Abstract
The B7 family member programmed death-1 ligand (PD-L1) has been shown to play an inhibitory role in the regulation of T cell responses in several organs. However, the role of PD-L1 in regulating tolerance to self-Ags of the small intestine has not been previously addressed. In this study, we investigated the role of PD-L1 in CD8+ T cell tolerance to an intestinal epithelium-specific Ag using the iFABP-tOVA transgenic mouse model, in which OVA is expressed as a self-Ag throughout the small intestine. Using adoptive transfer of naive OVA-specific CD8+ T cells, we show that loss of PD-1:PD-L1 signaling, by either Ab-mediated PD-L1 blockade or transfer of PD-1−/− T cells, leads to considerable expansion of OVA-specific CD8+ T cells and their differentiation into effector cells capable of producing proinflammatory cytokines. A fatal CD8+ T cell-mediated inflammatory response develops rapidly against the small bowel causing destruction of the epithelial barrier, severe blunting of intestinal villi, and recruitment and activation of myeloid cells. This response is highly specific because immune destruction selectively targets the small intestine but not other organs. Collectively, these results indicate that loss of the PD-1:PD-L1 inhibitory pathway breaks CD8+ T cell tolerance to intestinal self-Ag, thus leading to severe enteric autoimmunity.
Programmed cell death-1 (PD-1)3 and its ligands PD-L1 and PD-L2 are among the most recently characterized members of the B7 family of costimulatory molecules (1). Functional studies of the PD-1 ligands have revealed that PD-L1 and PD-L2 have overlapping functions and can act synergistically to inhibit T cell activation, proliferation, and cytokine production, with PD-L1 playing a predominant role in vivo (1–20). PD-L1 and PD-L2 have been shown to have differential expression on hematopoietic and nonhematopoietic cells. PD-L2 expression is restricted to cells of hematopoietic origin including activated dendritic cells (DCs) and macrophages (1–3, 6–8, 21, 22). In contrast, PD-L1 is more broadly expressed on both hematopoietic cells, including resting and activated T cells, B cells, DCs, macrophages, and bone marrow-derived mast cells, and on nonhematopoietic organs including vascular endothelium, epithelium, muscle, liver, heart, pancreas, placenta, skin, and eye (1–4, 6–10, 21–24). Studies using animal models of autoimmune disease have implicated the PD-1:PD-L1 pathway in peripheral tolerance and protection against autoimmunity. For example, studies using either PD-L1 blocking Ab or mice genetically deficient in PD-1 or PD-L1 have shown that loss of PD-1:PD-L1 signaling can increase the incidence of autoimmune diabetes (4, 6, 12, 17), exacerbate autoimmune kidney disease (18), myocarditis (9), experimental autoimmune encephalomyelitis (19, 20, 25), and autoimmune hepatitis (26), as well as impair fetomaternal tolerance (11) and prevent allograft survival (27).
Despite an extensive number of studies on the regulation of T cell responses by PD-L1, nothing is known about its role in the small intestine. The small intestine is a particularly interesting locale to address the role of PD-L1 because this tissue and the immune cells that reside there are constantly bathed in a myriad of bacterial species as well as other microbes, dietary factors, and environmental Ags, all of which can provoke unwanted immunity and inflammation (28–32). Furthermore, intestinal epithelial cells (IECs) turn over at a relatively high rate, and fragments of dying IECs are constitutively phagocytosed and carried to mesenteric lymph nodes by DCs (33). Together, these dynamics make the intestine potentially susceptible to autoimmune reactivity; surprisingly, however, autoimmune inflammatory disorders are uncommon in the small bowel, which is likely due to elaborate regulatory mechanisms that maintain tolerance to intestinal self-Ags.
In this study, we address the role of PD-L1 in controlling CD8+ T cell responses to the small intestine. We used the iFABP-tOVA transgenic mouse model in which the promoter for intestinal fatty acid binding protein (iFABP) drives expression of a truncated cytosolic form of OVA (tOVA136–336) (34). Thus, OVA is expressed as a self-Ag by mature IECs throughout the small intestine of iFABP-tOVA mice. We previously demonstrated that, in iFABP-tOVA mice, OVA peptide is cross-presented by CD8α+ DCs within mesenteric lymph nodes and directly presented by nonhematopoietic lymph node stromal cells (LNSCs) in all lymph nodes (35). Adoptive transfer of naive OT-I TCR transgenic CD8+ T cells, which recognize a complex of the OVA peptide (OVA257–264) with the MHC class I molecule H-2Kb, into iFABP-tOVA mice, leads to their proliferation and activation in all lymph nodes. However, this proliferative response is abortive, ultimately leading to deletion of the donor OT-I T cells. Thus, we reasoned that if PD-L1 plays a general role in restricting self-reactive CD8+ T cell expansion, then loss of PD-L1 signaling might allow gut-specific T cells to expand and execute their effector functions. In this study, we demonstrate that loss of the PD-1:PD-L1 inhibitory pathway leads to the robust expansion of gut-specific CD8+ effector T cells and lethal and selective destruction of the small intestinal epithelium in iFABP-tOVA mice. Our results provide evidence that the PD-1:PD-L1 inhibitory pathway plays a critical role in maintaining mucosal tolerance by preventing naive, gut-specific CD8+ T cells from accumulating and acquiring pathogenic potential.
Materials and Methods
Mice
Transgenic iFABP-tOVA mice (34) were provided by L. Lefrançois (University of Connecticut, Farmington, CT) and were maintained on a C57BL/6 background. C57BL/6 mice were obtained from The Jackson Laboratory. OT-I CD45.1, iFABP-tOVA, and C57BL/6 mice were bred and maintained under pathogen-free conditions in the Dana-Farber Cancer Institute Animal Research Facility. OT-I PD-1−/− Thy1.1+ mice (6) were bred and maintained on a C57BL/6 background under pathogen-free conditions at Harvard Medical School. All mice were maintained and used in accordance with institutional and National Institutes of Health guidelines. Harvard Medical School and Dana-Farber Cancer Institute are accredited by the American Association for the Accreditation of Laboratory Animal Care.
Abs and flow cytometry
Abs to CD11b (M1/70), CD25 (PC61), CD40 (HM40-3), CD44 (IM7), CD45.1 (Ly5.1, A20), CD62L (MEL-14), CD69 (H1.2F3), and IFN-γ (XMG1.2) were purchased from BioLegend, PD-1 (J43), TNF-α (MP6-XT22), and Thy1.1/CD90.1 (HIS51) were purchased from eBioscience, Vα2 (B20.1) was purchased from Caltag Laboratories, and CD8α (53-6.7), CD45 (30-F11), anti-mouse MHC class II (I-A/I-E, clone 2G9), and PD-L1 (MIH5) were purchased from BD Pharmingen. The lectin Ulex europaeus agglutinin (UEA)-I was purchased from Vector Laboratories and 7-aminoactinomycin D was purchased from BD Pharmingen. For flow cytometry, single cell suspensions were prepared from lymph nodes (pooled axial, brachial, cervical, inguinal, and mesenteric), small intestine, or spleen, washed, counted by trypan blue exclusion, and stained with directly conjugated Abs in PBS supplemented with 0.1% BSA. Data were acquired with a BD FACSCalibur using CellQuest software. Data were analyzed with the FlowJo program (Tree Star).
