Abstract
The treatment of castration-resistant prostate cancer (CRPC) remains palliative. Immunotherapy offers a potentially effective therapy for CRPC; however, its advancement into the clinic has been slow, in part because of the lack of representative in vitro tumor models that resemble the in vivo tumor microenvironment for studying interactions of CRPC cells with immune cells and other potential therapeutics. This study evaluates the use of 3D porous chitosan-alginate (CA) scaffolds for culturing human prostate cancer (PCa) cells and studying tumor cell interaction with human peripheral blood lymphocytes (PBLs) ex vivo. CA scaffolds and Matrigel matrix samples supported in vitro tumor spheroid formation over 15 days of culture, and CA scaffolds supported live cell fluorescence imaging with confocal microscopy using stably transfected PCa cells for 55 days. PCa cells grown in Matrigel matrix and CA scaffolds for 15 days were co-cultured with PBLs for 2 and 6 days in vitro and evaluated with scanning electron microscopy (SEM), immunohistochemistry (IHC), and flow cytometry. Both the Matrigel matrix and CA scaffolds supported interaction of PBLs with PCa tumors, with CA scaffolds providing a more robust platform for subsequent analyses. This study demonstrates the use of 3D natural polymer scaffolds as a tissue culture model for supporting long-term analysis of interaction of prostate cancer tumor cells with immune cells, providing an in vitro platform for rapid immunotherapy development.
Keywords: immunotherapy, NK cells, T cells, tumor microenvironment, tumor spheroids
1. Introduction
Prostate cancer (PCa) is the most common and second deadliest cancer in men in the US with an estimated 217,730 new cases and 32,050 deaths in 2010.[1] Castration-resistant prostate cancer (CRPC) represents the most deadly form of PCa in which the average survival is a dismal 2–3 years.[2] Even with front-line chemotherapy, disease progression occurs within 7 months for most patients.[2] Immunotherapy represents an ideal strategy for CRPC therapy since the body would use its natural defenses to actively destroy the cancer. Unfortunately, the rapid development of effective immunotherapies has been hindered by the lack of in vitro tumor models that accurately mimic the human disease.[3]
In cancer patients, the tumor escapes immunosurveillance-based cancer elimination through immunoediting.[4,5] During this process, the cancer cells acquire mutations which allow them to evade recognition by immune cells and secrete signaling molecules into the tumor microenvironment and blood which inactivate immune cells. In immunotherapy, the body’s immune system is reactivated against antigens on the tumor cell surface either through immunological adjuvants or ex vivo activation of autologous peripheral blood lymphocytes (PBLs) that are injected back into the patient. Surprisingly, vaccine optimization usually occurs in small phase I/II clinical trials,[6] which is likely due to the poor translation of in vitro efficacy to clinical response.[3,7,8] For example, sipuleucel-T (Provenge; Dendreon), the first autologous cellular immunotherapy for CRPC approved by the FDA, prolongs median survival of CRPC patients by only 4.1 months as compared to placebo.[9] The use of a representative model of the native tumor microenvironment in vitro will allow for better prediction of clinical response, which will reduce long-term costs associated with product development and generate higher quality therapeutics.
In vitro trials of activated PBL interaction with cancer cell suspensions or monolayers have shown high anti-cancer activity of immunotherapy. Activated PBLs show a high propensity for recognizing and eliminating target cancer cells. However, when the cancer cells are arranged in a three-dimensional (3D) architecture such as in tumor spheroids or 3D gel matrix, the activated PBLs show dramatically reduced affinity towards and cytotoxicity against target cancer cells.[3,7,10–17] Therefore, the development of 3D tissue culture models is expected to improve the relevance of in vitro immunotherapy results to clinical response by enhancing the ability to study the interaction of immune components with cancer cells and providing a platform for screening immunotherapies.[3] These in vitro trials using 3D tissue culture models could systematically identify tumor response to specific immune cells and reveal the components of the tumor microenvironment that aid in or inhibit immune therapies.
In this study, we investigated the use of 3D porous chitosan-alginate (CA) scaffolds to support the growth of PCa cells and formation of PCa tumor spheroids, and the feasibility of using the 3D co-culture system to study the interaction between human PCa cells and PBLs. Chitosan and alginate are both natural polymers that are widely used in biomedical applications due to their excellent biological properties and limited immunogenicity. [18–20] Both chitosan and alginate have the proxy structure of glycosaminoglycans (GAGs),[21] a major component of the native extracellular matrix (ECM).[22] The GAG hyaluronan, to which the CA complex is most chemically similar, makes up a significant portion of PCa ECM and promotes malignancy.[23,24] The 3D porous CA scaffolds developed by our research group have served as an effective mimic of the tumor microenvironment for human glioblastoma and human hepatocellular carcinoma, and supported the culture of these cells with performance that better replicates the in vivo system compared to 2D and Matrigel cultures.[25,26] Unlike scaffolds made from synthetic polymers, CA scaffolds can be easily dissolved in cell-compatible solution to release cultured cells for easy collection for subsequent analysis.[27] Porous CA scaffolds can sustain long-term stability in tissue culture media and provide a reliable and cost-effective platform for study of cancer biology.
Here human PCa cells (LNCaP, C4-2, and C4-2B) were seeded on Matrigel matrix and CA scaffolds and cultured in vitro for 15 days. The expansion of red fluorescent protein (RFP) expressing mouse PCa cells (TC2-RFP) in Matrigel matrix and CA scaffold samples was used to monitor in situ cell-cell interactions by confocal microscopy. After 15 days of in vitro culture, the Matrigel and CA scaffold human PCa samples were co-cultured with human PBLs for 2 and 6 days to observe the interaction of immune cells with PCa cells, and were characterized using SEM imaging, immunostaining, and flow cytometry.
