Abstract
BACKGROUND & AIMS
Aspirin reduces the incidence of and mortality from colorectal cancer (CRC) by unknown mechanisms. Cancer cells have defects in signaling via the mechanistic target of rapamycin (mTOR), which regulates proliferation. We investigated whether aspirin affects adenosine monophosphate–activated protein kinase (AMPK) and mTOR signaling in CRC cells.
METHODS
The effects of aspirin on mTOR signaling, the ribosomal protein S6, S6 kinase 1 (S6K1), and eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1) were examined in CRC cells by immunoblotting. Phosphorylation of AMPK was measured; the effects of loss of AMPKα on the aspirin-induced effects of mTOR were determined using small interfering RNA (siRNA) in CRC cells and in AMPKα1/α2−/− mouse embryonic fibroblasts. LC3 and ULK1 were used as markers of autophagy. We analyzed rectal mucosa samples from patients given 600 mg aspirin, once daily for 1 week.
RESULTS
Aspirin reduced mTOR signaling in CRC cells by inhibiting the mTOR effectors S6K1 and 4E-BP1. Aspirin changed nucleotide ratios and activated AMPK in CRC cells. mTOR was still inhibited by aspirin in CRC cells after siRNA knockdown of AMPKα, indicating AMPK-dependent and AMPK-independent mechanisms of aspirin-induced inhibition of mTOR. Aspirin induced autophagy, a feature of mTOR inhibition. Aspirin and metformin (an activator of AMPK) increased inhibition of mTOR and Akt, as well as autophagy in CRC cells. Rectal mucosal samples from patients given aspirin had reduced phosphorylation of S6K1 and S6.
CONCLUSIONS
Aspirin is an inhibitor of mTOR and an activator of AMPK, targeting regulators of intracellular energy homeostasis and metabolism. These could contribute to its protective effects against development of CRC.
Keywords: Chemoprevention, Colon Cancer, Oncogene, Tumor Suppressor
Colorectal cancer (CRC) is common, with a worldwide incidence estimated at more than 1 million cases annually. Overall survival is poor, underscoring the rationale for new preventative approaches. Aspirin, a nonsteroidal anti-inflammatory drug (NSAID), reduces cancer risk, particularly CRC.1,2 Primary prevention with aspirin is not currently recommended because the risk:benefit ratio is finely balanced. NSAIDs inhibit cell growth and induce apoptosis at various disease stages, from initiation to progression. Although evidence that aspirin prevents cancer is compelling, the underlying molecular mechanism remains enigmatic. Numerous molecular targets have been implicated but the antitumor activity of aspirin cannot be attributed wholly to a single target. It is likely that aspirin influences several molecular pathways and that the nonspecific nature of the effect may be key to cancer prevention. Hence, the complex signaling effects of aspirin that lead to CRC cell death require further elucidation.
Signaling via the serine/threonine kinase mechanistic target of rapamycin (mTOR) controls cell survival and regulation of metabolism.3 mTOR is pivotal in assimilating growth factor, nutrient, and signaling stimuli that regulate protein synthesis and growth.4 mTOR forms the catalytic core of 2 distinct complexes, mTORC1 and mTORC2, both containing mLST8 and DEPTOR proteins. In addition, mTORC1 consists of raptor and PRAS40, whereas mTORC2 includes rictor, mSIN1, and protor. mTORC1 integrates growth factor and nutrient signals to influence protein synthesis, growth, autophagy, and ribosomal biogenesis. The role of mTORC2 is less well defined, involving cell survival and cytoskeleton regulation. Furthermore, mTORC1 regulates mTORC2 through rictor phosphorylation by S6 kinase 1 (S6K1), adding further complexity to mTOR regulation.5,6
Substantial evidence implicates dysregulated phosphoinositide-3-kinase (PI3K)/mTOR signaling in cancer development, including CRC. Mutations in PI3K signaling genes occur in 40% of CRCs.7 Raptor, rictor, and mTOR itself are overexpressed in CRCs.8 The role of mTOR in cancer biology is strengthened by evidence that negative regulators of mTOR are tumor suppressors. PTEN, which down-regulates mTOR, is inactivated in 30%–40% of CRCs.9 Unconstrained mTOR signaling, via effectors S6K1 and 4E-BP1, promotes tumor growth by enhancing translation and protein synthesis. Activation of the adenosine monophosphate–activated protein kinase (AMPK), a critical cellular energy sensor, leads to mTOR suppression. AMPK is activated by liver kinase B1 (LKB1), a tumor-suppressor gene inactivated by germline mutations in Peutz–Jeghers syndrome, a CRC susceptibility disorder.10 LKB1 tumor-suppressor activity is caused partly by AMPK-mediated inhibition of inappropriate mTOR activation.11 Indeed, AMPK activation by pharmacologic activators 5-Aminoimidazole-4-carboxyamide ribonucleoside (AICAR) and metformin inhibits growth in several cancers.12 Furthermore, treatment of tumor-prone PTEN+/− / LKB1 hypomorphic mice with AMPK activators including A-769662, metformin, and phenformin delays tumor onset.13
Clinical trials of mTOR inhibitors have been disappointing, especially for solid tumors. Studies using rapamycin, mainly targeting mTORC1, have highlighted feedback signaling, which counters mTOR inhibition by increasing Akt via S6K/IRS-1.14 Adenosine triphosphate (ATP)-competitive inhibitors targeting both mTORC1 and mTORC2 catalytic sites have been developed, but some increase Akt despite S6K1 inhibition, suggesting that increased Akt signaling as a result of mTORC1 inhibition overwhelms mTORC2 inhibition.15 Hence, the latest generation inhibitors specifically target mTORC2 to avoid feedback caused by mTORC1 inhibition.16 However, the very specificity of such agents may be problematic, whereas drugs targeting several components within the same pathway may circumvent signaling redundancy.17 Furthermore, the long-term safety profile of such drugs is unknown, and so their use for chemoprevention is not appropriate.18
Much available evidence supports AMPK/mTOR signaling as a chemoprevention target. We hypothesize that pathway modulation is a mechanism by which aspirin exerts antitumor effects. Here, we investigate the effects of aspirin on AMPK/mTOR signaling and provide novel insight into the mechanism of action of aspirin as a chemopreventive agent in CRC.
Materials and Methods
Antibodies and Reagents
Details are provided in Supplementary Table 1.
Cell Line Culture and Treatment
CRC cell lines (RKO, SW480, and HCT116) are available from the American Type Culture Collection (Middlesex, UK). Professor Bert Vogelstein kindly provided HCT116 Akt1/Akt2 knockout cells.19 Dr Benoit Viollet kindly provided AMPK α1/α2 knockout mouse embryonic fibroblasts (MEFs).20 Cells grown as monolayers in respective media supplemented with 10% fetal calf serum and 1% penicillin/streptomycin were treated at 60%–70% confluence.