In vivo PD-L1 blockade and T cell transfers
Monoclonal Ab against murine PD-L1 (10F.9G2, rat IgG2b) was produced and purified as previously described (7, 10). Isotype control rat IgG2b (LTF-2) was obtained from BioXCell. PD-L1 blockade experiments were performed as shown (see Fig. 2). Briefly, C57BL/6 and iFABP-tOVA mice (CD45.2+) were injected i.p. with 200 μg of anti-PD-L1 or isotype control Ab in 100 μl of PBS i.p. every other day throughout the experiment. OVA-specific CD8+ T cell responses to PD-L1 blockade were analyzed by transferring ~1 × 106 CD45.1+ naive OT-I T cells i.v. 24 h after administration of blocking Ab or isotype control. Mice were weighed on a daily basis starting on the day of T cell transfer and until the mice were euthanized 5–7 days later. Lymph nodes (pooled axial, brachial, cervical, inguinal, and mesenteric), spleen, and intestine were then collected for enumeration and characterization of donor T cells (CD45.1+ CD8α+ Vα2+ cells) by flow cytometry. For T cell transfers without Ab treatment, ~1 × 106 Thy1.1+ PD-1−/− naive OT-I T cells were transferred i.v. into Thy1.2+ recipients. Mice were monitored and the tissues were harvested as described for characterization of donor T cells (Thy1.1+ CD8α+ Vα2+ cells) by flow cytometry.
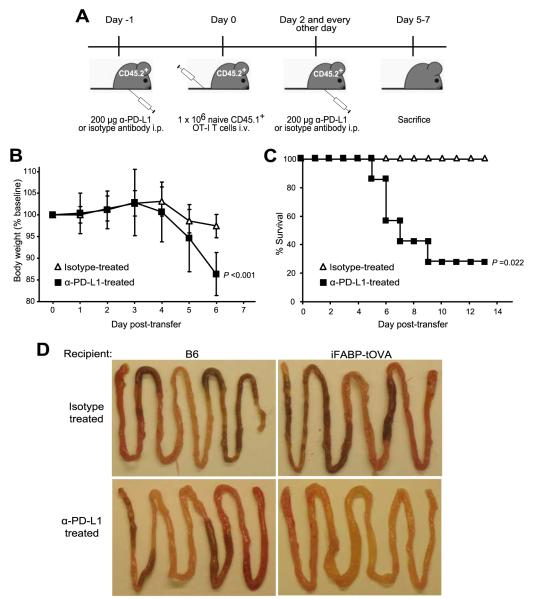
FIGURE 2.
PD-L1 blockade leads to significant weight loss, severe intestinal inflammation, and death. A, Schematic of Ab treatment and T cell transfer regimen. Mice were injected i.p. with 200 μg of anti-PD-L1 Ab or isotype control. One day later, naive congenic CD45.1+ OT-I T cells were transferred i.v. into CD45.2+ C57BL/6 or iFABP-tOVA recipients. Mice were weighed starting on the day of transfer (day 0) and daily thereafter until the mice were euthanized 5–7 days posttransfer. Mice were treated with Ab every other day throughout the experiment. B, Impact of anti-PD-L1 treatment on body weight. Data represent mean ± SD of body weight plotted as a percentage of baseline weight at day 0 for isotype-treated mice (n = 7) and anti-PD-L1-treated iFABP-tOVA mice (n = 6). C, Effect of anti-PD-L1 treatment on the survival of iFABP-tOVA mice. Survival percentage of anti-PD-L1-treated iFABP-tOVA mice (n = 7) was compared with isotype-treated iFABP-tOVA mice (n = 4). D, Whole mounts of the small intestine from isotype-treated and anti-PD-L1-treated C57BL/6 and iFABP-tOVA mice. Images show representative tissues excised from isotype and anti-PD-L1-treated mice 5–7 days after T cell transfer.
Intestinal cell isolation and infiltrate characterization
The small intestine was removed and flushed with 20 ml of cold HBSS supplemented with 20 mM HEPES. Following removal of Peyer’s patches, the tissue was cut open longitudinally, cut again into 0.5- to 1-cm pieces, and transferred into a 50-ml tube with 20 ml of cold HBSS. Intestine fragments were then washed one to two times with 20 ml of cold HBSS each time and passed through a coarse metal strainer. The tissue was then resuspended in 25 ml of RPMI 1640 supplemented with 2% FBS and 20 mM HEPES and incubated at 250 rpm for 30 min at 37°C. Following this incubation, the tubes containing the intestinal fragments were manually shaken vigorously for 30 s to release intestinal cells and then filtered again through a coarse metal strainer. The cell suspension was centrifuged at 300 × g for 5 min at 4°C, resuspended in a 44% Percoll solution (GE Healthcare) at room temperature in 15-ml tubes, and then underlayed with a layer of 67% Percoll. The samples were centrifuged at 600 × g for 20 min at 20°C with smooth acceleration and no brake. IEL were isolated from the gradient interface, washed in RPMI 1640 supplemented with 2% FBS and 20 mM HEPES, centrifuged at 300 × g for 5 min at 4°C, resuspended in PBS supplemented with 0.1% BSA, and processed as described for flow cytometry and cytokine analysis.
For characterization of the leukocytic infiltrate, the IEL suspension was processed in a Percoll gradient as described to remove excess epithelial cells. In addition, the remaining intestinal tissue was digested in 25 ml of RPMI 1640 supplemented with 2% FBS, 20 mM HEPES, 1 mg/ml Collagenase-P (Roche), and 50 μg/ml DNase I (Roche) at 250 rpm for 30 min at 37°C. The cell suspension was then mixed vigorously, filtered, washed, pooled with the IEL fraction, resuspended in PBS supplemented with 0.1% BSA, and processed for flow cytometry.
For isolation of IECs, the small intestine was flushed, Peyer’s patches were removed, and the tissue was washed and cut as described. After washing with cold HBSS, the tissue fragments were resuspended in 20 ml of cold HBSS containing 1 mM DTT, incubated for 45 min at room temperature with moderate shaking, and excess mucus removed with forceps. The tissue was then resuspended in 25 ml of RPMI 1640 supplemented with 2% FBS and 20 mM HEPES, incubated at 250 rpm for 30 min at 37°C, vigorously shaken manually for 30 s, filtered through a coarse metal strainer, centrifuged at 50 × g for 10 min at 4°C, washed and resuspended in PBS supplemented with 0.1% BSA, and processed as described for flow cytometry.
Intracellular cytokine staining
Single cell suspensions were prepared from lymph nodes and small intestine. Cells were plated at 2.5 × 106/ml in RPMI 1640 supplemented with 10% FBS and activated with 2 μg/ml SIINFEKL peptide for 5 h with 0.75 μl/ml GolgiStop (BD Pharmingen) added in the last 4 h. A portion of the samples was activated by PMA (5 ng/ml) and ionomycin (500 ng/ml), or left unactivated, and served as controls. After cell surface staining with Abs to Vα2, CD8α, or CD45.1, cells were fixed with Cytofix solution (BD Pharmingen), permeabilized with CytoPerm solution (BD Pharmingen), stained with Abs to IFN-γ, TNF-α, or appropriate isotype control, and analyzed by flow cytometry.