2. Results and Discussion
2.1. CA Scaffolds for Supporting Prostate Tumor Growth
In vitro trials are widely used to elucidate the behavior of cancer cells, the mechanisms that control their behavior, and potential clinical treatments. Many of these trials are conducted with cells cultured on 2D surfaces (polystyrene tissue culture plates or wells), leading to discordant cell responses when applied to an in vivo system resulting from differences between 2D and 3D environments.[28] The use of 3D structures for in vitro trials allows for a more relevant model of the in vivo system and increases the chances of success in later, more costly stages of clinical trials. Biomaterial scaffolds (hydrogels or 3D porous scaffolds) are used to replicate the natural extracellular matrix in tissue engineering applications and have been demonstrated for tumor microenvironment applications.[25,26,28,29] Here, CA scaffolds were evaluated as an in vitro culture model of PCa through cell proliferation and morphology assessment. The three human cell lines used are variants of the LNCaP line, with the C4-2 and C4-2B having increased metastatic potential and reduced androgen sensitivity, more closely resembling CRPC.[30] Human PCa cells were cultured in 2D culture wells (12-well plates), 3D Matrigel matrix, and 3D porous CA scaffold in vitro to examine the ability of CA scaffolds to support cell proliferation. Proliferation of the cells in each culture environment (2D, Matrigel, CA) was evaluated over 5, 10, and 15 days of in vitro culture using the Alamar blue assay (Figure 1). The 2D and Matrigel matrix samples had the greatest cell numbers in all cell lines, with both samples in each cell line having similar cell populations at 15 days. The CA scaffold samples demonstrated a lag in cell growth compared to other culture environments in all PCa cell lines as expected for 3D culture.[25,26,31–34] Studies have shown that the 3D microenvironment provided by porous scaffolds promotes greater malignancy of tumor cells than Matrigel controls both in vitro and in vivo.[25,26,31–34] This greater malignancy contributes to decreased proliferation, which more closely mimics the in vivo conditions than the rapidly proliferating cells in standard 3D Matrigel culture.[25,26,31–34]
Figure 1.

Proliferation PCa cells cultured on different substrates. The growth of (a) LNCaP, (b) C4-2, and (c) C4-2B human prostate cancer cells grown on 2D culture plates, Matrigel matrix, and CA scaffolds after 5, 10, and 15 days of culture was determined by the Alamar blue assay.
The PCa cell morphology in the three culture environments was observed with scanning electron microscopy (SEM). The SEM images (Figure 2) demonstrate the influence of culture environment on cell morphology. The 2D samples had flat layers of cells with little three-dimensional structure and did not form tumor spheroids. The Matrigel samples formed tumor spheroids within the gel matrix, while the cells on the plate surface (under the Matrigel matrix) had a linear and elongated morphology and formed dense, thick cell sheets in some regions. The CA scaffold samples demonstrated tumor spheroid formation within the scaffold pores. The cell morphology in these three culture environments indicates that the 3D environments promoted tumor spheroid formation, while the 2D surface did not. The ability of cells to form tumor spheroids in 3D culture environments provides a better replica of the in vivo tumor structure.[25,26,28,29] This shows that the 3D environments should be better models for studying PBL interaction with tumor cells in vitro than 2D monolayer cultures.
Figure 2.

SEM images of PCa cells cultured on various substrates. (a) LNCaP, (b) C4-2, and (c) C4-2B human prostate cancer cells were grown on 2D culture plates, Matrigel matrix, and CA scaffolds for 15 days before analysis; scale bars are 40 μm.
2.2. Live Cell Tumor Monitoring
The CA scaffolds were further evaluated as an in vitro culture platform by demonstrating live cell fluorescence imaging of cells cultured within the scaffolds. Mouse prostate tumor TRAMP-C2 (TC2) cells stably transfected with red fluorescent protein (TC2-RFP) were seeded in Matrigel matrix and CA scaffolds and were monitored with confocal microscopy at various times during culture (Figure 3). The Matrigel matrix and CA scaffold samples imaged one day after cell seeding (Figure 3a and 3b, respectively) showed a few cells within the matrices. CA scaffold samples at 14 days (Figure 3c) and 55 days (Figure 3d) after cell seeding showed the presence of large tumor spheroids within the CA scaffolds. The 55 day CA scaffold samples had several large tumor spheroids in the samples, which could be readily observed due to the stable RFP transfection. The Matrigel matrix became highly degraded after 7 days and could no longer be imaged. This occurred in all Matrigel samples (n = 6), regardless of the ratio of Matrigel to cell solution (samples were cultured at 1:1 and 4:1 Matrigel:cell solution ratios). This matrix degradation is due to the high invasive nature of TC2 cells[29] and reveals the limitations of Matrigel for in vitro trials. Furthermore, Matrigel can commonly maintain in vitro culture for only 10 days.[35] The CA scaffolds supported cultures for longer periods of time (up to 55 days in this study) indicating they are more robust than Matrigel, allowing for prolonged analyses.
Figure 3.
Live cell images of TC2-RFP cells grown on various substrates. Cells were grown for 1 day in (a) Matrigel matrix and (b) CA scaffolds. Cells were grown in CA scaffolds for (c) 14 days and (d) 55 days before imaging. Scale bars are 20 μm. Left: fluorescence; middle: differential interference contrast (DIC); right: overlay.
