Abstract
Introduction
Hyper- and hypoglycemia are strongly associated with adverse outcomes in critical care. Neurologically injured patients are a unique subgroup, where optimal glycemic targets may differ, such that the findings of clinical trials involving heterogeneous critically ill patients may not apply.
Methods
We performed a systematic review and meta-analysis of randomized controlled trials (RCTs) comparing intensive insulin therapy with conventional glycemic control among patients with traumatic brain injury, ischemic or hemorrhagic stroke, anoxic encephalopathy, central nervous system infections or spinal cord injury.
Results
Sixteen RCTs, involving 1248 neurocritical care patients, were included. Glycemic targets with intensive insulin ranged from 70-140 mg/dl (3.9-7.8 mmol/L), while conventional protocols aimed to keep glucose levels below 144-300 mg/dl (8.0-16.7 mmol/L). Tight glycemic control had no impact on mortality (RR 0.99; 95% CI 0.83-1.17; p = 0.88), but did result in fewer unfavorable neurological outcomes (RR 0.91; 95% CI 0.84-1.00; p = 0.04). However, improved outcomes were only observed when glucose levels in the conventional glycemic control group were permitted to be relatively high [threshold for insulin administration > 200 mg/dl (> 11.1 mmol/L)], but not with more intermediate glycemic targets [threshold for insulin administration 140-180 mg/dl (7.8-10.0 mmol/L)]. Hypoglycemia was far more common with intensive therapy (RR 3.10; 95% CI 1.54-6.23; p = 0.002), but there was a large degree of heterogeneity in the results of individual trials (Q = 47.9; p<0.0001; I2 = 75%). Mortality was non-significantly higher with intensive insulin in studies where the proportion of patients developing hypoglycemia was large (> 33%) (RR 1.17; 95% CI 0.79-1.75; p = 0.44).
Conclusions
Intensive insulin therapy significantly increases the risk of hypoglycemia and does not influence mortality among neurocritical care patients. Very loose glucose control is associated with worse neurological recovery and should be avoided. These results suggest that intermediate glycemic goals may be most appropriate.
Introduction
A key paradigm in the care of patients with acute brain and spinal cord injury is prevention of physiological abnormalities that may contribute to secondary neurological damage. Hyperglycemia is common in critically ill patients, and has been associated with worsened outcomes in the setting of traumatic brain injury (TBI) [1-9], aneurysmal subarachnoid hemorrhage (SAH) [10-19], spontaneous intracerebral hemorrhage (ICH) [20-26], ischemic stroke [27-35] and anoxic brain injury [36-38]. The mechanisms whereby hyperglycemia could be harmful are complex. Contributing factors may include free radical formation and oxidative injury, activation of N-methyl-D-aspartate receptors, raised intracellular calcium, triggering of inflammatory and apoptotic pathways, and alterations in lactate metabolism with reduced tissue pH [39]. Despite these observations, it remains unclear from human studies whether hyperglycemia is simply a marker for a greater severity of neurological damage or truly contributes to secondary injury in a causative fashion. Hypoglycemia may also be deleterious, since neurocritical care patients are dependent on sufficient glucose as an energy source for the central nervous system (CNS) [40,41]. Even moderate reductions in serum glucose can result in pronounced neuroglycopenia [42-44].
Numerous randomized controlled trials (RCTs) have assessed the efficacy and safety of intensive insulin therapy and tight glycemic control regimens in the care of critically ill patients. Despite initial enthusiasm based on the results of single-center RCTs [45,46], more recent multi-center RCTs have been unable to confirm any benefit, and have even suggested harm [47-49]. Similarly, meta-analyses have not demonstrated a reduction in mortality with tight versus conventional glycemic control [50-53].
Neurocritical care patients are a unique subgroup, in which the association between hyperglycemia and adverse outcomes in observational studies has been particularly strong. Although RCTs of tight glycemic control in critically ill patients have focused largely on mortality as the primary outcome, functional recovery is an especially meaningful endpoint in the neurologically injured. Even if an intervention does not impact on mortality, it may still be efficacious at improving functional and cognitive outcomes among survivors. Thus, the findings of RCTs involving heterogeneous populations of critically ill patients may not necessarily apply.
Some meta-analyses have pooled results in specific subgroups of brain-injured patients [54-56]. However, results from several RCTs were not included in these reviews. A comprehensive overview of all clinical trials involving neurocritical care patients has never been performed, and the optimal approach to glycemic control remains largely unknown. Therefore, we performed a systematic review and meta-analysis to assess whether tight glycemic control reduces mortality and improves outcomes in neurocritical care patients. We also conducted stratified analyses and meta-regression in an attempt to determine whether particular clinical or study-design characteristics influence the relationship between tight glycemic control and patient outcomes.
Materials and methods
A written protocol, with a pre-specified analysis plan, was developed prior to study initiation in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [57].
Search strategy
Using the OVID interface, we conducted unrestricted searches in MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL) and the Cochrane Database of Systematic Reviews from their inception date until the first week of November 2011. To identify RCTs involving neurocritical care patients, the Boolean operator AND was used to combine three search concepts: intensive glycemic control, neurocritical care (defined below) and clinical trials. These concepts were created using a combination of Medical Subject Heading (MeSH) terms and keywords, and were combined using the Boolean operator OR (Additional file 1, Appendix).
A separate search was performed to identify clinical trials involving general critical care patients with heterogeneous diagnostic categories that were cared for in multi-system ICUs. Four published meta-analyses were used to identify relevant manuscripts [50-53], and the search strategy from one of these was repeated from March 2008 to November 2011 [51]. The manuscripts of retrieved studies were reviewed to determine if separate results were reported specifically for neurocritical care patients. We also searched the references of included RCTs and previous systematic reviews relating to intensive insulin therapy.
Study selection
Article selection was performed in two sequential steps. First, one investigator (AHK) screened the title, abstract and keywords of all records retrieved using the search strategy. This stage was intended to be inclusive, and identified all RCTs involving hospitalized patients that compared at least two regimens of insulin administration or glycemic control. Second, the resultant, shorter list was reviewed independently and in duplicate by two investigators (AHK, and DJR).
Studies were considered eligible based on the following inclusion criteria: (1) study design (RCTs only); (2) target population (adults with at least one of the following conditions: TBI, SAH, ICH, ischemic stroke, anoxic injury, spinal cord injury or CNS infection); (3) intervention (comparing an intensive glycemic control protocol with a conventional (less tight) strategy); and (4) outcome (documentation of at least one of the primary or secondary outcomes (see below) in the target population).
Studies were excluded if other aspects of care, besides glycemic control, differed between groups. Thus, RCTs assessing the efficacy of glucose-potassium-insulin (GKI) regimens were not eligible, but were included in a planned sensitivity analysis. For RCTs involving mixed populations, but not presenting separate data for neurocritical care patients, we included the pooled results only if >75% of patients had a neurocritical care diagnosis. Studies consisting largely of non-emergent, perioperative neurosurgical patients were excluded, since these patients did not have an acute neurological injury.
Data abstraction and assessment for risk of bias
Independently and in duplicate, two investigators (AHK, and DJR) abstracted data in an unblinded fashion, using a standardized form [58]. A translator was consulted to assist with papers published in a foreign language. Risk of bias among included RCTs was assessed using the following criteria: adequacy of allocation concealment, blinding of subjects and clinicians to treatment groups, blinding during outcome adjudication (for studies reporting neurological outcomes in addition to mortality), use of an intention-to-treat analysis, loss to follow-up, and baseline differences in important prognostic variables. In each case, we also assigned a Jadad score, which grades studies' descriptions of randomization (two points), blinding (two points) and attrition information (one point) [59]. Studies with an appropriate randomization strategy that prevented investigators or clinicians from predicting subsequent treatment allocation were considered to have adequate concealment [60]. For subsequent analyses, we categorized studies as having a relatively lower risk of bias if the Jadad score was >3 and there was adequate concealment of allocation. For studies reporting neurological outcomes, we also required outcome adjudication to have been performed in a blinded fashion.
Primary outcomes included: (1) 6-month mortality; if this was not specifically presented, we used the available time frame closest to 6 months, and (2) poor neurological recovery, as defined in individual studies. If a full range of outcomes was presented, we considered a Glasgow Outcome Scale (GOS) score of 1 to 3 (death, vegetative state or severe disability), a modified Rankin Scale (mRS) score of 4 to 6 (moderately severe disability, severe disability, death) or a cerebral performance category (CPC) of 3 to 5 (severe disability, coma or vegetative state, death) to represent poor outcomes.