Histology
Tissue samples were collected, directly fixed in Bouin’s solution, paraffin embedded, and processed for H&E staining at the Dana-Farber/Harvard Cancer Center Rodent Histopathology Core. Histologic analysis was performed in a blinded fashion. Representative images are shown for each experimental condition.
Statistical analysis
All data are presented as the mean ± SD. Statistical analysis used the one-tailed, unpaired Student’s t test for comparison of two experimental groups, and the log-rank test for comparison of survival in a Kaplan-Meier plot. A value for p < 0.05 was considered significant.
Results
Expression of PD-L1 by cells involved in intestinal tolerance
Initially, we sought to determine whether PD-L1 was expressed by the cell populations involved in promoting peripheral CD8+ T cell tolerance to the small intestinal epithelium. We and others have previously reported that tolerogenic CD8α+ DCs constitutively cross-present IEC-derived OVA in gut-draining lymph nodes of iFABP-tOVA mice (34–38). In addition, we and others have shown that a population of CD45− stromal cells restricted to LNSCs can directly present peripheral tissue Ags, including OVA in iFABP-tOVA mice, on MHC class I molecules to CD8+ T cells (35, 39–41). Furthermore, direct presentation of Ag by LNSCs was shown to promote deletional tolerance among CD8+ T cells (35, 39–41). Therefore, we initially sought to examine the expression of PD-L1 by DCs and LNSCs, the APCs that present OVA peptide-MHC class I complexes to naive T cells, in lymph nodes of healthy iFABP-tOVA mice. In agreement with previous reports showing expression of PD-L1 by DCs (3, 13, 15, 22, 42), we found that surface PD-L1 was uniformly expressed by CD11chigh DCs in lymph nodes (Fig. 1). Similarly, we found that a subset of CD45− LNSCs expressed surface PD-L1, although the PD-L1+ cells could be divided into PD-L1int and PD-L1high subsets (Fig. 1). Because PD-L1 can also be expressed by resting T cells (3, 15, 18, 22, 42), we also analyzed CD8+ T cells from C57BL/6 mice and naive OT-I TCR transgenic T cells and found that both populations of CD8+ T cells expressed surface PD-L1 (Fig. 1).
FIGURE 1.
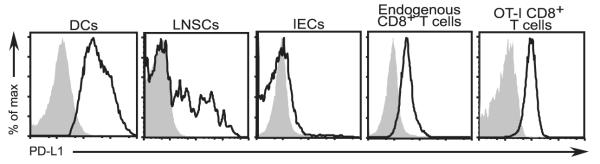
Surface PD-L1 is expressed by DCs, LNSCs, and T cells, but not IECs. Expression of PD-L1 on DCs, LNSCs, IECs, polyclonal CD8+ T cells, and OT-I TCR transgenic T cells (gated on 7-aminoactinomycin D (7AAD)−CD45+CD11chigh, 7AAD−CD45−, 7AAD−CD45− UEA-I+, CD45+CD8+, and CD8+Vα2+ cells, respectively) was analyzed by flow cytometry. Overlays depict staining with anti-PD-L1 Ab for the indicated cell-type (open histogram) compared with isotype control (gray-filled histogram). For each cell type, tissues from n = 3–6 individual mice were analyzed and representative results are shown.
Self-reactive CD8+ T cells may also encounter their cognate Ag at the surface of the intestinal epithelial cell once they infiltrate the small intestine. In iFABP-tOVA recipients, transferred OT-I T cells will initially encounter their cognate Ag in peripheral lymph nodes while circulating in the blood between secondary lymphoid organs (35). However a small number of these cells, which infiltrate the small intestine 4–5 days after transfer without causing damage (34), may encounter their cognate Ag presented by IECs. Thus, we wanted to know whether IECs in the small intestine might also express the co-inhibitory molecule, PD-L1. To address this question, epithelial cells were isolated from the small intestine of C57BL/6 mice and analyzed by flow cytometry. Unexpectedly, we found that IECs, which were identified by their lack of CD45 expression and positive staining with the fucose-binding lectin UEA-I (43), were essentially negative for surface PD-L1 (Fig. 1).
PD-L1 blockade leads to CD8+ T cell-mediated destruction of the small intestine
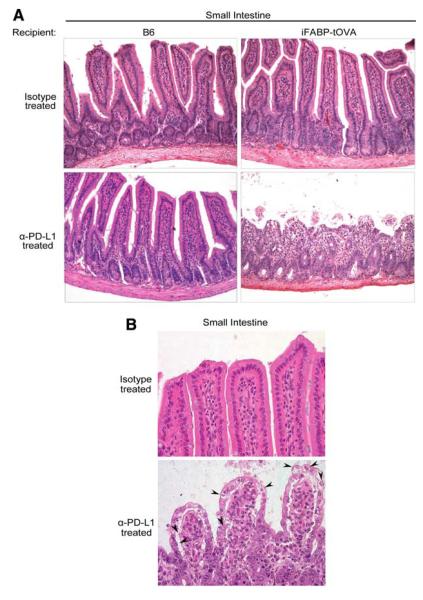
Next, we wanted to determine whether PD-L1 signaling impinges on the response of CD8+ T cells to intestinal self-Ag in iFABP-tOVA mice. To this end, we injected either PD-L1 blocking Ab or an isotype control into CD45.2+ iFABP-tOVA and CD45.2+ C57BL/6 controls i.p. (Fig. 2A) followed by i.v. injection of naive, congenic CD45.1+ OT-I T cells 1 day later. Ab injections were repeated every other day following T cell transfer and mice were monitored daily for weight loss. All anti-PD-L1-treated iFABP-tOVA mice showed significant weight loss within 4–5 days after T cell transfer (p < 0.001) (Fig. 2B) and 71% of mice died by day 9 posttransfer (p = 0.022) (Fig. 2C). Isotype-treated iFABP-tOVA mice maintained normal weight and remained healthy throughout the experiment. Macroscopically, the small intestine in anti-PD-L1-treated iFABP-tOVA mice showed gross alterations including excessive luminal fluid and a lack of digested chow (Fig. 2D), suggesting that the weight loss observed might be due to nutrient malabsorption. In contrast to mice with ulcerative colitis, anti-PD-L1-treated iFABP-tOVA mice did not exhibit intestinal bleeding. Histologic analysis of intestinal sections from anti-PD-L1-treated iFABP-tOVA mice exhibited several hallmarks of enteritis including sloughing off of the epithelial layer, severe villous blunting, an increase in mitotic figures in the crypts (Fig. 3A and data not shown) and elevated numbers of apoptotic bodies in the lamina propria (Fig. 3B, arrowhead). The major histologic alterations were localized primarily at the tips of villi which correlate with the localization of mature, OVA-expressing IECs in the iFABP-tOVA mouse model.
FIGURE 3.
Lack of PD-L1 signaling leads to altered intestinal histology. A, Histology of the small intestine from isotype and anti-PD-L1-treated C57BL/6 and iFABP-tOVA mice 5–7 days posttransfer of T cells. Tissues were fixed and processed for H&E staining. Images at a magnification of ×20 show representative histology of the small intestine for each condition. B, Images at a magnification of ×40 show representative histology of intestinal villi for each condition with apoptotic epithelial cells indicated (arrowhead).
