Abstract
Background
Isoflurane exposure causes improvement in long-term neurocognitive function in young adult rats, this is associated with an increase in dentate gyrus progenitor proliferation 4 days after anesthesia. However, the number of new neurons, that were born from cells that incorporated bromodeoxyuridine 4 days after anesthesia is not affected by anesthesia. We tested the hypothesis that progenitor proliferation continues to increase past 4 days, which would imply the possibility that the number of new neurons after anesthesia could be increased if bromodeoxyuridine labeling occurred at a later time point.
Methods
Bromodeoxyuridine was injected at 0, 1, 2, 4, 9, 16 days after 4 hours of isoflurane exposure to 60-day old rats. Brains were harvested 2 hours later, immunohistochemically stained, and the number of bromodeoxyuridine-positive cells in the dentate gyrus was assessed microscopically.
Results
After 4 hours of exposure to isoflurane in 60-day old rats, the number of bromodeoxyuridine-positive cells decreased on days 0–2, then increased on day 4 significantly, and regressed toward the control level on day 9 and 16.
Conclusions
Anesthesia-induced progenitor proliferation in the dentate gyrus was not sustained 9 days after anesthesia. We interpret these results to signify that an anesthetic effect on neurogenesis likely does not play a critical role in the previously observed isoflurane-induced long-term improvement in neurocognitive function in 60-day old rats and that the transient increase in progenitor proliferation serves to replenish the pool of neural stem cells. The mechanism of anesthesia-induced improvement in cognition of young adult rats remains elusive.
Keywords: Cell Proliferation, Dentate Gyrus, Hippocampus, Isoflurane, Neurogenesis
Introduction
Anesthetic neurotoxicity has been described at the extremes of age 1–3. It is now widely accepted that a single episode of anesthetic exposure in the immature animal brain causes widespread neurodegeneration and persistent neurocognitive problems 4–9. In aged animals, anesthesia may cause biochemical changes that are similar to those occurring in Alzheimer’s disease10, 11. Curiously, in young adult rats (2–3 months of age), none of these cellular or neurobehavioral sequelae are observed. In fact, anesthesia has been shown to improve long-term cognitive function in this age group9, 12. Both Culley et al. 12 and our this author 9 have shown that young adult rats that received a single anesthetic had improvements in learning and memory tasks weeks to months later, compared to unanesthetized controls. The mechanism of this effect is not yet well understood but might reflect the known action of γ-amino butyric acid (GABA) on the differentiation of neural progenitor cells13. A GABA-ergic anesthetic such as isoflurane should briefly inhibit proliferation and stimulate neuronal differentiation14.
We have previously demonstrated an isoflurane-induced decrease in proliferation and an increase in neuronal differentiation in vitro in cultured progenitor cells 15. Similarly, in vivo, there is a decrease in progenitor proliferation at the time of the anesthetic in 60-day old rats but a subsequent increase in proliferation was seen 4 days later 9. A sustained increase in dentate gyrus progenitor number or proliferation rate may enhance the number or quality of new neurons or increase the number of immature cells available for incorporation thereby leading to improved neurocognitive function. The surge of proliferation 4 days after anesthesia didn’t result in an increase of new neurons 4 weeks after anesthesia9, but neurocognitive function was improved in the isoflurane-treated animals 9.
The isoflurane-induced increase in proliferation on post-anesthesia day 4 could not have mediated isoflurane-induced cognitive improvement, unless the effect of isoflurane on progenitor proliferation persisted or accelerated beyond the single time point at which we previously chose to observe it. We tested the hypothesis that the increase in isoflurane-induced dentate gyrus progenitor cell proliferation persists beyond day 4 by conducting a longitudinal 16 day assessment of progenitor proliferation after a 4 hours isoflurane anesthetic in 60-day old rats.