Secondary outcomes, in each case using the definitions provided within individual studies, included the following: (1) hypoglycemia (if several definitions were provided, we utilized the threshold closest to 60 mg/dl); (2) nosocomial pneumonia; (3) other nosocomial infections.
Data synthesis
Studies were pooled using Comprehensive Meta-Analysis (version 2.0, Biostat Inc., Englewood, NJ, USA). The risk ratio was chosen as the summary measure of association. Random effects models were used to pool risk ratios across studies and secondary analyses were performed using fixed effects models. Statistical heterogeneity was assessed with the I2 statistic and Q-test (with a P-value < 0.10 considered significant) [61].
Potential reasons for variability in study results were anticipated in advance, and explored using pre-planned random effects meta-regression, in which patients were pooled a priori according to the following factors: glycemic targets in the control group (defined as loose if insulin was only initiated for glucose concentrations >200 mg/dl, and moderate if insulin was initiated for lower glucose concentrations); incidence of hypoglycemia (studies were dichotomized based on the median incidence, and the two groups were then compared); diagnosis (subgroups of studies involving patients with TBI or stroke were assessed separately); risk of bias (higher vs. lower, as defined above); and duration of intensive glycemic control (> 72 hours vs. <72 hours). We also planned sensitivity analyses with inclusion of studies involving GKI regimens or perioperative patients.
Results
Selection of studies
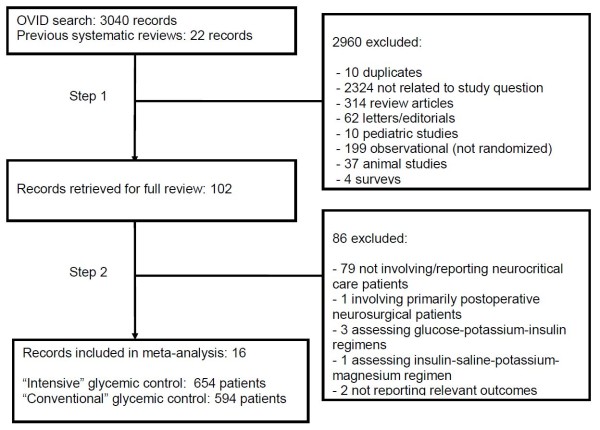
Selection of studies is shown in Figure 1. Our initial search strategy identified 3,040 references. Of these, 90 involved a comparison of two insulin or glycemic control strategies in acute care patients. Another 22 papers, published prior to March 2008, were identified through previous meta-analyses of general critical care patients [50-53]. After removal of 10 duplicates, a list of 102 studies was reviewed in full during the second stage of article selection. Of these, 78 were excluded, leaving a total of 23 RCTs specifically assessing neurocritical care patients.
Figure 1.
Selection of randomized controlled trials comparing intensive and conventional glycemic control protocols in neurocritical care patients.
Of the 23 trials, one study involving perioperative neurosurgical patients was excluded because some of the data had already previously been published in two papers that were included in the meta-analysis. Moreover, the remaining patients primarily had brain tumors, which were treated with semi-elective surgery [62-64]. However, because this was a relatively large study, and the appropriateness of excluding elective neurosurgical patients is somewhat debatable, these results were incorporated into a secondary sensitivity analysis, from which the redundant data from the two other trials were removed [63,64]. Three RCTs involving patients with ischemic stroke used GKI regimens rather than only intensive insulin as their experimental treatment [65-67]. Another trial used an insulin-saline-potassium-magnesium infusion [68]. These four studies were excluded from the primary analysis, but their results were incorporated into a secondary analysis. Two additional RCTs were identified, but did not report any of our primary or secondary outcomes in the manuscript [69,70]. Thus, 16 studies, involving 1,248 patients (654 treated with intensive vs. 594 with conventional glycemic control), were retained for the determination of primary pooled outcomes [47,63,64,71-83].
Characteristics of included studies
The target glucose concentration among patients treated with intensive insulin therapy was most often 80 to 110 mg/dl (4.4 to 6.1 mmol/L), but did vary slightly across RCTs, ranging from 70 to 150 mg/dl (3.9 to 8.3 mmol/L). Glucose goals were more variable in the conventional treatment groups. In the most extreme case, insulin therapy was only initiated when glucose levels exceeded 300 mg/dl (16.7 mmol/L), which was, at the time, consistent with AHA Guidelines for the management of ischemic stroke [76]. At the opposite extreme, one study had a conventional glucose target of 110 to 144 mg/dl (6.1 to 8.0 mmol/L) [77]. In most cases, insulin was only initiated in control patients when glucose levels exceeded 180 to 200 mg/dl. The duration of treatment varied from as short as 24 hours to the entire duration of the ICU admission. Definitions of hypoglycemia ranged from less than 40 to 80 mg/dl (2.2 to 4.4 mmol/L). The frequency of glucose monitoring for patients receiving intravenous (IV) insulin ranged from every 1 to 4 hours. Neurological outcomes were generally reported using the mRS, GOS or extended GOS. Relatively little information was provided on the provision of nutrition; in most cases tube feeding appeared to have been initiated as soon as possible to patients who could not eat (Table 1).
Table 1.
Characteristics of studies comparing intensive and conventional glycemic control in neurocritical care patients
| Author, year | Patients, number | Diagnosis | Intensive definition | Conventional definition |
Duration of protocol | Definition of hypoglycemia | Definition of poor outcome | Timing of nutrition |
|---|---|---|---|---|---|---|---|---|
| Staszewski, 2011 | 50 | IS | 81-126 mg/dl (iv insulin) | < 180 mg/dl (sc insulin) | 24 hours | < 60 mg/dl | mRS 3-6 (30 days) |
Deferred 24 hours |
| Green, 2010 | 81 | Mixed | 80-110 mg/dl (iv insulin) | < 150 mg/dl (sc insulin) | ICU stay | < 60 mg/dl | mRS 3-6 (3 months) |
EN within 24 hours |
| Coester, 2010 | 88 | TBI | 80-110 mg/dl | < 180 mg/dl | ICU stay | < 80 mg/dl | GOS 1-3 (6 months) |
EN within 24-48 hours |
| Johnston, 2009 | 74 | IS | 70-110 mg/dl | < 200 mg/dl (loose) < 300 mg/dl (usual) | 5 days | < 55 mg/dl | mRS 2-6 (3 months) |
PO or EN ASAP |
| Azevedo, 2009 | 34 | IS | < 140 mg/dl (IV insulin) | < 150 mg/dl (SC insulin) | NR | NR | eGOS (Hospital dc) |
Not specified; carbohydrate restriction in controls |
| Meng, 2009 | 240 | TBI | 80-110 mg/dl | 180-200 mg/dl | ICU stay | < 40 mg/dl | GOS 1-3 (6 months) |
IV glucose for 24 hours then EN or PN |
| Yang, 2009 | 110 | ICH, IS, SAH | 80-150 mg/dl (IV insulin) | Treated with twice daily insulin 30/70 | ICU stay | < 80 mg/dl | mRS 4-6 (time frame unclear) |
Not specified |
| Bilotta, 2008 | 97 | TBI | 80-120 mg/dl | < 220 mg/dl | ICU stay | < 80 mg/dl | GOS 1-3 (6 months) |
EN or PN ASAP |
| Kreisel, 2008 | 40 | IS | 80-110 mg/dl | < 200 mg/dl | 5 days | < 60 mg/dl | RS > 2 | Not specified |
| Arabi, 2008 | 94 | TBI | 80-110 mg/dl | 180-200 mg/dl | ICU stay | < 40 mg/dl | NR | EN ASAP |
| Bruno, 2008 | 46 | IS | 90-130 mg/dl | < 200 mg/dl | 72 hours | < 60 mg/dl | mRS 3-6 (3 months) |
Not specified |
| Oksanen, 2007 | 90 | AI | 80-110 mg/dl | 110-144 mg/dl | 48 hours | < 55 mg/dl | NR | Not specified |
| Azevedo, 2007 | 48 | Mixed | 80-120 mg/dl | < 180 mg/dl | ICU stay | < 40 mg/dl | eGOS (3 months) |
IV glucose for 48 hours then EN; carbohydrate restriction in controls |
| Bilotta, 2007 | 78 | SAH | 80-120 mg/dl | < 220 mg/dl | ICU stay | < 80 mg/dl | mRS 4-6 (6 months) |
EN or PN ASAP |
| Walters, 2006 | 25 | IS | 90-144 mg/dl | < 270 mg/dl | 48 hours | NR | NR | Deferred 48 hours |
| Van den Berghe, 2005 | 63 | Mixed | 80-110 mg/dl | < 200 mg/dl | ICU stay | <40 mg/dl | Karnofsky > 60 (12 months) |
IV glucose for 24 hours then EN or PN |
AI, anoxic brain injury; ASAP, as soon as possible; eGOS, extended GOS; EN, enteral nutrition; GCS, Glasgow Coma Scale; GOS, Glasgow Outcome Scale; ICH, intracerebral hemorrhage; IS, ischemic stroke; mRS, modified Ranking Scale; NR, not reported; PN, parenteral nutrition; RS, Rankin score; SAH, subarachnoid hemorrhage; TBI, traumatic brain injury.