Immunoblotting
Cells were lysed in ice-cold, whole-cell lysis buffer (buffer details are available in the Supplementary Materials and Methods section). For cytoplasmic and nuclear extraction, cells were lysed in cytoplasmic lysis buffer, nuclei pelleted, and lysed in hypotonic buffer (30 minutes). Protein was measured by the Bradford method (Bio-Rad, Hemel Hempstead, UK). Lysates separated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis were transferred to a polyvinylidene difluoride membrane and blocked in 4% nonfat milk with 0.3% Tween20 (Sigma-Aldrich, St. Louis, MO). Antigen-antibody complexes were visualized with chemiluminescence (Amersham, GE Healthcare, Little Chalfont, UK).
AMPK Activity Assay
AMPK was immunoprecipitated from 50 μg lysate with antibodies against AMPK α1 and assayed for phosphotransferase activity toward AMARA peptide using [γ-32P]ATP, as previously described.21
Nucleotide Measurements
Cells were rinsed with ice-cold phosphate-buffered saline (PBS) before addition of 5% perchloric acid to extract nucleotides and centrifuged to remove debris. An equal volume of a 1:1 mix of 1,1,2 trichlorotrifluoroethane and trioctylamine was added to supernatant. Samples were vortexed, centrifuged, and the upper aqueous layer was collected. This organic extraction was repeated twice before separation by capillary electrophoresis with oncolumn isotachophoretic concentration.22
Small Interfering RNA
Two small interfering RNA (siRNA) corresponding to bases 238–256 and 267–285 of human AMPKα1 open reading frame (NCBI Reference Sequence: NM_006251.5): 5′-CCU CAA GCU UUU CAG GCA Utt-3′ and 5′-UUA AAC UGU ACC AGG UCA Utt-3′ were co-transfected to maximize knockdown (Ambion, Austin, TX). Nonspecific siRNA was used as negative control. At 60% confluence cells were transfected in antibiotic-free McCoy’s 5A medium (10% fetal calf serum). Transfection (50 or 100 pmol/mL) was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) and Opti-MEM (Invitrogen). Fresh medium was added 6 hours after transfection. Transfection medium was removed after 48 hours and experiments were performed 16 hours later.
Immunofluorescence
Cells grown to 60%–70% confluence on glass coverslips in 6-well plates were treated with aspirin, metformin, or carrier for 16 hours. After treatment, cells were washed with PBS, fixed with acetone:methanol (−20°C, 10 minutes), and blocked in 10% nonimmune goat serum (Sigma) for 1 hour. LC-3 antibody (Nanotools, Teningen, Germany) was applied (1 h) followed by incubation (1 hr) with Alexa Fluor 594 F(ab′)2 fragment of goat anti-mouse IgG (Invitrogen). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Animal Studies
All animal studies were approved by the University of Dundee Ethics Committee and performed under a UK Home Office project license. Groups of 4 female C57BL/6J mice were given aspirin or phenformin in drinking water (aspirin, 400 mg/kg; phenformin, 300 mg/kg/day). Drugs were dissolved from powder every day into drinking water, taking into account mouse body weight and the volume of water consumed each day. No other water was available, and the volume of water consumed by each mouse per day was noted and used to calculate the dose of drugs required. The mice were maintained under standard husbandry conditions and monitored for body weight and water and food intake (Supplementary Figure 1). The aspirin dose was escalated to 100% by day 5 and phenformin by day 15. At day 21 mice were euthanized and organs were harvested. All animal experiments were performed in accordance with UK Coordinating Committee on Cancer Research (UKCCR) guidelines.23
Patient Studies
Three patients were treated with 600 mg aspirin once daily for 7 days. The exclusion criteria were current/recent ingestion of NSAIDs or any contraindication to NSAIDs. The study had ethical and management approval (local research ethics committee number 99/5/8 and clinical trial authorisation number 17844/0001/001). After informed consent, biopsy samples were taken from normal rectal mucosa before, and at 4 hours, 24 hours, and after 7 days of aspirin treatment.
Results
Aspirin Inhibits mTOR Signaling and Induces AMPK in CRC Cells
We investigated aspirin’s effects on the mTORC1 target proteins S6K1, its substrate S6 ribosomal protein (S6), and 4E-BP1 in 3 CRC cell lines: RKO, SW480, and HCT116. These cell lines represent CRC as a whole and differ in their mutation profile with respect to mTOR pathway genes (Supplementary Table 2).
We used 5 mmol/L aspirin for stimulation having previously observed apoptosis with this concentration in CRC cells.24 There was a striking decrease in S6K1 phosphorylation at 10 minutes and an overall decrease at 16 hours in all CRC cell lines after aspirin (Figure 1A). Aspirin decreased S6 phosphorylation at sites specific to S6K1 at 8 and 16 hours in all cell lines (Figure 1A). Decreased S6K1 phosphorylation at 10 minutes did not translate into an immediate decrease in S6 phosphorylation, suggesting the presence of intermediate steps. We examined aspirin effects on the other well-characterized mTORC1 target 4E-BP1. When phosphorylated by mTORC1, 4E-BP1 dissociates from eIF4E which initiates cap-dependent translation. Hypophosphorylated 4E-BP1 binds eIF4E, thereby inhibiting translation. Aspirin decreased phosphorylation of 4E-BP1 in CRC cells (Figure 1B). These results suggest that aspirin exerts an inhibitory effect on mTORC1 signaling. We confirmed increased expression of phosphorylated proteins of S6K1, S6, and total 4E-BP1 in CRC tissue, compared with matched normal tissue (Supplementary Figure 2).
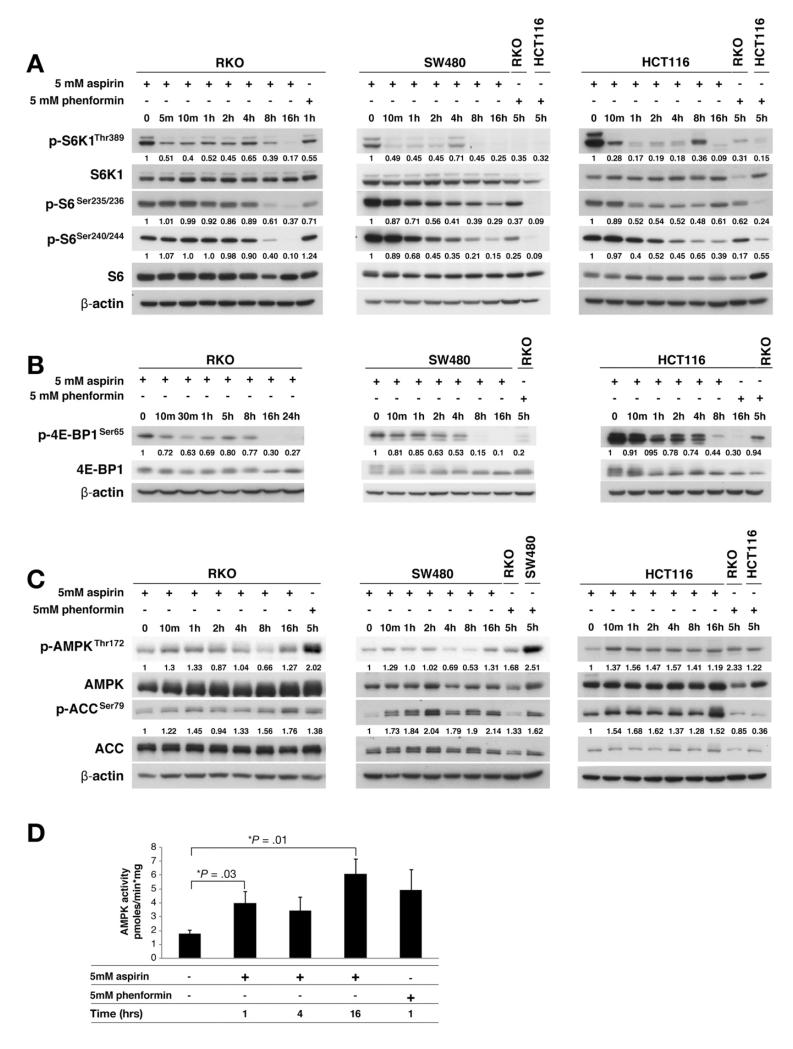
Figure 1.