Materials and Methods
Animals
With approval from the Institutional Animal Care and Use Committee of University of California San Francisco (Animal care approval number is AN080240-01C), male 60-day old Sprague Dawley rats (n= 58) (from Charles River Laboratories, Gilroy, CA) were included in this experiment. Rats were randomly divided into 6 isoflurane anesthesia treatment groups (n=46) or a sham anesthesia group (n=12). After 4 hours of isoflurane anesthesia or sham anesthesia, rats were randomly assigned to injection with the S-phase marker BrdU at one of six time points (0, 1, 2, 4, 9, 16 days after anesthesia). Day 0 is defined as the end of the 4-hour anesthetic, day 1 is defined as 24 hours after the end of the anesthetic, and so on.
Isoflurane Anesthesia
Anesthesia was carried out as previously described 9, 16. Isoflurane was administered to the rats in a preheated, humidified glove box with FiO2 0.5 (O2 2.5 L/min mixed with air 3.5L/min). Isoflurane was titrated to 1 minimum alveolar concentration (MAC) using an algorithm based on the cohort’s response to tail clamping every 15 minutes during anesthesia 16. Pericranial temperature was computer-controlled at 36.5±1 degrees Celsius by a custom-made heater/cooler system as previously described 8, 16. We have previously demonstrated that physical parameters including PH, arterial oxygen, carbon dioxide, blood hemoglobin and blood glucose are stable in P60 rats and didn’t result in cell death9, 17, so they were not repeated in this study.
Sham Anesthesia
Control rats were placed in the same glove box in which the anesthetic was administered, with the same inspired gases at FiO2 0.5 at the same flow rates for 4 hours but without exposure to anesthetic vapor.
BrdU injection and Tissue Preparation
Bromodeoxyuridine (BrdU; Sigma, St. Louis, MO; 15mg/ml) was injected at a dose of 150 mg/kg intraperitoneally 2 hours before transcardiac perfusion with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS), pH 7.4 under deep isoflurane anesthesia.
Brains were removed, postfixed overnight in 4% PBS, and placed in 20% sucrose until they sunk. Coronal sections (40 μm) were cut on a microtome and stored in PBS. For immunocytochemical detection of BrdU-labeled nuclei, DNA was denatured to expose the antigen. Incubation with 50% formamide in PBS for 2 h at 65°C preceded 2-normal hydrochloric acid incubation for 30 min at 37°C and neutralization with 0.5 M boric acid (pH 8.5) for 10 min at room temperature. In-between each step, 3 washes with PBS for 10 min were performed. Blocking of nonspecific epitopes with 3% serum and 0.1% bovine serum albumin in PBS with 0.3% Triton-X (Fisher Scientific, Pittsburg, PA) for 30 min at room temperature preceded incubation overnight at 4°C with the primary antibody at 1:400 dilution (Clone BMC9318; Roche Applied Science) in PBS and bovine serum albumin 0.2%. On day 2 of the stain, incubation with a biotinylated antibody (sheep anti-mouse IgG, 1:200; Amersham [GE Healthcare], Piscattaway, NJ) for 2 h at room temperature was performed. Streptavidin-biotin treatment for 2 h at room temperature (Vectastain, ABC kit, Catalogue # PK 6100; Vector Laboratories, Burlingame, CA) was followed by three thorough washes to eliminate residual peroxidase activity. This was followed by incubation with diaminobenzidine + urea (Fast DAB tablets, Sigma, St. Louis, Missouri) with nickel chloride augmentation (7.5 μl of 8% stock in 25 ml) for 5 min followed by mounting and coverslipping with Depex mounting medium (Electronmicroscopy Sciences, Fort Washington, PA). Twelve coronal slices for immunocytochemical staining were obtained from each animal and examined for bromodeoxyuridine staining of the dentate gyrus.
Microscopy
BrdU-positive cells were detected using brightfield microscopy with a 4x, 20x, and 100x objective lenses to distinguish cells within tightly packed cell conglomerates. The target area was the granule cell layer and the subgranular zone of each DG, which was traced using StereoInvestigator® software (MicroBrightField, Williston, VT). An observer blinded to group assignments determined the number of BrdU+ cells per target area in every twelfth slice for each animal.
