Abstract
Thyroid hormone receptors, THRA and THRB, together with the TSH receptor, TSHR, are key regulators of thyroid function. Alterations in the genes of these receptors (THRA, THRB and TSHR) have been related to thyroid diseases, including thyroid cancer. Moreover, there is evidence suggesting that predisposition to differentiated thyroid cancer (DTC) is related to common genetic variants with low penetrance that interact with each other and with environmental factors. In this study, we investigated the association of single nucleotide polymorphisms (SNPs) in the THRA (one SNP), THRB (three SNPs) and TSHR (two SNPs) genes with DTC risk. A case–control association study was conducted with 398 patients with sporadic DTC and 479 healthy controls from a Spanish population. Among the polymorphisms studied, only THRA-rs939348 was found to be associated with an increased risk of DTC (recessive model, odds ratio=1.80, 95% confidence interval=1.03–3.14, P=0.037). Gene–gene interaction analysis using the genotype data of this study together with our previous genotype data on TG and TRHR indicated a combined effect of the pairwises: THRB-TG (P interaction=0.014, THRB-rs3752874 with TG-rs2076740; P interaction=0.099, THRB-rs844107 with TG-rs2076740) and THRB-TRHR (P interaction=0.0024, THRB-rs3752874 with TRHR-rs4129682) for DTC risk in a Spanish population. Our results confirm that THRA is a risk factor for DTC, and we show for the first time the combined effect of THRB and TG or TRHR on DTC susceptibility, supporting the importance of gene–gene interaction in thyroid cancer risk.
Keywords: thyroid-function genes, association, thyroid cancer risk, cancer susceptibility
Introduction
Thyroid cancer is of special concern in endocrinology practice, accounting for more than 90% of all endocrine cancers. Moreover, its incidence is increasing in developed countries (1, 2). Differentiated thyroid carcinoma (DTC) represents more than 90% of thyroid neoplasms, and it comprises papillary (PTC) and follicular (FTC) histological subtypes (3). The genetic predisposition to thyroid cancer is estimated to be higher than in other common cancers and, together with environmental factors, is considered the main component in the aetiology of thyroid cancer (4, 5, 6, 7).
Association studies have identified several genetic variants related to thyroid cancer risk, including 8q24, 1p12, 9q22.23, 14p13.3 and 5q33 (8, 9, 10, 11, 12). Polymorphic variations in the FOXE1 and NKX2-1 genes, both involved in thyroid function and development of the thyroid gland, have also been reported to be associated with the risk of thyroid cancer (10, 13, 14). Furthermore, common genetic variants associated with low levels of TSH and increased thyroid cancer risk suggest the role of thyroid-related hormones in thyroid cancer susceptibility (15), including thyroid hormone receptors (TRs) and TSH receptor (TSHR), which are key proteins in the regulation of the thyroid function. Although mutations in the TR genes, THRA and THRB, are not common in thyroid tumours (16, 17, 18), a decreased expression of these genes has been found in thyroid cancer (19).
These observations together with a study using mouse models with deleted Thra and Thrb genes support the tumour suppressor functions of the TR genes (20). Three studies have reported the association of genetic variants in the THRA gene with thyroid cancer risk, but the conclusions are limited by the small size of the populations studied (21, 22, 23). In the case of THRB, there is no reported data about THRB genetic variants related to the thyroid cancer risk. The implication of TSHR in thyroid cancer is unclear (24, 25). While epigenetic silencing of TSHR in thyroid carcinomas has been reported (26), two other studies have shown a lack of association of TSHR polymorphisms with thyroid cancer risk (27, 28). On the contrary, TSHR is associated with other thyroid diseases (29).
Given the limited information concerning the implication of genes involved in the thyroid function on thyroid cancer risk, in this study, we have analysed the risk association between common genetic variants in the THRA, THRB and TSHR genes and DTC in a large Spanish population. In addition, we have examined the combined effect of the variants of these three genes and the TG and TRHR genes on DTC susceptibility, using data from our previous association studies (12).
Materials and methods
Subjects
The study was carried out on a Spanish population with a total of 877 unrelated subjects who were recruited during a 5-year period (2004–2008). A group of 398 newly diagnosed patients with DTC (309 females and 89 males) with a mean age ±s.d. of 46.99±15.45 years was recruited at Vall d'Hebron Hospital (Barcelona) and at Josep Trueta Hospital (Girona). Thyroid cancer patients were classified as papillary cancer (85%) or follicular cancer (15%). The control group consisted of 479 healthy cancer-free volunteers (283 females and 196 males) with a mean age ±s.d. of 45.99±17.26 years. All subjects were Caucasians with Spanish ancestry and from the same geographic area (Table 1).
Table 1.
General characteristics of the studied population: control and thyroid cancer groups.
| Control | Cases | PTC | FTC | |
|---|---|---|---|---|
| Total | 479 | 398 | 339 | 59 |
| Male, n (%) | 196 (41) | 89 (22) | 75 (22) | 14 (24) |
| Female, n (%) | 283 (59) | 309 (78) | 264 (78) | 45 (76) |
| Age: mean±s.d. | 45.9±17.3 | 46.9±15.4 | 46.3±15.1 | 50.7±17.1 |
| Male | 49.9±18.1 | 47.5±14.3 | 46.7±14.0 | 51.9±15.7 |
| Female | 43.2±16.1 | 46.8±15.8a | 46.2±15.4a | 50.3±17.6a |
| Mean age at diagnostic | 41.7±15.1 | 40.9±14.6 | 46.2±16.8b |
PTC, papillary thyroid cancer; FTC, follicular thyroid cancer.
T-test, P<0.05, between control and cases.
