Abstract
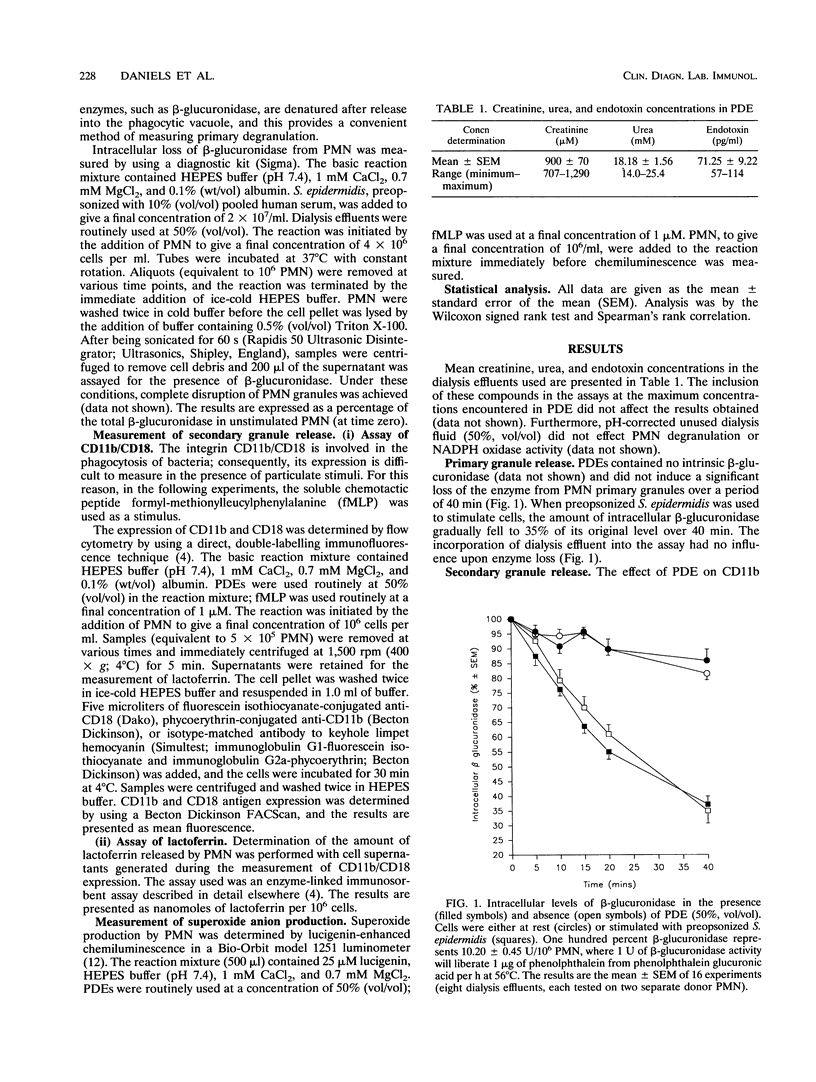
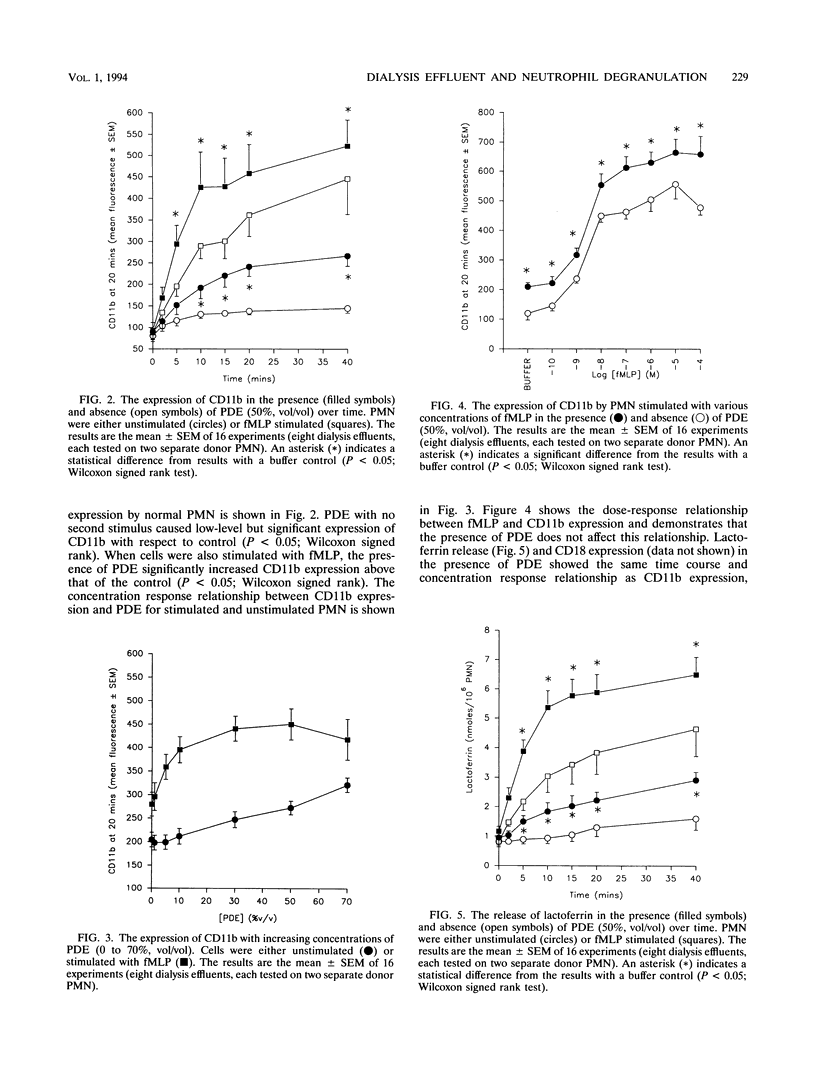
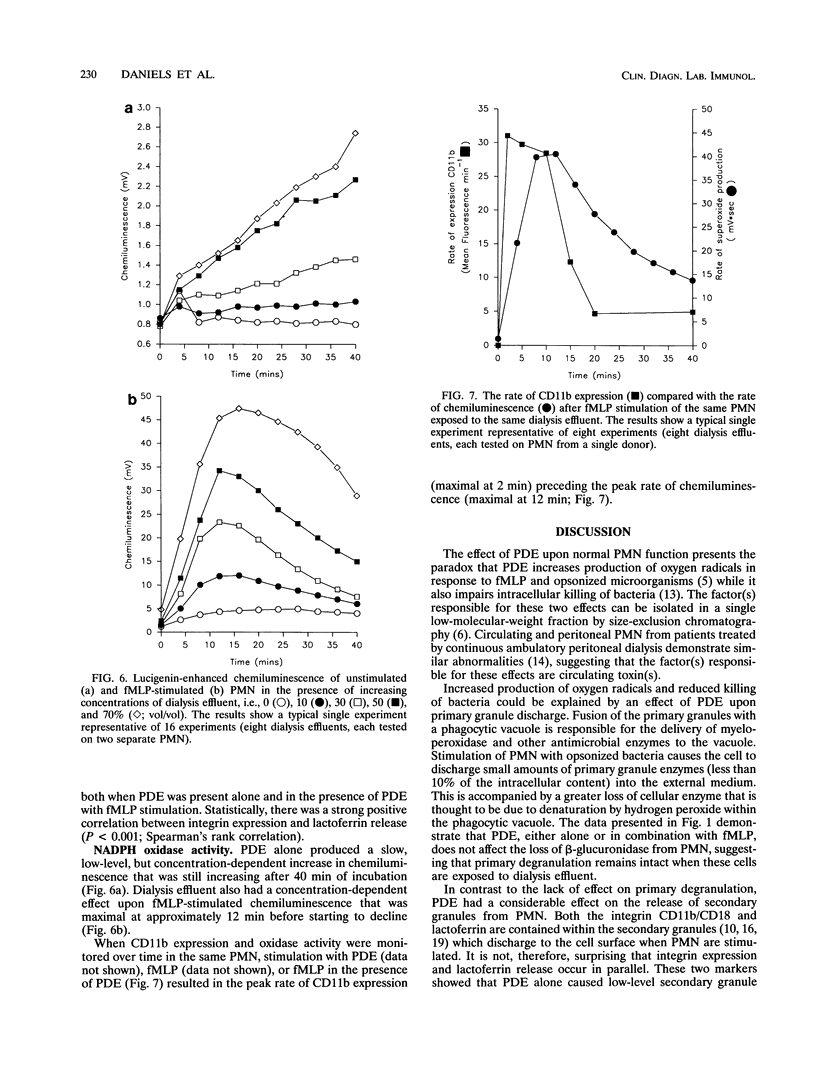
Peritoneal dialysis effluent from patients with end-stage renal failure contains a low-molecular-weight solute that inhibits the killing of phagocytosed Staphylococcus epidermidis by polymorphonuclear leukocytes (PMN). This observation has been investigated by using luciginen-enhanced chemiluminescence to measure PMN NADPH oxidase activity, CD11b/CD18 expression and lactoferrin release to measure secondary granule discharge, and cellular levels of beta-glucuronidase (EC 3.2.1.31) to measure changes in primary granules. Peritoneal dialysis effluent had no effect on the loss of intracellular beta-glucuronidase from normal unstimulated PMN or from PMN stimulated with S. epidermidis. It did, however, cause a concentration-dependent (0 to 70%; vol/vol) increase in expression of CD11b/CD18 and NADPH oxidase activity. CD11b/CD18 expression increased over 20 min before starting to plateau. Release of lactoferrin by the same cells demonstrated a strong positive correlation with integrin expression (P < 0.001, Spearman's rank correlation coefficient). When dialysis effluent-treated PMN were stimulated with formyl-methionylleucylphenylalanine, integrin expression, release of lactoferrin, and NADPH oxidase activity were greater than in PMN