Abstract
Pattern separation plays an important role in perception and memory. In olfaction, pattern separation is critical component of piriform cortical odor processing contributing to behavioral perception of overlapping odor mixtures. Previous work has demonstrated that odor discrimination ability is modulated by acetylcholine. Here, we extended this previous work by using a distinct, well characterized complex odor stimulus set that has been shown to differentially involve pattern separation processes within piriform cortex. We find that the cholinergic muscarinic receptor agonist oxotremorine facilitates the acquisition of odor discrimination. Furthermore, the muscarinic receptor antagonist scopolamine impairs acquisition of odor discrimination even if the antagonist is limited to the piriform cortex. Finally, acetylcholine effects are most robust during discrimination acquisition, with minimal effects during expression.
Keywords: Odor discrimination, pattern separation, acetylcholine, piriform cortex, odor memory, perceptual learning
INTRODUCTION
Pattern separation plays an important role in both perception and memory [19, 24]. Pattern separation allows discrimination of overlapping patterns of activity, resulting in unique representations of very similar stimuli or memories. Pattern separation can be disrupted in aging and dementia, contributing to impairment in memory and cognition [7, 27]. The result is a shift toward pattern completion, resulting in disrupted storage of discrete memories and enhanced proactive interference from pre-existing memories [7, 11, 27]. Given the importance of acetylcholine in modulating the balance of pattern separation and completion [11], impairments may be mediated in part by changes in cholinergic modulation.
Pattern separation and completion also play important roles in piriform cortical odor processing and behavioral perception of overlapping odor mixtures. For example, cortical pattern separation promotes behavioral discrimination of even highly overlapping mixtures, while cortical pattern completion promotes perceptual stability of familiar mixtures despite slight variation in component make-up [5, 8]. Training can shift the threshold between separation and completion, either enhancing perceptual acuity or enhancing generalization depending on task demands [8, 10]. These conditioning-induced changes are associated with robust changes in piriform cortical single-unit receptive fields and in single-unit ensemble decorrelation of the overlapping mixtures [8, 10]. Many studies have demonstrated that acetylcholine modulates odor discrimination learning [11, 14, 21, 23], and have identified both the olfactory bulb [9, 12, 14, 23] and piriform cortex [17, 26] as important cholinergic targets in these effects. In the piriform cortex, acetylcholine arising from the horizontal limb of the diagonal band selectively modulates intra-cortical association fiber synapses and pyramidal cell excitability [3]. Furthermore, acetylcholine modulates plasticity of intra-cortical association fiber synapses which is hypothesized to underlie the binding of distributed piriform cortical neurons responding to an odor, and thus promotes learning of input patterns [16].
In the present work we focused on examining the role of acetylcholine in learned perceptual odor discrimination using well characterized [5, 8, 10] odor mixtures. The mixtures can be morphed in ways that either challenge cortical pattern separation, and thus are difficult to discriminate, or that promote pattern separation and are relatively easy to behaviorally discriminate [5, 8]. The results demonstrate that the cholinergic muscarinic receptor agonist oxotremorine facilitates the acquisition of fine odor discrimination, presumably through enhancement of cortical pattern separation. Furthermore, the muscarinic receptor antagonist scopolamine impairs acquisition of odor discrimination even if the antagonist is limited to the piriform cortex. Finally, the role of acetylcholine is most robust during acquisition of discrimination, with minimal effects during expression. The results may have relevance for understanding impairment in odor discrimination in disorders impacting cholinergic release, such as Alzheimer’s Disease.