Aspirin inhibits mTOR and activates AMPK in CRC cells. Aspirin-treated CRC whole-cell lysates were immunoblotted. Aspirin inhibits phosphorylation of (A) S6K1, S6 ribosomal protein (S6), and (B) 4E-BP1 in CRC cells. (C) Aspirin induces phosphorylation of AMPK and its substrate ACC in CRC cells. Imagequant software (GE Healthcare, Little Chalfont, UK) was used for densitometry, which is presented as the ratio of phosphorylated to total protein corrected for actin. Phenformin-treated CRC cells were used as controls. AMPK was immunoprecipitated from aspirin-treated whole-cell lysates and assayed with the AMARA peptide. (D) Aspirin increases AMPK activity in HCT116 cells (mean of 3 independent experiments).
We next examined whether aspirin’s inhibitory effects on mTOR signaling were explained by AMPK activation. Aspirin increased phosphorylation of AMPK at Thr172, which reflects AMPK activity, at 10 minutes in all CRC cell lines (Figure 1C). This generally was sustained for 1–2 hours, with a further increase at 16 hours. Phosphorylation of acetyl-CoA carboxylase (ACC), a well-established AMPK substrate, may be a more accurate representation of AMPK enzyme activity. Enhanced AMPK phosphorylation was paralleled by increased phosphorylation of ACC that was sustained up to 16 hours (Figure 1C). We further confirmed that aspirin stimulates AMPK activity by performing quantitative kinase assays, which reflected the phosphorylation state of AMPK and ACC (Figure 1D). We confirmed that salicylate (aspirin metabolite) induces AMPK and ACC phosphorylation at 1 and 5 hours. There is evidence of decreased S6 phosphorylation at 8 and 16 hours and decreased 4E-BP1 at 16 hours (Supplementary Figure 3).
Nucleotide fluctuation increases the AMP:ATP ratio and activates AMPK, therefore we assessed whether aspirin influences nucleotides in CRC. Consistent with previous data,22 basal AMP levels were below capillary electrophoresis detection limits but AMP was evident in aspirin-treated CRC cells. In line with previous work,22 we used the adenosine diphosphate (ADP):ATP ratio as a surrogate for AMP:ATP variation. If the ADP:ATP ratio increases by 5-fold, the AMP:ATP ratio increases by 25-fold, providing the adenylate kinase reaction is in equilibrium.25 There is a 2.8-fold increase in the ADP:ATP ratio and a 10-fold increase in the derived AMP:ATP ratio after 4 hours of aspirin exposure (Supplementary Table 3). The magnitude of increase in the ADP:ATP ratio with aspirin is similar to that with mitochondrial and glycolytic 2-deoxyglucose (2-DG) inhibitors.22 Taken together with the effects on AMPK and ACC phosphorylation and AMP kinase activity, these results definitively show that aspirin activates AMPK in CRC cells.
Dependency of Aspirin-Mediated mTOR Inhibition on AMPK Activation
To investigate whether aspirin-induced mTOR inhibition is caused by AMPK activation, we aimed to abrogate the aspirin-induced AMPK response in CRC cells using siRNA to silence the AMPKα catalytic subunits. Given AMPKα1 was the predominant isoform in RKO cells (Figure 2A), transfection was performed with 2 siRNAs to AMPKα1 that knockdown both AMPK and ACC.26 Although siRNA inhibition of AMPKα1 reduced both AMPK and ACC phosphorylation in response to aspirin, this did not attenuate aspirin-induced inhibition of S6K1 and S6 phosphorylation (Figure 2B). However, total AMPK was not completely silenced, raising the possibility of residual kinase activity. The response to AMP is finely tuned and small increases in AMP lead to large changes in AMPK signaling. Nonetheless, these findings suggest that attenuating aspirin-induced AMPK activation does not exert equivalent abrogation of aspirin’s inhibitory effects on mTOR signaling.
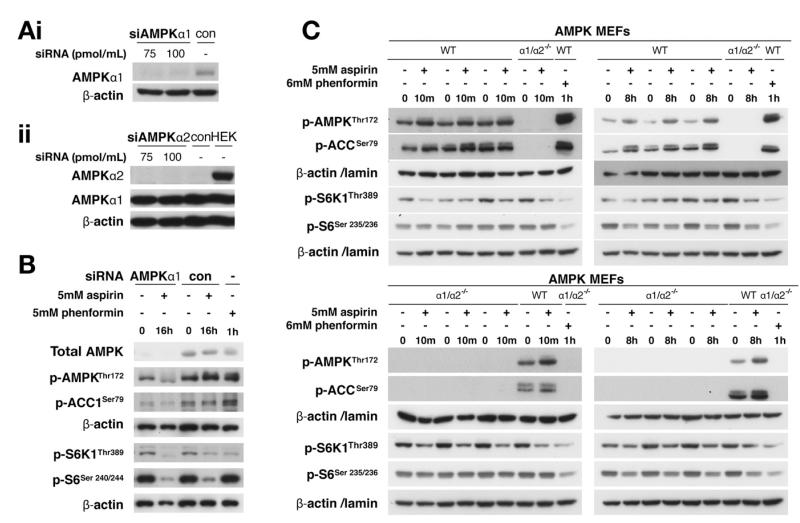
Figure 2.
AMPK depletion, siRNA-mediated or genetic, does not attenuate aspirin-induced mTOR inhibition. RKO cells, transfected with siRNA against (Ai) AMPK α1, (Aii) α2, or control (con) for 48 hours, were probed as noted. (B) Lysates from aspirin-treated RKO cells transfected with AMPKα1 siRNA were immunoblotted. (C) Lysates from aspirin-treated AMPKα1/α2−/− knockout MEFs and parental cells (wild type [WT]) were immunoblotted.