T-test, P<0.05, between PTC and FTC.
Individual information was obtained by personal interview, and clinical information of patients was obtained from medical history. All participants in the study gave written informed consent. The study was approved by the ethics committees of all the institutions involved.
Single nucleotide polymorphisms selection
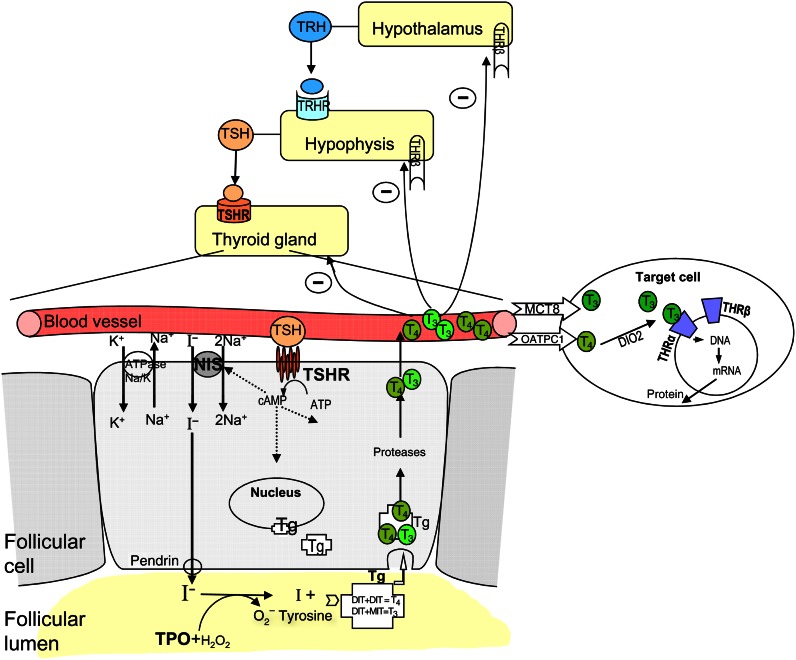
THRA and THRB and TSHR receptors are critical for the function of the thyroid gland via the pituitary–thyroid axis (see Fig. 1). The selection of the single nucleotide polymorphisms (SNPs) in these genes was based on the information available in the literature and public databases. The selection criteria were based on the minor allele frequency >0.1. A total of six tagSNPs at the THRA, THRB and TSHR genes were selected for the study. More detailed information of the studied SNPs is described in Table 2.
Figure 1.
Scheme of the thyroid hormone production and regulation on the hypothalamus–pituitary–thyroid axis.
Table 2.
Information of the studied SNPs and allele frequencies between cases and controls in a Spanish population.
| Chromosome | MAF | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene | SNP ID | Number | Positiona | Nucleotide change | Gene polymorphism | Control n=479 | Cases n=398 | P value b |
| THRA | rs939348 | 17 | 35485379 | C/T | Intron 2 | 0.25 | 0.28 | 0.25 |
| THRB | rs3752874 | 3 | 24159999 | C/T | Phe245Phe | 0.16 | 0.14 | 0.23 |
| rs826377 | 3 | 24143435 | T/C | Intron 9 | 0.18 | 0.19 | 0.91 | |
| rs844107 | 3 | 24138025 | A/G | 3′-UTR | 0.38 | 0.37 | 0.60 | |
| TSHR | rs11845164 | 14 | 80601507 | C/T | Intron 2 | 0.14 | 0.15 | 0.60 |
| rs8019570 | 14 | 80619348 | A/G | Intron 3 | 0.14 | 0.15 | 0.58 | |
n, number of subjects; MAF, minor allele frequency; SNP, single nucleotide polymorphisms.
Position according to NCBI reference sequence.
Two-sided χ 2 test for distribution of allele frequencies.
DNA preparation and genotyping
DNA for both cases and controls was isolated preferentially from peripheral blood mononuclear cells using the standard phenol–chloroform method. For some controls, DNA was obtained from saliva samples using the Oragene DNA Self-Collection kit (DNA Genotek, Ottawa, Ontario, Canada). DNA concentration and purity were measured with a Nanodrop 1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). The samples were stored at −20 °C until use.
All genotype analyses were performed at the Spanish National Genotyping Centre (CeGen, Santiago de Compostela, Spain) using MassArray Sequenom technology (Genome, San Diego, CA, USA) with the iPLEX strategy. SNP genotyping was performed using a custom-made SNP panel. Genotyping reliability was guaranteed through double genotyping by replicating 10% of samples at random in multiple 96-well plates. In addition, two HapMap reference trios were incorporated in the plates, and the genotype concordance and correct Mendelian inheritance were verified.
Statistical analysis
The SNPStats software (30), SPSS (PASW statistics v17.0) and GraphPad software were used to perform all the statistical analyses. Comparison of the sex proportion between cases and control groups and the Hardy–Weinberg equilibrium test of the genotype distribution in the control population were examined using the χ 2 test with a 5% level of significance. Mean ages of cases and controls were compared by the T-test. The thyroid cancer risk was assessed using unconditional logistic regression analyses, adjusted for gender and age, to determine the odds ratios (OR) and 95% confidence intervals (CI). Tests for interaction were performed including the cross product of the respective variant alleles. The Haploview software (31) was used to examine the linkage disequilibrium (LD) between adjacent SNPs and to define haplotype block structures. The entire tests were two sided and P<0.05 was considered statistically significant.
Results
The characteristics of the population used in this study are summarized in Table 1. The gender distribution in patients and controls was different (78 and 59% females respectively, P<0.0001). Further analysis of the genotype distribution within gender in the control group has showed no statistically significant differences between males and females (data not shown). Thus, in our population, the different sex proportion in patients and control groups did not influence the successive association analysis.