Figure 4 shows an expanded z-series confocal image illustrating an individual TC2-RFP tumor spheroid from cells cultured in CA scaffolds at day 55. The entire tumor spheroid was imaged with the z-stack at 5 μm intervals, which had a total height of 80 μm. The image labeled “Z-stack” is the projection of the individual RFP images comprising the entire z-series. The use of live cell fluorescence imaging allows for real-time monitoring of the external surface of a tumor spheroid and its interactions with the matrix and other cells. This could allow for real-time imaging of immune cell interaction and infiltration with tumor spheres within the 3D microenvironment with tracker dyes.[36] The ability to monitor specific cell-cell interactions can only be studied directly in vitro. The interplay between tumor cells and specific immune cells in vivo can be confounded by the presence of other stromal cells. Therefore, in vitro tests can be used to specifically dissect the interaction of immune cells with tumor cells and extrapolate tumor-specific elements that may inhibit immune-based therapies. CA scaffolds provide such an in vitro model which is suitable for long-term trials and convenient for down-stream analyses.
Figure 4.

Z-series confocal imaging of a tumor sphere in CA scaffolds. Expanded live cell confocal fluorescence z-stack images of 55 day in vitro culture CA scaffold samples, with each image of z-stack shown individually then combined in the z-stack projection. Total z-stack height is 80 μm with images at 5 μm increments. The DIC image is a representative image from the z-stack and the merged image is the combination of z-stack projection and DIC images. Scale bars are 20 μm.
2.3. Interaction of Human PBLs with In Vitro Tumors
2.3.1. SEM Imaging of PBLs Interacting with In Vitro Tumors
Human PCa cells (LNCaP, C4-2, and C4-2B) cultured in Matrigel matrix and CA scaffolds for 15 days to allow tumor spheroid formation were then co-cultured with human PBLs for 2 and 6 days to demonstrate the use of CA scaffolds for supporting interaction of primary lymphocytes with tumor cells in vitro. The cell-cell interactions were first analyzed with SEM imaging to determine the cell morphology. The SEM images indicated that PBLs could readily interact with in vitro tumors in both Matrigel and CA scaffold samples at the 2 day time point (Figure 5). There is heterogeneity in the PBL population, so there are several different types of cells with different sizes observed interacting with the tumors. In SEM images, these immune cells were distinguished from tumor cells by their morphology and size, and by comparing the SEM images of co-cultured samples (Figure 5) with the images of samples cultured with PCa cells only (Figure 2). The arrows in the high magnification images indicate PBLs present on the surface of the PCa in vitro tumor spheroids. Physical interaction of PBLs with cancer cells in both the Matrigel matrix and CA scaffold samples was observed for all three cell lines. These images indicate that the in vitro culture of PCa cells in CA scaffolds is sufficient to allow immune cells to home to the tumor spheroids developed within the 3D structures. Both 3D structures supported interaction with immune cells, however, the CA scaffolds provided a more convenient model for SEM and IHC analyses (detailed in Section 2.3.2). Matrigel samples were challenging to analyze with SEM to determine cell morphology due to their fine 2 μm pore structure,[28,37] while the CA scaffolds provided an easily analyzed SEM sample in which the tumor spheroids could be imaged within the 100 μm pores.
Figure 5.
SEM images PCa cells grown on various substrates co-cultured with PBLs. (a) LNCaP, (b) C4-2, and (c) C4-2B human prostate cancer cells in Matrigel matrix and CA scaffolds were co-cultured with PBLs for 2 days. The boxes indicate the higher magnification region shown to the right and the arrows indicate PBLs. Scale bars are 10 μm.
2.3.2. Identification of PBLs Interacting with In Vitro Tumors
PBLs are comprised of T cells, B cells, and natural killer (NK) cells. T cells, the component of the adaptive immune system, are the major responders to vaccine-based therapies. NK cells are known to be the innate first defense against tumors. B cells are the component of the humoral immunity and also serve as antigen presenting cells. In order to assess the specific types of PBLs that migrate and infiltrate to tumor spheroids and potentially interact with the human PCa in vitro tumors, cultured scaffolds were sectioned and analyzed by IHC with antibodies to specific lymphocytes.
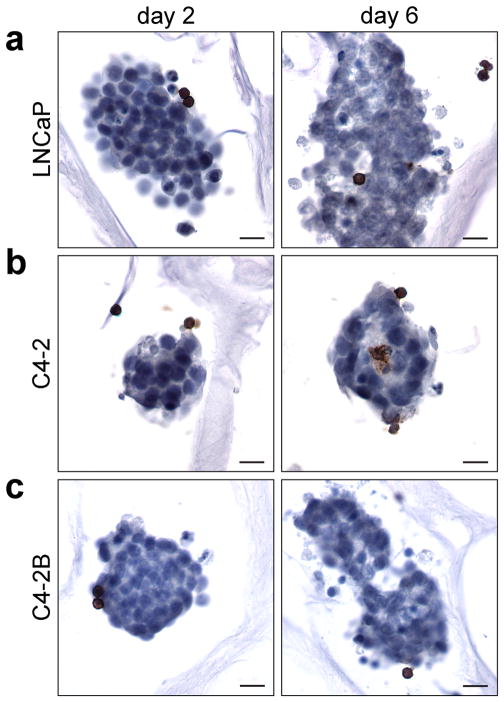
The localization of specific PBLs on the PCa seeded scaffolds was first assessed through IHC analysis of CD45R staining. CD45R denotes B cells and activated T cells. CD45R+ cells were observed bound to the in vitro tumors in CA scaffolds at the 2 day time point (Figure 6). Furthermore, the CD45R+ cells penetrated the tumor spheres after 6 days (Figure 6). There was not increase in the number of CD45+ cells between 2 and 6 days indicating these cells are likely B cells. T cells would start to become activated in the tumor microenvironment due to the presence of NK T cells, discussed below. This shows the ability of the in vitro tumors to promote the binding of PBLs
Figure 6.