MATERIAL AND METHODS
Experiments were approved by the Institutional Animal Care and Use Committees at the Nathan Kline Institute and the New York University Medical School. Male Long-Evans hooded rats (n=38) obtained from Charles River Laboratories (~200g at arrival) were used. The behavioral procedure has been detailed previously [5, 8]. Animals were given limited access to water during behavioral training. Odor discrimination ability was assessed in a two-alternative, Go-Left, Go-Right odor discrimination task for water reward. Animals were trained in sessions of 30 min, 5 days/week. The behavioral apparatus was a Plexiglas box (30x30x40 cm) with a central odor port and two water ports on the left and right walls. Rats initiated a trial by poking their nose in the odor port (at least 350 ms) then, depending on odor identity, made a choice of a left or right reward port to initiate water delivery. All the animals first learned to perform the task with an easily discriminable pair of odors (vanilla vs. peppermint) until criterion (performance >80%) was attained during three successive sessions. Animals reached this criterion in (mean ± SD) 14±3 days. During the testing sessions, animals were presented with complex, 10 component, odorant mixtures (see Barnes et al. (2008) for the detail of the mixture’s preparation). The full 10 component mixture (10c) included the following monomolecular odorants: isoamyl acetate, nonane, ethyl valerate, 5-methyl-2-hexanone, isopropylbenzene, 1-pentanol, 1,7-octadiene, 2-heptanone, heptanal, 4-methyl-3-penten-2-one. Odorants were diluted in mineral oil to obtain a concentration for each component of 100 PPM, based on vapor pressure. The 10c mixture was manipulated to produce two similar stimuli; the first one was the 10c original mix with one component (isoamyl acetate) missing (10c-1). The second one was the 10c original mix with one component (isoamyl acetate) replaced by a novel component (2-methyl-2-buten-1-ol) (10cR1). Previous work has demonstrated that learning to discriminate 10c from 10c-1 is significantly more difficult than learning to discriminate 10c from 10cR1 with these odorant configurations [8, 22]. Furthermore, the difficult training induces changes in single-unit, ensemble and local field potential responses in the anterior piriform cortex that do not emerge after the simpler 10c vs 10cR1 training [8]. Based on piriform cortical physiology, the difficulty of discriminating 10c from 10c-1 is due to a pattern completion process, which must be shifted to pattern separation through training [8].
During the mixture discrimination training rats were either directly treated by systemic drug injections (Figs. 1 and 2) or received intra-piriform cortical injections of the drugs (Fig. 3). For the systemic manipulation of ACh, all drugs (obtained from Sigma-Aldrich) were dissolved in sterile physiological saline and injected into the intraperitoneal cavity at a volume of 1 ml/kg. The doses were selected based on the ability of scopolamine to impair olfactory learning and discrimination [14, 23] and of oxotremorine to improve memory performance in various tasks [2].
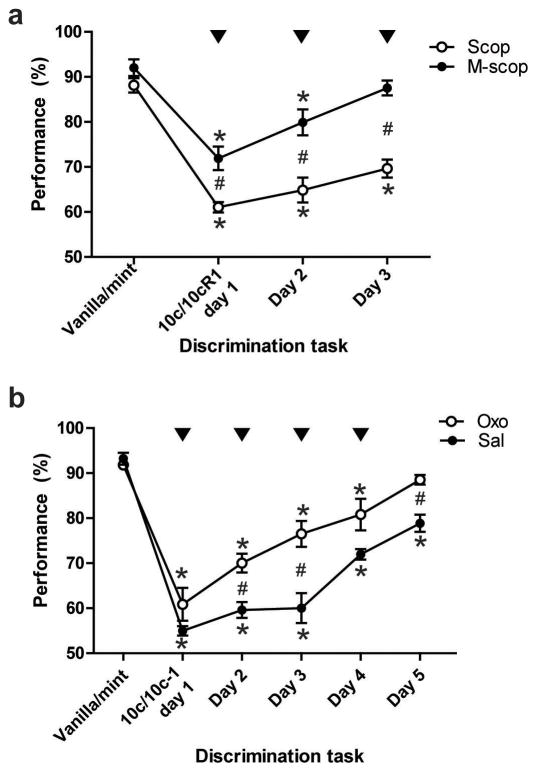
Figure 1.