To further explore the dependency of aspirin-induced mTOR inhibition on AMPK activation, we used AMPK MEFs with both catalytic subunits genetically deleted. Notably, the cellular energy status is not affected in AMPK knockout compared with wild-type MEFs.27 Similar to CRC cells, aspirin increased AMPK and ACC phosphorylation in parental MEFs (AMPKα1/α2+/+), although there were no detectable signals in AMPKα1/α2−/− knockout MEFs (Figure 2C and Supplementary Figure 4). However, aspirin decreased both S6K1 and S6 phosphorylation in parental and AMPKα1/α2−/− MEFs (Figure 2C and Supplementary Figure 4). Together with siRNA results, these findings indicate that aspirin may induce mTOR inhibition through both AMPK-dependent and AMPK-independent mechanisms.
Influence of Akt on AMPK Activation and mTOR Inhibition and Effects on mTORC2
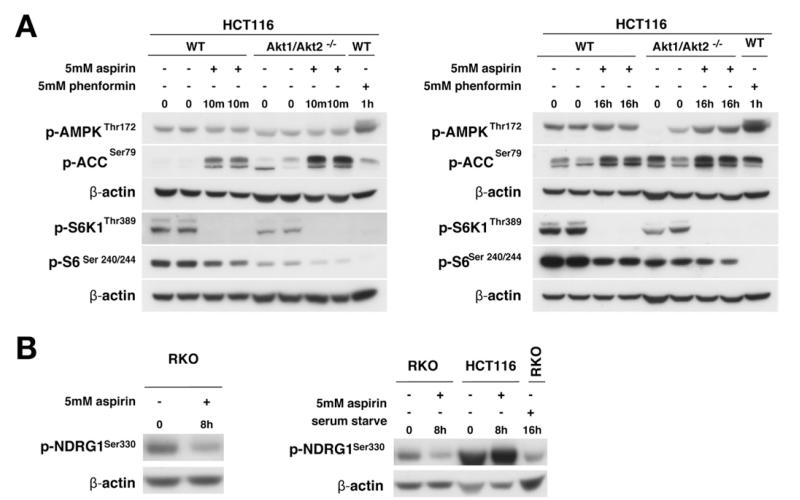
Given that Akt may affect both AMPK and mTOR, we investigated whether Akt signaling influences aspirin-induced AMPK activation and mTOR inhibition using cells with AKT1 and 2 deleted (HCT116 Akt1/2−/−).19 Akt expression was confirmed (Supplementary Figure 5). Aspirin increased AMPK and ACC phosphorylation in both parental and HCT116 Akt1/2−/− cells (Figure 3A). Indeed, the effect on AMPK/ACC is greater in the absence of Akt. We next examined whether Akt influenced aspirin-mediated effects on mTOR signaling. Although there was less phosphorylated S6K1 in untreated HCT116 Akt1/2−/− cells compared with parental cells, aspirin decreased S6K1 and S6 phosphorylation in both cell lines at 10 minutes and 16 hours (Figure 3A). These results indicate that aspirin-induced AMPK activation and mTOR inhibition are not secondary to Akt signaling. Phosphorylation of the SGK1 substrate, NDRG1, is a robust marker of mTORC2. Aspirin decreased NDRG1 phosphorylation in RKO cells but not in HCT116 cells (Figure 3B). Further experimentation is required to establish whether the effects of aspirin on mTORC2 are cell-type specific.
Figure 3.
Influence of Akt on AMPK activation and mTOR inhibition and effects on mTORC2. Whole-cell lysates from (Ai) 10-minute or (Aii) 16-hour aspirintreated HCT116 Akt1/2 knockout and parental (wild type [WT]) cells immunoblotted (duplicate experiments presented). (B) Aspirin-treated CRC cells immunoblotted for p-NDRG1.
Aspirin Combined With Metformin Enhances AMPK Activation and mTOR Inhibition
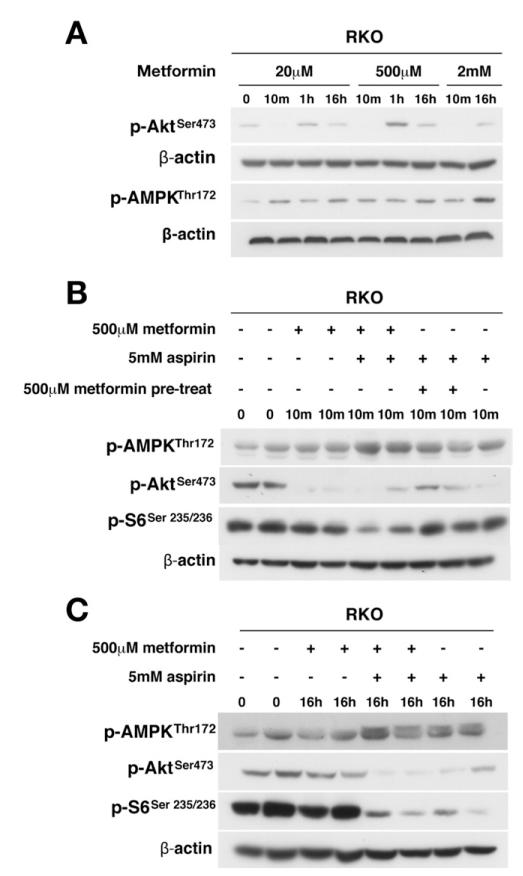
Results thus far establish that aspirin acts on AMPK and mTOR, both key regulators of cellular energy and metabolism. We next investigated whether aspirin combined with a known AMPK activator would have an additive effect on mTOR inhibition because aspirin effects may not saturate the potential AMPK response. Metformin, a known AMPK activator, inhibits Akt28 and this was confirmed in RKO cells (Figure 4A). Aspirin and metformin combination treatment resulted in greater AMPK activation than either agent alone after 10 minutes, and activation was attenuated only marginally at 16 hours (Figure 4B and C). AMPK activation was paralleled by a marked decrease in Akt phosphorylation at 10 minutes, remaining detectable at 16 hours. Neither agent alone decreased S6 phosphorylation, examined as an end point of mTOR signaling, at 10 minutes, but there was a substantial decrease with combination treatment, which was sustained at 16 hours (Figure 4B and C). These results show that the combination of aspirin and metformin has a striking additive effect on AMPK activation and mTOR inhibition.
Figure 4.
Dual targeting of AMPK, Akt, and mTOR signaling with aspirin and metformin. (A) Metformin decreases Akt phosphorylation and increases AMPK phosphorylation in RKO cells. RKO cells were treated with metformin alone, metformin and aspirin, aspirin after pretreatment with metformin (10 minutes), or aspirin alone at concentrations indicated for (B) 10 minutes or (C) 16 hours. Representative results are presented in duplicate.
