Abstract
Background
Data suggest that differences in patient preferences may account for racial disparities in the use of medical interventions. Racial disparities have also been noted in outcomes and the delivery of healthcare services in chronic disease. Whether treatment preferences in chronic disease differ by race is not known.
Methods
We elicited treatment preferences for aggressive therapy in patients with rheumatoid arthritis (RA) who identified themselves as being Black or White.
Results
One hundred fifty consecutive eligible patients were invited to participate. Of these, 136 subjects completed the interview. In unadjusted analysis, 51% of White participants preferred aggressive therapy compared to 16% of Blacks (p<0.0001). Subjects who were married and reported having at least some college education had stronger preferences for aggressive therapy compared to their respective counterparts. After adjusting for covariates, race remained the strongest predictor of aggressive therapy examined in this study [adjusted odds ratio (95% CI) = 11.2 (1.9-64.9)].
Conclusions
In this study, fewer Blacks patients preferred aggressive treatment compared to White patients with similar disease severity. These results have important clinical implications because use of aggressive treatment improves both short and long-term outcomes in RA. Efforts to improve patient education and physician communication should be made to ensure that all patients have an accurate understanding of the benefits, as well as risks, associated with the best available treatment options.
Racial disparities in the delivery of healthcare have been well documented across many disorders (1). Current efforts are now focused on understanding the reasons why minority patients often receive less aggressive care compared to Whites. While unwanted variability in healthcare utilization may be due to both system and provider factors, data suggest that differences in patient preferences may account for some of the differential use of healthcare services across persons of different racial backgrounds. For example, both Byrne et al. (2) and Ibrahim et al. (3) found that Black patients with moderate to severe osteoarthritis were less willing to consider total joint arthroplasty compared to White patients with similar disease severity. Similarly, Whittle et al. (4) found that White patients were more likely to be willing to undergo coronary artery bypass grafting compared to Black patients. Among seriously ill hospitalized patients, preferences for discussions related to resuscitation efforts also differs by race (5). These studies suggest that racial disparities in the use of relatively high risk medical interventions may be partially explained by patient preferences.
In contrast, less is known regarding whether variability in patient preferences influences racial disparities in chronic disease. Cooper et al. (6) found that treatment preferences differ significantly among White, Black and Hispanic patients meeting criteria for major depression. Other studies examining chronic diseases including osteoporosis (7), osteoarthritis (8), diabetes (9) and hypertension (10) have failed to find an association between sociodemographic characteristics and treatment preferences; however, these studies were not designed, nor powered, to examine the impact of race or ethnicity on outcomes.
Rheumatoid arthritis (RA) is the most common type of inflammatory arthritis affecting 1% of the world's population. This disorder results in significant disability in most patients within two decades from symptom onset and is associated with two-fold increased mortality rate (11). The economic impact of RA is comparable to that of coronary artery disease in large part due to the loss of work productivity (12). Some studies suggest that minority RA patients have worse outcomes compared to their counterparts. Specifically, greater levels of pain (p<0.05) and higher rates of disability have been reported in Black RA patients compared to their White counterparts (13,14).
The care of patients with RA has changed dramatically over the past two decades, and now emphasizes the early introduction of aggressive therapies to suppress disease activity. This shift in treatment paradigm is supported by studies indicating that early suppression of disease activity improves both short and long-term clinical outcomes (15-17). Emerging data suggest that minority RA patients, with access to care and insurance, may be less likely to receive aggressive therapy compared to White patients. In a large retrospective cohort study of over 44,000 patients, Berrios-Rivera et al. (18) found that Black patients were about half as likely to use a biologic agent (the newest class of disease modifying agents) than were White patients of similar disease severity. Similarly, using data abstracted from a large national prospective cohort study of community-based RA patients, Head et al. (19) also found that Blacks were less likely to have been prescribed a biologic agent compared to White patients after adjusting for sociodemographic characteristics, disease severity, prior medication use and current health status.
