Abstract
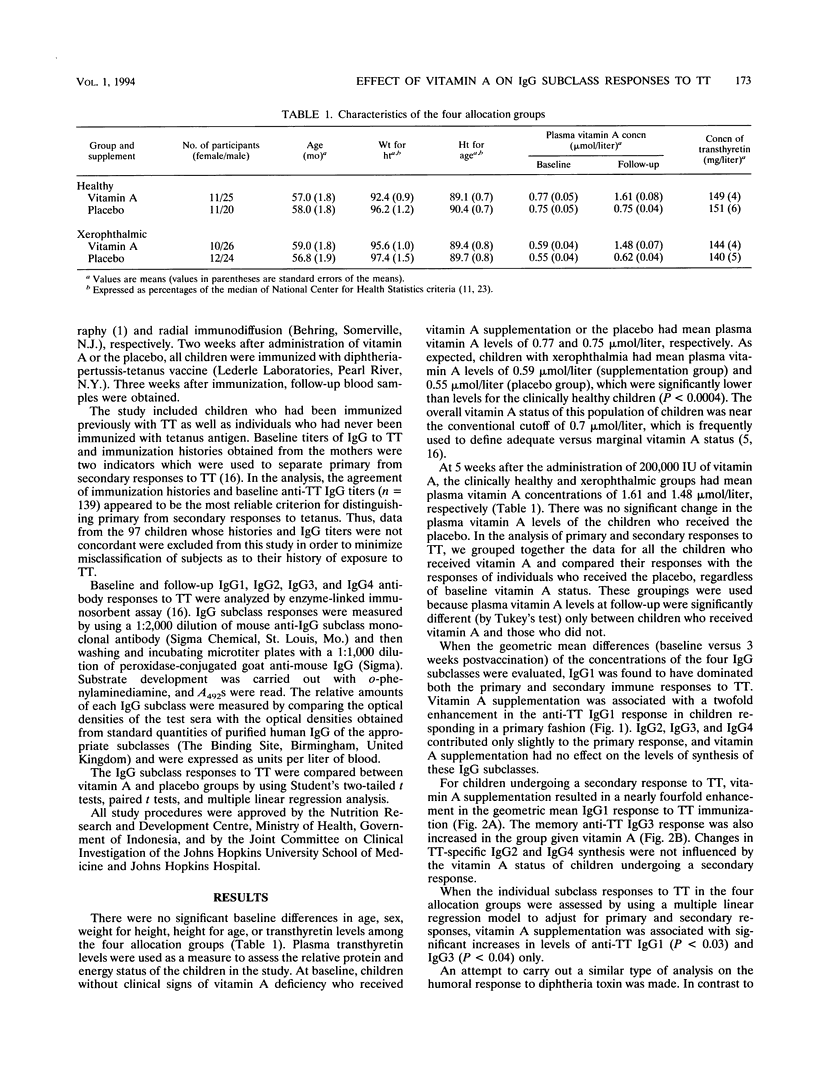
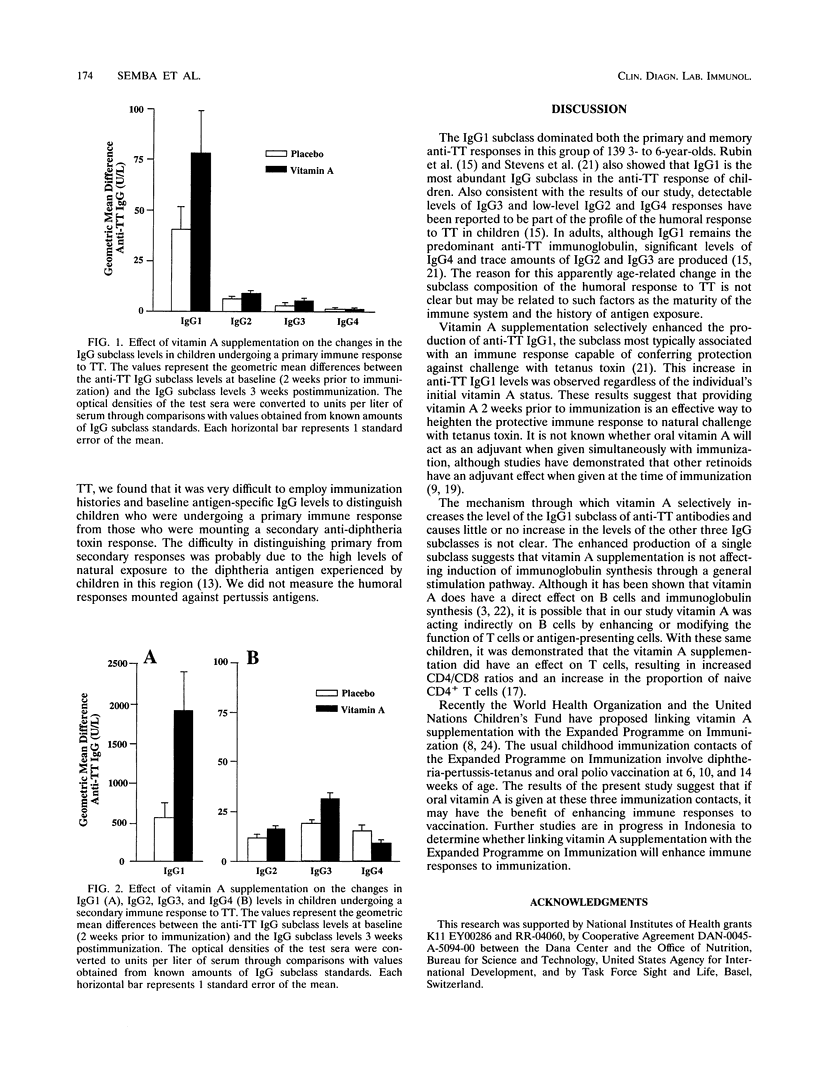
Previously, we demonstrated that administering vitamin A supplements to children resulted in a significant increase in the immunoglobulin G (IgG) response generated against a vaccine dose of tetanus toxoid (TT) (R. D. Semba et al., J. Nutr. 122:101-107, 1991). However, from these analyses we could not determine whether there was an increase in levels of IgG of the subclass presumed to be important for protection against challenge by the toxin or whether there was simply a general increase in the levels of all the IgG subclasses expressing anti-TT activity. The goal of this study was to determine the profile of the anti-TT IgG subclasses in children receiving vitamin A supplementation or a placebo in order to assess the potential utility of the enhanced anti-TT response. In a randomized, double-masked, placebo-controlled clinical trial, the levels of the different anti-TT IgG subclasses were measured in 139 Indonesian preschool children (3 to 6 years of age) 2 weeks before and 3 weeks after immunization. Baseline anti-TT levels and immunization histories were used to separate those children who were responding to TT for the first time from those who responded in a secondary fashion because of previous exposure to TT. Children who were given vitamin A prior to immunization had significant increases in IgG1 levels regardless of whether they were undergoing primary or memory reactions. In the group of individuals who underwent a secondary response to TT, vitamin A supplementation was also associated with a modest but significant change in the levels of anti-TT IgG3. There were only minor changes in the levels of anti-TT IgG2 and IgG4. Since IgG1 is the subclass associated with a protective response to TT immunization, these results suggest that vitamin A supplementation may be a safe and effective intervention to enhance the relevant humoral response to TT and other vaccine antigens.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blomhoff H. K., Smeland E. B., Erikstein B., Rasmussen A. M., Skrede B., Skjønsberg C., Blomhoff R. Vitamin A is a key regulator for cell growth, cytokine production, and differentiation in normal B cells. J Biol Chem. 1992 Nov 25;267(33):23988–23992. [PubMed] [Google Scholar]