Aspirin Induces Autophagy in CRC Cells
Having established that aspirin modulates mTOR signaling in CRC cells through composite effects on pathway components, we explored resultant cell biological outcomes. Aspirin inhibits cell proliferation and induces apoptosis.24,29 As expected, aspirin increased cleaved caspase-3 and reduced proliferating cell nuclear antigen (PCNA) levels in CRC cells (Figure 5A and B), consistent with apoptosis and inhibition of proliferation. We also examined the RNA binding protein human antigen R (HuR) given its relevance to CRC cell proliferation. HuR cellular localization determines its ability to influence messenger RNA (mRNA) stability by binding adenylateuridylate–rich elements of labile mRNAs. HuR is located in nuclei of unstimulated cells and mRNA-stabilizing properties rely on cytoplasmic translocation. AMPK decreases cytoplasmic HuR and binding to target transcripts30 and HuR regulates stability of cyclins.31 Aspirin decreased cytoplasmic HuR and cyclin A in CRC cells (Figure 5B). Taken together these results confirm that aspirin inhibits proliferation and induces apoptosis.
Figure 5.
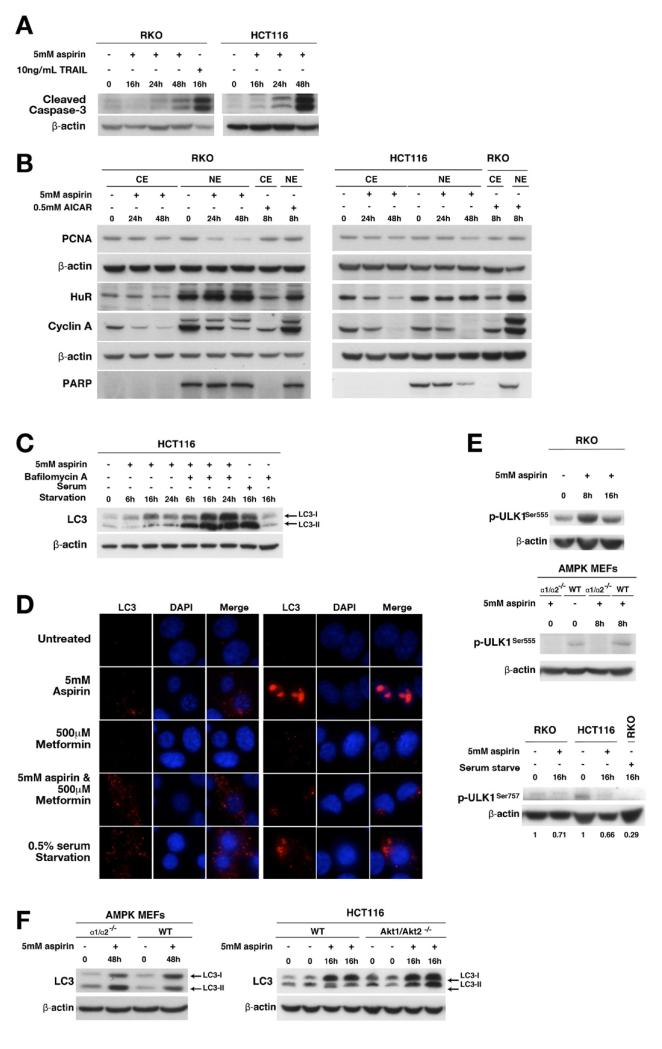
Aspirin induces apoptosis, inhibition of cell proliferation, and autophagy in CRC cells. Aspirin- or TNF-related apoptosis-inducing ligand (TRAIL) (10 ng/mL) treated CRC cells were lysed and whole-cell, cytoplasmic (CE), or nuclear (NE) extracts were immunoblotted. (A) Aspirin increases cleaved caspase-3 in CRC cells. (B) Aspirin decreases proliferating cell nuclear antigen (PCNA) in both cytoplasmic and nuclear CRC cell extracts. Aspirin decreases cytoplasmic HuR and cyclin A in CRC cells. (C) Aspirin increases LC3-II in HCT116 cells pretreated with bafilomycin A. (D) Increased endogenous LC3 staining in aspirin-, metformin-, or combination-treated RKO cells confirms autophagy at 16 hours. Aspirin induces ULK1 Ser555 phosphorylation in RKO cells but not in AMPKα1/α2−/− knockout MEFs. DAPI, 4′,6-diamidino-2-phenylindole. (E) Aspirin decreases ULK1 Ser757 phosphorylation in HCT and RKO cells. (F) Aspirin increases LC3-II in both AMPKα1/α2−/− MEFs and HCT116 Akt1/2 knockout and respective parental cells.
mTOR negatively regulates autophagy and therefore we assessed aspirin’s effects on autophagy. LC3 is a commonly used autophagy marker and its processed form, LC3-I, resides in cytoplasm. After autophagy induction, LC3-II, the conjugated form of LC3, associates with autophagosomes. However, an increase in autophagosomes alone, suggested by increased LC3-II, does not necessarily indicate increased autophagy.32 Increases in LC3-II after pretreatment with a lysosomal inhibitor, such as bafilomycin A, signify a true increase in autophagic flux. Aspirin increased LC3-II in HCT116 cells, which is increased further with bafilomycin A pretreatment, suggesting induction of autophagy (Figure 5C). Immunofluorescence confirmed increased LC3 detection after aspirin alone and in combination with metformin (Figure 5D). AMPK phosphorylates ULK1, the mammalian homologue of Atg1, which initiates autophagy.33,34 We found that aspirin induces ULK1 phosphorylation at Ser555 in RKO cells (Figure 5E). Aspirin-induced ULK1 phosphorylation was abrogated in AMPKα1/α2−/− MEFs, indicating AMPK dependency (Figure 5E). Aspirin decreases phosphorylation of ULK at serine 757, suggesting inhibition of mTOR also may contribute to autophagy induction in CRC cells (Figure 5E). However, aspirin induced autophagy, evidenced by increased LC3, in AMPKα1/α2−/− MEFs, indicating an AMPK-independent contribution (Figure 5F). Notably, aspirin also induces autophagy in HCT116 Akt1/2−/− cells (Figure 5F). These results show that aspirin induces autophagy in CRC cells, likely through both direct AMPK-mediated ULK1 phosphorylation and by inhibiting mTOR signaling.