One explanation for these results is that patient preferences for aggressive treatment of RA differ by race. In order to examine this hypothesis we administered a conjoint analysis survey to RA patients under the care of a rheumatologist. Conjoint analysis is a well-validated tool originally developed to understand consumer preferences and predict market shares of innovative products (20-22). This method is strongly based on seminal work in mathematical psychology (23). It has a strong theoretical basis, obtains high levels of internal consistency, is able to predict future choices, and works in real world settings (20, 21, 24, 25). This approach has been used across diverse clinical settings in patients from varied sociodemographic backgrounds, including those with lower levels of education, to elicit preferences for healthcare (7, 8, 26-30). When faced with complex decisions, people typically evaluate a number of attributes and then make trade-offs to arrive at a final choice. Conjoint analysis evaluates these trade-offs to determine which combination of attributes are most preferred. Using this information, preferences for specific options can be calculated.
Conjoint analysis is a decompositional technique that is based on the premise that respondents' preferences can be calculated based on the value that respondents attach to the specific attributes of the product under consideration. For example, consider having to choose from four insurance plans which differ on four attributes: co-pays, access to subspecialists, drug coverage, and deductibles. By asking subjects to evaluate these characteristics, using for example rating and paired comparison tasks (described in detail below), conjoint analysis can determine which plan is preferred by each individual subject. This method minimizes the influences associated with the context in which choices are presented, eliminates ordering effects by presenting treatment characteristics in random order, and makes trade-offs between competing options explicit. Careful consideration of the trade-offs involved in complex decisions has been shown to improve the quality of decision making (31). Because conjoint analysis elicits individual patient preferences based on how they value treatment characteristics, it is not biased by physicians' preferences, recognition of a treatment name, or personal experiences with specific medications.
Patients and Methods
Participants
We recruited RA patients from Washington Hospital Center, Washington, DC (N=57 patients) and Virginia Commonwealth University, Richmond, VA (N=79 patients). Consecutive patients were recruited and interviewed after their appointments in the outpatient rheumatology clinics. Inclusion criteria were RA diagnosed by, and currently under the care of a rheumatologist, a positive serum test for at least one of the RA-associated autoantibodies (rheumatoid factor or anti-cyclic citrullinated peptide), self-identified as Black or White, and able to read and write English. The research protocol was approved by the Washington Hospital Center, Washington, DC and Virginia Commonwealth University, Richmond, institutional review boards and all subjects provided verbal informed consent.
Data Collection
We elicited treatment preferences using Adaptive Conjoint Analysis (ACA, Sawtooth Software ®). ACA is a specific type of conjoint analysis that elicits preferences using an interactive computer program. The method uses individual respondent's answers to update and refine upcoming questions through a series of graded-paired comparisons. Because it is interactive, ACA is more efficient than other techniques and allows a large number of attributes to be evaluated without resulting in information overload or respondent fatigue. This is an important advantage, since complex treatment decisions often require multiple trade-offs between competing risks and benefits. In addition, studies have demonstrated that ACA's computer-based format engages participants, minimizes interviewer biases, and facilitates data collection and management (22, 32).
We composed an ACA questionnaire to elicit preferences for aggressive (but more toxic) therapy versus a less aggressive but less toxic regimen. These two choices were composed to represent two clinical strategies commonly used by rheumatologists in clinical practice, the former representing regimens including a biologic tumor necrosis factor inhibitor (either alone or in combination with methotrexate) and the latter representing monotherapy with methotrexate. Treatment characteristics included in the ACA questionnaire were: benefits (chance of remission, symptom improvement, and radiographic progression), route of administration, and risks (injection reaction, nausea, lung or liver injury, tuberculosis, neurological disease, and theoretical risk of cancer). All characteristics were defined by a range of probabilities based on the best available data from the literature (Appendix A) (33-36) and a list of standardized descriptions were provided (Appendix B).