Association of individual SNPs with risk of DTC
The allele frequencies for the six SNPs studied are shown in Table 2. No significant differences in the allele frequency were observed between cases and controls for any of the SNPs studied, although for the THRA-rs939348 a slight difference in cases with respect to controls was observed (0.28 and 0.25 respectively). The Hardy–Weinberg analysis of the control group showed that the genotype distribution was in equilibrium for the six genotyped polymorphisms (P=0.07–0.92).
Table 3 shows the genotype distribution of the SNPs studied and the association analysis with DTC, in the total patient group, and in PTC and FTC subgroups. No significant differences in the ORs were observed between controls and patients for either of the three SNPs genotyped in THRB or the two genotyped SNPs in TSHR.
Table 3.
Genotype frequencies of the selected SNPs and their association with differentiated thyroid cancer in a Spanish population.
| SNP | Genotype | Control, n (%) | DTC, n (%) | Odds ratio a (95% CI) | P value b | PTC, n (%) | Odds ratio a (95% CI) | P value b | FTC, n (%) | Odds ratio a (95% CI) | P value b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| THRA | |||||||||||
| rs939348 | C/C | 259 (54.2) | 210 (53.2) | 1.00 | 180 (53.6) | 1.00 | 30 (50.9) | 1.00 | |||
| C/T | 196 (41.0) | 151 (38.2) | 0.89 (0.67–1.18) | 130 (38.7) | 0.90 (0.67–1.21) | 21 (35.6) | 0.84 (0.46–1.53) | ||||
| T/T | 23 (4.8) | 34 (8.6) | 1.71 (0.97–3.03) | 0.082 | 26 (7.7) | 1.59 (0.87–2.91) | 0.19 | 8 (13.6) | 2.51 (1.01–6.24) | 0.098 | |
| C/C+C/T−T/T | 23 (4.8) | 34 (8.6) | 1.80 (1.03–3.14) | 0.037 c | 26 (7.7) | 1.66 (0.92–3.01) | 0.092 c | 8 (13.6) | 2.70 (1.12–6.49) | 0.037 c | |
| THRB | |||||||||||
| rs3752874 | C/C | 331 (69.2) | 287 (72.7) | 1.00 | 246 (73.0) | 1.00 | 41 (70.7) | 1.00 | |||
| C/T | 138 (28.9) | 103 (26.1) | 0.85 (0.63–1.16) | 86 (25.5) | 0.83 (0.60–1.14) | 17 (29.3) | 1.05 (0.57–1.93) | ||||
| T/T | 9 (1.9) | 5 (1.3) | 0.63 (0.21–1.95) | 0.45 | 5 (1.5) | 0.73 (0.24–2.25) | 0.47 | 0 (0.0) | 0.00 (0.00–NA) | 0.39 | |
| rs826377 | T/T | 320 (67.0) | 260 (65.8) | 1.00 | 223 (66.4) | 1.00 | 37 (62.7) | 1.00 | |||
| T/C | 141 (29.5) | 124 (31.4) | 1.06 (0.79–1.43) | 103 (30.6) | 1.03 (0.75–1.41) | 21 (35.6) | 1.29 (0.72–2.30) | ||||
| C/C | 17 (3.6) | 11 (2.8) | 0.77 (0.35–1.69) | 0.72 | 10 (3.0) | 0.83 (0.37–1.87) | 0.87 | 1 (1.7) | 0.50 (0.06–3.93) | 0.50 | |
| rs844107 | A/A | 178 (38.5) | 158 (40.8) | 1.00 | 135 (40.7) | 1.00 | 23 (41.1) | 1.00 | |||
| A/G | 219 (47.4) | 176 (45.1) | 0.89 (0.66–1.21) | 155 (46.7) | 0.93 (0.68–1.26) | 21 (37.5) | 0.79 (0.42–1.49) | ||||
| G/G | 65 (14.1) | 54 (14.1) | 0.92 (0.60–1.41) | 0.76 | 42 (12.7) | 0.83 (0.52–1.31) | 0.71 | 12 (21.4) | 1.47 (0.68–3.14) | 0.31 | |
| TSHR | |||||||||||
| rs11845164 | T/T | 347 (73.0) | 287 (72.7) | 1.00 | 242 (71.8) | 1.00 | 45 (77.6) | 1.00 | |||
| T/C | 122 (25.7) | 97 (24.6) | 0.97 (0.71–1.33) | 86 (25.5) | 1.02 (0.74–1.42) | 11 (19.0) | 0.71 (0.35–1.44) | ||||
| C/C | 6 (1.3) | 11 (2.8) | 2.22 (0.79–6.24) | 0.29 | 9 (2.7) | 2.08 (0.71–6.06) | 0.4 | 2 (3.5) | 2.66 (0.50–14.10) | 0.32 | |
| rs8019570 | G/G | 352 (73.6) | 288 (72.9) | 1.00 | 243 (72.3) | 1.00 | 45 (76.3) | 1.00 | |||
| G/A | 120 (25.1) | 97 (24.6) | 1.00 (0.73–1.37) | 85 (25.3) | 1.04 (0.75–1.44) | 12 (20.3) | 0.80 (0.41–1.58) | ||||
| A/A | 6 (1.3) | 10 (2.5) | 2.08 (0.73–5.96) | 0.38 | 8 (2.4) | 1.90 (0.64–5.70) | 0.51 | 2 (3.4) | 2.70 (0.51–14.26) | 0.42 |
PTC, papillary thyroid cancer; FTC, follicular thyroid cancer.
Adjusted for age and gender.
P value corresponding to co-dominant model.
P value corresponding to recessive model. P<0.05.