Aspirin Affects AMPK and mTOR Signaling In Vivo
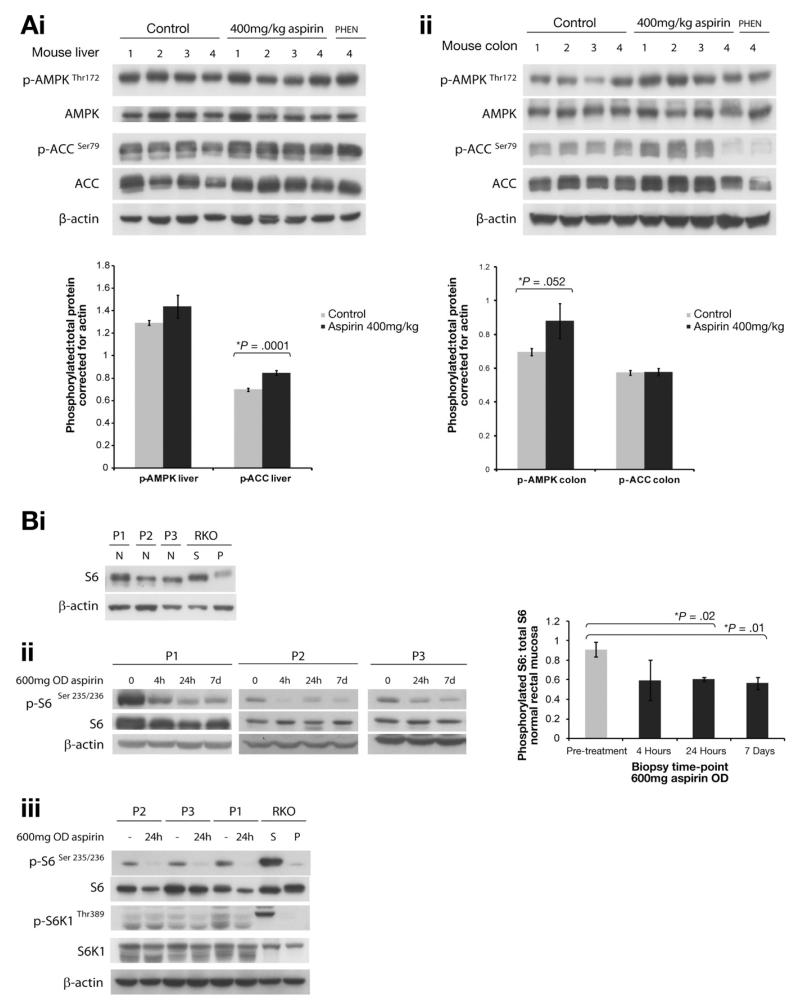
We performed a short-term experiment over 21 days in control mice to investigate whether aspirin induces AMPK activation in vivo. We found evidence of both AMPK and ACC phosphorylation in livers of aspirin-treated mice (Figure 6Ai). Aspirin increased AMPK phosphorylation in the colon of treated mice. Increased ACC phosphorylation was detectable in 3 of 4 mouse colons.
Figure 6.
Aspirin activates AMPK and inhibits mTOR in vivo. Aspirin activates AMPK and ACC phosphorylation in (Ai) liver and (Aii) colon from mice treated with aspirin or phenformin for 21 days (aspirin, 400 mg/kg; phenformin [PHEN], 300 mg/kg body weight). (Bi) Basal untreated total S6 levels in normal rectal mucosa of 3 patients. (Bii & Biii) Aspirin-treated patients (600 mg once-daily [OD], 7 days) show decreased S6 and S6K1 phosphorylation in normal rectal mucosa. Respective graphs show mean densitometry values of phosphorylated:total protein, corrected for actin ± standard error. P, 5mM phenformin; S, serum shock.
We also undertook a short-term biological-response study in normal rectal mucosa of patients treated with aspirin. S6 was the most robust marker of mTOR inhibition with some variation in basal levels in untreated patients (Figure 6Bi). Three patients were given 600 mg aspirin orally once daily for 7 days. Normal rectal mucosa was biopsied before treatment and at 4 hours, 24 hours, and 7 days. We found that aspirin decreases S6 phosphorylation in normal rectal mucosa (Figure 6Bii) and there is some decrease in phosphorylation of S6K1 (Figure 6Biii). These results suggest that aspirin when ingested orally can modulate effectors of mTOR in vivo.
Discussion
Here, we show that aspirin inhibits mTOR signaling in CRC cells, as evidenced by inhibition of phosphorylation of S6K1, 4E-BP1, and S6. We show that aspirin activates AMPK in CRC cells. Furthermore, we show that aspirin induces autophagy in CRC cells, a response characteristic of mTOR inhibition. Our results support the concept that aspirin affects multiple components of the AMPK/mTOR signaling pathway.
mTORC1 plays a key role in protein synthesis regulation via its effectors S6K1 and 4E-BP1. Constitutively activated mTOR signaling has been shown previously in CRC. Indeed, several ribosomal proteins are up-regulated in CRC, including the S6K1 target S6.35 Targeted mTOR inhibition decreases adenoma formation in a mouse familial adenomatous polyposis model36 and also inhibits CRC cell growth. Our results show (to our knowledge, for the first time), in CRC cells, that aspirin inhibits the downstream effectors of mTORC1: S6K1, 4E-BP1, and S6. These results are consistent with microarray data showing that aspirin induces the greatest changes in ribosome biogenesis genes.37 S6K1 deletion in mice results in defective ribosomal biogenesis and disruption of a single ribosomal protein shuts down ribosomal synthesis.38 Given the striking decrease in S6K1, it will be important to evaluate whether aspirin affects ribosomal synthesis both in normal colon and in CRCs from both chemopreventive and adjuvant perspectives.
It was important to determine whether aspirin-mediated mTOR inhibition was related to the upstream kinase AMPK. AMPK can inhibit mTORC1 through phosphorylation of TSC2, which enhances GAP activity toward Rheb, or via a TSC2-independent mechanism by direct phosphorylation of raptor, which induces 14-3-3 binding to raptor.39,40 Treatment with known AMPK activators leads to mTOR inhibition and decreased growth in CRC cells and mouse adenoma models.13,41 Our results show (to our knowledge, for the first time) that aspirin activates AMPK in CRC cells, confirmed by kinase assays and demonstration of ACC phosphorylation, supporting the perception that AMPK has a tumor-suppressor role.12 Furthermore, phosphorylated AMPK expression has been shown to be associated with improved survival in a subset of CRCs.42
Our data from siRNA experiments in CRC cells and AMPK MEFs indicate that aspirin-induced AMPK activation is not the sole determinant of observed mTOR inhibition. Although the effects of aspirin on AMPK/mTOR signaling in MEFs may not be representative of all somatic mutations arising in human CRCs, these experiments provide a model for so called normal cells representing normal colonic epithelium, which is relevant to chemoprevention. Our data are consistent with mTOR effects being AMPK-independent but we cannot exclude an AMPK-dependent contribution.
Recently, it was shown that AMPK is not required for mTORC1 inhibition after glycolytic blockage by energy-depleting agents (2DG) or known AMPK activators.27 An alternative mechanism of mTORC1 inhibition via RAG guanosine triphosphatase inhibition is suggested. Indeed, aspirin may mediate mTORC1 inhibition via similar effects on RAG function. Combination treatment with 2-DG and metformin led to apoptosis in prostate cancer cells via ATP depletion and AMPK activation.43 Similarly, aspirin may induce changes in ATP with ensuing alterations in AMP:ATP ratios, and/or inhibition of the mitochondrial chain complex. Both aspirin and salicylate have been shown to uncouple mitochondrial oxidative phosphorylation. The results presented here show that aspirin increases the ADP:ATP ratio, an established surrogate for the AMP:ATP ratio. It is clear that the upstream mechanisms underlying aspirin-induced AMPK activation merit further investigation.