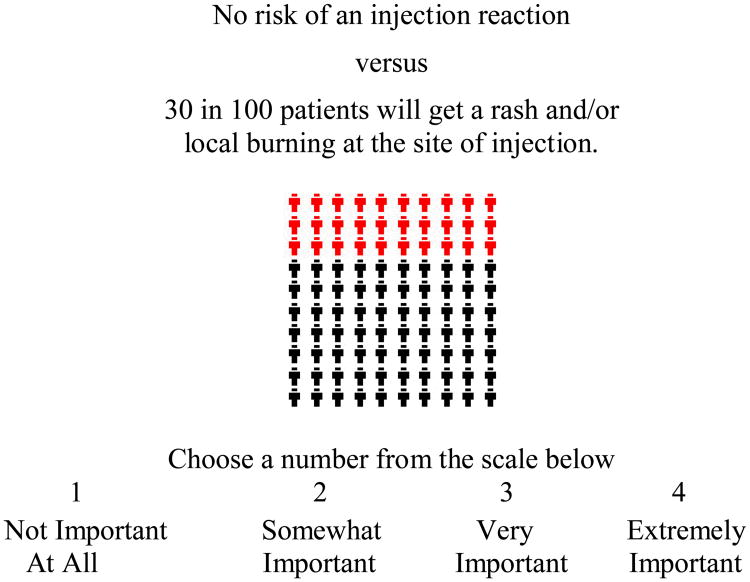
ACA is a hybrid approach in that it uses both self-explicated ratings and pairwise comparisons to predict preferences (see examples below). In contrast to random-utility theory based discrete choice experiments (37-40) ACA uses rating tasks and does not require that respondents evaluate all characteristics at the same time. The ACA task included three groups of questions. Participants were first asked to rank the estimate for “route of administration” since this characteristic does not have a priori (or natural) ranking, that is, an obvious preference from one level to the next. Respondents then rated the importance of the difference between best and worst estimates of each characteristic on a four-point scale. For example:
This set of questions enables the software to calculate a preliminary estimate of utilities for each treatment characteristic. In this context “utility” is a number that represents the value a respondent associates with a particular characteristic, with higher utilities indicating increased value.
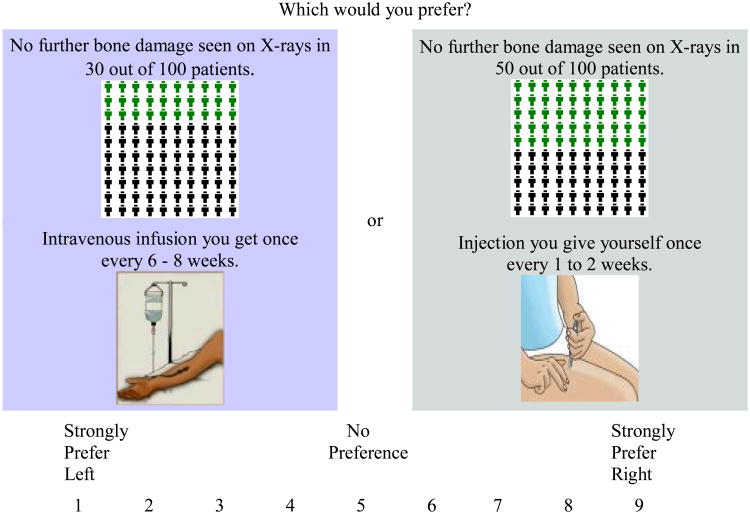
Respondents then completed a series of paired-comparisons. For example:
As with Thurstone scaling, ACA is based on the premise that measurement of differences by presenting two objects simultaneously is more efficient than by presenting the objects separately. Thurstone scaling assumes that the scale value of an object is measured imperfectly, with the error distributed normally. In contrast, ACA, and other conjoint methods, assume a “composition rule” which says that every attribute level has some value, and that the value of the bundle is equal to the sum of its part values (32, 41, 42).
ACA is interactive, in that it uses the information obtained from each new paired comparison to update utility estimates and to select the next pair of options. Utility measures become more precise as subjects are asked to discriminate among competing risks and benefits in successive pairs. The software continues presenting the subject with paired comparisons until enough data have been collected to estimate utilities for each estimate of each characteristic.
Participants also completed a questionnaire to ascertain gender, race, maximum level of education obtained, marital status, employment status, annual household income, insurance (Medicare, Medicaid, Private, or combination), functional status [Health Assessment Questionnaire (HAQ) (43)], and current and prior medication use related to RA.
Statistical Analyses
Patient characteristics were entered into SAS computer files (SAS Software, version 6.12, SAS Institute, Inc., Cary, North Carolina). Preference data derived from ACA were imported into SAS (version 8.0) and merged with the patient characteristics data set. Conjoint models are regression models. The coefficients from the model are the utilities. In ACA, regression models are constructed for each individual respondent. The models are constructed based on individual respondent's ratings to the questions described above. This approach assumes that respondent's ratings reveal some information about how they value the specific characteristics included in the survey. Characteristics which are rated higher are presumed to be of greater value or utility. A respondent's utility is therefore a measure of their relative preference for each estimate of each characteristic. Utilities are calculated using a least squares updating algorithm. The final utility estimates reflect true least squares (32).