The risk of bias varied across studies. In no study were clinicians blinded to glucose levels. For this reason, the Jadad score was never > 3. Most studies used an intention-to-treat analysis and loss to follow-up was relatively uncommon. Baseline characteristics among patients in the two groups were largely similar. Individuals adjudicating neurological outcomes were not always blinded with respect to the treatment group (Table 2).
Table 2.
Risk of bias in studies comparing intensive and conventional glycemic control in neurocritical care patients
| Author (year) | Concealed allocation | Description of random allocation method | Double-blind | ITT analysis | All patients accounted for | Major baseline differences | Blinded outcome adjudication | Jadad score |
|---|---|---|---|---|---|---|---|---|
| Staszewski, 2011 | Unclear | No | No | Yes | Yes | Mean age higher in conventional group (87 vs. 68 yrs; NS) | Yes | 2 |
| Green, 2010 | Adequate | No | No | Yes | 7 patients lost | No | Yes | 2 |
| Coester, 2010 | Adequate | No | No | No† | Yes | More poly-trauma, normal CT scans in control patients | Unclear | 2 |
| Johnston, 2009 | Adequate | No | No | Yes | 1 patient lost (incarcerated) | No | Yes | 2 |
| Azevedo, 2009 | Unclear | No | No | Yes | No‡ | Unclear | Unclear | 1 |
| Meng, 2009 | Adequate | No | No | Yes | 7 patients lost | No | Yes | 2 |
| Yang, 2009 | Unclear | No | No | Yes | Yes | No | Unclear | 2 |
| Bilotta, 2008 | Adequate | Yes | No | Yes | Yes | No | Yes | 3 |
| Kreisel, 2009 | Adequate | Yes | No | Yes | 3 patients lost | More males in intensive group | No | 3 |
| Arabi, 2008 | Unclear | Yes | No | Yes | Yes | Unclear | Not relevant | 3 |
| Bruno, 2008 | Adequate | No (although done by "data management center") | No | Yes | Yes | More patients with diabetes mellitus, treated with tPA in intensive group | Yes | 2 |
| Oksanen, 2007 | Adequate | No (although done by "independent statistician" | No | Yes | Yes | More patients male in ITT groups. Lower MAP in ITT group. | Not relevant | 2 |
| Azevedo, 2007 | Adequate | Yes | No | Yes | No* | No | No | 2 |
| Bilotta, 2007 | Adequate | Yes | No | Yes | Yes | No | Yes | 3 |
| Walters, 2006 | Unclear | No (although done by pharmacy using "standard algorithm") | No | Yes | Unclear | More patients with high HbA1C in treatment group | Not relevant | 1 |
| Van den Berghe, 2005 | Adequate | No | No | Yes | Yes | More males, patients diabetes mellitus, malignancy, ICH, SAH in intensive group | Yes | 2 |
† Although authors stated they used intention-to-treat (ITT) analysis, description of patient flow suggests otherwise. Eight patients did not receive their allocated treatment; their results were not presented or analyzed (largely because unable to obtain consent after randomization); ‡ 20 patients randomized to conventional group; 6 died; functional outcome information only described for 12 (rather than 14); *numbers in Table 3 of manuscript do not account for all patients. NS, not significant; CT, computer tomography; ICH, intracerebral hemorrhage; MAP, mean arterial pressure; SAH, subarachnoid hemorrhage; tPA, tissue plasminogen activator.
Effect of intensive glycemic control on mortality and poor neurological outcomes
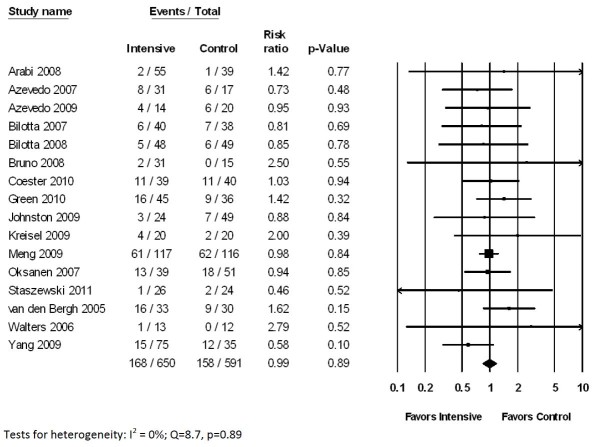
There was no statistically significant difference in mortality between patients treated with intensive (26%) versus conventional glycemic targets (27%) (relative risk, RR 0.99, 95% CI 0.83 to 1.17, P = 0.89) (Figure 2). There was little heterogeneity in study results (Q = 8.7, P = 0.89; I2 = 0%). Findings were consistent in five RCTs involving patients with TBI (RR 0.99, 95% CI 0.79 to 1.22, P = 0.89), six RCTs of patients with ischemic stroke (RR 1.10, 95% CI 0.57 to 2.12, P = 0.78), and nine RCTs of patients with any type of stroke (ischemic or hemorrhagic; RR 0.91, 95% CI 0.61 to 1.34, P = 0.63).
Figure 2.
Impact of intensive glycemic control on mortality in neurocritical care patients.
In 13 RCTs reporting neurological recovery in 1,023 randomized patients, intensive glycemic control resulted in a lower risk of poor neurological outcomes (58% vs. 68%; RR 0.91, 95% CI 0.84 to 1.00, P = 0.04) (Figure 3). There was no significant heterogeneity (Q = 9.6, P= 0.65; I2 = 0%). A comparable trend was observed in four RCTs involving 449 patients with TBI (RR 0.91, 95% CI 0.80 to 1.02, P = 0.11) and in eight RCTs involving 457 patients with either ischemic or hemorrhagic stroke (RR 0.90, 95% CI 0.77 to 1.05, P = 0.19). Among 241 patients specifically with ischemic stroke, intensive insulin had no clear effect (RR 0.97, 0.83 to 1.14, P = 0.71).
Figure 3.
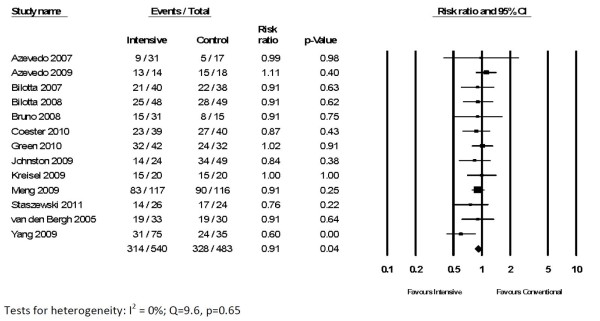
Impact of intensive glycemic control on poor functional recovery in neurocritical care patients.