- Buck J., Ritter G., Dannecker L., Katta V., Cohen S. L., Chait B. T., Hämmerling U. Retinol is essential for growth of activated human B cells. J Exp Med. 1990 May 1;171(5):1613–1624. doi: 10.1084/jem.171.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutsoudis A., Kiepiela P., Coovadia H. M., Broughton M. Vitamin A supplementation enhances specific IgG antibody levels and total lymphocyte numbers while improving morbidity in measles. Pediatr Infect Dis J. 1992 Mar;11(3):203–209. doi: 10.1097/00006454-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Dresser D. W. Adjuvanticity of vitamin A. Nature. 1968 Feb 10;217(5128):527–529. doi: 10.1038/217527a0. [DOI] [PubMed] [Google Scholar]
- Friedman A. Induction of immune response to protein antigens by subcutaneous co-injection with water-miscible vitamin A derivatives. Vaccine. 1991 Feb;9(2):122–128. doi: 10.1016/0264-410x(91)90268-b. [DOI] [PubMed] [Google Scholar]
- Garbe A., Buck J., Hämmerling U. Retinoids are important cofactors in T cell activation. J Exp Med. 1992 Jul 1;176(1):109–117. doi: 10.1084/jem.176.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Farley R., Soewarso T. I., Karyadi A., Adhyatma M. Assessing the impact of the expanded programme on immunization: the example of Indonesia. Bull World Health Organ. 1987;65(2):203–206. [PMC free article] [PubMed] [Google Scholar]
- Ross A. C. Vitamin A status: relationship to immunity and the antibody response. Proc Soc Exp Biol Med. 1992 Jul;200(3):303–320. doi: 10.3181/00379727-200-43436a. [DOI] [PubMed] [Google Scholar]
- Rubin R. L., Tang F. L., Lucas A. H., Spiegelberg H. L., Tan E. M. IgG subclasses of anti-tetanus toxoid antibodies in adult and newborn normal subjects and in patients with systemic lupus erythematosus, Sjogren's syndrome, and drug-induced autoimmunity. J Immunol. 1986 Oct 15;137(8):2522–2527. [PubMed] [Google Scholar]