We have shown that aspirin activates AMPK and inhibits mTOR signaling in CRC cells. The key question is where the balance lies in terms of mTOR inhibition and cellular response to aspirin. Here, we show in CRC cells that aspirin induces a cellular phenotype characteristic of mTOR inhibition, namely autophagy. During autophagy lysosomes digest their own cytoplasmic organelles to generate energy.44 Substantial evidence indicates that AMPK/mTOR signaling regulates autophagy.45 Some previous reports have suggested that some NSAIDs induce autophagy.46,47 We show that aspirin does induce autophagy, likely through AMPK phosphorylation of ULK1 and also an AMPK-independent mechanism of mTOR inhibition. That aspirin induces autophagy in AMPKα1/α2−/− MEFs strengthens the probability of AMPK-independent input. Concerns with mTOR inhibition are the potential for feedback-initiated Akt activation. Our results suggest that the predominant aspirin-induced cellular response is one of mTOR inhibition (autophagy) rather than Akt activation (cellular proliferation and inhibition of apoptosis). Signaling between mTOR and Akt appears to exist in balance and inter-regulatory pathways likely have evolved to restrain hyperactivation of both.48 Indeed, we show the added value, in terms of both mTOR and Akt inhibition, of combining aspirin with metformin.
Combination treatment is a particularly attractive strategy to combat the metabolic syndrome, characterized by hyperinsulinemia, insulin resistance, obesity, type 2 diabetes, and hypertension. There is a strong association between the metabolic syndrome and colorectal neoplasia.49 Furthermore, metabolic syndrome may adversely affect the propensity of CRC to metastasize and relapse, impacting survival.50 Considerable evidence indicates that physical inactivity is associated with increased cancer risk.51 Because exercise activates AMPK, we speculate that AMPK and mTOR may be linked mechanistically to the cancer-protection effects of exercise. Indeed, the absence of S6K1 protects mice from both age- and diet-related obesity and enhances insulin sensitivity.52 As master regulators of cellular energy and insulin signaling, both AMPK and mTOR highlight the association between the metabolic syndrome and CRC, and present ideal targets for intervention.
A small-molecule approach directed at a single target to effect cancer cure remains elusive, and may even activate signaling detrimentally through normally redundant pathways. It is recognized that mutations in genes encoding PI3K/mTOR and RAS pathways in CRC cell lines influence response and combined inhibition is required to inactivate mTOR.53 Thus, development of several agents, each targeting different signaling switches, may have greater efficacy with reduced side effects. We have shown that aspirin targets the AMPK/mTOR signaling pathway at several levels in CRC cells, thus gaining new understanding of the molecular mechanisms underlying the antitumor activity of aspirin (Figure 7). Furthermore, we have shown that metformin may be used in a concerted manner to inhibit the mTOR pathway in CRC.
Figure 7.
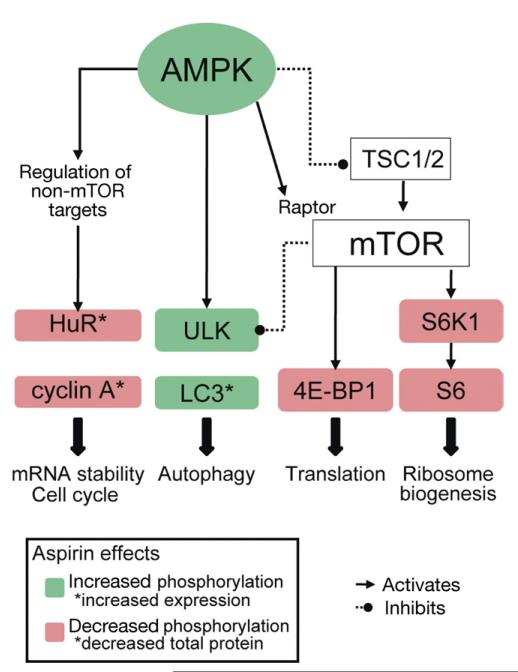
Aspirin targets multiple components of the AMPK/mTOR signaling pathway in colorectal cancer. Aspirin increases phosphorylation of AMPK and ULK1. Aspirin inhibits mTOR signaling as evidenced by decreased phosphorylation of S6K1, S6, and 4E-BP1. Hence, aspirin is targeting multiple cellular pathways involved in mRNA stability, cell cycle, autophagy, protein translation, and ribosome biogenesis.
Supplementary Material
Acknowledgments
The authors thank Professor Grahame Hardie (Dundee), who kindly provided the AMPKα1 antibody, discussion, and expertise; Dr Kevin Green for performing nucleotide assays; Dr Beniot Viollet (INSERM), who kindly provided AMPKα1/α2−/− knockout mouse embryonic fibroblasts; Professor Bert Vogelstein (Johns Hopkins) for providing HCT116 Akt1/Akt2 knockout cells; Dr Masashi and Masako Narita (Cambridge) for advice about endogenous LC-3 staining; and Professor Nick Hastie (MRC HGU, Edinburgh), who provided invaluable critical manuscript review.
Funding This work was funded by a clinician scientist fellowship to FVND from Cancer Research UK (C26031/A11378) and additional funding from a Centre grant to MGD from CORE Charity.
Abbreviations used in this paper
- ACC
acetyl-CoA carboxylase
- ADP
adenosine diphosphate
- AMPK
adenosine monophosphate–activated protein kinase
- ATP
adenosine triphosphate
- CRC
colorectal cancer
- eIF4E
eukaryotic translation initiation factor 4E
- 4E-BP1
4E, binding protein 1
- HuR
human antigen R
- LC3
light chain 3
- MEF
mouse embryonic fibroblast
- mRNA
messenger RNA
- mTOR
mechanistic target of rapamycin
- NDRG1
N-myc downstream regulated 1
- NSAID
nonsteroidal anti-inflammatory drug
- PBS
phosphate-buffered saline
- PI3K
phosphoinositide-3-kinase
- siRNA
small interfering RNA
- S6
S6 ribosomal protein
- S6K1
S6 kinase 1
Footnotes
Supplementary Material Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2012.02.050.
Conflicts of interest The authors disclose no conflicts.
