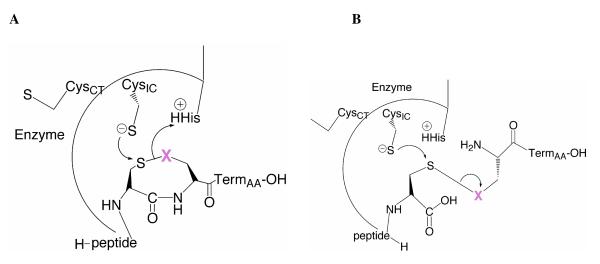
Figure 4.
Mechanism of truncated TR toward cyclic and acyclic peptide substrates. The peptide substrate is positioned in the active site such that nucleophilic CysIC thiolate can attack the Cys1 position, and cause the release of X (magenta). A) In the case of the cyclic peptide, when X = S, the ring is critical for positioning X near the conserved His, to receive a proton to stabilize the leaving group. When X = Se, the position of the ring near the His matters less due to the much lower pKa of a selenolate in comparison to that of a thiolate. B) In the case of the flexible, acyclic peptide, which has more degrees of motion in comparison the cyclic peptide, protonation of X is much less likely to occur due to the “floppy” nature of the peptide. However, when X = Se, protonation does not need to occur for transition-state stabilization due to its lower pKa. Experimentally we observe that when X = S, the acyclic peptides are extremely poor substrates.