In the case of the THRA gene, for the rs939348 polymorphism, the logistic regression analysis (co-dominant model) showed an increase in the risk of thyroid cancer. The ORs of the homozygous variant (TT) for this SNP were OR=1.71 (95% CI=0.97–3.03, P=0.082) for the total of cases, OR=1.59 (95% CI=0.87–2.91, P=0.19) for PTC and OR=2.51 (95% CI=1.01–6.24, P=0.098) for FTC. These risk estimates suggest that the variant allele (T) could act as a recessive risk factor for DTC. Thus, we next evaluated the risk assessment for this polymorphism according to the recessive model. As shown in Table 3, a statically significant association of the homozygous variant was observed with DTC (OR=1.80, 95% CI=1.03–3.14, P=0.037); moreover, the association of the homozygous variant was also significant for the FTC subgroup (OR=2.70, 95% CI=1.12–6.49, P=0.037) and marginally significant for PTC (OR=1.66, 95% CI=0.92–3.01, P=0.092). The lack of significant association for the papillary type is most likely due to the low number of homozygous individuals for the variant allele in our population.
Analysis of gene–gene interactions and risk of DTC
Given the functional relationship between THRA, THRB, TSHR and TRHR, we decided to evaluate the combined effect of common genetic variants of these genes on thyroid cancer susceptibility. We performed gene–gene interaction analysis on the pairwise SNPs of either THRA or THRB with TSHR, TG and TRHR. For the interaction analysis, we used the genotype results of this study together with the TG and TRHR genotype data of our previous association study (12). In both the studies, the same Spanish population was genotyped. No significant interaction for risk of DTC was observed in the pairwise SNPs that included the THRA-rs939348 polymorphism (data not shown), although, as indicated before, this polymorphism showed an individual association with thyroid cancer in the study population (see Table 3).
Because two of the three THRB SNPs, rs3752874 and rs826377, are in LD (D′=0.98, r 2=0.36), we explored the interaction of THRB-rs3752874 and the third THRB SNP, rs844107, with TSHR, TG and TRHR genetic variants. In the interaction analysis of two genes, the stratification of the population leads to a reduction of the number of individuals, decreasing the statistical power of the analysis. Therefore, to eliminate such effect for each THRB polymorphism (rs3752874 or rs844107), we combined the heterozygous and the homozygous variant individuals as the variant carriers.
Certain combinations between the THRB polymorphisms (rs3752874 or rs844107) and polymorphisms in TG (rs2076740) or TRHR (rs4129682) genes were significantly associated with the risk of DTC. However, neither of these markers, individually, contribute to the DTC risk (see Table 3 and (12)). The rest of the combinations between the THRB polymorphisms and TSHR-rs8019570, TSHR-rs11845164, TG-rs180223, TG-rs853326 or TRHR-rs7823804 showed no statistically significant association with DTC risk (data not shown).
An interaction effect between genetic variants of THRB and TG was found for thyroid cancer risk. Table 4 illustrates that THRB-rs3752874 (exon 7) showed a significant interaction with TG-rs2076740 (P interaction=0.014). The variant allele carriers for THRB-rs3752874 (CT+TT) together with the homozygous variant for TG-rs2076740 manifested a 0.035-fold decrease in DTC risk (95% CI=0.16–0.79). A combination effect with borderline significance (P interaction=0.099) was also observed between THRB-rs844107 and TG-rs2076740.
Table 4.
Risk of DTC associated with the combination of rs3752874 and rs844107 of THRB and different TG and TRHR polymorphisms.
| THRB rs3752874 | |||||
|---|---|---|---|---|---|
| CC | CT+TT | ||||
| Controls/cases | OR (95% CI) | Controls/cases | OR (95% CI) | P for interaction | |
| TG | |||||
| rs2076740 | 0.014 | ||||
| CC | 128/106 | 1.00 | 44/46 | 1.17 (0.71–1.93) | |
| CT | 147/123 | 0.94 (0.65–1.34) | 65/43 | 0.77 (0.48–1.23) | |
| TT | 40/44 | 1.30 (0.78–2.16) | 30/9 | 0.35 (0.16–0.79) | |
| TRHR | |||||
| rs4129682 | 0.0024 | ||||
| CC | 85/83 | 1.00 | 44/17 | 0.41 (0.21–0.76) | |
| CT | 163/154 | 0.93 (0.63–1.37) | 77/63 | 0.76 (0.48–1.20) | |
| TT | 80/50 | 0.57 (0.36–0.92) | 25/28 | 1.14 (0.61–2.15) | |
| THRB rs844107 | |||||
| AA | AG+GG | ||||
| Controls/cases | OR (95% CI) | Controls/cases | OR (95% CI) | P for interaction | |
| TG | |||||
| rs2076740 | 0.099 | ||||
| CC | 66/55 | 1.00 | 95/95 | 1.11 (0.69–1.78) | |
| CT | 81/75 | 0.97 (0.59–1.59) | 127/86 | 0.76 (0.48–1.21) | |
| TT | 18/23 | 1.58 (0.76–3.29) | 51/30 | 0.65 (0.36–1.17) | |
| TRHR | |||||
| rs4129682 | 0.11 | ||||
| CC | 47/51 | 1.00 | 76/46 | 0.58 (0.33–1.01) | |
| CT | 87/81 | 0.84 (0.50–1.40) | 146/132 | 0.79 (0.49–1.26) | |
| TT | 42/26 | 0.52 (0.27–1.00) | 60/52 | 0.74 (0.42–1.29) | |
OR, odds ratio adjusted for age and gender; CI, confidence interval.