a. Systemic blockade of cholinergic muscarinic transmission impairs acquisition of a simple odor mixture pattern separation task. Arrowheads indicate daily systemic injections of Scopolamine (Scop) or Methyl-scopolamine (M-scop, control) (0.5 mg/kg) 20 min prior the mixtures discrimination task. b. Systemic enhancement of muscarinic neurotransmission improves acquisition of a difficult odor mixture pattern separation task. Arrowheads indicate daily systemic injections of Oxotremorine (Oxo) (0.1 mg/kg) or Saline (Sal, control) 20 min prior the mixtures discrimination task. Performances are showed as mean ± SEM. *Post-hoc Fisher tests revealed significant decrease of performance compared to baseline (vanilla-mint), p<0.01; # significant difference between Scop and M-scop groups (a.) or between Oxo and Sal groups (b.), p<0.01.
Figure 2.
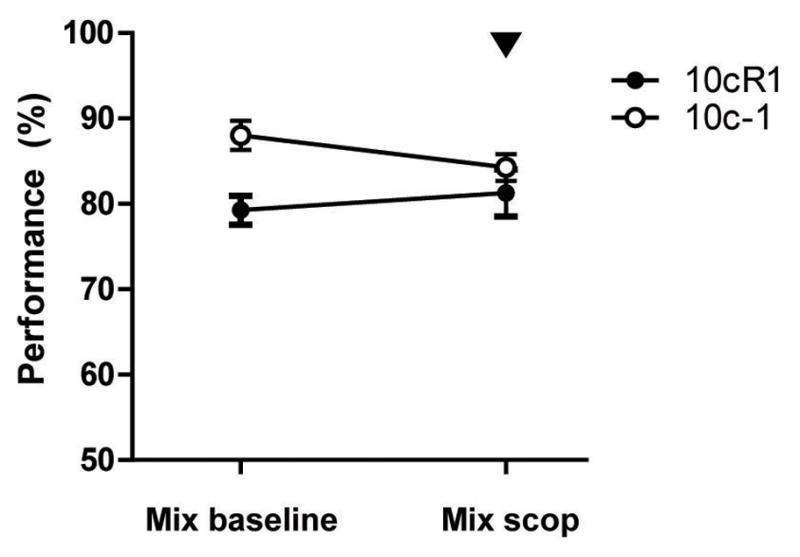
Systemic blockade of cholinergic muscarinic transmission does not disturb performances of rats expert at odor mixture discrimination. Animals first trained to discriminate the 10c core mixture from its morph versions (either 10cR1 or 10c-1) and showing performances >75% for over a week received systemic injection of Scopolamine (0.5 mg/kg) 20 min prior testing. Performances are showed as mean ± SEM.
Figure 3.
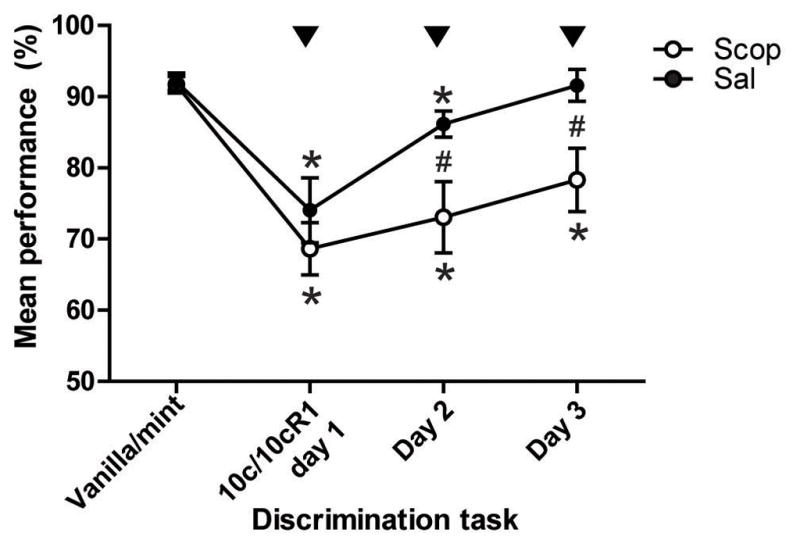
Piriform cortical blockade of cholinergic muscarinic transmission impairs odor mixture perceptual learning. Arrowheads indicate daily microinjection of Scopolamine (Scop, 30 μg/hemisphere) or Saline (Sal, control) into anterior piriform cortex 20 min prior the mixture discrimination task. Performances are showed as mean ± SEM. *Significant decrease of performance compared to baseline (vanilla-mint), p<0.05; # significant difference between Scop and M-scop groups, p<0.05.