Adoptive transfer of OT-I T cells into anti-PD-L1-treated C57BL/6 or into isotype-treated iFABP-tOVA mice did not lead to enteritis indicating that the autoimmune response that we observe is dependent on both Ag expression and the inhibition of PD-L1 signaling (Fig. 3A). In addition, anti-PD-L1 treatment alone, in the absence of OT-I T cells, did not lead to enteritis, indicating that the observed intestinal damage required the presence of gut-specific CD8+ T cells (see Supplemental Fig. 1).4 Cytofluorimetric analysis of the small intestine of anti-PD-L1-treated iFABP-tOVA mice revealed a significant leukocytic infiltrate as indicated by an average 2-fold increase in CD45+ cells compared with isotype-treated controls (p ≤ 0.008) (see Supplemental Fig. 2A).4 Consistent with an ongoing inflammatory reaction, we found that CD11b+ myeloid cells increased from 30% to 50% among total leukocytes in the small intestine of anti-PD-L1-treated iFABP-tOVA mice (p ≤ 0.001) (see Supplemental Fig. 2A).4 Furthermore, the proportion of activated CD11b+ cells in the small intestine of anti-PD-L1-treated iFABP-tOVA mice was significantly increased compared with isotype-treated iFABP-tOVA mice as indicated by the percentage of CD40+ (p = 0.001) and MHC class II+ (p = 0.006) myeloid cells (see Supplemental Fig. 2B).4
Extraintestinal tissues and organs in anti-PD-L1-treated iFABP-tOVA mice were also examined histologically for signs of tissue destruction and inflammation. Remarkably, the tissue damage was found to be highly specific to the small intestine as other nonlymphoid organs examined including (but not limited to) colon, liver, lung, kidney, and pancreas were healthy and devoid of leukocytic infiltrates (Supplemental Fig. 3).4 Taken together, these results indicate that blocking PD-L1 signaling in iFABP-tOVA mice leads to a lethal autoimmune enteritis that is dependent on the presence of OVA-specific CD8+ T cells and the expression of their cognate self-Ag in the intestinal epithelium.
PD-L1 signaling prevents the accumulation of OVA-specific effector CD8+ T cells
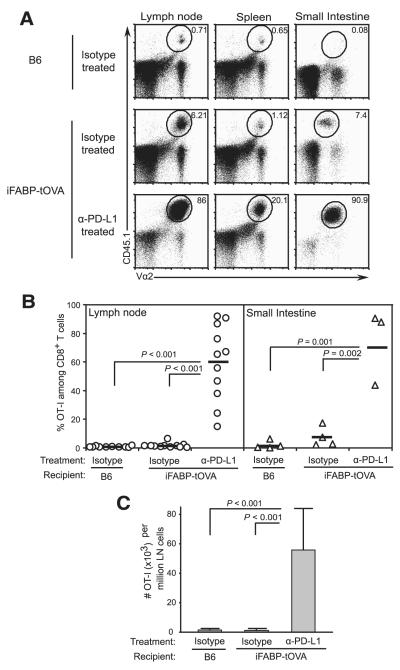
In light of previous reports that PD-L1 signaling can inhibit the expansion and survival of effector T cells, we sought to determine whether blocking PD-L1 signaling would permit the accumulation of OVA-specific CD8+ effector T cells in the iFABP-tOVA mouse model. To this end, we transferred naive CD45.1+ OT-I T cells into anti-PD-L1-treated CD45.2+ iFABP-tOVA mice and examined their fate 5–7 days later by flow cytometry. Upon treatment of iFABP-tOVA mice with PD-L1 blocking Ab, the percentage of OT-I T cells increased an average 37-fold in lymph nodes and 10-fold in the small intestine compared with isotype-treated iFABP-tOVA mice (p ≤ 0.002) (Fig. 4, A and B). The relative increase in OT-I T cells in anti-PD-L1-treated iFABP-tOVA mice corresponded to an average 45-fold increase in their absolute number in lymph nodes compared with isotype-treated iFABP-tOVA mice (p < 0.001) (Fig. 4C).
FIGURE 4.
PD-L1 inhibits the accumulation of gut-reactive CD8+ T cells. A, Accumulation of transferred CD45.1+ OT-I T cells in lymphoid tissues and small intestines of isotype-treated CD45.2+ C57BL/6 and iFABP-tOVA mice and anti-PD-L1-treated iFABP-tOVA mice. Donor-derived (CD45.1+) OT-I T cells were enumerated 5–7 days posttransfer by flow cytometry. Representative dot plots are shown and values at gate indicate percentage of OT-I T cells among total CD8+ cells. B, Graphic representation of data shown in A for lymph nodes (circles) and intestine (triangles). Data represent the percentage of donor OT-I T cells among total CD8+ T cells as mean ± SD for lymph nodes and small intestine. C, Absolute number of OT-I T cells in lymph nodes from data shown in B. Data represent the mean number ± SD of donor OT-I T cells per 106 lymph node cells for each tissue.
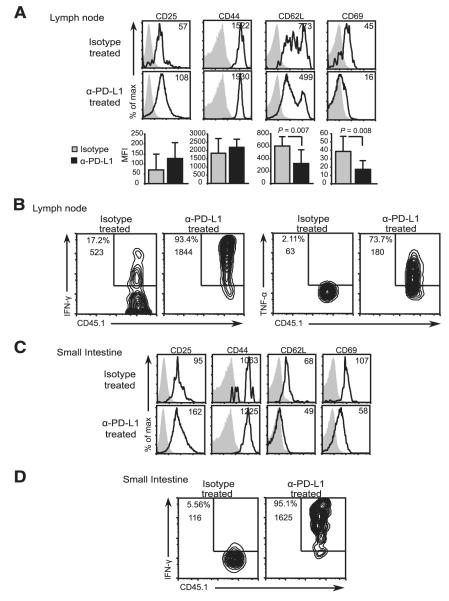
Next we evaluated the differentiation status of the OVA-specific CD8+ T cells that accumulate in lymph nodes and in the gut of anti-PD-L1-treated iFABP-tOVA mice. Initially, the surface expression of several prototypical activation markers by donor OT-I T cells in lymph nodes was evaluated by flow cytometry. OVA-specific CD8+ T cells in lymph nodes of both isotype and anti-PD-L1-treated iFABP-tOVA mice displayed an activated phenotype as indicated by the up-regulation of CD25 and CD44, and down-regulation of CD62L and the early activation marker, CD69, relative to C57BL/6 control recipients (Fig. 5A and data not shown). However, differences in the expression levels of these markers between isotype and anti-PD-L1-treated iFABP-tOVA mice were only statistically significant (p = 0.007 and p = 0.008) for CD62L and CD69, respectively (Fig. 5A). We then ascertained whether the expanded population of OVA-specific OT-I T cells in anti-PD-L1-treated iFABP-tOVA mice had differentiated into effector CD8+ T cells by examining their expression of the effector cytokines IFN-γ and TNF-α by intracellular cytokine staining. The donor OT-I T cells in lymph nodes of anti-PD-L1-treated iFABP-tOVA mice expressed IFN-γ and TNF-α upon in vitro restimulation with SIINFEKL peptide (Fig. 5B), whereas OT-I T cells from isotype-treated C57BL/6 (data not shown) and iFABP-tOVA mice had limited effector function as indicated by the minimal production of IFN-γ and TNF-α (Fig. 5B). Finally, we evaluated the effector status of the donor OT-I T cells in the small intestine of anti-PD-L1-treated iFABP-tOVA mice and the relevant controls. Gut-infiltrating OT-I T cells displayed an activated phenotype in both isotype and anti-PD-L1-treated iFABP-tOVA mice (Fig. 5C). However, only gut-infiltrating OT-I T cells from anti-PD-L1-treated mice expressed intracellular IFN-γ production upon in vitro restimulation with OVA peptide (Fig. 5D). Taken together, these results indicate that systemic PD-L1 blockade releases the brakes on gut-specific CD8+ T cells, allowing these to accumulate and differentiate into potent effector cells following activation by their cognate Ag.