Effect of intensive glycemic control on secondary outcomes
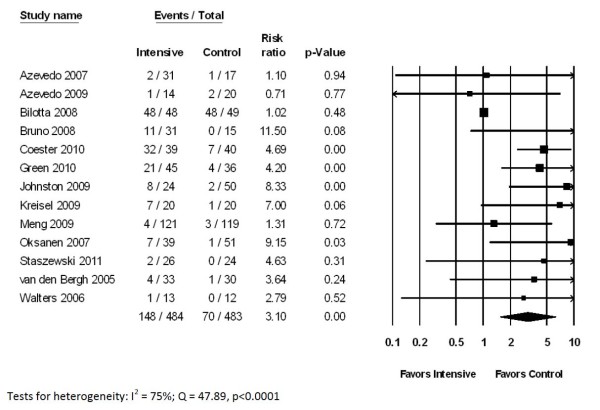
Thirteen trials, involving 967 patients, reported the incidence of hypoglycemia. The proportion of patients treated with intensive insulin who developed hypoglycemia varied greatly between studies, ranging from 3 to 100%, with a median value of 18 to 33%. Although definitions varied, the incidence of hypoglycemia was markedly greater among patients treated with intensive insulin protocols (30% vs. 14%; RR 3.10, 95% CI 1.54 to 6.23, P = 0.002) (Figure 4). However, there was a large degree of heterogeneity between studies (Q = 47.9, P < 0.0001, I2 = 75%).
Figure 4.
Impact of intensive glycemic control on incidence of hypoglycemia in neurocritical care patients.
Six RCTs reported the incidence of pneumonia. Intensive glycemic control did not have any protective effect (RR 1.04, 95% CI 0.82 to 1.32, P = 0.73). Mild to moderate heterogeneity between studies was observed (Q = 6.0, P = 0.31, I2 = 17%). Other nosocomial infections were infrequently reported, such that we did not pool the results.
Meta-regression & sensitivity analyses
Results of subgroup analysis and meta-regression are shown in Table 3. Of the 13 studies reporting the occurrence of neurological outcomes, eight used a control group where glycemic control could be considered, according to our a priori definition, to have been very loose, with insulin administered only if glucose was >200 mg/dl (11.1 mmol/L). Five studies used a design where even the control group received insulin to maintain glucose levels within a relatively narrow range, with a threshold for insulin administration of 144 to 180 mg/dl (8.0 to 10.0 mmol/L). An improvement in outcomes was only observed in the subgroup of studies where control group glucose levels were allowed to be relatively high (RR 0.88, 95% CI 0.79 to 0.98, P = 0.02), but not in those where there was a less extreme difference (RR 0.99, 95% CI 0.85 to 1.14, P = 0.84). The difference between these two categories of studies was statistically significant (P = 0.04).
Table 3.
Subgroup analysis and meta-regression of studies assessing the efficacy of intensive glycemic control in neurocritical care patients
| Comparison | Mortality | Poor neurological outcome | ||||
|---|---|---|---|---|---|---|
| Studies, number | Risk ratio (95% confidence intervals) | P-value for comparison | Studies, number | Risk ratio (95% confidence intervals) | P-value for comparison | |
| Control group | ||||||
| Very loose | 10 | 0.98 (0.80-1.20) | 8 | 0.88 (0.79-0.98) | ||
| Moderate | 6 | 1.00 (0.72-1.39) | 0.89 | 5 | 0.99 (0.85-1.14) | 0.04 |
| Hypoglycemia† | ||||||
| Uncommon | 7 | 1.00 (0.86-1.24) | 5 | 0.94 (0.84-1.06) | ||
| Common | 6 | 1.17 (0.78-1.76) | 0.72 | 6 | 0.94 (0.81-1.08) | 0.94 |
| Duration of tight control | ||||||
| > 72 hours | 12 | 0.99 (0.83-1.18) | 11 | 0.92 (0.84-1.01) | ||
| <72 hours | 4 | 0.97 (0.56-1.67) | 0.37 | 2 | 0.81 (0.57-1.15) | 0.04 |
| Risk of bias | ||||||
| Higher | 11 | 1.03 (0.86-1.24) | 10 | 0.94 (0.85-1.04) | ||
| Lower | 4 | 1.00 (0.52-1.91) | 0.76 | 3 | 0.94 (0.76-1.17) | 0.17 |
| Timing of nutrition | ||||||
| As soon as possible | 6 | 1.07 (0.73-1.57) | 5 | 0.93 (0.80-1.08) | ||
| Deferred > 24 hours | 5 | 1.01 (0.81-1.26) | 0.82 | 4 | 0.90 (0.79-1.03) | 0.79 |
| Definition | ||||||
| Hypoglycemia | 8 | 0.94 (0.67-1.31) | 8 | 0.88 (0.77-1.00) | ||
| 56-80 mg/dl 55 mg/dl or below |
6 | 1.01 (0.82-1.23) | 0.72 | 4 | 0.91 (0.79-1.03) | 0.72 |
† As per a priori plan, patients were dichotomized based on the median prevalence of hypoglycemia across studies; common, hypoglycemia occurred in 33-100% of patients; uncommon, hypoglycemia occurred in 3 to 18% of patients.
As per our a priori plan, studies were dichotomized into those having a high (33 to 100%) or a low incidence (3 to 18%) of hypoglycemia. A non-significant increment in mortality was seen in studies where the incidence of hypoglycemia was high (RR 1.17, 95% CI 0.78 to 1.76, P = 0.44). However, this result did not differ statistically when compared with studies where the incidence of hypoglycemia was low.
Twelve studies assessed the efficacy of intensive insulin administered for more than 72 hours. In four studies, intensive insulin was used more briefly, for time intervals ranging from 24 to 72 hours. No differences in mortality were observed based on the duration of time that intensive insulin was administered. In 11 of the 12 studies using more prolonged regimens of intensive insulin, neurological outcomes were reported and there was a trend towards an improvement with intensive therapy (RR 0.92, 95% 0.84 to 1.01, P = 0.07).
Inclusion of the trial that involved postoperative neurosurgical patients (and exclusion of patients for whom there were redundant data) had little impact on the results [62-64]. On combining the studies assessing the impact of GKI or insulin-saline-potassium-magnesium infusions in ischemic stroke patients, there was no improvement in mortality (reported in three studies; RR 1.08, 95% CI 0.89 to 1.32, P = 0.43) or neurological recovery (reported in three studies; RR 1.02, 95% CI 0.94 to 1.10, P = 0.63). When these four RCTs were combined with all other RCTs, the improvement in functional outcomes associated with intensive glycemic control was no longer present (RR 0.95, 95% CI 0.89 to 1.01, P = 0.11).
Five studies deferred nutritional supplementation for 24 to 48 hours, of which three explicitly mentioned providing intravenous glucose during this time (Table 1). No difference in mortality or unfavourable outcomes was observed in comparison to RCTs where enteral nutrition was not delayed. Three RCTs explicitly mentioned providing intravenous glucose supplementation to patients who were not receiving any other nutrition; in contrast to most other studies, intensive insulin did not significantly increase the incidence of hypoglycemia in these trials (RR 1.64, 0.56 to 4.80, P = 0.37) [71,79,81].
We also assessed outcomes of studies based on the definition of hypoglycemia that was used. Eight RCTs defined hypoglycemia using a relatively high threshold of glucose ≤ 60 to 80 mg/dl (3.3 to 4.4 mmol/L) and six studies used a low threshold of glucose ≤ 40 to 55 mg/dl (2.2 to 3.1 mmol/Ll). There were no differences in mortality, neurological recovery or the incidence of hypoglycemia based on these thresholds.
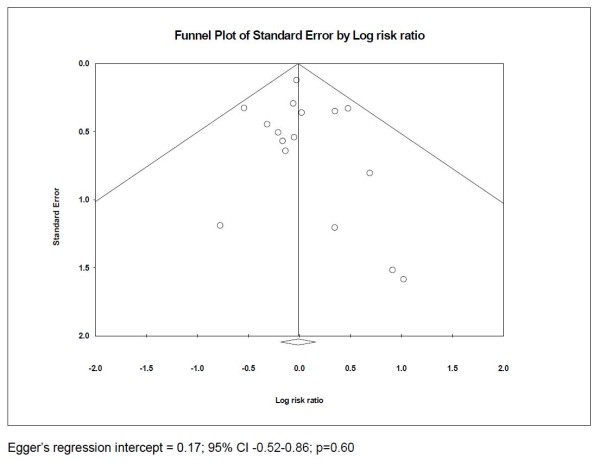
Publication bias
Visual inspection of a funnel plot revealed relative symmetry, arguing against the presence of publication bias (Figure 5). Similarly, there was no evidence of publication bias using Egger's test (intercept 0.17, 95% CI -0.52 to 0.86 P = 0.60).
Figure 5.