Prior to mixture discrimination training, a subset of animals were implanted with guide cannulas aimed at their anterior piriform cortex, and cemented to their skulls. The implantation was performed under aseptic conditions under isoflurane anesthesia. Guide cannulas (26 gauge; Plastics One, Roanoke, VA) were inserted bilaterally into the aPCX at the following coordinates: +2 mm AP; ±4 mm ML; −5 mm DV relative to bregma. The tips of the guide cannulas were positioned 1 mm dorsal to the target infusion site. Four stainless steel screws and dental cement were used to secure the guide cannulas to the skull. Dummy infusion cannulas were then placed into the guide cannulas to prevent clogging and infection. At least one week after surgery, their ability to perform the initial odor discrimination was confirmed before switching them to the mixture discrimination task. The muscarinic receptor antagonist scopolamine hydrobromide trihydrate (30 μg/side; Sigma-Aldrich) was infused in aPCX 20 min prior to behavioral training (10c/10cR1 discrimination). Rats (n=6) were microinjected as follows: while gently restrained, the dummy cannulas were removed and injection cannulas (33 gauge) were inserted to a depth of 1 mm beyond the tips of the guide cannulas. Injection cannulas were connected via polyethylene tubing to two 250 μl Hamilton syringes driven by an automated syringe pump (Harvard Apparatus, MA). The drug was dissolved in sterile saline 0.9% and delivered bilaterally over a period of 5 min (1 μl/hemisphere). The infusion cannulas remained in place for an additional 30 s to allow a correct diffusion of the solution. In order to habituate the animals to the infusion procedure, rats experienced during vanilla-mint training sessions sham infusions identical in all aspects to the procedure described above except that no liquid was injected. The mixture discrimination test began once the animals had recovered their baseline level. Control animals (n=6) received equivalent microinjections of 0.9% saline following identical procedure. The injections were repeated daily until the control group reached criterion performance. After completion of the experiment, implanted animals were overdosed with urethane, transcardially perfused with 0.9 % saline and 10% paraformaldehyde. Coronal brain sections (40 μm thick) were performed and stained with cresyl violet to ensure that all the cannulas were positioned at the vicinity of the aPCX.
RESULTS
The aim of the first experiment was to test whether the blockade of cholinergic muscarinic transmission could disturb the learning of odor mixtures discrimination (Fig. 1.a.). Rats (n=6) received an injection of the muscarinic receptor antagonist scopolamine hydrobromide trihydrate (0.5 mg/kg) 20 min before behavioral testing (10c vs. 10cR1 ‘easy’ discrimination). The control group (n=4) received an equivalent dose of methyl-scopolamine (0.5 mg/kg), a muscarinic receptor antagonist replicating scopolamine’s peripheral effects without crossing the blood brain barrier [13]. The injections were repeated in this same manner the two following days until the animals of the control group reached criterion (>80% correct) performance. Repeated-measures ANOVA revealed a significant effect of the training (F1,3=80.713, p<0.0001), a significant effect of the drug treatment (F1,8=25.237, p=0.0010) and a significant training x drug interaction (F1,3=7.280, p=0.0012), indicating that though cholinergic disruption did not completely inhibit the learning, animals injected with scopolamine were significantly poorer (post-hoc Fisher tests) than controls to discriminate the mixtures. Both groups showed a significant reduction of the number of trials generated during the 30 min training session with drug treatment compared to their initial vanilla-mint baseline level (Group Scop: 106±25 (mean±SD) trials for baseline, 74±12 trials with drug treatment, paired t test t5=3.252, p=0.0226; Group M-scop: 93±19 trials for baseline, 63±19 trials with drug treatment t3=4.035, p=0.0274).