FIGURE 5.
Gut-specific CD8+ T cells acquire effector function upon PD-L1 blockade. Activation of CD45.1+ OT-I T cells in isotype and anti-PD-L1-treated iFABP-tOVA mice. Expression of CD25, CD44, CD62L, and CD69 by donor OT-I T cells (gated on CD8+Vα2+CD45.1+ cells) in lymph nodes (A) and small intestine (C) was analyzed by flow cytometry. Overlays depict the expression of the indicated markers by OT-I T cells (open histogram) from isotype and anti-PD-L1-treated iFABP-tOVA mice compared with isotype Ab staining (gray-filled histogram). Values indicate the mean fluorescence intensity (MFI) of OT-I T cells for the indicated marker. For each condition, n = 3–6 individual mice were analyzed, and representative histograms are shown. Results represent the mean fluorescence intensity ± SD for the indicated markers on donor OT-I T cells isolated from lymph nodes. Cytokine production by CD45.1+ OT-I T cells in lymph nodes (B) and small intestines (D) from isotype and anti-PD-L1-treated iFABP-tOVA mice analyzed by intracellular cytokine staining and flow cytometry. The n = 3–9 individual mice were analyzed for each condition and representative contour plots are shown. Values at gate indicate percentage of cells among CD8+Vα2+ cells and mean fluorescence intensity for IFN-γ or TNF-α.
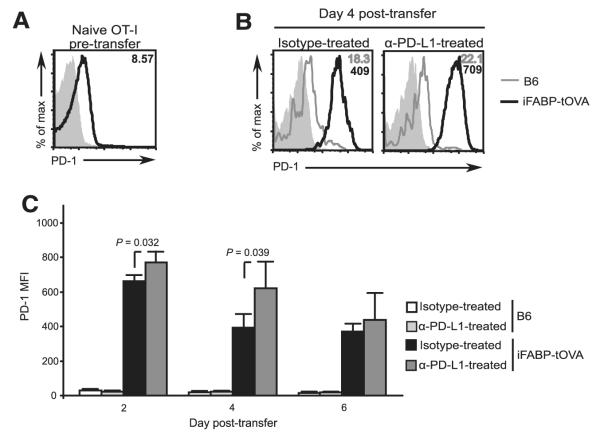
PD-L1 blockade can affect CD8+ T cells at early and late stages of activation
The receptor for PD-L1, PD-1, is up-regulated almost immediately upon Ag encounter (44) and is further increased upon T cell activation (1, 22, 44–47). Indeed, a recent report indicated that PD-1 is up-regulated on self-reactive T cells as soon as the first cell division after TCR engagement (44). Furthermore, it was shown that Ab-mediated blockade of the PD-1:PD-L1 pathway at the time of initial Ag encounter was sufficient to block this inhibitory pathway and endow the self-reactive T cells with the capacity to differentiate into effector cells. To better understand how PD-L1 blockade confers its effect on adoptively transferred naive OT-I T cells in our experimental system, we first examined the surface expression of PD-1 on the donor cells. We found that naive OT-I T cells display surface PD-1, albeit at low levels, before (Fig. 6A) and following transfer into C57BL/6 control mice (Fig. 6, B and C). Upon transfer into iFABP-tOVA recipients, in which OVA is presented in all lymph nodes, OT-I T cells displayed significantly higher PD-1 expression (Fig. 6B). A detailed kinetic analysis of surface PD-1 expression indicated that PD-1 is up-regulated ~29-fold by day 2 posttransfer on OT-I T cells isolated from lymph nodes of both isotype and anti-PD-L1-treated-iFABP-tOVA mice (Fig. 6C). The OT-I T cells in anti-PD-L1-treated iFABP-tOVA mice expressed significantly higher levels of PD-1 than OT-I T cells in isotype-treated controls at days 2 and 4 posttransfer (Fig. 6C), likely reflecting a more activated phenotype. Surface PD-1 levels remained relatively high for the duration of the experiment on OT-I T cells in both conditions in iFABP-tOVA mice though surface levels decreased somewhat between days 2 and 6. By day 6 posttransfer, there was no statistically significant difference in the expression of PD-1 on OT-I T cells between isotype and anti-PD-L1-treated iFABP-tOVA mice. The expression of PD-1 by both naive and activated OT-I T cells suggests that the PD-1: PD-L1 inhibitory pathway may regulate these cells as early as the first encounter with Ag within lymph nodes and throughout their life history, provided the ligand for this receptor is available. Thus, blockade of PD-L1 signaling prevents gut-specific CD8+ T cells from receiving a negative signal through PD-1 thereby allowing these cells to develop into effector cells with pathogenic function.
FIGURE 6.
PD-L1 blockade can affect self-reactive CD8+ T cell function early upon Ag encounter in iFABP-tOVA mice. A, Surface anti-PD-1 staining on naive OT-I T cells gated on CD8+Vα2+ CD45.1+ cells (open histogram) before transfer compared with isotype control (gray-filled histogram). Value in histogram indicates mean fluorescence intensity (MFI) for PD-1. B, Overlays depict PD-1 expression by OT-I T cells isolated from lymph nodes 4 days after adoptive transfer into isotype and anti-PD-L1-treated C57BL/6 (gray line histogram) and iFABP-tOVA (open histogram) mice compared with isotype control (gray-filled histogram). Values in histograms indicate mean fluorescence intensity for PD-1. C, Surface PD-1 expression over time on OT-I T cells isolated from lymph nodes of isotype and anti-PD-L1-treated C57BL/6 and iFABP-tOVA mice. Data represent the mean fluorescence intensity ± SD for PD-1 expression on OT-I T cells (n = 3 mice for each condition).