The interaction between THRB and TRHR showed that the THRB-rs3752874 (exon 7) polymorphism together with TRHR-rs4129682 also had a protective effect for DTC risk (P interaction=0.0024) (see Table 4). The highest protective interaction was found between the THRB-rs3752874 variant allele carriers and the TRHR-rs4129682 common allele homozygous (CC) that showed a 0.41-fold decrease in DTC risk (95% CI=0.21–0.76).
Discussion
TRs together with the TSHR have crucial roles in the pituitary–thyroid axis (see Fig. 1). The TSHR gene is expressed in the thyroid follicular cells. The THRA and THRB genes are not only ubiquitous transcription factors but also key regulators of the thyroid function (32, 33). Therefore, we postulated that genetic variants in THRA, THRB and TSHR genes individually or in combination with genetic variants in other thyroid function/regulator genes (i.e. TG and TRHR) could be important in DTC development. In this study, we provide evidence for the association of THRA with DTC. In addition, we show that an interaction exists between THRB and TSHR, TG or TRHR that modifies the risk of developing DTC.
In the population studied, the THRA-rs939348 in the 5′-noncoding region (intron 2) acted as a possible marker for thyroid cancer susceptibility. According to the co-dominant model, the homozygous variant showed an increased risk of 1.71 (95% CI=0.97–3.03; P=0.082). Although the statistical significance with the recessive model was higher than with the co-dominant model (P=0.037; see Table 3), these results should be taken with caution, as the number of the homozygous variant was small in our population. Limited information is available about genetic variants in THRA related to thyroid cancer. In three earlier reports (21, 22, 23), the dinucleotide CA repeated in the 3′-noncoding region of THRA was found to be related to thyroid cancer. Two of these studies were based in a case–control design (22, 23), but the conclusions were limited by the small size of the population used. To some extent, our study, using a population of 877 individuals, confirms the above previous results. In this study, we have examined a THRA polymorphism that maps in the 5′-UTR region, far away from the 3′-UTR CA repeat analysed in the previous studies, and our results also indicated a role for the THRA gene in thyroid cancer risk. Moreover, Onda et al. (21) have reported a correlation of the 3′-UTR CA repeat with THRA expression and thyroid tumour aggressiveness. In contrast, no mutations in the THRA gene have been described in thyroid tumours (18); thus, the specific function of THRA in thyroid cancer remains unclear. More recently, Zhu et al. (20) have shown that a mouse model with deleted Thra and Thrb genes developed FTC, suggesting a tumour suppressor function of these genes in FTC.
Regarding the association of TSHR with thyroid cancer, none of the two genetic variants of TSHR included in our study (rs8019570 and rs11845164) modified the susceptibility to DTC significantly, suggesting a lack of influence of these polymorphisms on the development of DTC. Indeed, the role of TSHR in thyroid cancer is not clear; however, it is a major thyroid autoantigen, and genetic variants in the TSHR gene have been described to be associated with the risk of thyroid diseases (29). TSHR mutations in DTC are unusual (29), but Xing et al. (26) reported epigenetic silencing of TSHR in thyroid carcinomas. Two studies investigated TSHR polymorphisms related to DTC, although no association was found (27, 28). Altogether, our results confirm previous studies supporting the lack of association between common genetic variants of TSHR and DTC risk.
In this study, we found no significant association of any of the three THRB SNPs with thyroid cancer when analysed individually. Of these SNPs, rs3752874 and rs826377 map in the ligand binding domain of the THRB gene and they showed LD (D′=0.98, r 2=0.36) in our population. The third THRB SNP maps in the 3′-UTR region of the gene. Genetic variants in the ligand binding domain of the THRB gene have been reported to be associated with serum TSH levels (32, 34), while the 3′-UTR is involved in the silencing of THRB by microRNAs in PTC (19). It is also well established that THRB is important in thyroid cancer development (33). Recently, the activity of THRB as a tumour suppressor has been reported (20, 35), which is correlated with a reduced THRB expression found in renal cancer (36, 37, 38). In mice, certain Thrb mutations also promoted the development of mammary tumours (39). As a further contribution to all this evidence supporting the role of THRB in cancer and particularly in thyroid cancer, the present results represent, to our knowledge, the first study investigating the relationship between THRB variants and DTC risk and suggest that individual genetic variants of THRB are not related to DTC risk. Nevertheless, it is important to replicate these results in other populations and evaluate other genetic variants of THRB to ascertain the implication of individual THRB polymorphisms in thyroid cancer susceptibility.
Furthermore, increasing evidence indicates that, together with environmental factors, the interaction of common genetic variants of low penetrance could be major determinants for thyroid cancer susceptibility (3, 40). Thus, we have explored the interaction of the THRB gene and other thyroid function-related loci for DTC risk. The reasoning behind these analyses was based on the well-known relevance of THRB in DTC, together with the fact that in this study neither of the individually analysed THRB SNPs showed association with DTC. We found a gene–gene interaction of THRB with TG or TRHR for thyroid cancer susceptibility in the population studied. The significant P interaction values found in this population support the general notion that often no association is detected for individual SNPs, but their effect is manifested in combination with other SNPs. The THRB SNP that maps in the 3′-UTR region (rs844107) suggests a combined effect with TG for thyroid cancer risk. As the THRB 3′-UTR is involved in the silencing of the THRB gene (19), we can speculate that genetic variants in the gene encoding the TG protein may produce a small variation in the function of the protein that, in combination with a decrease in the TR, would alter the susceptibility to thyroid cancer. The TG polymorphism (exon 33, rs2076740), which has been shown to interact with the 3′-UTR THRB polymorphism for DTC risk, maps in a cysteine-repetitive element in TG that has been suggested to be involved in intracellular hormone transport (41). This TG polymorphism is associated with autoimmune thyroid disease (42, 43) but showed no association with DTC (12). Therefore, another possible explanation for the combined effect of TG and THRB for thyroid cancer susceptibility could reside in the alteration of the transport of the thyroid hormone together with a decrease in the hormone receptor in the follicular cells of the thyroid gland. On the other hand, the genotyped THRB SNP that maps in the hormone binding domain (rs3752874) has shown a combined effect with either the TG-rs2076740 or the TRHR-rs4129682 for thyroid cancer risk, and the interaction effect was more than multiplicative (P interaction<0.05 and <0.01 respectively). The combination of TG and THRB variants could alter the hormone–receptor interaction for DTC risk. Although no functional interaction is expected between THRB and TRHR, we suggest that variations in the function of these proteins could alter the regulation of the thyroid function via the hypothalamic–pituitary–thyroid axis with consequences in the risk for DTC.