Immunohistochemical analysis of CD45R expressing lymphocytes in CA scaffold co-cultured in vitro tumors. (a) LNCaP, (b) C4-2, and (c) C4-2B human prostate cancer cells in CA scaffolds were co-cultured with PBLs for 2 and 6 days; scale bars are 20 μm. The brown color denotes positive staining of B cells and the cell nuclei are counterstained blue.
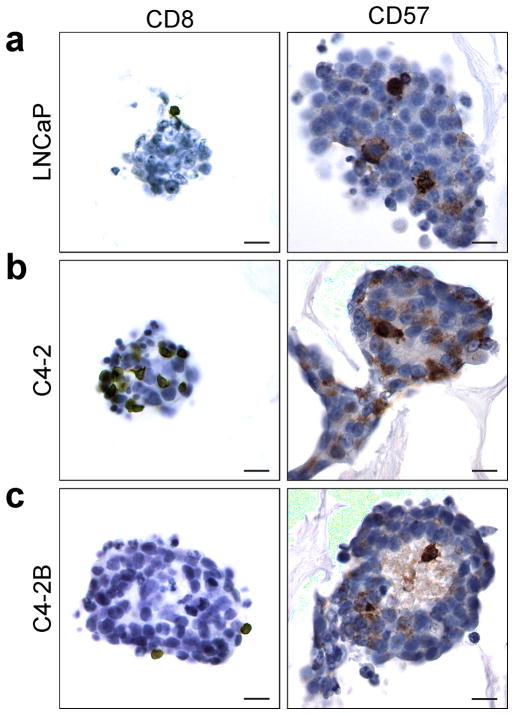
Further characterization of PBLs on the PCa seeded scaffolds was performed after 2 day co-culture through IHC staining of the CD8 and CD57 (Figure 7). Positive staining was observed for both CD8 and CD57, with significant CD57+ staining (Figure 7). In PBLs CD8 is present on T cells (30–40%), NK cells (30–40%), and more rarely (< 5%) on monocytes and neutrophils.[38] The observed interaction of CD8+ cells with the CA scaffold cultured in vitro tumors is intriguing and mimics the homing of CTLs observed in the in vivo setting.[39] CD57, or human natural killer-1 (HNK-1), is one of several markers used to denote NK cells with immunohistochemistry. NK cells are a type of lymphocyte that perform functions similar to both helper and cytotoxic T cells in that they both produce cytokines for immune activation and are directly involved in cell killing. Different from T cells, the recognition of tumor cells and activation of NK cells is through direct contact with tumor cells and does not require the participation of antigen-presenting cells. The CD57+ NK cells in the 2 day samples have penetrated the tumor spheres indicating rapid migration into the in vitro tumors. This is especially apparent in the C4-2 and C4-2B in vitro tumors where the CD57+ cells can be observed in the light colored areas of the tumor spheres, which likely corresponds to its necrotic center, a common phenomenon in tumor spheres and tumors in vivo.[28] High levels of NK cells were expected in the in vitro tumors since the cancer cells and PBLs are from different donors, and therefore have different major histocompatibility complex (MHC) class I markers.
Figure 7.
Immunohistochemical analysis of CD8 (T and NK cells), and CD57 (natural killer cells) in CA scaffold co-cultured in vitro tumors. (a) LNCaP, (b) C4-2, and (c) C4-2B human prostate cancer cells in CA scaffolds co-cultured with PBLs for 2 days; scale bars are 20 μm. The brown color denotes positive staining and the cell nuclei are counterstained blue.
2.3.3. Quantification of PBLs Interacting with In Vitro Tumors
The 6 day co-cultured CA scaffold human PCa samples were degraded, stained with CD3 (indicates T cells) and CD56, or neural cell adhesion molecule (NCAM, indicates NK cells), and analyzed with flow cytometry (Figure 8). The CA scaffold samples yielded a large number of tumor cells and PBLs; the gated regions denote the lymphocyte population, which was further analyzed for CD3 and CD56 positivity (Figure 8). Cell numbers required for accurate flow cytometry analysis could not be collected from Matrigel samples due to the difficulty in processing the gel matrix. CD3+CD56+ double positive lymphocytes are defined as NK-T cells (NKT) a subpopulation of T cells which have properties of NK cells.[40,41] CD3−CD56− double negative lymphocytes are likely B cells.[42] CD3−CD56+ lymphocytes are defined as NK cells and CD3+CD56− cells are T cells such as CTLs.[43] All CA scaffold samples had approximately 17% CD3−CD56+ NK cells (Figure 8) which confirms that NK cells were interacting with the tumor spheroids, as indicated in Figure 7 with CD57 IHC staining. Both CD56 and CD57 are NK cell surface markers, with CD56 expressed in the majority of NK cells (> 95%), while CD57 is expressed in ~50 – 60% of NK cells.[38] Furthermore, CD3+CD56− T cells were observed at a similar level between the different tumor spheres indicating similar tumor cell recognition regardless of malignancy. Interestingly, there was a positive correlation between the proportion of NKT cells incorporated with the tumor sphere and PCa cell malignancy. While the activity of NKT cells collected from PCa patients is compromised,[40] NKT cells from healthy donors in this study showed highly effective recognition of malignant PCa. This suggests the NKT cell inactivation, which occurs in tumor evasion, is systemic rather than a result of the local tumor microenvironment, in agreement with previous studies;[40,44] inactivation of NKT cells occurs in the blood rather than in the local tumor microenvironment. This highlights the utility of the CA scaffolds for studying tumor cell-lymphocyte interactions in PCa progression and therapy.
Figure 8.