Funnel plot showing standard error of studies assessing efficacy of intensive glycemic control in neurocritical care patients in relation to log of calculated risk ratio.
Discussion
Our results provide the most contemporary and comprehensive overview of RCTs involving different glycemic control strategies in neurocritical care patients. Previous quantitative systematic reviews have been published [54,55], but they did not include multiple relevant publications [47,72-78,83], they were based in part on redundant data [62-64], and they did not perform stratified analyses and meta-regression in order to explain heterogeneity in RCT results.
Our findings suggest that intensive glycemic control does not reduce mortality among neurocritical care patients. This observation is consistent with the results of recent large, multi-center RCTs performed in critically ill patients with more heterogeneous, and not necessarily neurological, diagnostic categories [47-53].
In contrast, we did observe intensive glycemic control to reduce the occurrence of poor neurological outcomes. This finding was largely limited to the subgroup of studies where target glucose concentrations in the control group were very loose (insulin initiated only when glucose concentration exceeded 200 mg/dl). A benefit was not observed when intensive treatment was compared with more intermediate glycemic targets (110 to 180 mg/dl). This observation suggests that some of the benefit from intensive insulin may instead have been related to harm attributable to loose glucose control. Thus, glucose concentrations in excess of 180 mg/dl should be avoided in neurocritical care patients. This finding is consistent with a large number of animal experiments and human observational studies.
We found that the incidence of hypoglycemia was markedly increased by intensive insulin therapy. However, the rate of hypoglycemia varied greatly across RCTs. Patients treated with intensive treatment had somewhat higher mortality in studies where the incidence of hypoglycemia was high (>33%), although this result was not statistically significant. Hypoglycemia has been shown to be a strong predictor of mortality in critically ill patients [47,84]. In brain-injured patients, microdialysis studies have demonstrated that reductions in serum glucose concentration may produce profound neuroglycopenia, which in turn may contribute to metabolic distress and secondary brain injury [43,85-88]. Hypoglycemia may also help explain why the introduction of an intensive insulin protocol has been associated with worse outcomes at some centers [89].
One of the proposed complications of hyperglycemia is an increased vulnerability to nosocomial infections. Only a small proportion of studies involving neurocritical care patients reported infection rates. When the results were combined, we could not find any impact of glycemic control on the incidence of hospital-acquired pneumonia.
We did not identify one subgroup of neurocritical care patients in whom intensive insulin therapy was associated either with any particular benefit or harm. The relationship between tighter glycemic control and improved neurological recovery was, however, stronger among patients with TBI, ICH or SAH than it was for patients with ischemic stroke. This finding is consistent with the lack of benefit observed in RCTs assessing the efficacy of GKI infusions, all of which exclusively involved patients with ischemic stroke [65-67]. Our findings should not necessarily be applied to patients undergoing semi-elective neurosurgical procedures, such as resection of a brain tumor, since these were not included in the analysis.
We believe that RCTs are consistent with a U-shaped relationship between serum glucose concentration and neurological outcomes [43]. Both hypoglycemia and extreme hyperglycemia are likely to be harmful. Comparable observations have also been made in medical and surgical critical care patients [90]. The optimal glucose target for neurocritical care patients is likely to fall between 80 and 180 mg/dl (4.4 and 10.0 mmol/L). Given that RCTs suggest a relatively high incidence of hypoglycemia when clinicians attempt to maintain glucose levels between 80 and 110 mg/dl (4.4 to 6.1 mmol/L), we consider a more conservative approach to be most appropriate, for example, 110 to 180 mg/dl (6.1 to 10.0 mmol/L).
Some large RCTs involving heterogeneous populations of critically ill patients have not yet published results for their subgroup of neurological patients, and were therefore excluded from this analysis. Most importantly, the NICE-SUGAR trial included more than 6,000 critically ill patients [49]. The GLUCONTROL trial designated 142 of 1,078 patients (13%) as having a neurological diagnostic category, but did not provide results for this subgroup [91]. Another trial, involving 1,200 medical ICU patients, reported hospital mortality among 61 patients with neurologic conditions. Because the specific disorders were not described, it was unclear if these patients met our eligibility criteria [46]. We were unable to obtain this information from the authors. However, a sensitivity analysis performed with inclusion of these patients did not change our results (RR 0.99, 0.84 to 1.17, P = 0.90).
There are further limitations to this meta-analysis. Although there were many similarities to the methodology of the included RCTs, there was also some variability. This variability is especially reflected by the wide range of hypoglycemia (3 to 100%) among patients randomized to intensive insulin protocols. Any future RCTs of intensive insulin should therefore first carefully pilot their protocol to ensure that hypoglycemia can be minimized. There was some heterogeneity in the provision and reporting of nutritional supplementation, which may have influenced the results. Neurological outcomes reported in this meta-analysis were relatively crude; it remains possible that glycemic control could have a greater influence on more subtle neurocognitive or indices of quality of life. Finally, although we have clustered various neurocritical care conditions, there may be significant differences across disease states, or based on brain-injury severity, that may influence the pathophysiology and implications of hyperglycemia.
Conclusions
In summary, a growing number of RCTs, involving many hundreds of patients, cumulatively demonstrate that intensive glycemic control does not reduce mortality in neurocritical care patients. A unique benefit in certain subgroups cannot be excluded, but no such trend was observed in our analysis. Very loose glycemic control with a target of > 180 mg/dl (10 mmol/L) appears to be harmful and should be avoided. Intensive control with target glucose of 80 to 110 mg/dl (4.4 to 6.1 mmol/L) greatly increases the risk of hypoglycemia. Thus, at present, the literature supports targeting more intermediate glucose levels.
Key messages
• Intensive glycemic control does not appear to improve mortality in neurocritical care patients.
• Very loose glycemic control with insulin initiated only for glucose concentrations >200 mg/dl (11.1 mmol/L) is associated with poor neurological outcomes in neurocritical care patients, compared with either intensive insulin therapy with a target glucose concentration of 80 to 110 mg/dl (4.4 to 6.1 mmol/L), or more modest glycemic control with a target glucose concentration of 110 to 180 mg/dl (6.1 to 10.0 mmol/L).
• Intensive glycemic control greatly increases the risk of hypoglycemia in neurocritical care patients.
Abbreviations
CNS: central nervous system; CPC: Cerebral Performance Category; GOS: Glasgow Outcome Scale; GKI: glucose potassium insulin; ICH: intracerebral hemorrhage; IV: intravenous; mRS: modified Rankin Scale; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analysis; RCT: randomized controlled trial; RR: relative risk; SAH: subarachnoid hemorrhage; TBI: traumatic brain injury.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AK conceived the study and performed the background literature review. All three authors reviewed the protocol prior to study initiation. AK and DR were responsible for searching the literature, selecting manuscripts and critically appraising them. AK and DR performed the statistical analysis. All three authors assisted in interpreting the results and writing the final manuscript. All authors have read and approved the final manuscript.
Acknowledgements
The authors acknowledge Dr Candace Poon, who assisted with the translation of one of the manuscripts.
Supplementary Material
Appendix - OVID Search Strategies.
See related commentary by Bilotta and Rosa, http://ccforum.com/content/16/5/163.
Contributor Information
Andreas H Kramer, Email: Andreas.Kramer@AlbertaHealthServices.ca.
Derek J Roberts, Email: Derek.Roberts@Dal.Ca.
David A Zygun, Email: dzygun@ucalgary.ca.