Market simulators are used to convert the raw utilities into preferences for specific options. Preferences are calculated by first summing the utilities of the levels corresponding to each option. The utilities are then exponentiated and rescaled so that they sum to 100. This model is based on the assumption that subjects' prefer the option with the highest utility. Details related to this model have been previously published (32). Treatment preference was treated as a dichotomous variable with subjects in the upper tertile classified as preferring aggressive treatment.
We examined the association between subjects' characteristics and treatment preference using the chi-square statistic and t-test for categorical and continuous variables respectively. The number of patients recruited was sufficient to detect a 25% difference in proportions between groups with 80% power assuming a two-sided α 0.05 significance level. We subsequently used logistic regression to ascertain associations between respondent characteristics (found to be significant at p<0.05 in bivariate analyses) and preference for aggressive therapy. In the logistic regression, not preferring aggressive therapy was designated as the reference group and no selection procedures were utilized, i.e. all variables found to be significant at p<0.05 in bivariate analyses were included in the logistic regression model. We used the post-estimation Wald test to assess the individual contribution of each variable.
Results
Participants' Characteristics (Table 1)
One hundred fifty consecutive patients willing to hear about the study agreed to participate. Of these, nine refused to complete the questionnaire and five could not complete the computer survey due to time constraints, resulting in a total of 136 subjects (67 Blacks, 69 Whites). The mean age of the study sample was 55 years (range 22-84) and 83% percent were women. Characteristics for Black and White subjects are further described in Table 1.
Participants' Treatment Preferences (Tables 2 and 3)
In unadjusted analysis, 51% of White participants preferred aggressive therapy compared to 16% of Blacks (p<0.0001). Associations between the remaining covariates and treatment preference for aggressive therapy are presented in Table 2. Subjects who were married and reported having at least some college education had stronger preferences for aggressive therapy compared to their respective counterparts. We found no associations between age (mean difference = 1.0, p=0.7) and duration of RA (mean difference = 1.5, p = 0.5) or functional status (mean difference = 0.02, p=0.9) and treatment preference.
In the logistic regression model adjusting for the covariates associated with treatment preference (p < 0.05 in bivariate analyses), race remained the strongest predictor of aggressive therapy examined in this study (Table 3).
Given the strong association between education and race we performed subgroup analyses to determine whether our findings persisted in subjects with high and low levels of education. Among patients without a college education, 32% of White subjects preferred aggressive treatment compared to 3% of Black subjects (p=0.002). Among those with at least some college education, preference for aggressive treatment was 61% among White subjects and 30% among Black subjects (p=0.007). Subgroup analyses by location also demonstrated stronger preferences for aggressive treatment in White versus Black subjects for both the Virginia (51% versus 25%, p=0.04) and Washington (50% versus 13% p=0.007) sites.
Discussion
In this study we found that patient preferences for aggressive treatment of RA differ by race. Specifically, Black patients had weaker preferences for aggressive treatment compared to their White counterparts. This study suggests that variability in patient preferences may account for some of the differential use of newer and more aggressive therapeutic regimens in RA. To the best of our knowledge this is the first study designed and powered to examine the impact of race on patients' assessment of competing treatment options for a chronic disease.
Our findings are consistent with studies demonstrating weaker preferences among minority patients for more invasive procedures. Studies attempting to explain racial variability in patient preferences have found that differences in spirituality, health beliefs, perceptions of benefit, and trust all influence patients' treatment preferences for medical interventions (44-47). Further research is needed to determine whether similar factors account for the racial differences in preference for treatment of chronic disease.
To quantify preferences, we used ACA which is a robust preference measurement tool. ACA allows a large number of characteristics to be evaluated without resulting in information overload or respondent fatigue, and is therefore particularly well-suited towards measuring preferences for complex treatment options. An important advantage of using ACA in this setting is that preferences are quantified based on trade-offs between specific risks and benefits, and therefore are not biased by physicians' preferences or previously formed opinions based on external sources of information. We chose to omit the names of medications from the conjoint questionnaire to ensure that preferences were based on values for specific risks and benefits and not biased by recognition of specific brand names. To facilitate understanding of probabilistic information, we used natural frequencies instead of probabilities (e.g. 1 in 100 instead of 1%) and human figure charts in the ACA survey.