Sample Size Calculation and Statistical Methods
On the basis of previous work (effect size of isoflurane on dentate gyrus progenitor proliferation in young adult rats of 43% (bromodeoxyuridine positive cells/DG isoflurane 1499; control 871; SD isoflurane 569; control 589)9), a minimal group size of 6 animals was required to detect a difference between means of 40% with 80% power. P value less than 0.05 were considered statistically significant. SAS Version 9 Proc Power (SAS Institute, Cary, NC) was used for sample size estimation.
Data were expressed as medians and interquartile ranges after determining that not all groups met parametric assumptions using d’Agostino’s and Pearson’s omnibus normality tests. A Kruskall Wallis test with Dunn’s correction for multiple comparisons was performed to determine between-group differences. The number of BrdU+ cells in the anesthesia groups representing the various time points after anesthesia was compared to the number of BrdU+ cells in the control group. Prism5 for Mac OS X (GraphPad, San Diego, CA) was used for statistical analysis.
Results
The inspired anesthetic concentration reached a stable plateau of 1.6% isoflurane after 75 minutes of anesthesia. All animals survived to completion of this experiment. The time course of proliferative activity of neural progenitors, as measured by incorporation of the S-phase marker BrdU (Fig. 1.A), showed a biphasic response (P <0.0001, Kruskall Wallis test). The median number of BrdU+ cells detected in the control group was 1156.08 (IQR 646.2–1488) per dentate gyrus. In isoflurane-treated animals, progenitor proliferation tended to decrease initially (Fig. 1.B). On day 0, a median of 321.12 (IQR: 158.04–363.24) BrdU+ cells per dentate gyrus were detected, i.e., 28% of control values (rank sum difference: 17.25, P>0.05 using Dunn’s correction for multiple comparisons). On day 1, the median number of BrdU+ cells per dentate gyrus was 209.52 (IQR: 110.41–404.52) or 18% of control values (rank sum difference: 18.25, P>0.05, Dunn’s correction for multiple comparisons). BrdU+ cells then increased on day 2 (median 879.48, IQR 693.00–1096.08), to 76.07% of control values (rank sum difference: 3.417, P>0.05, Dunn’s correction for multiple comparisons). By day 4 BrdU+ cell number had increased to 366% of control values (median 4228.8, IQR 3238.8 –6278.4, rank sum difference: −123.58, P<0.05, Dunn’s correction for multiple comparisons), and then slowly regressed towards control values by day 16. On day 9 (median 2452.8, IQR 1902–4070.4, rank sum difference: −17.46, P>0.05, Dunn’s correction for multiple comparisons) and day 16 (median 2013.6, IQR 1032.72–3320.4 rank sum difference: −9.58, P>0.05, Dunn’s correction for multiple comparisons), the percentage of BrdU+ cell were still somewhat elevated compare to control values, 212.17% and 174.17% respectively, albeit not statistically significantly so.
Fig. 1.
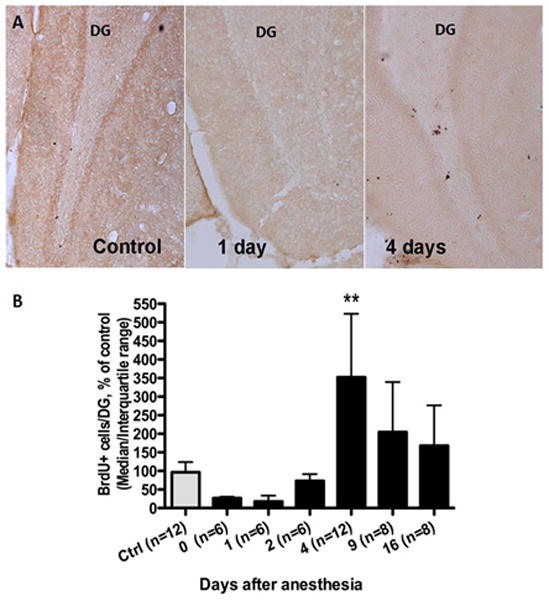
Hippocampal DG progenitor proliferation after 4 hours of isoflurane in P60 rats. BrdU (150mg/kg) was injected 2 hours before brain tissue harvest at various time points after the anesthetic. The percentage of BrdU positive cells (A) relative to control values showed a biphasic response with an initial decrease followed by a sustained increase (B). *P<0.05, BrdU bromodeoxyuridine, DG Dentate Gyrus.