References
- Lam AM, Winn HR, Cullen BF, Sundling. Hyperglycemia and neurological outcome in patients with head injury. J Neurosurg. 1991;16:545–551. doi: 10.3171/jns.1991.75.4.0545. [DOI] [PubMed] [Google Scholar]
- Yang SY, Zhang S, Wang ML. Clinical significance of admission hyperglycemia and factors related to it in patients with acute severe head injury. Surg Neurol. 1995;16:373–377. doi: 10.1016/0090-3019(96)80243-6. [DOI] [PubMed] [Google Scholar]
- Rovlias A, Kotsou S. The influence of hyperglycemia on neurological outcome in patients with severe head injury. Neurosurgery. 2000;16:335–342. doi: 10.1097/00006123-200002000-00015. [DOI] [PubMed] [Google Scholar]
- Cochran A, Scaife ER, Hansen KW, Downey EC. Hyperglycemia and outcomes from pediatric traumatic brain injury. J Trauma. 2003;16:1035–1038. doi: 10.1097/01.TA.0000031175.96507.48. [DOI] [PubMed] [Google Scholar]
- Jeremitsky E, Omert LA, Dunham CM, Wilberger J, Rodriguez A. The impact of hyperglycemia on patients with severe brain injury. J Trauma. 2005;16:47–50. doi: 10.1097/01.TA.0000135158.42242.B1. [DOI] [PubMed] [Google Scholar]
- Liu-DeRyke X, Collingridge DS, Orme J, Roller D, Zurasky J, Rhoney DH. Clinical impact of early hyperglycemia during acute phase of traumatic brain injury. Neurocrit Care. 2009;16:151–157. doi: 10.1007/s12028-009-9228-6. [DOI] [PubMed] [Google Scholar]
- Griesdale DE, Tremblay MH, McEwen J, Chittock DR. Glucose control and mortality in patients with severe traumatic brain injury. Neurocrit Care. 2009;16:311–316. doi: 10.1007/s12028-009-9249-1. [DOI] [PubMed] [Google Scholar]
- Salim A, Hadjizacharia P, Dubose J, Brown C, Inaba K, Chan LS, Margulies D. Persistent hyperglycemia in severe traumatic brain injury: an independent predictor of outcome. Am Surg. 2009;16:25–29. [PubMed] [Google Scholar]
- Smith RL, Lin JC, Adelson PD, Kochanek PM, Fink EL, Wisniewski SR, Bayir H, Tyler-Kabara EC, Clark RS, Brown SD, Bell MJ. Relationship between hyperglycemia and outcome in children with severe traumatic brain injury. Pediatr Crit Care Med. 2012;16:85–91. doi: 10.1097/PCC.0b013e3182192c30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzino G, Kassell NF, Germanson T, Truskowski L, Alves W. Plasma glucose levels and outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 1993;16:885–891. doi: 10.3171/jns.1993.79.6.0885. [DOI] [PubMed] [Google Scholar]
- Dorhout Mees SM, van Dijk GW, Algra A. Glucose levels and outcome after subarachnoid hemorrhage. Neurology. 2003;16(61):1132–1133. doi: 10.1212/01.wnl.0000090466.68866.02. [DOI] [PubMed] [Google Scholar]
- Badjatia N, Topcuoglu MA, Buonanno FS, Smith EE, Nogueira RG, Rordorf GA, Carter BS, Ogilvy CS, Singhal AB. Relationship between hyperglycemia and symptomatic vasospasm after subarachnoid hemorrhage. Crit Care Med. 2005;16:1603–1609. doi: 10.1097/01.CCM.0000168054.60538.2B. [DOI] [PubMed] [Google Scholar]
- Wartenberg KE, Schmidt JM, Claassen J, Temes RE, Frontera JA, Ostapkovich N, Parra A, Connolly ES, Mayer SA. Impact of medical complications on outcome after subarachnoid hemorrhage. Crit Care Med. 2006;16:617–623. doi: 10.1097/01.ccm.0000201903.46435.35. [DOI] [PubMed] [Google Scholar]
- Frontera JA, Fernandez A, Claassen J, Schmidt M, Schumacher HC, Wartenberg K, Temes R, Parra A, Ostapkovich ND, Mayer SA. Hyperglycemia after SAH: predictors, associated complications, and impact on outcome. Stroke. 2006;16:199–203. doi: 10.1161/01.STR.0000194960.73883.0f. [DOI] [PubMed] [Google Scholar]
- McGirt MJ, Woodworth GF, Ali M, Than KD, Tamargo RJ, Clatterbuck RE. Persistent perioperative hyperglycemia as an independent predictor of poor outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;16:1080–1085. doi: 10.3171/JNS-07/12/1080. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lim JS, Kim N, Yoon BW. Effects of admission glucose level on mortality after subarachnoid hemorrhage: a comparison between short-term and long-term mortality. J Neurol Sc. 2008;16:18–21. doi: 10.1016/j.jns.2008.05.024. [DOI] [PubMed] [Google Scholar]
- Schlenk F, Vajkoczy P, Sarrafzadeh A. Inpatient hyperglycemia following aneurysmal subarachnoid hemorrhage: relation to cerebral metabolism and outcome. Neurocrit Care. 2009;16:56–63. doi: 10.1007/s12028-009-9222-z. [DOI] [PubMed] [Google Scholar]
- Latorre JG, Chou SH, Nogueira RG, Singhal AB, Carter BS, Ogilvy CS, Rordorf GA. Effective glycemic control with aggressive hyperglycemia management is associated with improved outcome in aneurysmal subarachnoid hemorrhage. Stroke. 2009;16:1644–1652. doi: 10.1161/STROKEAHA.108.535534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruyt ND, Biessels GJ, de Haan RJ, Vermeulen M, Rinkel GJ, Coert B, Roos YB. Hyperglycemia and clinical outcome in aneurysmal subarachnoid hemorrhage: a meta-analysis. Stroke. 2009;16:424–430. doi: 10.1161/STROKEAHA.108.529974. [DOI] [PubMed] [Google Scholar]
- Passero S, Ciacci G, Ulivelli M. The influence of diabetes and hyperglycemia on clinical course after intracerebral hemorrhage. Neurology. 2003;16:1351–1356. doi: 10.1212/01.WNL.0000094326.30791.2D. [DOI] [PubMed] [Google Scholar]
- Song EC, Chu K, Jeong SW, Jung KH, Kim SH, Kim M, Yoon BW. Hyperglycemia exacerbates brain edema and perihematomal cell death after intracerebral hemorrhage. Stroke. 2003;16:2215–2220. doi: 10.1161/01.STR.0000088060.83709.2C. [DOI] [PubMed] [Google Scholar]
- Juvela S, Siironen J, Kuhmonen J. Hyperglycemia, excess weight, and history of hypertension as risk factors for poor outcome and cerebral infarction after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2005;16:998–1003. doi: 10.3171/jns.2005.102.6.0998. [DOI] [PubMed] [Google Scholar]
- Kimura K, Iguchi Y, Inoue T, Shibazaki K, Matsumoto N, Kobayashi K, Yamashita S. Hyperglycemia independently increases the risk of early death in acute spontaneous intracerebal hemorrhage. J Neurol Sci. 2007;16:90–94. doi: 10.1016/j.jns.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Godoy DA, Piñero GR, Svampa S, Papa F, Di Napoli M. Hyperglcyemia and short-term outcome in patients with spontaneous intracerebral hemorrhage. Neurocrit Care. 2008;16:217–229. doi: 10.1007/s12028-008-9063-1. [DOI] [PubMed] [Google Scholar]
- Stead LG, Jain A, Bellolio MF, Odufuye A, Gilmore RM, Rabinstein A, Chandra R, Dhillon R, Manivannan V, Serrano LA, Yerragondu N, Palamari B, Jain M, Decker WW. Emergency department hyperglycemia as a predictor of early mortality and worse functional outcome after intracerebral hemorrhage. Neurocrit Care. 2010;16:67–74. doi: 10.1007/s12028-010-9355-0. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Palesch YY, Martin R, Novitzke J, Cruz-Flores S, Ehtisham A, Ezzeddine MA, Goldstein JN, Kirmani JF, Hussein HM, Suri MF, Tariq N, Liu Y. ATACH Investigators. Association of serum glucose concentrations during acute hospitalization with hematoma expansion, perihematomal edema, and three month outcome among patients with intracerebral hemorrhage. Neurocrit Care. 2011;16:428–435. doi: 10.1007/s12028-011-9541-8. [DOI] [PubMed] [Google Scholar]
- Berger L, Hakim AM. The association of hyperglycemia with cerebral edema in stroke. Stroke. 1986;16:865–871. doi: 10.1161/01.STR.17.5.865. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lim LL, Levi C, Heller RF, Fisher J. Influence of hyperglycemia on stroke mortality. J Stroke Cerebrovasc Dis. 2001;16:11–18. doi: 10.1053/jscd.2001.20976. [DOI] [PubMed] [Google Scholar]