Flow cytometry analysis of CA scaffold co-cultured PCa cells and PBLs. (a) LNCaP, (b) C4-2, and (c) C4-2B human prostate cancer cells grown on CA scaffolds co-cultured with PBLs for 6 days were stained for CD3 and CD56. The left column shows the analyzed population and lymphocyte gates, while the right column shows the expression of CD3 and CD56 among lymphocytes. Population designations: CD3+CD56+, NKT cells; CD3−CD56−, B cells; CD3−CD56+, NK cells; CD3+CD56−, T cells.
These results indicate the ability to recover the co-cultured cells from CA scaffolds for analysis with flow cytometry. Flow cytometry is a valuable tool to investigate cell populations and could enable high throughput screening and sorting of immunotherapies tested against cultures on the CA scaffolds. This flow cytometry analysis also demonstrates a use of the controlled degradation of the natural polymer CA scaffolds to allow for studying the functional interaction of co-cultured cells, as we have shown previously with embryonic stem cell cultures.[27] The use of an ionic scaffold degradation solution disrupts the polyelectrolyte complex formed between chitosan and alginate, leading to the separation of chitosan and alginate molecules and the degradation of the 3D porous CA scaffold and release of the cells. The EDTA in the scaffold degradation solution further helps break up the in vitro tumors into single cell suspensions.
The use of 3D biomaterial scaffolds for immunological or immunotherapy studies provide a more relevant model that should accelerate the successful translation of new immunotherapies into the clinic.[3,7,8,10,36,45,46] From current immunotherapy clinical trials it is clear that a better understanding of PBL interactions with PCa cells is required to develop more effective therapies.[47] These PCa tumors cultivated on CA scaffolds could be used as an in vitro platform for testing immunotherapies, potentially providing tumors that can be tested in a standard, high throughput method, without relying on advanced CRPC patients.
3. Conclusion
We have demonstrated that CA scaffolds provide an in vitro culture environment for culture and study of PCa cells. Tumor spheroids formed in the CA scaffolds during 15 days of in vitro culture. Long-term culture (55 days) of PCa cells in CA scaffolds could be monitored in situ through fluorescence microscopy. Furthermore, the CA scaffolds supported the interaction of immune cells, including tumor-infiltrating B cells, T lymphocytes, and natural killer cells, with tumor cells and present a potential in vitro experimental model for screening immunotherapies to treat CRPC, presumably other malignancies as well. The CA scaffolds also provided a more convenient platform than Matrigel or other hydrogels for subsequent analyses and supported prolonged culture. The enhanced in vitro tumor microenvironment model that the CA scaffold provides generates more clinically relevant responses as compared to 2D monolayer cultures due to the formation of tumor spheroids in the 3D cultures. The use of CA scaffolds as an in vitro tumor platform can be undoubtedly expanded to investigate the interaction between immune cells and other cancer cells, and will hopefully aid in the development of more effective therapies to treat various cancers.
4. Experimental Section
Materials
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. Chitosan (practical grade, > 75% deacetylated, MW = 190,000 – 375,000) and alginate (alginic acid from brown seaweed) were used as received. Reduced growth factor Matrigel matrix was purchased from BD Biosciences (San Jose, CA). RPMI Medium 1640, Dulbecco’s Modified Eagle Medium (DMEM), antibiotic-antimycotic (AA), Dulbecco’s phosphate buffered saline (D-PBS), Versene and AlamarBlue reagent were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Atlanta, GA). LNCaP, C4-2, and C4-2B human PCa cell lines and TRAMP-C2 (TC2) mouse PCa cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA). Human PCa cells were maintained according to manufacturer’s instruction in fully supplemented RPMI with 10% FBS and 1% AA at 37°C and 5% CO2 in a fully humidified incubator. TC2 cells were transfected with pDsRed-Max-N1 (Addgene plasmid 21718, Addgene Inc., Cambridge, MA) plasmid using the Lipofectamine 2000 transfection reagent (Invitrogen) following the manufacturer’s protocol. Cells stably expressing RFP were selected for neomycin resistance with G418 (1 mg mL−1) in fully supplemented DMEM (10% FBS, 1% AA) for 2 weeks followed by FACS to obtain a pure population of TC2-RFP cells. TC2-RFP cells were maintained in fully supplemented DMEM containing 1 mg mL−1 G418 at 37°C and 5% CO2 in a fully humidified incubator.
CA Scaffold Synthesis
CA scaffolds were prepared as previously reported.[48] Briefly, a 4 wt% chitosan solution was prepared by dissolving chitosan in 1 wt% acetic acid solution and a 4 wt% alginate solution was prepared by dissolving alginate in deionized (DI) water. Porous scaffolds were prepared by combining equal amounts of chitosan and alginate solutions and mixing for 10 minutes, followed by mixing under constant stirring for 5 minutes in a blender. The CA solution was cast in molds, refrigerated at 4°C for 12 hours, frozen at −20°C overnight, and lyophilized for 24 hours. The scaffolds were sectioned into 2 mm thick, 13 mm diameter discs, then crosslinked with a 0.2 M CaCl2 solution for 10 minutes under vacuum, washed 3 times with excess DI water to remove any remaining salt, and sterilized in 70 v% ethanol for 2 hours under vacuum. The scaffolds were then washed 3 times with D-PBS, immersed in 500 mL D-PBS, and shaken on an orbital shaker at 100 rpm overnight to remove any remaining ethanol.