These results suggest that a cholinergic muscarinic receptor antagonist can impair learning of a simple odor discrimination. In the second experiment we tested whether a more difficult pattern separation task could be improved by the enhancement of muscarinic transmission (Fig. 1.b.). 20 min before behavioral testing (10c vs. 10c-1 ‘difficult’ discrimination), rats (n=5) received an injection of the muscarinic receptor agonist oxotremorine (0.1 mg/kg). Importantly, and so as to annul the invalidating peripheral effects of oxotremorine (in particular nasal secretions) but spare its central effects, rats were co-treated with methyl-scopolamine (0.5 mg/kg)[15]. Control rats (n=4) received an equivalent volume of physiological 0.9% saline. The injections were repeated daily until the control group reached criterion performance. Repeated-measures ANOVA revealed a significant effect of the training (F1,4=80.137, p<0.0001), a significant effect of the drug treatment (F1,7=11.286, p=0.0121) and a significant interaction (F1,4=4.882, p=0.0041). Post-hoc tests revealed a significant improvement of the rats’ discrimination capacity due to enhancement of cholinergic transmission. No effect of the drug injection was observed on the animals’ activity (Group Oxo: 93±17 trials/30 min session for baseline, 90±8 trials with drug treatment, t4=0.689, p=0.5286; Group Sal: 122±43 trials for baseline, 137±13 trials with injection t3=−0.993, p=0.3938).
These results suggest that acquisition of a simple or difficult olfactory pattern separation task can be modulated by muscarinic receptor activity. To test if cholinergic suppression alters expression of the discrimination of complex odorant mixtures in animals already proficient to perform the task (fig. 2.), scopolamine (0.5 mg/kg) was injected (20 min prior testing) in different rats previously trained to criterion in either the easy (10c vs. 10cR1, n=4) or difficult (10c vs. 10c-1, n=3) task. Disturbing cholinergic transmission in these expert animals had no effect on expression of previously learned discrimination (repeated-measures ANOVA, F1,1=0.117, p=0.7461).
Finally, as suggested by previous electrophysiological data [5, 8, 10], the piriform cortex could be a key neural substrate for pattern recognition processes required for the learned performance described here. To determine if cortical cholinergic transmission can control this process, we replicated the experiment described in Fig 1.a using microinjections of scopolamine (or vehicle) directly into the aPCX rather than systemic injections. The results revealed a similar disturbance of perceptual learning observed previously with the systemic injections (fig. 3). Repeated-measures ANOVA indicated a significant effect of the training (F1,4=28.454, p<0.0001), no significant effect of the drug treatment (F1,10=4.419, p=0.0619) but a significant training x drug interaction (F1,4=3.715, p=0.0220); animals injected with scopolamine were significantly slower than controls to discriminate the mixtures, especially at day 2 and day 3 (post-hoc tests). The microinjections had no effect on the number of trials performed by the animals (Group Scop: 95±14 trials/30 min for baseline, 97±21 trials with drug treatment, t5=−0.284, p=0.7875; Group Sal: 94±9 trials for baseline, 84±14 trials with injection t5=1.205, p=0.2821).