Discussion
The main finding of this study is that in young adult rats, after isoflurane exposure, progenitor proliferation initially decreased and then significantly increased on postanesthesia day 4. This was followed by regression towards baseline over the next 12 days. We did not observe a sustained proliferative response. This study excludes one possible explanation (sustained increased in the proliferation rate of progenitor cells in the dentate gyrus) for the anesthesia-induced neurocognitive improvement seen after anesthesia in young adult rats9, 12.
The dentate gyrus (DG), a part of the hippocampal formation, retains an actively dividing pool of progenitor cells into adulthood18–20. Neuronal progenitor cells can stop proliferating, exit the cell cycle and differentiate into new neurons which become dentate granule cells. Alternatively, they may divide, giving rise to two new progenitor cells, or to one progenitor and one cell that differentiates to become a neuron. Inhibition of proliferation and differentiation in the dentate gyrus decreases the generation of granule cells and impairs hippocampal function7. Dentate granule cells are particularly important in spatial or episodic memory and in pattern separation21–23.
We have previously reported an increase in progenitor proliferation 4 days after isoflurane exposure, similar to our findings on day 4 in the current study. We also reported that cells labeled with BrdU immediately before isoflurane exposure are more likely to express a neuronal marker weeks later and that cells labeled with BrdU 4 days after isoflurane (during the increased rate of proliferation) were less likely to express a neuronal marker one month later. At the same time found the total number of NeuN+ cells in the dentate was not different in anesthetized or control animals9. This left open the questions: why is there an increase in proliferation 4 days after anesthesia if those cells do not becoming new neurons, and if that increase were sustained could it account for the improved cognitive function we and others have observed9, 12? The result from the current study begins to fill in those gaps.
In this study, dentate gyrus progenitor proliferation showed a transient decrease on post anesthesia day 1 and day 2. During the cell cycle, progenitors can either re-enter the cell cycle generating growth of the population or exit the cell cycle terminating the proliferative lineage and following a neuronal lineage. Our finding of a decrease in proliferation may be due to an increase in differentiation. This is consistent with our previous findings that isoflurane acutely increased neuronal differentiation and decreased progenitor proliferation in vivo and in vitro9, 15. Isoflurane’s GABAergic action likely plays a role in promoting neuronal differentiation of progenitors in the adult brain. Higher intracellular chloride concentration leads to GABA receptor mediated depolarization of progenitor cells, subsequent calcium influx, and induction of the transcription factor NeuroD which promotes neuronal differentiation14, 24.
This finding of decreased proliferation is not consistent with the results of Zhu et al. 7, who found that proliferation did not change after isoflurane exposure in 60-day old rats. The discrepancy between these findings and the combination of our current and previous findings might be explained by the different duration and frequency of isoflurane exposure and BrdU labeling strategy. In our studies, we administered a single isoflurane anesthetic at 1MAC for four hours and a single BrdU injection was given to each animal allowing for precise analysis of what occurs at specific time points following isoflurane exposure. In Zhu’s study, isoflurane was administered at the fixed concentration of 1.7% for 35 minutes daily for four days, and the BrdU injections were repeated daily which might have masked the events that occur after a single isoflurane exposure in P60 animals.
At 4 weeks after isoflurane, only 10% of proliferating cells turn into new neurons versus greater than 50% in control animals9 which is in agreement with the findings of Zhu. We speculate that the increase in proliferation on post-anesthesia day 4 in our current and previous study serves to replenish the pool of proliferating progenitors after its temporary decrease. Based on our previous studies and the current data we hypothesize that isoflurane induces progenitors to leave the cell cycle and differentiate at an inappropriate time. This is reflected as a decrease in BrdU incorporation on post anesthesia day 1 and 2. A compensatory increase in proliferation rate then occurs to return the progenitor pool to its normal size. This is observed as an increase in BrdU incorporation around post anesthesia day 4. Several weeks later, a lower proportion of BrdU labeled cells express NeuN in anesthetized animals because, although the rate of proliferation was increased when the BrdU was incorporated into the cells, most of those new cells remained in the pool of progenitors rather than differentiating to become new neurons.