In conclusion, this study confirms the association of THRA with thyroid cancer in a Spanish population, and it supports the lack of association of TSHR with thyroid cancer as previously reported. Furthermore, this is the first study reporting on the analysis of the association of genetic variants in THRB with thyroid cancer. The essential role of THRB in thyroid function has been well documented. Here, our results suggest the importance of genetic variants in THRB in combination with polymorphisms in other genes of the thyroid function (i.e. TG or TRHR) in determining thyroid cancer risk. In line with other authors, our study indicates that the role of gene–gene interaction could be crucial in cancer susceptibility.
Acknowledgements
The authors thank all the subjects participating in this study as well as to the members of the Nuclear Medicine Service, Hospital Vall d'Hebron (Barcelona) and the Endocrinology Unit of the Hospital Josep Trueta (Girona) for providing patient blood samples, Aida Baida, Esteban-Mariano Giménez, Wilser García-Quispes and Cristian Valiente for collecting and preparing the samples.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was partially funded by the Spanish Ministry of Education and Science (project SAF2007-6338) and the Generalitat de Catalunya (CIRIT; 2009SGR-725). A Akdi was supported by a predoctoral fellowship from the Universitat Autònoma de Barcelona and E R González-Flores by a predoctoral fellowship from the Carolina Foundation.
References
- 1.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 2.Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid. 2011;21:125–134. doi: 10.1089/thy.2010.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nature Reviews. Cancer. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 4.Czene K, Lichtenstein P, Hemminki K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish Family-Cancer Database. International Journal of Cancer. 2002;99:260–266. doi: 10.1002/ijc.10332. [DOI] [PubMed] [Google Scholar]
- 5.Hrafnkelsson J, Tulinius H, Jonasson JG, Sigvaldason H. Familial non-medullary thyroid cancer in Iceland. Journal of Medical Genetics. 2001;38:189–191. doi: 10.1136/jmg.38.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. Journal of the National Cancer Institute. 1994;86:1600–1608. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- 7.Amundadottir LT, Thorvaldsson S, Gudbjartsson DF, Sulem P, Kristjansson K, Arnason S, Gulcher JR, Bjornsson J, Kong A, Thorsteinsdottir U, Stefansson K. Cancer as a complex phenotype: pattern of cancer distribution within and beyond the nuclear family. PLoS Medicine. 2004;1:e65. doi: 10.1371/journal.pmed.0010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He H, Nagy R, Liyanarachchi S, Jiao H, Li W, Suster S, Kere J, de la Chapelle A. A susceptibility locus for papillary thyroid carcinoma on chromosome 8q24. Cancer Research. 2009;69:625–631. doi: 10.1158/0008-5472.CAN-08-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baida A, Akdi M, Gonzalez-Flores E, Galofre P, Marcos R, Velazquez A. Strong association of chromosome 1p12 loci with thyroid cancer susceptibility. Cancer Epidemiology, Biomarkers & Prevention. 2008;17:1499–1504. doi: 10.1158/1055-9965.EPI-07-0235. [DOI] [Google Scholar]
- 10.Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Sigurdsson A, Bergthorsson JT, He H, Blondal T, Geller F, Jakobsdottir M, Magnusdottir DN, Matthiasdottir S, Stacey SN, Skarphedinsson OB, Helgadottir H, Li W, Nagy R, Aguillo E, Faure E, Prats E, Saez B, Martinez M, Eyjolfsson GI, Bjornsdottir US, Holm H, Kristjansson K, Frigge ML, Kristvinsson H, Gulcher JR, Jonsson T, Rafnar T, Hjartarsson H, Mayordomo JI, de la Chapelle A, Hrafnkelsson J, Thorsteinsdottir U, Kong A, Stefansson K. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nature Genetics. 2009;41:460–464. doi: 10.1038/ng.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jazdzewski K, Murray EL, Franssila K, Jarzab B, Schoenberg DR, de la Chapelle A. Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. PNAS. 2008;105:7269–7274. doi: 10.1073/pnas.0802682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akdi A, Perez G, Pastor S, Castell J, Biarnes J, Marcos R, Velazquez A. Common variants of the thyroglobulin gene are associated with differentiated thyroid cancer risk. Thyroid. 2011;21:519–525. doi: 10.1089/thy.2010.0384. [DOI] [PubMed] [Google Scholar]