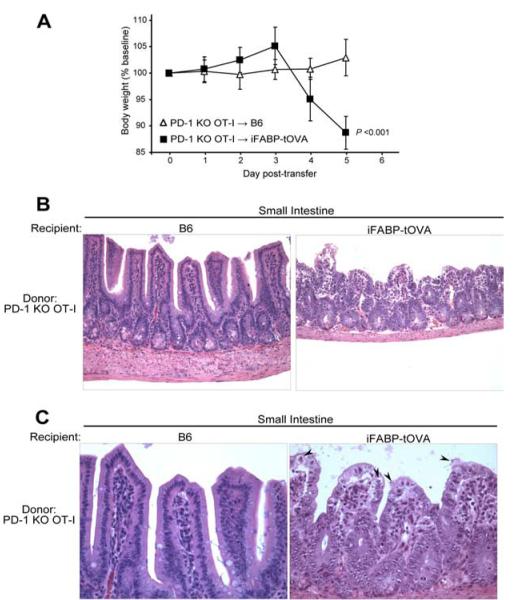
PD-1 deficiency allows gut-specific CD8+ T cells to attack the small intestine
Recently, it was shown that PD-L1 can interact with CD80 and that this interaction diminished T cell activation, reduced proliferation, and decreased cytokine production (42). In addition, it was shown that the PD-L1 Ab clone used in this study could block the interaction of PD-L1 with both PD-1 and CD80. To ascertain whether loss of the PD-1:PD-L1 inhibitory pathway was responsible for the autoimmune response observed in our studies we performed similar transfer experiments using PD-1−/− OT-I T cells. For this naive, Thy1.1+ marked, PD-1−/− OT-I T cells were injected i.v. into Thy1.2+ C57BL/6 and iFABP-tOVA mice. Significant weight loss was observed within 4–5 days in 100% of iFABP-tOVA mice that received PD-1−/− OT-I T cells (p < 0.001) (Fig. 7A), whereas C57BL/6 control mice maintained normal weight and remained healthy. Histologic analysis of intestinal sections 5 days posttransfer revealed that iFABP-tOVA mice, but not C57BL/6 controls, exhibited the same hallmarks of enteritis previously observed in anti-PD-L1-treated iFABP-tOVA mice (Fig. 7B) with most histologic alterations concentrated at the villus tips. Consistent with our experiments using PD-L1 blocking Ab, extraintestinal organs were devoid of leukocytic infiltrates and histologic alterations (data not shown).
FIGURE 7.
PD-1 deficiency in gut-specific CD8+ T cells leads to autoimmune enteritis. A, Weight of mice following transfer of PD-1−/− OT-I T cells. Mice were weighed starting on the day of transfer (day 0) and daily thereafter until the mice were euthanized. Data represent mean body weight ± SD plotted as a percentage of baseline weight at day 0 for C57BL/6 mice (n = 6) and iFABP-tOVA mice (n = 12). B, Histology of the small intestine from C57BL/6 and iFABP-tOVA mice 5 days after transfer of PD-1−/− OT-I T cells. Tissues were fixed and processed for H&E staining. Images at a magnification of ×20 show representative histology of the small intestine for each condition. C, Histology of intestinal villi from small intestines of C57BL/6 and iFABP-tOVA mice 5 days after transfer of PD-1−/− OT-I T cells. Tissues were fixed and processed for H&E staining. Images at a magnification of ×40 show representative histology of intestinal villi for each condition (arrowhead), indicating apoptotic epithelial cells.
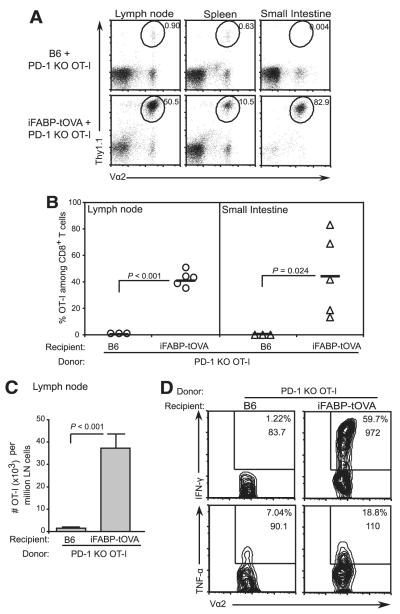
Next, we sought to determine whether transfer of PD-1−/− OT-I T cells into iFABP-tOVA mice led to an accumulation of OVA-specific CD8+ effector T cells as observed in our experiments with PD-L1 blockade. Flow cytometric analysis of tissues from iFABP-tOVA mice 5 days posttransfer indicated that the relative abundance of donor PD-1−/− OT-I T cells increased 50-fold in lymph nodes and 4500-fold in the small intestine (p ≤ 0.02) relative to C57BL/6 control mice (Fig. 8, A and B). The relative increase in PD-1−/− OT-I T cells in iFABP-tOVA mice corresponded to an average 25-fold increase in their absolute number in lymph nodes (p < 0.001) compared with C57BL/6 controls (Fig. 8C). Furthermore, in lymph nodes from iFABP-tOVA mice, the PD-1−/− OT-I T cells exhibited a marked increase in IFN-γ expression and a modest increase in TNF-α production upon in vitro restimulation with OVA peptide (Fig. 8D). These data indicate that PD-1 deficiency in gut-reactive CD8+ T cells can lead to an autoimmune response against the small intestine characterized by accumulation of self-reactive CD8+ T cells with effector function and subsequent destruction of the gut epithelium. Importantly, the events leading to the development of autoimmune enteritis and the morphology of the inflammatory lesions were nearly identical in iFABP-tOVA mice that received PD-L1 blocking Ab and those that received PD-1−/− OT-I T cells. In sum, our results suggest that loss of signaling via PD-1:PD-L1 is sufficient to avert tolerance induction among gut-specific CD8+ T cells, thus allowing a destructive autoimmune response against the small intestine to ensue.
FIGURE 8.
PD-1 deficiency allows gut-specific CD8+ T cells to accumulate, and acquire effector function. A, Accumulation of transferred Thy1.1+ PD-1−/− OT-I T cells in lymphoid tissues and small intestines of C57BL/6 and iFABP-tOVA mice. OT-I T cell accumulation was assessed 5 days posttransfer by enumerating donor-derived (Thy1.1+) PD-1−/− OT-I T cells. Values at gate indicate the percentage of OT-I T cells among total CD8+ cells. B, Graphic of data shown in A for lymph nodes (circles) and intestine (triangles). Data represent mean ± SD of the percentage of donor PD-1−/− OT-I T cells among total CD8+ T cells. C, Absolute number of PD-1−/− OT-I T cells in lymph nodes from data shown in B. Data represent the mean number ± SD of donor OT-I T cells per 106 lymph node cells. D, Cytokine production by PD-1−/− OT-I T cells in lymph nodes from C57BL/6 and iFABP-tOVA mice analyzed by intracellular cytokine staining and flow cytometry. For each condition, n = 3–5 individual mice were analyzed and representative contour plots are shown. Values in gate indicate the percentage of cells among CD8+ cells and the mean fluorescence intensity for IFN-γ or TNF-α.
Discussion
In this study, we have shown that PD-1:PD-L1 interactions play a critical role in preventing CD8+ T cell-mediated autoimmunity against intestinal self-Ag. Treatment of iFABP-tOVA mice with anti-PD-L1 Ab led to the development of lethal enteritis within 5–7 days of OT-I T cell transfer. The intestinal pathology was characterized by apoptosis of epithelial cells, sloughing off of the villous epithelium, severe blunting of the villi, increased cell division in the crypts, and leukocytic infiltration. The villi appeared to be the primary target of the T cells as the crypts and the submucosa were largely intact and devoid of leukocytic infiltrates. Interestingly, the intestinal pathology observed in our study was reminiscent of the clinical picture in human celiac disease, an autoinflammatory condition of the small intestine resulting from an inappropriate immune response to ingested gluten (48, 49). The autoimmune reactivity required the presence of gut-specific CD8+ T cells and selectively targeted the small intestine, corresponding with the pattern of OVA expression in iFABP-tOVA mice (34, 35). Following transfer into anti-PD-L1-treated iFABP-tOVA mice, OT-I T cells expanded and accumulated within lymphoid tissues and small intestine and differentiated into potent, cytokine-producing effector cells. Furthermore, we found that transfer of PD-1−/− OT-I T cells into iFABP-tOVA mice led to autoimmune enteritis which developed with similar kinetics, similar pathologic features, and a nearly indistinguishable CD8+ T cell response compared with PD-L1 blockade. Thus, our study demonstrates that loss of PD-1:PD-L1 signaling leads to the differentiation of naive CD8+ T cells, which would otherwise be tolerized in this model (34, 35), into potent effector cells that accumulate in a large number in the small intestine and cause lethal damage to the epithelial barrier.