- Semba R. D., Muhilal, Scott A. L., Natadisastra G., Wirasasmita S., Mele L., Ridwan E., West K. P., Jr, Sommer A. Depressed immune response to tetanus in children with vitamin A deficiency. J Nutr. 1992 Jan;122(1):101–107. doi: 10.1093/jn/122.1.101. [DOI] [PubMed] [Google Scholar]
- Sidell N., Chang B., Bhatti L. Upregulation by retinoic acid of interleukin-2-receptor mRNA in human T lymphocytes. Cell Immunol. 1993 Jan;146(1):28–37. doi: 10.1006/cimm.1993.1003. [DOI] [PubMed] [Google Scholar]
- Sidell N., Connor M. J., Chang B., Lowe N. J., Borok M. Effects of 13-cis retinoic acid therapy on human antibody responses to defined protein antigens. J Invest Dermatol. 1990 Nov;95(5):597–602. doi: 10.1111/1523-1747.ep12505593. [DOI] [PubMed] [Google Scholar]
- Sommer A., Tarwotjo I., Hussaini G., Susanto D. Increased mortality in children with mild vitamin A deficiency. Lancet. 1983 Sep 10;2(8350):585–588. doi: 10.1016/s0140-6736(83)90677-3. [DOI] [PubMed] [Google Scholar]
- Stevens R., Dichek D., Keld B., Heiner D. IgG1 is the predominant subclass of in vivo- and in vitro- produced anti-tetanus toxoid antibodies and also serves as the membrane IgG molecule for delivering inhibitory signals to anti-tetanus toxoid antibody-producing B cells. J Clin Immunol. 1983 Jan;3(1):65–69. doi: 10.1007/BF00919140. [DOI] [PubMed] [Google Scholar]
- Wang W., Napoli J. L., Ballow M. The effects of retinol on in vitro immunoglobulin synthesis by cord blood and adult peripheral blood mononuclear cells. Clin Exp Immunol. 1993 Apr;92(1):164–168. doi: 10.1111/j.1365-2249.1993.tb05964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West K. P., Jr, Djunaedi E., Pandji A., Kusdiono, Tarwotjo I., Sommer A. Vitamin A supplementation and growth: a randomized community trial. Am J Clin Nutr. 1988 Nov;48(5):1257–1264. doi: 10.1093/ajcn/48.5.1257. [DOI] [PubMed] [Google Scholar]