- Williams LS, Rotich J, Qi R, Fineberg N, Espay A, Bruno A, Fineberg SE, Tierney WR. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology. 2002;16:67–71. doi: 10.1212/WNL.59.1.67. [DOI] [PubMed] [Google Scholar]
- Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, Tress BM, Davis SM. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;16:20–28. doi: 10.1002/ana.10241. [DOI] [PubMed] [Google Scholar]
- Alvarez-Sabín J, Molina CA, Ribó M, Arenillas JF, Montaner J, Huertas R, Santamarina E, Rubiera M. Impact of admission hyperglycemia on stroke outcome after thrombolysis: risk stratification in relation to time to reperfusion. Stroke. 2004;16:2493–2498. doi: 10.1161/01.STR.0000143728.45516.c6. [DOI] [PubMed] [Google Scholar]
- Ribo M, Molina CA, Delgado P, Rubiera M, Delgado-Mederos R, Rovira A, Munuera J, Alvarez-Sabin J. Hyperglycemia during ischemia rapidly accelerates brain damage in stroke patients treated with tPA. J Cereb Blood Flow Metab. 2007;16:1616–1622. doi: 10.1038/sj.jcbfm.9600460. [DOI] [PubMed] [Google Scholar]
- Young M, Kaste M. Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS-II trial. Stroke. 2008;16:2749–2755. doi: 10.1161/STROKEAHA.108.514307. [DOI] [PubMed] [Google Scholar]
- Poppe AY, Majumdar SR, Jeerakathil T, Ghali W, Buchan AM, Hill MD; Canadian Alteplase for Stroke Effectiveness Study Investigators. Admission hyperglycemia predicts a worse outcome in stroke patients treated with intravenous thrombolysis. Diabetes Care. 2009;16:617–622. doi: 10.2337/dc08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes B, Ortega-Casarrubios MA, Sanjosé B, Castillo J, Leira R, Serena J, Vivancos J, Dávalos A, Gil-Nuñez A, Egido J, Díez-Tejedor E; Stroke Project of the Cerebrovascular Diseases Study Group Spanish Society of Neurology. Persistent hyperglycemia > 155 mg/dL in acute ischemic stroke patients: how well are we correcting it? Implications for outcome. Stroke. 2010;16:2362–2365. doi: 10.1161/STROKEAHA.110.591529. [DOI] [PubMed] [Google Scholar]
- Langhelle A, Tyvold SS, Lexow K, Hapnes SA, Sunde K, Steen PA. In-hospital factors associated with improved outcome after out-of-hospital cardiac arrest. A comparison between four regions in Norway. Resuscitation. 2003;16:247–263. doi: 10.1016/S0300-9572(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Beiser DG, Carr GE, Edelson DP, Peberdy MA, Hoek TL. Derangements in blood glucose following initial resuscitation from in-hospital cardiac arrest: a report from the national registry of cardiopulmonary resuscitation. Resuscitation. 2009;16:624–630. doi: 10.1016/j.resuscitation.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrifvars MB, Pettila V, Rosenberg PH, Castren M. A multiple logistic regression analysis of in-hospital factors related to survival at six months in patients resuscitated from out-of-hospittal ventricular fibrillation. Resuscitation. 2003;16:319–328. doi: 10.1016/S0300-9572(03)00238-7. [DOI] [PubMed] [Google Scholar]
- Godoy DA, Di Napoli M, Rabinstein AA. Treating hyperglycemia in neurocritical patients: benefits and perils. Neurocrit Care. 2010;16:425–438. doi: 10.1007/s12028-010-9404-8. [DOI] [PubMed] [Google Scholar]
- Bergsneider M, Hovda DA, Shalmon E, Kelly DF, Vespa PM, Martin NA, Phelps ME, McArthur DL, Caron MJ, Kraus JF, Becker DP. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J Neurosurg. 1997;16:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- Vespa PM, McArthur D, O'Phelan K, Glenn T, Etchepare M, Kelly D, Bergsneider M, Martin NA, Hovda DA. Persistently low extracellular glucose correlates with poor outcome 6 months after human traumatic brain injury despite a lack of increased lactate: a microdialysis study. J Cereb Blood Flow Meab. 2003;16:865–877. doi: 10.1097/01.WCB.0000076701.45782.EF. [DOI] [PubMed] [Google Scholar]
- Vespa P, Boonyaputthikul R, McArthur DL, Miller C, Etchepare M, Bergsneider M, Glenn T, Martin N, Hovda D. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. 2006;16:850–856. doi: 10.1097/01.CCM.0000201875.12245.6F. [DOI] [PubMed] [Google Scholar]
- Oddo M, Schmidt JM, Carrera E, Badjatia N, Connolly ES, Presciutti M, Ostapkovich ND, Levine JM, Le Roux P, Mayer SA. Impact of tight glycemic control on cerebral glucose metabolism after severe brain injury: a microdialysis study. Crit Care Med. 2008;16:3233–3238. doi: 10.1097/CCM.0b013e31818f4026. [DOI] [PubMed] [Google Scholar]
- Schlenk F, Nagel A, Graetz D, Sarrafzadeh AS. Hyperglycemia and cerebral glucose in aneurysmal subarachnoid hemorrhage. Intensive Care Med. 2008;16:1200–1207. doi: 10.1007/s00134-008-1044-5. [DOI] [PubMed] [Google Scholar]
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;16:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;16:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- Arabi YM, Dabbagh OC, Tamim HM, Al-Shimemeri AA, Memish ZA, Haddad SH, Syed SJ, Giridhar HR, Rishu AH, Al-Daker MO, Kahoul SH, Britts RJ, Sakkijha MH. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical criticially ill patients. Crit Care Med. 2008;16:3190–197. doi: 10.1097/CCM.0b013e31818f21aa. [DOI] [PubMed] [Google Scholar]
- Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. N Engl J Med. Vol. 16. German Competence Network Sepsis (SepNet); 2008. Intensive insulin therapy and pentastarch resuscitation in severe sepsis; pp. 125–139. [DOI] [PubMed] [Google Scholar]
- NICE-SUGAR Study Investigators. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;16:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008;16:933–944. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;16:831–837. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik PE, Preiser JC. Toward understanding tight glycemic control in the ICU: a systematic review and meta-analysis. Chest. 2010;16:544–551. doi: 10.1378/chest.09-1737. [DOI] [PubMed] [Google Scholar]
- Kansagara D, Fu R, Freeman M, Wolf F, Helfand M. Intensive insulin therapy in hospitalized patients: a systematic review. Ann Intern Med. 2011;16:268–282. doi: 10.7326/0003-4819-154-4-201102150-00008. [DOI] [PubMed] [Google Scholar]
- Zafar SN, Iqbal A, Frez MF. et al. Intensive insulin therapy in brain injury: a meta-analysis. J Neurotrauma. 2011;16:1307–1317. doi: 10.1089/neu.2010.1724. [DOI] [PubMed] [Google Scholar]
- Shan L, Hao PP, Chen YG. Efficacy and safety of intensive insulin therapy for critically ill neurologic patients: a meta-analysis. J Trauma. 2011;16:1460–1464. doi: 10.1097/TA.0b013e3182250515. [DOI] [PubMed] [Google Scholar]
- Bellolio MF, Gilmore RM, Stead LG. Insulin for glycaemic control in acute ischaemic stroke. Cochrane Database Syst Rev. 2011;16:CD005346. doi: 10.1002/14651858.CD005346.pub3. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;16:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- Berlin JA. Does blinding of readers affect the results of meta-analyses? Lancet. 1997;16:185–186. doi: 10.1016/s0140-6736(05)62352-5. [DOI] [PubMed] [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trial. 1996;16:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;16:408–412. doi: 10.1001/jama.1995.03520290060030. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;16:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Bilotta F, Caramia R, Paoloni FP, Delfini R, Rosa G. Safety and efficacy of intensive insulin therapy in critical neurosurgical patients. Anesthesiology. 2009;16:611–619. doi: 10.1097/ALN.0b013e318198004b. [DOI] [PubMed] [Google Scholar]
- Bilotta F, Caramia R, Cernak I, Paoloni FP, Doronzio A, Cuzzone V, Santoro A, Rosa G. Intensive insulin therapy after severe traumatic brain injury: a randomized clinical trial. Neurocrit Care. 2008;16:159–166. doi: 10.1007/s12028-008-9084-9. [DOI] [PubMed] [Google Scholar]