Although this represents the first study of PD-1:PD-L1 signaling in the regulation of immunity in the small intestine our results are mostly in agreement with previous studies showing that loss of PD-L1 signaling, either from genetic deficiency or Ab blockade, leads to inflammation, increased leukocytic infiltration, and T cell-mediated tissue damage, resulting in the loss of vital organ function (4, 6, 9, 12, 17–20, 25, 26). Though it has been shown in a different transgenic model that expression of self-Ag alone by the intestinal epithelium is sufficient to stimulate adoptively transferred CD8+ T cells to differentiate into potent effector cells and provoke autoimmune-mediated intestinal inflammation (50), we and others have found this not to be the case (34, 35, 40). Rather we find that interactions between PD-1 and PD-L1 occurring before T cell entry into the intestine prevent naive CD8+ T cells from developing into gut-specific effectors that can mediate intestinal damage.
Goldberg and colleagues (44) recently reported that naive self-reactive CD8+ T cells express high levels of PD-1 as early as their first cell division after Ag encounter and therefore can become tolerized immediately after recognition of self-Ag in secondary lymphoid organs. Most importantly, they showed that blockade of the PD-1:PD-L1 signaling pathway during initial Ag encounter inhibited tolerance induction and instead allowed self-reactive CD8+ T cells to gain effector potential. We also find that PD-1 is expressed by naive OT-I T cells and that this molecule is up-regulated within 48 h after transfer into iFABP-tOVA mice. Given these results and our observation that the APCs presenting OVA in lymph nodes of iFABP-tOVA mice display surface PD-L1, it seems likely that PD-L1 blockade is influencing gut-specific CD8+ T cells during their initial Ag encounter in lymph nodes. Thus, the presentation of OVA peptide-MHC class I complexes to circulating, naive CD8+ T cells by lymph node APCs in untreated iFABP-tOVA mice would normally be accompanied by a potent co-inhibitory signal provided by PD-1:PD-L1 interactions. T cell activation in this setting would lead to an abortive proliferative response, thereby preventing the responding self-reactive CD8+ T cells from surviving and differentiating into potentially hazardous effector cells.
Our finding that IECs lack PD-L1 under steady state conditions was rather surprising given the broad expression of PD-L1 on both hematopoietic cells and parenchymal tissues (1–4, 6–10, 21–24). However, it remains to be determined whether PD-L1 can be up-regulated on IECs upon inflammation or infection as has been previously shown with human gastric epithelial cells upon infection with Helicobacter pylori (51). The lack of PD-L1 on IECs under steady state conditions could be potentially dangerous if gut-specific CD8+ T cells had immediate access to the small intestinal mucosa. However, we and others have shown that adoptively transferred, naive OT-I T cells initially enter the blood and circulate between secondary lymphoid organs for several days. Though most of the donor cells are eventually deleted under steady state conditions, a fraction of these cells can enter the small intestine by day 4 posttransfer (34, 35). However, these gut-infiltrating CD8+ T cells lack effector function and do not cause intestinal damage (34, 35). As shown in our current studies, only OT-I T cells from anti-PD-L1-treated iFABP-tOVA mice showed increased production of IFN-γ and TNF-α and mounted a pathological response against the small intestine. This observation suggests that self-reactive CD8+ T cells may receive extensive and perhaps irreversible inhibitory signals from PD-L1-expressing APCs during their first 3- to 4-day passage through lymph nodes. The time that gut-reactive CD8+ T cells spend interacting with APCs within lymph nodes might be critical for their receipt of inhibitory signals via PD-L1 while being imprinted to migrate to the gut, thus preventing these gut-infiltrating T cells from becoming pathogenic upon interaction with PD-L1− IECs.
We recently reported that PD-L1 and CD80 could interact specifically as binding partners with an affinity that was intermediate to that of CD80 with CD28 and CD80 with CTLA-4 (42). Importantly, the CD80:PD-L1 interaction impaired T cell activation, proliferation, and cytokine production. Furthermore, we found that both the PD-1:PD-L1 and CD80:PD-L1 interactions could be inhibited using a well-characterized blocking Ab to PD-L1 (7, 10). The same Ab was used in the current study to block PD-L1 interactions. Our results showing that transfer of PD-1−/− OT-I T cells into iFABP-tOVA mice leads to the same autoimmune enteritis observed with Ab-mediated PD-L1 blockade suggests that the PD-1:PD-L1 signaling pathway is sufficient to protect the small intestine from CD8+ T cell-mediated autoimmunity. However, this mechanism does not preclude a contribution of the CD80:PD-L1 pathway and further work will be needed to evaluate a possible role for this pathway in the maintenance of intestinal tolerance.
In conclusion, we have found that loss of PD-1:PD-L1 signaling can break intestinal tolerance in iFABP-tOVA mice leading to CD8+ T cell-mediated autoimmune enteritis. Although the regulation of T cell responses in the small intestine is undoubtedly controlled by myriad complex mechanisms, this study reveals the importance of the PD-1:PD-L1 pathway in maintaining intestinal tolerance. Such safeguards are essential given the degree of bacterial and environmental provocation to which the small intestine is subjected. Elucidating the mechanistic details of T cell responses to innocuous and potentially harmful intestinal Ags will be important for understanding immunologic tolerance and applying this knowledge to treat inflammatory bowel diseases.
Supplementary Material
Acknowledgments
We thank members of the Turley lab for helpful discussions, Jose G. Martinez for discussions and critical review of this manuscript, Melissa Yowono Tjota for mouse cartoon and for proofreading of this manuscript, Vijay K. Vanguri for assistance with PD-1−/− OT-I mice, and Etienne Gagnon, J. Rodrigo Mora, and Jessica Wagner for technical advice.
Footnotes
This work was supported in part by a grant from the Claudia Adams Barr Program for Innovative Cancer Research (to S.J.T.), the Baruj Benacerraf Fellowship in Immunology (to K.G.E.), and National Institutes of Health Grants R01 DK074500 (to S.J.T.) and AI56299 (to A.H.S. and G.J.F.).
Abbreviations used in this paper: PD-1, programmed cell death-1; IEC, intestinal epithelial cell; IEL, intestinal epithelial lymphocyte; DC, dendritic cell; iFABP, intestinal fatty acid binding protein; UEA, Ulex europaeus agglutinin; LNSC, lymph node stromal cell.
The online version of this article contains supplemental material.
Disclosures Dr. Gordon J. Freeman and Dr. Arlene H. Sharpe have received licensing fees on patents in the PD-1 pathway. All other authors have no financial conflict of interest.