Our hypothesis that dentate progenitor proliferation is sustained beyond 4 days post anesthesia is false. We observed that dentate progenitor proliferation actually decreased back to baseline by 9 days after anesthesia, which leaves the remote possibility that we missed a peak that may have occurred between day 4 and 9.
New neurons make a unique contribution to memory processing in the dentate gyrus25 and need to be integrated into the existing network for dentate gyrus plasticity and memory formation 25, 26. This leaves us at a loss for a mechanistic explanation for the previously observed9 long-term improvement of neurocognitive function after isoflurane exposure in young adult rats. The number of new dentate gyrus neurons7, 9, the number of progenitor cells7, and the rate of progenitor proliferation (present study) are all unchanged 4 weeks after isoflurane exposure and thus cannot explain the improved cognitive function previously observed weeks after isoflurane 9, 12.
Although highly speculative, it is possible that the number of progenitors available and the quality of new neurons27 somehow has improved, possibly by having a larger selection of newly formed neurons available for integration into the neural network. In control animals, neurons integrated into the dentate gyrus network may not have been optimally suited for a given functional demand 28, 29. The luxurious supply of progenitors in isoflurane-treated rats, in contrast, may have resulted in a dentate gyrus network that was better suited for the task the rats were trained on.
Limitations
The use of a single control group at postnatal day 60, as opposed to multiple control groups at each time point after anesthesia was chosen because after 2 months of age dentate gyrus progenitor proliferation declines only very slowly over time30. This relative stability in the rate of dentate gyrus progenitor proliferation does not, however, rule out the possibility that the increases in progenitor proliferation observed at later time points might have been more pronounced had progenitor proliferation been measured directly in unanesthetized rats at each time point. The current findings were generated in 60 day old rats and therefore have no bearing on the anesthetic neurotoxicities that have been demonstrated in immature rats4 or those that have been proposed to occur in the elderly brain31.
BrdU incorporation was presumed to be a result of cell division of neural progenitor or stem cells in the dentate gyrus of the hippocampus. It is possible that other proliferating cells such as microglia could account for the BrdU labeling we observed and no co-stain for markers of proliferating progenitors was performed in order to confirm the cell type. However, this scenario is very unlikely given that BrdU incorporation occurred almost exclusively in the dentate gyrus and sub-ventricular zones – two areas well known to contain neural stem cells. In the absence of some other injury it would be unusual for an immune type reaction to occur in only these areas of the brain.
Conclusion
We conclude that after 4 hours exposure to isoflurane, dentate gyrus progenitor proliferation displays a biphasic response, characterized by an acute decrease and subsequent increase of limited duration. For reasons discussed above, the lack of a more sustained increase in progenitor proliferation makes it unlikely that the effect of isoflurane on dentate gyrus progenitor proliferation plays a role in the previously observed isoflurane-induced long term improvement in neurocognitive function in 60-day old rats.
Acknowledgments
Funding
This work was supported by John Severinghaus Research Award (GS) and NIH grant GM086511 (JWS)
We thank to the financial assistance from Chinese Society of Anesthesiology for Young Investigator’s Award and Beijing universities joint post-graduate training for international projects. We are also grateful for Professor Adrian Gelb’s valuable suggestion and insight during the writing of this manuscript.
Footnotes
This study was carried out in the Dept. of Anesthesia and Perioperative Care, University of California San Francisco
Conflicts of interest: none
This study has been presented at the Annual Meeting of the SNACC 2012.