Cell Seeding on Scaffolds
PCa cells were seeded onto 12-well plate wells and D-PBS damp CA scaffolds in 12-well plates at 50,000 cells per sample in 100 μL fully supplemented media. The samples were incubated at 37°C and 5% CO2 in a fully humidified incubator to allow cell adhesion to the substrate for 2 hours before 1.5 mL fully supplemented media was added to each well. For Matrigel cultured samples, 50,000 cells in 200 μL fully supplemented media were mixed with 200 μL Matrigel of reduced growth factor and added to 12-well plate wells. Samples were incubated at 37°C and 5% CO2 in a fully humidified incubator for 2 hours to allow the Matrigel matrix to gel before 1.5 mL fully supplemented media was added to each well. Matrigel and CA scaffold samples were cultured for 15 days with regular media changes. The RFP transfected cells (TC2-RFP) were seeded on CA scaffolds in the same manner shown above. The TC2-RFP Matrigel samples were seeded at a concentration of 50,000 cells in 50 μL fully supplemented media mixed with 150 μL Matrigel of reduced growth factor. The cells in CA scaffolds were cultured for up to 55 days with regular media changes.
Cell Proliferation Analysis
Proliferation of human PCa cells cultured on 2D wells, Matrigel matrix, and CA scaffolds was assessed with the AlamarBlue assay following the manufacturer’s protocol. Briefly, 1 mL of AlamarBlue solution (10% AlamarBlue reagent in fully supplemented RPMI 1640) was added to each well. The samples were incubated at 37°C for a predetermined time for each cell line (2.25 hours for LNCaP, 2.33 hours for C4-2, and 3 hours for C4-2B), then the AlamarBlue solution was transferred to a 96-well plate to obtain fluorescence values using a microplate reader (Molecular Devices) at excitation wavelength of 570 nm and fluorescence emission read at 585 nm. The cell number was calculated based on previously created standard curves. Excess AlamarBlue solution was aspirated and fresh fully supplemented media was added to each well.
Live Cell Imaging
TC2-RFP seeded Matrigel matrix and CA scaffold samples were transferred to 110 mm diameter Petri dishes customized for confocal microscopy (part of the dish bottom was removed and replaced with a coverslip). Media was added to the Petri dish to cover the substrate material and keep the cells viable. The samples were imaged with a confocal laser scanning microscope (Zeiss 510 META LSM, Carl Zeiss AG, Oberkochen, Germany). The samples were imaged at 100× and 200× magnification with red fluorescence and DIC.
Human Peripheral Blood Lymphocytes (PBLs) Collection and Co-Culture
The study was carried out with the approval of the University of Washington Research Subjects Review Board and with informed signed consent. Blood samples were obtained from healthy donors. Peripheral blood lymphocytes (PBLs) were separated from the whole blood by Ficoll gradient. Briefly, the blood sample was first diluted twice in sterile D-PBS and overlaid onto Ficoll-Paque PLUS (GE Healthcare, Piscataway, NJ, USA), and soft spun at 2200 rpm for 20 minutes at room temperature without brake. PBLs were collected at the plasma/ficoll interface and washed once with D-PBS. Erythrocytes were lysed with ACK lysing buffer (BioWhittaker, Walkersville, MD, USA) and the remaining cells were washed twice with D-PBS in preparation for culture. The concentrated PBLs were resuspended in fresh RPMI media and added to Matrigel matrix or CA scaffold samples in a volume of 100 μL (2.5 × 105 PBLs per 100 μL). The PBLs were added to the samples after aspirating the culture media and they were incubated for 1 hour before adding fresh fully supplemented RPMI media. The samples were cultured for 2 and 6 days and the media was changed regularly every 2 day.
SEM Imaging
Samples for scanning electron microscopy (SEM) analysis were fixed with 2.5% glutaraldehyde in fully supplemented media for 30 minutes at 37°C. The samples were then fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer overnight at 4°C. The samples were dehydrated in a series of ethanol washes (0%, 30%, 50%, 70%, 85%, 95%, 100%), with each wash performed twice. The samples were critical point dried, sectioned, mounted, and sputter coated with platinum, then imaged with a JSM-7000F SEM (JEOL, Tokyo, Japan).
Immunohistochemical Staining
Samples were fixed in 4 w% formaldehyde, embedded in paraffin, and sectioned into 15 μm thick sections for immunohistochemical (IHC) staining. Sections were stained with: 1) anti-CD8 (rabbit IgG, 1:100; Neomarkers); 2) anti-CD45 antibody (rat IgG; 1:100; eBioscience); and 3) anti-mouse CD57 antibody (mouse IgM; 1:200; Neomarkers). A standard IHC staining procedure was used. Briefly, sections were deparaffinized, incubated for 10 minutes in 10 mM citrate buffer (pH 6.0) at 95°C for antigen retrieval. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide. After quenching endogenous peroxidase activity and blocking nonspecific binding, slides were incubated with specific primary antibody overnight at 4°C followed by subsequent incubation with the appropriate biotinylated secondary antibody: goat anti–rabbit IgG (Vector, Burlingame, CA); rabbit anti–mouse IgM and rabbit anti-rat IgG (Vector) at a 1:200 dilution for 30 minutes at RT. Immunoreactive antigens were detected using the Vectastain Elite ABC Immunoperoxidase Kit and DAB. All slides were counterstained with hematoxylin (Vector) and mounted with Permount (Fisher Scientific). The samples were imaged with optical microscopy at 400× magnification.