References
- 1.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 2.Ishida M, Iwai Y, Tanaka Y, Okazaki T, Freeman GJ, Minato N, Honjo T. Differential expression of PD-L1 and PD-L2, ligands for an inhibitory receptor PD-1, in the cells of lymphohematopoietic tissues. Immunol. Lett. 2002;84:57–62. doi: 10.1016/s0165-2478(02)00142-6. [DOI] [PubMed] [Google Scholar]
- 3.Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur. J. Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- 4.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keir ME, Latchman YE, Freeman GJ, Sharpe AH. Programmed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytes. J. Immunol. 2005;175:7372–7379. doi: 10.4049/jimmunol.175.11.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keir ME, Freeman GJ, Sharpe AH. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J. Immunol. 2007;179:5064–5070. doi: 10.4049/jimmunol.179.8.5064. [DOI] [PubMed] [Google Scholar]
- 7.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 9.Grabie N, Gotsman I, DaCosta R, Pang H, Stavrakis G, Butte MJ, Keir ME, Freeman GJ, Sharpe AH, Lichtman AH. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. 2007;116:2062–2071. doi: 10.1161/CIRCULATIONAHA.107.709360. [DOI] [PubMed] [Google Scholar]
- 10.Eppihimer MJ, Gunn J, Freeman GJ, Greenfield EA, Chernova T, Erickson J, Leonard JP. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation. 2002;9:133–145. doi: 10.1038/sj/mn/7800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, Noelle RJ, Coyle A, Mellor AL, Khoury SJ, Sayegh MH. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 2005;202:231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Orozco N, Wang YH, Yagita H, Dong C. Cutting edge: programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J. Immunol. 2006;177:8291–8295. doi: 10.4049/jimmunol.177.12.8291. [DOI] [PubMed] [Google Scholar]
- 13.Selenko-Gebauer N, Majdic O, Szekeres A, Hofler G, Guthann E, Korthauer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H, et al. B7–H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J. Immunol. 2003;170:3637–3644. doi: 10.4049/jimmunol.170.7.3637. [DOI] [PubMed] [Google Scholar]
- 14.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J. Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 16.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat. Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 17.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menke J, Lucas JA, Zeller GC, Keir ME, Huang XR, Tsuboi N, Mayadas TN, Lan HY, Sharpe AH, Kelley VR. Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: distinct roles. J. Immunol. 2007;179:7466–7477. doi: 10.4049/jimmunol.179.11.7466. [DOI] [PubMed] [Google Scholar]
- 19.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc. Natl. Acad. Sci. USA. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salama AD, Chitnis T, Imitola J, Ansari MJ, Akiba H, Tushima F, Azuma M, Yagita H, Sayegh MH, Khoury SJ. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J. Exp. Med. 2003;198:71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc. Natl. Acad. Sci. USA. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 23.Muhlbauer M, Fleck M, Schutz C, Weiss T, Froh M, Blank C, Scholmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-α and -γ and mediates T cell apoptosis. J. Hepatol. 2006;45:520–528. doi: 10.1016/j.jhep.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu B, Guleria I, Khosroshahi A, Chitnis T, Imitola J, Azuma M, Yagita H, Sayegh MH, Khoury SJ. Differential role of programmed death-ligand 1 [corrected] and programmed death-ligand 2 [corrected] in regulating the susceptibility and chronic progression of experimental autoimmune encephalomyelitis. J. Immunol. 2006;176:3480–3489. doi: 10.4049/jimmunol.176.6.3480. [DOI] [PubMed] [Google Scholar]
- 26.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7–H1 determines accumulation and deletion of intrahepatic CD8+ T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 27.Koehn BH, Ford ML, Ferrer IR, Borom K, Gangappa S, Kirk AD, Larsen CP. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J. Immunol. 2008;181:5313–5322. doi: 10.4049/jimmunol.181.8.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 29.Nagler-Anderson C. Man the barrier! Strategic defences in the intestinal mucosa. Nat. Rev. Immunol. 2001;1:59–67. doi: 10.1038/35095573. [DOI] [PubMed] [Google Scholar]
- 30.Macpherson AJ, Uhr T. Compartmentalization of the mucosal immune responses to commensal intestinal bacteria. Ann. NY Acad. Sci. 2004;1029:36–43. doi: 10.1196/annals.1309.005. [DOI] [PubMed] [Google Scholar]
- 31.Uhlig HH, Powrie F. Dendritic cells and the intestinal bacterial flora: a role for localized mucosal immune responses. J. Clin. Invest. 2003;112:648–651. doi: 10.1172/JCI19545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanaway P. Balance of flora, galt, and mucosal integrity. Altern. Ther. Health Med. 2006;12:52–61. [PubMed] [Google Scholar]
- 33.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vezys V, Olson S, Lefrançois L. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 2000;12:505–514. doi: 10.1016/s1074-7613(00)80202-2. [DOI] [PubMed] [Google Scholar]
- 35.Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat. Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 36.Belz GT, Behrens GM, Smith CM, Miller JF, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, Heath WR. The CD8α+ dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J. Exp. Med. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 38.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 39.Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, Anderson MS. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magnusson FC, Liblau RS, von Boehmer H, Pittet MJ, Lee JW, Turley SJ, Khazaie K. Direct presentation of antigen by lymph node stromal cells protects against CD8 T-cell-mediated intestinal autoimmunity. Gastroenterology. 2008;134:1028–1037. doi: 10.1053/j.gastro.2008.01.070. [DOI] [PubMed] [Google Scholar]
- 41.Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J. Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- 42.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellinger A, Pavelka M. Localization of fucosyl residues in cellular compartments of rat duodenal absorptive enterocytes and goblet cells. Eur. J. Cell Biol. 1988;47:62–71. [PubMed] [Google Scholar]
- 44.Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, Harris TJ, Getnet D, Whartenby KA, et al. Role of PD-1 and its ligand, B7–H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vibhakar R, Juan G, Traganos F, Darzynkiewicz Z, Finger LR. Activation-induced expression of human programmed death-1 gene in T-lymphocytes. Exp. Cell Res. 1997;232:25–28. doi: 10.1006/excr.1997.3493. [DOI] [PubMed] [Google Scholar]
- 46.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 47.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Presutti RJ, Cangemi JR, Cassidy HD, Hill DA. Celiac disease. Am. Fam. Physician. 2007;76:1795–1802. [PubMed] [Google Scholar]
- 49.Liu C, Crawford JM. The gastrointestinal tract: small and large intestines. In: Kumar V, Abbas AK, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 7th ed Elsevier Saunders; Philadelphia: 2005. p. 843. [Google Scholar]
- 50.Westendorf AM, Fleissner D, Deppenmeier S, Gruber AD, Bruder D, Hansen W, Liblau R, Buer J. Autoimmune-mediated intestinal inflammation-impact and regulation of antigen-specific CD8+ T cells. Gastroenterology. 2006;131:510–524. doi: 10.1053/j.gastro.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 51.Das S, Suarez G, Beswick EJ, Sierra JC, Graham DY, Reyes VE. Expression of B7–H1 on gastric epithelial cells: its potential role in regulating T cells during Helicobacter pylori infection. J. Immunol. 2006;176:3000–3009. doi: 10.4049/jimmunol.176.5.3000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.