There are several limitations of this study. Consecutive RA patients were recruited, and the participants may not be representative of other community-based samples. In addition, we did not include out-of pocket costs in the ACA questionnaire as they differed markedly between insurance plans. While ACA is a powerful method of predicting preferences it does assume that all important characteristics can be identified, that individual respondents have unique values for each estimate of each characteristic, and that utilities can be summed across characteristics. The study was conducted in patients with prevalent disease currently taking medications, since recruiting treatment naïve patients with new onset disease would not have been feasible. While our methods do not replicate decision-making in clinical practice, the approach used in this study allowed us to conclude that the observed findings were due to differences at the patient and not provider level.
We conclude that preferences for more aggressive treatment in RA differ by race, with Blacks preferring less aggressive treatment compared to White patients with similar disease severity. Some data suggest that minority RA patients may be less aggressively treated than White patients (18, 19), and our results suggest that this disparity might be in part due to patient preferences. These results have important clinical implications because use of aggressive treatment improves both short and long-term outcomes in RA. Efforts to improve patient education and physician communication should be made to ensure that all patients have an accurate understanding of the benefits, as well as risks, associated with the best available treatment options. In order to ensure equitable care for patients, and to improve outcomes among those at highest risk for future disability, further research is needed to understand the reasons underlying systematic differences in patient preferences and systematic differences in drug utilization.
Figure 1. Example of an ACA Importance Question.
If two medications were acceptable in all other ways, how important would this difference be?
Figure 2. Example of an ACA Paired-Comparison Question.
Acknowledgments
We would like to thank Drs. Shoba Wani, Brian Walitt, Paul DeMarco, Lawrence Schwartz, Lenore Buckley, Christopher Wise, Neal Roberts, George Moxley, Francoise Mullen, and DeLona Norton for assisting with recruitment. Special thanks to Rich Johnson for his help reviewing the manuscript. In addition, we are indebted to all the patients who participated in the study for their time and interest.
Appendix A. Characteristics Included in the ACA Questionnaire
| Treatment Characteristics | Estimates |
| Remission | 45 out of 100 patients go into remission |
| 25 out of 100 patients go into remission | |
| 15 out of 100 patients go into remission | |
| Improvement | 70 out of 100 patients feel much better, but occasionally have some joint pain or swelling |
| 50 out of 100 patients feel much better, but occasionally have some joint pain or swelling | |
| 40 out of 100 patients feel much better, but occasionally have some joint pain or swelling | |
| Radiographic progression | No further bone damage seen on X-rays in 80 out of 100 patients |
| No further bone damage seen on X-rays in 50 out of 100 patients | |
| No further bone damage seen on X-rays in 30 out of 100 patients | |
| Route | Pill you take once a week |
| Injection you give yourself once every 1-2 weeks | |
| Intravenous infusion you get every 6-8 weeks | |
| Injection reaction | No injection reactions |
| 30 in 100 patients get a rash or local burning at the site of injection | |
| 3 in 100 patients will get a reaction during the infusion (headache, nausea, fever) | |
| Reversible adverse events | No increased risk of nausea, dizziness or unusual tiredness |
| 10 in 100 people will have nausea, dizziness or unusual tiredness | |
| Risk of lung injury | No increased risk of lung or liver injury |
| Rare risk of lung injury (about 2 in 100 patients) or liver injury (about 1 in 1000 patients) | |
| Risk of tuberculosis | No increased risk of tuberculosis |
| Extremely rare risk of tuberculosis (about 1 in 10,000 patients) | |
| Extremely rare adverse events | No increased risk of neurologic disease or heart failure |
| Extremely rare risk of neurologic disease or heart failure (about 1 in 10,000 patients) | |
| Risk of cancer | No increased risk of cancer |
| Possible increased risk of cancer (about 1 in 1000 patients) |
Appendix B. Standardized Explanations
Subcutaneous injection
An injection given right under the skin, like an insulin injection. You can give it yourself or have someone else do it. It can be given at home or in a clinic.