Flow Cytometry
Cells were recovered from PBL co-cultured samples after 6 days of culture. The cells were recovered from the CA scaffold samples through a scaffold degradation method whereby the CA scaffolds were incubated in 5 mL 30 mM sodium bicarbonate (NaHCO3) and 5 mM HEPES solution at 37°C for 10 minutes, followed by the addition of 2 mL 10 mM EDTA and 20 mM HEPES solution, which was incubated at 37°C for an additional 5 minutes. The samples were then pipetted up and down with a 10 mL pipette several times to breakdown the CA scaffolds. After the scaffolds degraded into debris, the degradation solution of each scaffold sample was passed through a 70 μm cell strainer followed by a 40 μm cell strainer to separate the scaffold debris from the cells. After the cells were recovered, each sample was pelleted at 2250 rpm for 5 minutes, then washed once in washing buffer (5% FBS in D-PBS). The samples were blocked with staining buffer (10% human serum, 0.02% NaNO3, in D-PBS) for 15 minutes at room temperature and stained with antibodies for CD3 and CD56 (BD Biosciences, San Jose, CA) in staining buffer for 30 minutes in dark on ice. The samples were washed twice with washing buffer, and resuspended in 200 μL washing buffer. The samples were analyzed on a FACSCanto flow cytometer (BD Biosciences, San Jose, CA). The data were analyzed and plotted with the FlowJo software package (Tree Star Inc., Ashland, OR).
Statistical Analysis
All of the data was statistically analyzed to express the mean ± standard deviation (SD) of the mean.
Acknowledgments
This work was supported in part by NIH grants R01CA134213 and R01EB006043 and Kyocera Professorship Endowment to Prof. M. Zhang and NIH/NCI R01CA149405 to Prof. J. Wu. FK acknowledges support from an NIH training grant (T32CA138312). We thank Greg Martin and Stephanie Lara for their assistance with live cell confocal imaging and critical point drying of SEM samples, respectively. We acknowledge the use of the SEM at the Dept of Materials Science and Engineering and the confocal and optical microscopes at Keck Microscopy Imaging Facility at the University of Washington. We thank Prof. Benjamin S. Glick for submitting the pDsRed-Max-N1 plasmid to Addgene.
Contributor Information
Stephen J. Florczyk, Department of Materials Science and Engineering, University of Washington, 302L Roberts Hall, Box 352120, Seattle, WA, 98195, USA
Dr. Gang Liu, Department of Medicine, School of Medicine, University of Washington, Seattle, WA 98195, USA
Dr. Forrest M. Kievit, Department of Materials Science and Engineering, University of Washington, 302L Roberts Hall, Box 352120, Seattle, WA, 98195, USA
Allison M. Lewis, Department of Materials Science and Engineering, University of Washington, 302L Roberts Hall, Box 352120, Seattle, WA, 98195, USA
Prof. Jennifer D. Wu, Department of Medicine, School of Medicine, University of Washington, Seattle, WA 98195, USA
Prof. Miqin Zhang, Email: mzhang@u.washington.edu, Department of Materials Science and Engineering, University of Washington, 302L Roberts Hall, Box 352120, Seattle, WA, 98195, USA
References
- 1.Jemal A, Siegel R, Xu J, Ward E. CA: A Cancer Journal for Clinicians. 2010;60:277. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Beltran H, Beer TM, Carducci MA, de Bono J, Gleave M, Hussain M, Kelly WK, Saad F, Sternberg C, Tagawa ST, Tannock IF. Eur Urol. 2011;60:279. doi: 10.1016/j.eururo.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 3.Feder-Mengus C, Ghosh S, Reschner A, Martin I, Spagnoli GC. Trends Mol Med. 2008;14:333. doi: 10.1016/j.molmed.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber RD, Old LJ, Smyth MJ. Science. 2011;331:1565. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 5.Vesely M, Kershaw M, Schreiber RD, Smyth MJ. Annu Rev Immunol. 2011;29:235. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 6.Romero P, Cerottini JC, Speiser DE. Cancer Immunol Immunother. 2004;53:249. doi: 10.1007/s00262-003-0473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dangles-Marie V, Richon S, El-Behi M, Echchakir H, Dorothee G, Thiery J, Validire P, Vergnon I, Menez J, Ladjimi M, Chouaib S, Bellet D, Mami-Chouaib F. Cancer Res. 2003;63:3682. [PubMed] [Google Scholar]
- 8.Gattinoni L, Powell DJ, Rosenberg SA, Restifo NP. Nat Rev Immunol. 2006;6:383. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheever MA, Higano CS. Clin Cancer Res. 2011;17:3520. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 10.Dangles V, Validire P, Wertheimer M, Richon S, Bovin C, Zeliszewski D, Vallancien G, Bellet D. Int J Cancer. 2002;98:51. doi: 10.1002/ijc.10140. [DOI] [PubMed] [Google Scholar]