References
- 1.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesthesia and analgesia. 2008;106:1681–1707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 2.Stratmann G. Review article: Neurotoxicity of anesthetic drugs in the developing brain. Anesthesia and analgesia. 2011;113:1170–1179. doi: 10.1213/ANE.0b013e318232066c. [DOI] [PubMed] [Google Scholar]
- 3.Hudson AE, Hemmings HC., Jr Are anaesthetics toxic to the brain? British journal of anaesthesia. 2011;107:30–37. doi: 10.1093/bja/aer122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredriksson A, Ponten E, Gordh T, Eriksson P. Neonatal exposure to a combination of n-methyl-d-aspartate and gamma-aminobutyric acid type a receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology. 2007;107:427–436. doi: 10.1097/01.anes.0000278892.62305.9c. [DOI] [PubMed] [Google Scholar]
- 6.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 7.Zhu C, Gao J, Karlsson N, Li Q, Zhang Y, Huang Z, Li H, Kuhn HG, Blomgren K. Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:1017–1030. doi: 10.1038/jcbfm.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stratmann G, May LD, Sall JW, Alvi RS, Bell JS, Ormerod BK, Rau V, Hilton JF, Dai R, Lee MT, Visrodia KH, Ku B, Zusmer EJ, Guggenheim J, Firouzian A. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110:849–861. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 9.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–848. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 10.Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104:988–994. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Culley DJ, Baxter M, Yukhananov R, Crosby G. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesthesia and analgesia. 2003;96:1004–1009. doi: 10.1213/01.ANE.0000052712.67573.12. table of contents. [DOI] [PubMed] [Google Scholar]
- 13.Yuan TF. Gaba effects on neurogenesis: An arsenal of regulation. Science signaling. 2008;1:jc1. doi: 10.1126/stke.115jc1. [DOI] [PubMed] [Google Scholar]
- 14.Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. Gabaergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Sall JW, Stratmann G, Leong J, McKleroy W, Mason D, Shenoy S, Pleasure SJ, Bickler PE. Isoflurane inhibits growth but does not cause cell death in hippocampal neural precursor cells grown in culture. Anesthesiology. 2009;110:826–833. doi: 10.1097/ALN.0b013e31819b62e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stratmann G, Sall JW, Eger EI, 2nd, Laster MJ, Bell JS, May LD, Eilers H, Krause M, Heusen F, Gonzalez HE. Increasing the duration of isoflurane anesthesia decreases the minimum alveolar anesthetic concentration in 7-day-old but not in 60-day-old rats. Anesthesia and analgesia. 2009;109:801–806. doi: 10.1213/ane.0b013e3181aff364. [DOI] [PubMed] [Google Scholar]
- 17.Stratmann G, Sall JW, Bell JS, Alvi RS, May LV, Ku B, Dowlatshahi M, Dai R, Bickler PE, Russell I, Lee MT, Hrubos MW, Chiu C. Isoflurane does not affect brain cell death, hippocampal neurogenesis, or long-term neurocognitive outcome in aged rats. Anesthesiology. 2010;112:305–315. doi: 10.1097/ALN.0b013e3181ca33a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: From precursors to network and physiology. Physiological reviews. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 19.Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135:1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- 20.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. The Journal of comparative neurology. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 21.Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous ca3 map. Learn Mem. 2007;14:745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- 22.Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nature reviews Neuroscience. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. Gaba: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiological reviews. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 25.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatialmemory networks in the dentate gyrus. Nature neuroscience. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 26.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nature neuroscience. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 27.Song H, Kempermann G, Overstreet Wadiche L, Zhao C, Schinder AF, Bischofberger J. New neurons in the adult mammalian brain: Synaptogenesis and functional integration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:10366–10368. doi: 10.1523/JNEUROSCI.3452-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song J, Christian K, Ming GL, Song H. Modification of hippocampal circuitry by adult neurogenesis. Developmental neurobiology. 2012 doi: 10.1002/dneu.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aimone JB, Deng W, Gage FH. Put them out to pasture? What are old granule cells good for, anyway..? Hippocampus. 2010;20:1124–1125. doi: 10.1002/hipo.20867. [DOI] [PubMed] [Google Scholar]
- 30.Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiology of aging. 2004;25:361–375. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 31.Culley DJ, Baxter MG, Crosby CA, Yukhananov R, Crosby G. Impaired acquisition of spatial memory 2 weeks after isoflurane and isoflurane-nitrous oxide anesthesia in aged rats. Anesthesia and analgesia. 2004;99:1393–1397. doi: 10.1213/01.ANE.0000135408.14319.CC. table of contents. [DOI] [PubMed] [Google Scholar]