Intravenous infusion
This means the medicine will be given to you through a needle placed in a vein in your arm. It is given by a nurse in a clinic. It will take about 2 hours to give you the full dose of medicine.
Remission
This means that you do not have any joint pain, swelling or stiffness, but you still need to continue to take your medications.
Reversible side effects
The arthritis medication can cause mild or moderate nausea and vomiting (you sometimes feel a little queasy and vomit about once a day). The nausea and vomiting go away after the dose of the medication is lowered, or if necessary, when the medication is stopped.
Liver damage
The arthritis medication can cause liver damage. People with liver damage may become tired, weak, and lose their appetite. Many patients don't get other symptoms, but in some, the liver damage gets worse, and can cause yellow skin, intense itching, and bloating of the stomach.
Lung damage
The arthritis medication can cause lung problems that cause a dry cough, shortness of breath, and fever. Patients with this side effect need to be admitted to the hospital for treatment with oxygen and intravenous medications (steroids by vein). Treatment takes an average of two weeks.
Risk of Tuberculosis (TB)
Before starting the infusion medication, patients will be examined for TB with a skin test. If this test is positive, you will take a medication for 9 months and this medication will decrease the risk of TB becoming active.
Heart Failure
Patients with heart failure have shortness of breath during activity or, sometimes, even without activity. It occurs when the heart doesn't pump as well as it should. Heart failure can cause fluid to build up in the legs and lungs.
Neurologic disease
There have been rare cases where people taking the medication have developed disorders that affected their nervous system. Signs that indicate that you might have a problem include: changes in your vision, weakness in your arms and/or legs, and numbness or tingling in any part of the body.
Risk of Cancer
Theoretical risk of cancer means that because the medication affects the immune system, it has the potential to increase cancer risk with long-term use. An increased risk has not been shown in studies of this drug in comparison with patients with RA not on medications, but the studies have followed patients for less than 5 years. If the medication does turn out to increase the risk of cancer after long-term use, the risk might be 1 in a 1000.
Contributor Information
Florina Constantinescu, Virginia Commonwealth University.
Suzanne Goucher, Washington Hospital Center.
Arthur Weinstein, Georgetown University, Washington Hospital Center.
Liana Fraenkel, Yale University School of Medicine; Clinical Epidemiology Research Center, VA CT Healthcare System, West Haven, CT.
References
- 1.Braverman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research. JAMA. 2005;294:2879–88. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 2.Byrne MM, Souchek J, Richardson M, Suarez-Almazor M. Racial/ethnic differences in preferences for total knee replacement surgery. J Clin Epidemiol. 2006;59:1078–86. doi: 10.1016/j.jclinepi.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim SA, Siminoff LA, Burant CJ, Kwoh CK. Differences in expectations of outcome mediate African American/white patient differences in “willingness” to consider joint replacement. Arthritis Rheum. 2002;46:2429–35. doi: 10.1002/art.10494. [DOI] [PubMed] [Google Scholar]
- 4.Whittle J. Racial differences in the use of invasive cardiovascular procedures in the Department of Veterans Affairs medical system. N Engl J Med. 1993;329:621–7. doi: 10.1056/NEJM199308263290907. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann JC, Wenger NS, Davis RB, et al. Patient preferences for communication with physicians about end-of-life decisions. Ann Intern Med. 1997;127:1–12. doi: 10.7326/0003-4819-127-1-199707010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Cooper LA, Gonzales JJ, Gallo JJ, et al. The acceptability of treatment for depression among African-American, Hispanic, and White primary care patients. Med Care. 2003;41:479–89. doi: 10.1097/01.MLR.0000053228.58042.E4. [DOI] [PubMed] [Google Scholar]