DISCUSSION
Together, these results support the hypothesis that olfactory cortical pattern separation processes can be modulated by acetylcholine, and that the piriform cortex is an important locus for this modulation. The results extend previous work that relied on discrimination of simple, monomolecular odors or binary odor mixtures [11, 21, 23], to discrimination of a well characterized [5, 8, 10, 22] set of overlapping complex mixtures. Previous single-unit ensemble recordings from anterior piriform cortex have demonstrated that discrimination of these mixtures involves cortical activity consistent with pattern separation processes [5, 8] that have been described in more detail in hippocampal networks [19, 20, 24]. The present results suggest that the olfactory cortical pattern separation underlying behavioral odor discrimination is modulated by acetylcholine. Specifically, learning a difficult discrimination task which requires enhanced cortical pattern separation (e.g., 10c vs 10c-1) is significantly improved by a muscarinic receptor agonist. Conversely, learning a simple pattern separation task is impaired by a muscarinic receptor antagonist, even if the drug is localized to the piriform cortex. Once learned, however, expression was not affected by muscarinic receptor blockade. This suggests that the behavioral impairment observed was not due to anosmia or dysosmia, but rather to modulation of the cortical plasticity required for the learned pattern recognition processes.
Acetylcholine plays a major role in odor processing and plasticity throughout the olfactory pathway from the OB [9, 23], to PCX [4, 26] to the EC [6]. In the piriform cortex, muscarinic receptor agonists suppress association fiber synaptic efficacy through a reduction in pre-synaptic glutamate release, with minimal effect on afferent fiber synapses from mitral cells [18, 25] and enhance long-term potentiation of association fiber synapses [17]. Together, these diverse cholinergic effects are hypothesized to result in strengthening of connections between co-active pyramidal cells (due to enhanced LTP) that is selective for only those activated cells (due to generalized association fiber synaptic depression). Only synapses of co-active cells are driven strongly enough to be strengthened. For optimal piriform cortex learning of odor patterns (objects), there must be a balance between synaptic potentiation and synaptic suppression, in part mediated by changing cholinergic input. Thus the current results may have relevance to the olfactory impairments observed in disorders that include a loss of cholinergic tone, such as Alzheimer’s Disease [1].
Highlights.
Acquisition of olfactory pattern separation requires acetylcholine.
Cholinergic blockade restricted to the piriform cortex impairs acquisition
Discrimination of well learned odors does not require acetylcholine
These results are interpreted in the context of piriform cortical circuit function
Acknowledgments
J.C. was funded in part by the Fyssen Foundation. D.A.W was funded by grants from NIDCD (R01- DC03906) and NIA (R01- AG037693).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Auld DS, Kar S, Quirion R. Beta-amyloid peptides as direct cholinergic neuromodulators: a missing link? Trends Neurosci. 1998;21:43–49. doi: 10.1016/s0166-2236(97)01144-2. [DOI] [PubMed] [Google Scholar]
- 2.Baratti CM, Huygens P, Mino J, Merlo A, Gardella J. Memory facilitation with posttrial injection of oxotremorine and physostigmine in mice. Psychopharmacology (Berl) 1979;64:85–88. doi: 10.1007/BF00427350. [DOI] [PubMed] [Google Scholar]
- 3.Barkai E, Hasselmo ME. Modulation of the input/output function of rat piriform cortex pyramidal cells. J Neurophysiol. 1994;72:644–658. doi: 10.1152/jn.1994.72.2.644. [DOI] [PubMed] [Google Scholar]
- 4.Barkai E, Hasselmo MH. Acetylcholine and associative memory in the piriform cortex. Mol Neurobiol. 1997;15:17–29. doi: 10.1007/BF02740613. [DOI] [PubMed] [Google Scholar]