- 13.Landa I, Ruiz-Llorente S, Montero-Conde C, Inglada-Perez L, Schiavi F, Leskela S, Pita G, Milne R, Maravall J, Ramos I, Andia V, Rodriguez-Poyo P, Jara-Albarran A, Meoro A, del Peso C, Arribas L, Iglesias P, Caballero J, Serrano J, Pico A, Pomares F, Gimenez G, Lopez-Mondejar P, Castello R, Merante-Boschin I, Pelizzo MR, Mauricio D, Opocher G, Rodriguez-Antona C, Gonzalez-Neira A, Matias-Guiu X, Santisteban P, Robledo M. The variant rs1867277 in FOXE1 gene confers thyroid cancer susceptibility through the recruitment of USF1/USF2 transcription factors. PLoS Genetics. 2009;5:e1000637. doi: 10.1371/journal.pgen.1000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi M, Saenko VA, Rogounovitch TI, Kawaguchi T, Drozd VM, Takigawa-Imamura H, Akulevich NM, Ratanajaraya C, Mitsutake N, Takamura N, Danilova LI, Lushchik ML, Demidchik YE, Heath S, Yamada R, Lathrop M, Matsuda F, Yamashita S. The FOXE1 locus is a major genetic determinant for radiation-related thyroid carcinoma in Chernobyl. Human Molecular Genetics. 2010;19:2516–2523. doi: 10.1093/hmg/ddq123. [DOI] [PubMed] [Google Scholar]
- 15.Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Masson G, He H, Jonasdottir A, Sigurdsson A, Stacey SN, Johannsdottir H, Helgadottir HT, Li W, Nagy R, Ringel MD, Kloos RT, de Visser MC, Plantinga TS, den Heijer M, Aguillo E, Panadero A, Prats E, Garcia-Castano A, De Juan A, Rivera F, Walters GB, Bjarnason H, Tryggvadottir L, Eyjolfsson GI, Bjornsdottir US, Holm H, Olafsson I, Kristjansson K, Kristvinsson H, Magnusson OT, Thorleifsson G, Gulcher JR, Kong A, Kiemeney LA, Jonsson T, Hjartarson H, Mayordomo JI, Netea-Maier RT, de la Chapelle A, Hrafnkelsson J, Thorsteinsdottir U, Rafnar T, Stefansson K. Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nature Genetics. 2012;44:319–322. doi: 10.1038/ng.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocha AS, Marques R, Bento I, Soares R, Magalhaes J, de Castro IV, Soares P. Thyroid hormone receptor β mutations in the ‘hot-spot region’ are rare events in thyroid carcinomas. Journal of Endocrinology. 2007;192:83–86. doi: 10.1677/JOE-06-0009. [DOI] [PubMed] [Google Scholar]
- 17.Takano T, Miyauchi A, Yoshida H, Nakata Y, Kuma K, Amino N. Expression of TRβ1 mRNAs with functionally impaired mutations is rare in thyroid papillary carcinoma. Journal of Clinical Endocrinology and Metabolism. 2003;88:3447–3449. doi: 10.1210/jc.2003-030012. [DOI] [PubMed] [Google Scholar]
- 18.Joseph B, Ji M, Liu D, Hou P, Xing M. Lack of mutations in the thyroid hormone receptor (TR) α and β genes but frequent hypermethylation of the TRβ gene in differentiated thyroid tumors. Journal of Clinical Endocrinology and Metabolism. 2007;92:4766–4770. doi: 10.1210/jc.2007-0812. [DOI] [PubMed] [Google Scholar]
- 19.Jazdzewski K, Boguslawska J, Jendrzejewski J, Liyanarachchi S, Pachucki J, Wardyn KA, Nauman A, de la Chapelle A. Thyroid hormone receptor β (THRB) is a major target gene for microRNAs deregulated in papillary thyroid carcinoma (PTC) Journal of Clinical Endocrinology and Metabolism. 2011;96:E546–E553. doi: 10.1210/jc.2010-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu XG, Zhao L, Willingham MC, Cheng SY. Thyroid hormone receptors are tumor suppressors in a mouse model of metastatic follicular thyroid carcinoma. Oncogene. 2010;29:1909–1919. doi: 10.1038/onc.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onda M, Li D, Suzuki S, Nakamura I, Takenoshita S, Brogren CH, Stampanoni S, Rampino N. Expansion of microsatellite in the thyroid hormone receptor-α1 gene linked to increased receptor expression and less aggressive thyroid cancer. Clinical Cancer Research. 2002;8:2870–2874. [PubMed] [Google Scholar]
- 22.Baida A, Farrington SM, Galofre P, Marcos R, Velazquez A. Thyroid cancer susceptibility and THRA1 and BAT-40 repeats polymorphisms. Cancer Epidemiology, Biomarkers & Prevention. 2005;14:638–642. doi: 10.1158/1055-9965.EPI-04-0424. [DOI] [PubMed] [Google Scholar]
- 23.Rebai M, Kallel I, Charfeddine S, Hamza F, Guermazi F, Rebai A. Association of polymorphisms in estrogen and thyroid hormone receptors with thyroid cancer risk. Journal of Receptor and Signal Transduction Research. 2009;29:113–118. doi: 10.1080/10799890902845682. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Jimenez C, Santisteban P. TSH signalling and cancer. Arquivos Brasileiros de Endocrinologia e Metabologia. 2007;51:654–671. doi: 10.1590/S0004-27302007000500003. [DOI] [PubMed] [Google Scholar]
- 25.Milas M, Shin J, Gupta M, Novosel T, Nasr C, Brainard J, Mitchell J, Berber E, Siperstein A. Circulating thyrotropin receptor mRNA as a novel marker of thyroid cancer: clinical applications learned from 1758 samples. Annals of Surgery. 2010;252:643–651. doi: 10.1097/SLA.0b013e3181f5ba51. [DOI] [PubMed] [Google Scholar]
- 26.Xing M, Usadel H, Cohen Y, Tokumaru Y, Guo Z, Westra WB, Tong BC, Tallini G, Udelsman R, Califano JA, Ladenson PW, Sidransky D. Methylation of the thyroid-stimulating hormone receptor gene in epithelial thyroid tumors: a marker of malignancy and a cause of gene silencing. Cancer Research. 2003;63:2316–2321. [PubMed] [Google Scholar]
