGC process specification. In conclusion, our results demonstrate that TAF1-mediated NFPC function is required in vivo for axon and dendrite initiation and elongation from RGCs, providing the first indication that this molecular couplet regulates these critical functions during nervous system development.
Footnotes
This work was funded by a Wellcome Trust Programme grant (C.H.). M.P. holds a National Health and Medical Research Council Howard Florey Centenary Research Fellowship, and L.L. holds a Medical Research Council studentship. We thank D. O'Connor, D. Pask, S. Shipway, S. Diamantakis, S. Cordiner-Lawrie, A. Bowden, and A. Curry for technical assistance and Julien Falk, Randal Moldrich, and Linda Richards for comments on this manuscript.
References
- Bradley RS, Espeseth A, Kintner C. NF-protocadherin, a novel member of the cadherin superfamily, is required for Xenopus ectodermal differentiation. Curr Biol. 1998;8:325–334. doi: 10.1016/s0960-9822(98)70132-0. [DOI] [PubMed] [Google Scholar]
- Chen X, Gumbiner BM. Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity. J Cell Biol. 2006;174:301–313. doi: 10.1083/jcb.200602062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Payer B, Cayouette M, Harris WA. In vivo time-lapse imaging of cell divisions during neurogenesis in the developing zebrafish retina. Neuron. 2003;37:597–609. doi: 10.1016/s0896-6273(03)00066-7. [DOI] [PubMed] [Google Scholar]
- Hahn CM, Kleinholz H, Koester MP, Grieser S, Thelen K, Pollerberg GE. Role of cyclin-dependent kinase 5 and its activator P35 in local axon and growth cone stabilization. Neuroscience. 2005;134:449–465. doi: 10.1016/j.neuroscience.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Heggem MA, Bradley RS. The cytoplasmic domain of Xenopus NF-protocadherin interacts with TAF1/set. Dev Cell. 2003;4:419–429. doi: 10.1016/s1534-5807(03)00036-4. [DOI] [PubMed] [Google Scholar]
- Hirano S, Suzuki ST, Redies C. The cadherin superfamily in neural development: diversity, function and interaction with other molecules. Front Biosci. 2003;8:d306–d355. doi: 10.2741/972. [DOI] [PubMed] [Google Scholar]
- Holt CE, Garlick N, Cornel E. Lipofection of cDNAs in the embryonic vertebrate central nervous system. Neuron. 1990;4:203–214. doi: 10.1016/0896-6273(90)90095-w. [DOI] [PubMed] [Google Scholar]
- Lee RC, Clandinin TR, Lee CH, Chen PL, Meinertzhagen IA, Zipursky SL. The protocadherin Flamingo is required for axon target selection in the Drosophila visual system. Nat Neurosci. 2003;6:557–563. doi: 10.1038/nn1063. [DOI] [PubMed] [Google Scholar]
- Li BS, Ma W, Jaffe H, Zheng Y, Takahashi S, Zhang L, Kulkarni AB, Pant HC. Cyclin-dependent kinase-5 is involved in neuregulin-dependent activation of phosphatidylinositol 3-kinase and Akt activity mediating neuronal survival. J Biol Chem. 2003;278:35702–35709. doi: 10.1074/jbc.M302004200. [DOI] [PubMed] [Google Scholar]
- Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271:11059–11062. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- Mao L, Yang L, Arora A, Choe ES, Zhang G, Liu Z, Fibuch EE, Wang JQ. Role of protein phosphatase 2A in mGluR5-regulated MEK/ERK phosphorylation in neurons. J Biol Chem. 2005;280:12602–12610. doi: 10.1074/jbc.M411709200. [DOI] [PubMed] [Google Scholar]
- Nam SC, Mukhopadhyay B, Choi KW. Antagonistic functions of Par-1 kinase and protein phosphatase 2A are required for localization of Bazooka and photoreceptor morphogenesis in Drosophila. Dev Biol. 2007;306:624–635. doi: 10.1016/j.ydbio.2007.03.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, III, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Anderson R, Dwivedy A, Weinl C, van Horck F, Leung KM, Cogill E, Holt C. Signaling mechanisms underlying Slit2-induced collapse of Xenopus retinal growth cones. Neuron. 2006;49:215–228. doi: 10.1016/j.neuron.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D, Li Q, Lim HY, Cheung NS, Li R, Wang JH, Qi RZ. The protein SET binds the neuronal Cdk5 activator p35nck5a and modulates Cdk5/p35nck5a activity. J Biol Chem. 2002;277:7324–7332. doi: 10.1074/jbc.M107270200. [DOI] [PubMed] [Google Scholar]
- Rashid D, Newell K, Shama L, Bradley R. A requirement for NF-protocadherin and TAF1/Set in cell adhesion and neural tube formation. Dev Biol. 2006;291:170–181. doi: 10.1016/j.ydbio.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Redies C, Vanhalst K, Roy F. delta-Protocadherins: unique structures and functions. Cell Mol Life Sci. 2005;62:2840–2852. doi: 10.1007/s00018-005-5320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehl R, Johnson K, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt CE. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- Ruchhoeft ML, Ohnuma S, McNeill L, Holt CE, Harris WA. The neuronal architecture of Xenopus retinal ganglion cells is sculpted by rho-family GTPases in vivo. J Neurosci. 1999;19:8454–8463. doi: 10.1523/JNEUROSCI.19-19-08454.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SB, McNamara P, Heo S, Turner A, Lane WS, Chakravarti D. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell. 2001;104:119–130. doi: 10.1016/s0092-8674(01)00196-9. [DOI] [PubMed] [Google Scholar]
- Tissir F, Bar I, Jossin Y, Goffinet AM. Protocadherin Celsr3 is crucial in axonal tract development. Nat Neurosci. 2005;8:451–457. doi: 10.1038/nn1428. [DOI] [PubMed] [Google Scholar]
- Uemura M, Nakao S, Suzuki ST, Takeichi M, Hirano S. OL-protocadherin is essential for growth of striatal axons and thalamocortical projections. Nat Neurosci. 2007;10:1151–1159. doi: 10.1038/nn1960. [DOI] [PubMed] [Google Scholar]
- Weiner JA, Wang X, Tapia JC, Sanes JR. Gamma protocadherins are required for synaptic development in the spinal cord. Proc Natl Acad Sci USA. 2005;102:8–14. doi: 10.1073/pnas.0407931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Watanabe M, Kato H, Dutta A, Sugano S. BH-protocadherin-c, a member of the cadherin superfamily, interacts with protein phosphatase 1 alpha through its intracellular domain. FEBS Lett. 1999;460:93–98. doi: 10.1016/s0014-5793(99)01309-5. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Arimura N, Kaibuchi K. Signaling networks in neuronal polarization. J Neurosci. 2006;26:10626–10630. doi: 10.1523/JNEUROSCI.3824-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]