- 7.Fraenkel L, Gulanski B, Wittink DR. Patient treatment preferences for osteoporosis. J Rheumatol. 2005;32:1086–92. [Google Scholar]
- 8.Fraenkel L, Bogardus ST, Felson DT, Wittink DR. Treatment options in knee osteoarthritis: the patient's perspective. Arch Intern Med. 2004;164:1299–1304. doi: 10.1001/archinte.164.12.1299. [DOI] [PubMed] [Google Scholar]
- 9.Montori VM, Bryant SC, O'Connor AM, Jorgensen NW, Walsh EE, Smith SA. Decisional attributes of patients with diabetes: The aspirin choice. Diabetes Care. 2003;26:2804–9. doi: 10.2337/diacare.26.10.2804. [DOI] [PubMed] [Google Scholar]
- 10.Okonofua EC, Cutler NE, Lackland DT, Egan BM. Ethnic differences in older Americans: awareness, knowledge, and beliefs about hypertension. Am J Hypertens. 2005;18:972–9. doi: 10.1016/j.amjhyper.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez A, Kremers HM, Crowson CS, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56:3583–7. doi: 10.1002/art.22979. [DOI] [PubMed] [Google Scholar]
- 12.Emery P, Breedveld FC, Dougados M, Kalden JR, Schiff MH, Smolen JS. Early referral recommendation for newly diagnosed rheumatoid arthritis: evidence based development of a clinical guide. Ann Rheum Dis. 2002;61:290–7. doi: 10.1136/ard.61.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce B, Fries JF, Murtagh KN. Health status disparities in ethnic minority patients with rheumatoid arthritis: a cross-sectional study. J Rheumatol. 2007;34:1475–9. [PubMed] [Google Scholar]
- 14.Iren UT. A pilot study to determine whether disability and disease activity are different in African-American and Caucasian patients with rheumatoid arthritis in St. Louis, Missouri, USA. J Rheumatol. 2005;32:602–8. [PubMed] [Google Scholar]
- 15.Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004;364:263–9. doi: 10.1016/S0140-6736(04)16676-2. [DOI] [PubMed] [Google Scholar]
- 16.Verstappen SMM, Jacobs JWG, van der Veen MJ, et al. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Ann Rheum Dis. 2007;66:1443–9. doi: 10.1136/ard.2007.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bijlsma JWJ, Weinblatt ME. Optimal use of methotrexate: the advantages of tight control. Ann Rheum Dis. 2007;66:1409–10. doi: 10.1136/ard.2007.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berrios-Rivera JP, Johnson ML, Huston DP, Suarez-Almazor ME. Disparities in the use of biologic therapies for the treatment of rheumatoid arthritis. Arthritis Rheum. 2006;54:S347. [Google Scholar]
- 19.Head AJ, Wang BJE, Michaud K, Wolfe F. Racial disparity in disability and pain self-report in rheumatoid arthritis persists despite controlling for sociodemographic factors. Arthritis Rheum. 2004;50:S296. [Google Scholar]
- 20.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000;320:1530–3. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wittink DR, Bergenstuen T. Forecasting with conjoint analysis. In: Armstrong JS, editor. Principles of forecasting: a handbook for researchers and practitioners. Norwell, MA: Kluwer Academic Publishers; 2001. [Google Scholar]
- 22.Green PE, Srinivasan V. Conjoint analysis in marketing: new developments with implications for research and practice. J Marketing. 1990;54:3–17. [Google Scholar]
- 23.Luce D, Tukey J. Simultaneous conjoint measurement: a new type of fundamental measurement. J Math Psychol. 1964;1:1–27. [Google Scholar]
- 24.Bridges JFP. Stated preference methods in health care evaluation: an emerging methodological paradigm in health economics. Appl Health Econ Health Policy. 2003;2:213–24. [PubMed] [Google Scholar]
- 25.Bridges JFP, Onukwugha E, Johnson FR, Hauber AB. Patient preference methods - A patient-centered evaluation paradigm. ISPOR Connections. 2007;13:4–7. [Google Scholar]
- 26.Beusterien KM, Dziekan K, Flood E, Harding G, Jordan JC. Understanding patient preferences for HIV medications using adaptive conjoint analysis: feasibility assessment. Value Health. 2005;8:453–61. doi: 10.1111/j.1524-4733.2005.00036.x. [DOI] [PubMed] [Google Scholar]