- 27.Lonn S, Bhatti P, Alexander BH, Pineda MA, Doody MM, Struewing JP, Sigurdson AJ. Papillary thyroid cancer and polymorphic variants in TSHR- and RET-related genes: a nested case–control study within a cohort of U.S. radiologic technologists. Cancer Epidemiology, Biomarkers & Prevention. 2007;16:174–177. doi: 10.1158/1055-9965.EPI-06-0665. [DOI] [PubMed] [Google Scholar]
- 28.Matakidou A, Hamel N, Popat S, Henderson K, Kantemiroff T, Harmer C, Clarke SE, Houlston RS, Foulkes WD. Risk of non-medullary thyroid cancer influenced by polymorphic variation in the thyroglobulin gene. Carcinogenesis. 2004;25:369–373. doi: 10.1093/carcin/bgh027. [DOI] [PubMed] [Google Scholar]
- 29.Davies TF, Yin X, Latif R. The genetics of the thyroid stimulating hormone receptor: history and relevance. Thyroid. 2010;20:727–736. doi: 10.1089/thy.2010.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sole X, Guino E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22:1928–1929. doi: 10.1093/bioinformatics/btl268. [DOI] [PubMed] [Google Scholar]
- 31.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen HG, van der Deure WM, Hansen PS, Peeters RP, Breteler MM, Kyvik KO, Sorensen TI, Hegedus L, Visser TJ. Identification and consequences of polymorphisms in the thyroid hormone receptor α and β genes. Thyroid. 2008;18:1087–1094. doi: 10.1089/thy.2008.0236. [DOI] [PubMed] [Google Scholar]
- 33.Cheng SY. Thyroid hormone receptor mutations and disease: beyond thyroid hormone resistance. Trends in Endocrinology and Metabolism. 2005;16:176–182. doi: 10.1016/j.tem.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Arnaud-Lopez L, Usala G, Ceresini G, Mitchell BD, Pilia MG, Piras MG, Sestu N, Maschio A, Busonero F, Albai G, Dei M, Lai S, Mulas A, Crisponi L, Tanaka T, Bandinelli S, Guralnik JM, Loi A, Balaci L, Sole G, Prinzis A, Mariotti S, Shuldiner AR, Cao A, Schlessinger D, Uda M, Abecasis GR, Nagaraja R, Sanna S, Naitza S. Phosphodiesterase 8B gene variants are associated with serum TSH levels and thyroid function. American Journal of Human Genetics. 2008;82:1270–1280. doi: 10.1016/j.ajhg.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Iglesias O, Garcia-Silva S, Tenbaum SP, Regadera J, Larcher F, Paramio JM, Vennstrom B, Aranda A. Thyroid hormone receptor β1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Research. 2009;69:501–509. doi: 10.1158/0008-5472.CAN-08-2198. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Sancho JM, Garcia V, Bonilla F, Munoz A. Thyroid hormone receptors/THR genes in human cancer. Cancer Letters. 2003;192:121–132. doi: 10.1016/S0304-3835(02)00614-6. [DOI] [PubMed] [Google Scholar]
- 37.Puzianowska-Kuznicka M, Nauman A, Madej A, Tanski Z, Cheng S, Nauman J. Expression of thyroid hormone receptors is disturbed in human renal clear cell carcinoma. Cancer Letters. 2000;155:145–152. doi: 10.1016/S0304-3835(00)00416-X. [DOI] [PubMed] [Google Scholar]
- 38.Master A, Wojcicka A, Piekielko-Witkowska A, Boguslawska J, Poplawski P, Tanski Z, Darras VM, Williams GR, Nauman A. Untranslated regions of thyroid hormone receptor β1 mRNA are impaired in human clear cell renal cell carcinoma. Biochimica et Biophysica Acta. 2010;1802:995–1005. doi: 10.1016/j.bbadis.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 39.Guigon CJ, Kim DW, Willingham MC, Cheng SY. Mutation of thyroid hormone receptor-β in mice predisposes to the development of mammary tumors. Oncogene. 2011;30:3381–3390. doi: 10.1038/onc.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sturgis EM, Li G. Molecular epidemiology of papillary thyroid cancer: in search of common genetic associations. Thyroid. 2009;19:1031–1034. doi: 10.1089/thy.2009.1597. [DOI] [PubMed] [Google Scholar]
- 41.Koch N, Lauer W, Habicht J, Dobberstein B. Primary structure of the gene for the murine Ia antigen-associated invariant chains (Ii). An alternatively spliced exon encodes a cysteine-rich domain highly homologous to a repetitive sequence of thyroglobulin. EMBO Journal. 1987;6:1677–1683. doi: 10.1002/j.1460-2075.1987.tb02417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins JE, Heward JM, Howson JM, Foxall H, Carr-Smith J, Franklyn JA, Gough SC. Common allelic variants of exons 10, 12, and 33 of the thyroglobulin gene are not associated with autoimmune thyroid disease in the United Kingdom. Journal of Clinical Endocrinology and Metabolism. 2004;89:6336–6339. doi: 10.1210/jc.2004-1336. [DOI] [PubMed] [Google Scholar]
- 43.Ban Y, Tozaki T, Taniyama M, Tomita M, Ban Y. Association of a thyroglobulin gene polymorphism with Hashimoto's thyroiditis in the Japanese population. Clinical Endocrinology. 2004;61:263–268. doi: 10.1111/j.1365-2265.2004.02096.x. [DOI] [PubMed] [Google Scholar]