References
- 1.Din FV, Theodoratou E, Farrington SM, et al. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut. 2010;59:1670–1679. doi: 10.1136/gut.2009.203000. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM, Wilson M, Elwin C-E, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 3.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29:5657–5670. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treins C, Warne PH, Magnuson MA, et al. Rictor is a novel target of p70 S6 kinase-1. Oncogene. 2010;29:1003–1016. doi: 10.1038/onc.2009.401. [DOI] [PubMed] [Google Scholar]
- 7.Parsons DW, Wang TL, Samuels Y, et al. Colorectal cancer: mutations in a signalling pathway. Nature. 2005;436:792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- 8.Johnson SM, Gulhati P, Rampy BA, et al. Novel expression patterns of PI3K/Akt/mTOR signaling pathway components in colorectal cancer. J Am Coll Surg. 2010;210:767–776. 776–778. doi: 10.1016/j.jamcollsurg.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naguib A, Cooke J, Happerfield L, et al. Alterations in PTEN and PIK3CA in colorectal cancers in the EPIC Norfolk study: associations with clinicopathological and dietary factors. BMC Cancer. 2011;11:123. doi: 10.1186/1471-2407-11-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 11.Shaw RJ, Bardeesy N, Manning BD, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Fogarty S, Hardie DG. Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta. 2010;1804:581–591. doi: 10.1016/j.bbapap.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Wullschleger S, Shapiro N, et al. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412:211–221. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- 14.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 16.Sparks CA, Guertin DA. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010;29:3733–3744. doi: 10.1038/onc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keith CT, Borisy AA, Stockwell BR. Multicomponent therapeutics for networked systems. Nat Rev Drug Discov. 2005;4:71–78. doi: 10.1038/nrd1609. [DOI] [PubMed] [Google Scholar]
- 18.Sankhala K, Mita A, Kelly K, et al. The emerging safety profile of mTOR inhibitors, a novel class of anticancer agents. Target Oncol. 2009;4:135–142. doi: 10.1007/s11523-009-0107-z. [DOI] [PubMed] [Google Scholar]
- 19.Ericson K, Gan C, Cheong I, et al. Genetic inactivation of AKT1, AKT2, and PDPK1 in human colorectal cancer cells clarifies their roles in tumor growth regulation. Proc Natl Acad Sci U S A. 2010;107:2598–2603. doi: 10.1073/pnas.0914018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laderoute KR, Amin K, Calaoagan JM, et al. 5′-AMP-activated protein kinase (AMPK) is induced by low-oxygen and glucose deprivation conditions found in solid-tumor microenvironments. Mol Cell Biol. 2006;26:5336–5347. doi: 10.1128/MCB.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamoto K, Zarrinpashneh E, Budas GR, et al. Deficiency of LKB1 in heart prevents ischemia-mediated activation of AMPKα2 but not AMPKα1. Am J Physiol Endocrinol Metab. 2006;290:E780–E788. doi: 10.1152/ajpendo.00443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawley SA, Ross FA, Chevtzoff C, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Workman P, Aboagye EO, Balkwill F, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102:1555–1577. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Din FV, Dunlop MG, Stark LA. Evidence for colorectal cancer cell specificity of aspirin effects on NF kappa B signalling and apoptosis. Br J Cancer. 2004;91:381–388. doi: 10.1038/sj.bjc.6601913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23:1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- 26.Levine YC, Li GK, Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK -> Rac1 -> Akt -> endothelial nitric-oxide synthase pathway. J Biol Chem. 2007;282:20351–20364. doi: 10.1074/jbc.M702182200. [DOI] [PubMed] [Google Scholar]
- 27.Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zakikhani M, Blouin MJ, Piura E, et al. Metformin and rapamycin have distinct effects on the AKT pathway and proliferation in breast cancer cells. Breast Cancer Res Treat. 2010;123:271–279. doi: 10.1007/s10549-010-0763-9. [DOI] [PubMed] [Google Scholar]
- 29.Shiff SJ, Koutsos MI, Qiao L, et al. Nonsteroidal antiinflammatory drugs inhibit the proliferation of colon adenocarcinoma cells: effects on cell cycle and apoptosis. Exp Cell Res. 1996;222:179–188. doi: 10.1006/excr.1996.0023. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, Fan J, Yang X, et al. AMP-activated kinase regulates cytoplasmic HuR. Mol Cell Biol. 2002;22:3425–3436. doi: 10.1128/MCB.22.10.3425-3436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, Caldwell MC, Lin S, et al. HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J. 2000;19:2340–2350. doi: 10.1093/emboj/19.10.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egan DF, Shackelford DB, Mihaylova MM, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2010;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai MD, Xu J. Ribosomal proteins and colorectal cancer. Curr Genomics. 2007;8:43–49. doi: 10.2174/138920207780076938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujishita T, Aoki K, Lane HA, et al. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proc Natl Acad Sci U S A. 2008;105:13544–13549. doi: 10.1073/pnas.0800041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin H, Xu H, Zhao Y, et al. Cyclooxygenase-independent effects of aspirin on HT-29 human colon cancer cells, revealed by oligonucleotide microarrays. Biotechnol Lett. 2006;28:1263–1270. doi: 10.1007/s10529-006-9084-9. [DOI] [PubMed] [Google Scholar]
- 38.Volarevic S, Stewart MJ, Ledermann B, et al. Proliferation, but not growth, blocked by conditional deletion of 40S ribosomal protein S6. Science. 2000;288:2045–2047. doi: 10.1126/science.288.5473.2045. [DOI] [PubMed] [Google Scholar]
- 39.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 40.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hosono K, Endo H, Takahashi H, et al. Metformin suppresses colorectal aberrant crypt foci in a short-term clinical trial. Cancer Prev Res (Phila) 2010;3:1077–1083. doi: 10.1158/1940-6207.CAPR-10-0186. [DOI] [PubMed] [Google Scholar]
- 42.Baba Y, Nosho K, Shima K, et al. Prognostic significance of AMP-activated protein kinase expression and modifying effect of MAPK3/1 in colorectal cancer. Br J Cancer. 2010;103:1025–1033. doi: 10.1038/sj.bjc.6605846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben Sahra I, Laurent K, Giuliano S, et al. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res. 2010;70:2465–2475. doi: 10.1158/0008-5472.CAN-09-2782. [DOI] [PubMed] [Google Scholar]
- 44.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu WK, Sung JJ, Wu YC, et al. Inhibition of cyclooxygenase-1 lowers proliferation and induces macroautophagy in colon cancer cells. Biochem Biophys Res Commun. 2009;382:79–84. doi: 10.1016/j.bbrc.2009.02.140. [DOI] [PubMed] [Google Scholar]
- 47.Huang S, Sinicrope FA. Celecoxib-induced apoptosis is enhanced by ABT-737 and by inhibition of autophagy in human colorectal cancer cells. Autophagy. 2010;6:256–269. doi: 10.4161/auto.6.2.11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Pelucchi C, Negri E, Talamini R, et al. Metabolic syndrome is associated with colorectal cancer in men. Eur J Cancer. 2010;46:1866–1872. doi: 10.1016/j.ejca.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Shen Z, Ye Y, Bin L, et al. Metabolic syndrome is an important factor for the evolution of prognosis of colorectal cancer: survival, recurrence, and liver metastasis. Am J Surg. 2010;200:59–63. doi: 10.1016/j.amjsurg.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Wallace K, Baron JA, Karagas MR, et al. The association of physical activity and body mass index with the risk of large bowel polyps. Cancer Epidemiol Biomarkers Prev. 2005;14:2082–2086. doi: 10.1158/1055-9965.EPI-04-0757. [DOI] [PubMed] [Google Scholar]
- 52.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 53.She QB, Halilovic E, Ye Q, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell. 2010;18:39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.