- 5.Barnes DC, Hofacer RD, Zaman AR, Rennaker RL, Wilson DA. Olfactory perceptual stability and discrimination. Nat Neurosci. 2008;11:1378–1380. doi: 10.1038/nn.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandon MP, Bogaard AR, Libby CP, Connerney MA, Gupta K, Hasselmo ME. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science. 2011;332:595–599. doi: 10.1126/science.1201652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke SN, Wallace JL, Hartzell AL, Nematollahi S, Plange K, Barnes CA. Age-associated deficits in pattern separation functions of the perirhinal cortex: a cross-species consensus. Behavioral neuroscience. 2011;125:836–847. doi: 10.1037/a0026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapuis J, Wilson DA. Bidirectional plasticity of cortical pattern recognition and behavioral sensory acuity. Nature Neuroscience. 2011 doi: 10.1038/nn.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhury D, Escanilla O, Linster C. Bulbar acetylcholine enhances neural and perceptual odor discrimination. J Neurosci. 2009;29:52–60. doi: 10.1523/JNEUROSCI.4036-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CF, Barnes DC, Wilson DA. Generalized versus stimulus-specific learned fear differentially modifies stimulus encoding in primary sensory cortex of awake rats. J Neurophysiol. 2011;106:3136–3144. doi: 10.1152/jn.00721.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Rosa E, Hasselmo ME. Muscarinic cholinergic neuromodulation reduces proactive interference between stored odor memories during associative learning in rats. Behavioral neuroscience. 2000;114:32–41. [PubMed] [Google Scholar]
- 12.Devore S, Linster C. Noradrenergic and cholinergic modulation of olfactory bulb sensory processing. Frontiers in behavioral neuroscience. 2012;6:52. doi: 10.3389/fnbeh.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira G, Poindron P, Levy F. Involvement of central muscarinic receptors in social and nonsocial learning in sheep. Pharmacol Biochem Behav. 2003;74:969–975. doi: 10.1016/s0091-3057(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher ML, Wilson DA. Experience modifies olfactory acuity: acetylcholine-dependent learning decreases behavioral generalization between similar odorants. J Neurosci. 2002;22:RC201. doi: 10.1523/JNEUROSCI.22-02-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Floody OR. Cholinergic control of male mating behavior in hamsters: effects of systemic agonist or antagonist treatment. Pharmacol Biochem Behav. 2011;100:289–298. doi: 10.1016/j.pbb.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- 17.Hasselmo ME, Barkai E. Cholinergic modulation of activity-dependent synaptic plasticity in the piriform cortex and associative memory function in a network biophysical simulation. J Neurosci. 1995;15:6592–6604. doi: 10.1523/JNEUROSCI.15-10-06592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasselmo ME, Bower JM. Acetylcholine and memory. Trends Neurosci. 1993;16:218–222. doi: 10.1016/0166-2236(93)90159-j. [DOI] [PubMed] [Google Scholar]
- 19.Hunsaker MR, Kesner RP. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neuroscience and biobehavioral reviews. 2012 doi: 10.1016/j.neubiorev.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 21.Linster C, Garcia PA, Hasselmo ME, Baxter MG. Selective loss of cholinergic neurons projecting to the olfactory system increases perceptual generalization between similar, but not dissimilar, odorants. Behavioral neuroscience. 2001;115:826–833. doi: 10.1037//0735-7044.115.4.826. [DOI] [PubMed] [Google Scholar]
- 22.Lovitz AM, Sloan AM, Rennaker RL, Wilson DA. Complex mixture discrimination and the role of contaminants. Chemical Senses. 2012 doi: 10.1093/chemse/bjs006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravel N, Vigouroux M, Elaagouby A, Gervais R. Scopolamine impairs delayed matching in an olfactory task in rats. Psychopharmacology (Berl) 1992;109:439–443. doi: 10.1007/BF02247720. [DOI] [PubMed] [Google Scholar]
- 24.Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang AC, Hasselmo ME. Selective suppression of intrinsic but not afferent fiber synaptic transmission by baclofen in the piriform (olfactory) cortex. Brain research. 1994;659:75–81. doi: 10.1016/0006-8993(94)90865-6. [DOI] [PubMed] [Google Scholar]
- 26.Wilson DA. Scopolamine enhances generalization between odor representations in rat olfactory cortex. Learn Mem. 2001;8:279–285. doi: 10.1101/lm.42601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]