- 11.Feder-Mengus C, Ghosh S, Weber WP, Wyler S, Zajac P, Terracciano L, Oertli D, Heberer M, Martin I, Spagnoli GC, Reschner A. Br J Cancer. 2007;96:1072. doi: 10.1038/sj.bjc.6603664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, Renner K, Timischl B, Mackensen A, Kunz-Schughart L, Andreesen R, Krause SW, Kreutz M. Blood. 2007;109:3812. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh S, Rosenthal R, Zajac P, Weber WP, Oertli D, Heberer M, Martin I, Spagnoli GC, Reschner A. Ann Surg. 2005;242:851. doi: 10.1097/01.sla.0000189571.84213.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budhu S, Loike JD, Pandolfi A, Han S, Catalano G, Constantinescu A, Clynes R, Silverstein SC. Journal of Experimental Medicine. 2010;207:223. doi: 10.1084/jem.20091279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weigelin B, Friedl P. Biochemical Pharmacology. 2010;80:2087. doi: 10.1016/j.bcp.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Tasaki A, Yamanaka N, Kubo M, Matsumoto K, Kuroki H, Nakamura K, Nakahara C, Onishi H, Kuga H, Baba E, Tanaka M, Morisaki T, Katano M. Journal of Immunological Methods. 2004;287:79. doi: 10.1016/j.jim.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. Journal of Biotechnology. 2010;148:3. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Bhattarai N, Gunn J, Zhang M. Adv Drug Deliv Rev. 2010;62:83. doi: 10.1016/j.addr.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Jayakumar R, Prabaharan M, Nair SV, Tamura H. Biotechnol Adv. 2010;28:142. doi: 10.1016/j.biotechadv.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal P, Strijkers GJ, Nicolay K. Adv Drug Deliv Rev. 2010;62:42. doi: 10.1016/j.addr.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Rinaudo M. Polym Int. 2008;57:397. [Google Scholar]
- 22.Lutolf MP, Hubbell JA. Nature Biotechnology. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 23.Lin SL, Chang D, Ying SY. Carcinogenesis. 2007;28:310. doi: 10.1093/carcin/bgl134. [DOI] [PubMed] [Google Scholar]
- 24.Josefsson A, Adamo H, Hammarsten P, Granfors T, Stattin P, Egevad L, Laurent AE, Wikstrom P, Bergh A. Am J Pathol. 2011;179:1961. doi: 10.1016/j.ajpath.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kievit F, Florczyk S, Leung MC, Veiseh O, Park JO, Disis M, Zhang M. Biomaterials. 2010;31:5903. doi: 10.1016/j.biomaterials.2010.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leung M, Kievit F, Florczyk SJ, Veiseh O, Wu J, Park JO, Zhang M. Pharm Res. 2010;27:1939. doi: 10.1007/s11095-010-0198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Leung M, Hopper R, Ellenbogen R, Zhang M. biomaterials. 2010;31:404. doi: 10.1016/j.biomaterials.2009.09.070. [DOI] [PubMed] [Google Scholar]
- 28.Hutmacher DW, Loessner D, Rizzi S, Kaplan DL, Mooney D, Clements JA. Trends in Biotechnology. 2010;28:125. doi: 10.1016/j.tibtech.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Benton G, Kleinman H, George J, Arnaoutova I. Int J Cancer. 2011;128:1751. doi: 10.1002/ijc.25781. [DOI] [PubMed] [Google Scholar]
- 30.Sobel RE, Sadar MD. J Urol. 2005;173:342. doi: 10.1097/01.ju.0000141580.30910.57. [DOI] [PubMed] [Google Scholar]
- 31.Chignola R, Schenetti A, Andrighetto G, Chiesa E, Foroni R, Sartoris S, Tridente G, Liberati D. Cell Prolif. 2000;33:219. doi: 10.1046/j.1365-2184.2000.00174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorlach A, Herter P, Hentschel H, Frosch PJ, Acker H. Int J Cancer. 1994;56:249. doi: 10.1002/ijc.2910560218. [DOI] [PubMed] [Google Scholar]
- 33.Marusic M, Bajzer Z, Freyer JP, Vukpavlovic S. Cell Prolif. 1994;27:73. doi: 10.1111/j.1365-2184.1994.tb01407.x. [DOI] [PubMed] [Google Scholar]
- 34.Fischbach C, Chen R, Matsumoto T, Schmelzle T, Brugge JS, Polverini PJ, Mooney DJ. Nature Methods. 2007;4:855. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 35.Lee GY, Kenny P, Lee E, Bissell M. Nat Meth. 2007;4:359. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weigelin B, Friedl P. Biochem Pharmacol. 2010;80:2087. doi: 10.1016/j.bcp.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Zaman MH, Trapani LM, Sieminski AL, Siemeski A, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Proc Natl Acad Sci USA. 2006;103:10889. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson MJ, Ritz J. Blood. 1990;76:2421. [PubMed] [Google Scholar]
- 39.Olson B, Mcneel D. Cancer Immunol Immunother. 2011;60:781. doi: 10.1007/s00262-011-0987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang HM, Yang D, Xu WY, Wang YQ, Ruan ZH, Zhao TT, Han JF, Wu YZ. Immunol Lett. 2008;120:65. doi: 10.1016/j.imlet.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Pievani A, Borleri G, Pende D, Moretta L, Rambaldi A, Golay J, Introna M. Blood. 2011;118:3301. doi: 10.1182/blood-2011-02-336321. [DOI] [PubMed] [Google Scholar]
- 42.Mendes R, Bromelow KV, Westby M, Galea-Lauri J, Smith IE, O’Brien ME, Souberbielle BE. Cytometry. 2000;39:72. doi: 10.1002/(sici)1097-0320(20000101)39:1<72::aid-cyto10>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 43.Doherty DG, Norris S, Madrigal-Estebas L, McEntee G, Traynor O, Hegarty JE, O’Farrelly C. J Immunol. 1999;163:2314. [PubMed] [Google Scholar]
- 44.Wu JD, Higgins LM, Steinle A, Cosman D, Haugk K, Plymate SR. J Clin Invest. 2004;114:560. doi: 10.1172/JCI22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berencsi KBK, Rani P, Zhang TQ, Gross L, Mastrangelo M, Meropol NJ, Herlyn D, Somasundaram R. J Transl Med. 2011;9:11. doi: 10.1186/1479-5876-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Budhu S, Loike JD, Pandolfi A, Han S, Catalano G, Constantinescu A, Clynes R, Silverstein SC. J Exp Med. 2010;207:223. doi: 10.1084/jem.20091279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gulley J, Drake CG. Clin Cancer Res. 2011;17:3884. doi: 10.1158/1078-0432.CCR-10-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Florczyk S, Kim D, Wood D, Zhang M. J Biomed Mater Res. 2011;98A:614. doi: 10.1002/jbm.a.33153. [DOI] [PubMed] [Google Scholar]