- 27.Dwight-Johnson M, Lagomasino IT, Aisenberg E, Hay J. Using conjoint analysis to assess depression treatment preferences among low-income Latinos. Psychiatric Serv. 2004;55:934–6. doi: 10.1176/appi.ps.55.8.934. [DOI] [PubMed] [Google Scholar]
- 28.Lee PY, Matchar DB, Clements DA, Huber J, Hamilton JD, Peterson ED. Economic analysis of influenza vaccination and antiviral treatment for healthy working adults. Ann Intern Med. 2002;137:225–31. doi: 10.7326/0003-4819-137-4-200208200-00005. [DOI] [PubMed] [Google Scholar]
- 29.Phillips KA, Maddala T, Johnson FR. Measuring preferences for health care interventions using conjoint analysis: an application to HIV testing. Health Serv Res. 2002;37:1681–705. doi: 10.1111/1475-6773.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraenkel L, Bogardus S, Wittink DR. Understanding patient preferences for the treatment of lupus nephritis with adaptive conjoint analysis. Med Care. 2001;39:1203–16. doi: 10.1097/00005650-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Janis IL, Mann L. Decision-making A psychological analysis of conflict, choice, and committment. New York: The Free Press; 1985. [Google Scholar]
- 32.Orme B. [Last accessed 07 2008];2008 http://www.sawtoothsoftware.com/download/techpap/acatech.pdf. [Google Scholar]
- 33.Breedveld F, Emery P, Keystone E, et al. Infliximab in active early rheumatoid arthritis. Ann Rheum Dis. 2004;63:149–55. doi: 10.1136/ard.2003.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37. doi: 10.1002/art.21519. [DOI] [PubMed] [Google Scholar]
- 35.Smolen JS, Van der Heijde D, St Clair EW, et al. Predictors of joint damage in patients with early rheumatoid arthritis treated with high-dose methotrexate with or without concomitant infliximab: results from the ASPIRE trial. Arthritis Rheum. 2006;54:702–10. doi: 10.1002/art.21678. [DOI] [PubMed] [Google Scholar]
- 36.van der Heijde D, K L, Rodriguez-Valverde V, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the TEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54:1063–74. doi: 10.1002/art.21655. [DOI] [PubMed] [Google Scholar]
- 37.Krabbe PFM. Thurstone scaling as a measurement method to quantify subjective health outcomes. Med Care. 2008;46:357–65. doi: 10.1097/MLR.0b013e31815ceca9. [DOI] [PubMed] [Google Scholar]
- 38.Maydeu-Olivares A, Böckenholt U. Modeling subjective health outcomes: top 10 reasons to use Thurstone's method. Med Care. 2008;46:357–65. doi: 10.1097/MLR.0b013e31816dd8d9. [DOI] [PubMed] [Google Scholar]
- 39.McFadden D. Conditional logit analysis of qualitative choice behavior. In: Zarembka P, editor. Frontiers in Econometrics. New York: Academic Press; 1974. pp. 105–42. [Google Scholar]
- 40.Swait J, Louviere J. The role of the scale parameter in the estimation and the use of multinomial logit models. J Marketing Res. 1993;30:305–14. [Google Scholar]
- 41.Green PE, Rao VR. Conjoint measurement for quantifying judgemental data. J Market Res. 1971;8:355–63. [Google Scholar]
- 42.Green PE, Srinivasan V. Conjoint analysis in consumer research: issue and outlook. J Consumer Res. 1978;5:102–23. [Google Scholar]
- 43.Fries JF, Spitz PW, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 44.Ang DC. Is there a difference in the perception of symptoms between African Americans and Whites with osteoarthritis? J Rheumatol. 2003;30:1305–10. [PubMed] [Google Scholar]
- 45.Suarez-Almazor ME. Unraveling gender and ethnic variation in the utilization of elective procedures: the case of total joint replacement. Med Care. 2002;40:447–50. doi: 10.1097/00005650-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Suarez-Almazor ME, Souchek J, Kelly PA, et al. Ethnic variation in knee replacement: patient preferences or uninformed disparity? Arch Intern Med. 2005;165:1117–24. doi: 10.1001/archinte.165.10.1117. [DOI] [PubMed] [Google Scholar]
- 47.Margolis ML, Christie JD, Silvestri GA, Kaiser L, Santiago S, Hansen-Flaschen J. Racial differences pertaining to a belief about lung cancer surgery: results of a multicenter survey. Ann Intern Med. 2003;139:558–63. doi: 10.7326/0003-4819-139-7-200310070-00007. [DOI] [PubMed] [Google Scholar]