- Bilotta F, Spinelli A, Giovannini F, Doronzio A, Delfini R, Rosa G. The effect of intensive insulin therapy on infection rate, vasospasm, neurologic outcome, and mortality in neurointensive care unit after intracranial aneurysm clipping in patients with acute subarachnoid hemorrhage: a randomized prospective pilot trial. J Neurosurg Anesthesiol. 2007;16:156–160. doi: 10.1097/ANA.0b013e3180338e69. [DOI] [PubMed] [Google Scholar]
- McCormick M, Hadley D, McLean JR, Macfarlane JA, Condon B, Muir KW. Randomized, controlled trial of insulin for acute poststroke hyperglycemia. Ann Neurol. 2010;16:570–578. doi: 10.1001/archneurol.2010.61. [DOI] [PubMed] [Google Scholar]
- Scott JF, Robinson GM, French JM, O'Connell JE, Alberti KG, Gray CS. Glucose potassium insulin infusions in the treatment of acute stroke patients with mild to moderate hyperglycemia: the Glucose Insulin in Stroke Trial (GIST) Stroke. 1999;16:793–799. doi: 10.1161/01.STR.30.4.793. [DOI] [PubMed] [Google Scholar]
- Gray CS, Hildreth AJ, Sandercock PA. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK) Lancet Neurol. 2007;16:397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- Vinychuk SM, Melnyk VS, Margitich VM. Hyperglycemia after acute ischemic stroke: prediction, significance and immediate control with insulin-potassium-saline-magnesium infusions. Heart Drug. 2005;16:197–204. doi: 10.1159/000089600. [DOI] [Google Scholar]
- Badenes R, Gonzalez P, Alcover L, Maruenda A, Belda J. Intensive insulin therapy in traumatic brain injury patients: a microdialysis study. Crit Care Med. 2009;16(Suppl 12):A46. [Google Scholar]
- Vriesendorp TM, Roos YB, Kruyt ND. Efficacy and safety of two 5 day insulin dosing regimens to achieve strict glycemic control in patients with acute ischemic stroke. J Neurol Neurosurg. 2009;16:1040–1043. doi: 10.1136/jnnp.2008.144873. [DOI] [PubMed] [Google Scholar]
- de Azevedo JRA, Lima ERM, Cossetti RJD, de Azevedo RP. Intensive insulin therapy versus conventional glycemic control in patients with acute neurological injury. Arq Neuropsiquiatr. 2007;16:733–738. doi: 10.1590/S0004-282X2007000500001. [DOI] [PubMed] [Google Scholar]
- Azevedo JRA, Azevedo MA, Miranda MN, Costa NNR, Araujo LO. Management of hyperglycemia in patients with acute ischemic stroke: comparison of two strategies. Crit Care. 2009;16:P48. [Google Scholar]
- Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, Kissela BM, Williams LS. Treatment of hyperglycemia in ischemic stroke (THIS)(: a randomized pilot trial. Stroke. 2008;16:384–389. doi: 10.1161/STROKEAHA.107.493544. [DOI] [PubMed] [Google Scholar]
- Coester A, Neumann CR, Schmidt MI. Intensive insulin therapy in severe traumatic brain injury: a randomized trial. J Trauma. 2010;16:904–911. doi: 10.1097/TA.0b013e3181c9afc2. [DOI] [PubMed] [Google Scholar]
- Green DM, O'Phelan KH, Bassin SL, Chang CW, Stern TS, Asai SM. Intensive versus conventional insulin therapy in critically ill neurologic patients. Neurocrit Care. 2010;16:299–306. doi: 10.1007/s12028-010-9417-3. [DOI] [PubMed] [Google Scholar]
- Johnston KC, Hall CE, Kissela BM, Bleck TP, Conaway MR. GRASP Investigators. Glucose regulation in acute stroke patients (GRASP) trial: a randomized pilot trial. Stroke. 2009;16:3804–3809. doi: 10.1161/STROKEAHA.109.561498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksanen T, Skrifvars MB, Varpula T, Kuitunen A, Pettilä V, Nurmi J, Castrén M. Strict versus moderate glucose control after resuscitation from ventricular fibrillation. Intensive Care Med. 2007;16:2093–2100. doi: 10.1007/s00134-007-0876-8. [DOI] [PubMed] [Google Scholar]
- Staszewski J, Brodacki B, Kotowicz J, Stepien A. Intravenous insulin therapy in the maintenance of strict glycemic control in nondiabetic acute stroke patients with mild hyperglycemia. J Stroke and Cerebrovasc Dis. 2011;16:150–154. doi: 10.1016/j.jstrokecerebrovasdis.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;16:1348–1353. doi: 10.1212/01.WNL.0000158442.08857.FC. [DOI] [PubMed] [Google Scholar]
- Walters MR, Weir CJ, Lees KR. A randomized, controlled pilot study to investigate the potential benefit of intervention with insulin in hyperglycaemic acute ischaemic stroke patients. Cerebrovasc Dis. 2006;16:116–122. doi: 10.1159/000093239. [DOI] [PubMed] [Google Scholar]
- Yang M, Guo Q, Zhang X, Sun S, Wang Y, Zhao L, Hu E, Li C. Intensive insulin therapy on infection rate, days in NICU, in-hospital mortality and neurological outcome in severe traumatic brain injury patients: a randomized controlled trial. Int J Nursing Stud. 2009;16:753–758. doi: 10.1016/j.ijnurstu.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Kreisel SH, Berschin UM, Hammes HP. Pragmatic management of hyperglycemia in acute ischemic stroke: safety and feasibility of intensive intravenous insulin treatment. Cerebrovasc Dis. 2009;16:167–175. doi: 10.1159/000185608. [DOI] [PubMed] [Google Scholar]
- Yang Z, Liu W, Ding Y, Liu X, Zhang Y, Niu H. Comatose stroke patients complicated with hyperglycemia: a study of realtime insulin titration model. Chin J Cerebrovasc Dis. 2009;16:113–118. [Google Scholar]
- Hermanides J, Bosman RJ, Vriesendorp TM, Dotsch R, Rosendaal FR, Zandstra DF, Hoekstra JB, DeVries JH. Hypoglycemia is associated with intensive care unit mortality. Crit Care Med. 2010;16:1430–1434. doi: 10.1097/CCM.0b013e3181de562c. [DOI] [PubMed] [Google Scholar]
- Zetterling M, Hillered L, Englad P, Karlsson T, Ronne-Engstrom E. Relation between brain interstitial and systemic glucose concentrations after subarachnoid hemorrhage. J Neurosurg. 2011;16:66–74. doi: 10.3171/2011.3.JNS10899. [DOI] [PubMed] [Google Scholar]
- Meierhans R, Béchir M, Ludwig S, Sommerfeld J, Brandi G, Haberthür C, Stocker R, Stover JF. Brain metabolism is significantly impaired at blood glucose below 6 mM and brain glucose below 1 mM in patients with severe traumatic brain injury. Crit Care. 2010;16:R13. doi: 10.1186/cc8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenk F, Graetz D, Nagel A, Schmidt M, Sarrafzadeh AS. Insulin-related decrease in cerebral glucose despite normoglycemia in aneurysmal subarachnoid hemorrhage. Crit Care. 2008;16:R9. doi: 10.1186/cc6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa P, Boonyaputthikul R, McArthur DL, Miller C, Etchepare M, Bergsneider M, Glenn T, Martin N, Hovda D. Intensive insulin therapy reduces microdialysis glucose values without altering glucose utilization or improving the lactate/pyruvate ratio after traumatic brain injury. Crit Care Med. 2006;16:850–856. doi: 10.1097/01.CCM.0000201875.12245.6F. [DOI] [PubMed] [Google Scholar]
- Graffagnino C, Gurram AR, Kolls B, Olson DM. Intensive insulin therapy in the neurocritical care setting is associated with poor clinical outcomes. Neurocrit Care. 2010;16:307–312. doi: 10.1007/s12028-010-9469-4. [DOI] [PubMed] [Google Scholar]
- Siegelaar SE, Hermanides J, Oudemans-van Straaten HM, van der Voort PH, Bosman RJ, Zandstra DF, DeVries JH. Mean glucose during ICU admission is related to mortality by a U-shaped curve in surgical and medical patients: a retrospective cohort study. Crit Care. 2010;16:R224. doi: 10.1186/cc9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiser JC, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chioléro R. A prospective randomized multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;16:1738–1748. doi: 10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix - OVID Search Strategies.