treated with formyl-methionylleucylphenylalanine alone. Under these conditions, a concentration-dependent increase in CD11b/ CD18 and lactoferrin release were observed only at a concentration between 0 and 30% (vol/vol) dialysis effluent, while a concentration-dependent increase in oxidase activity was seen at a concentration between 0 and 70% (vol/vol). The results suggest that dialysis effluent does not affect PMN primary granule release but does cause increased release of secondary granules and an increase in NADPH oxidase activity in both unstimulated and stimulated PMN.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bengtsson T., Dahlgren C., Stendahl O., Andersson T. Actin assembly and regulation of neutrophil function: effects of cytochalasin B and tetracaine on chemotactic peptide-induced O2- production and degranulation. J Leukoc Biol. 1991 Mar;49(3):236–244. doi: 10.1002/jlb.49.3.236. [DOI] [PubMed] [Google Scholar]
- Berkow R. L., Wang D., Larrick J. W., Dodson R. W., Howard T. H. Enhancement of neutrophil superoxide production by preincubation with recombinant human tumor necrosis factor. J Immunol. 1987 Dec 1;139(11):3783–3791. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Crouch S. P., Fletcher J. Effect of ingested pentoxifylline on neutrophil superoxide anion production. Infect Immun. 1992 Nov;60(11):4504–4509. doi: 10.1128/iai.60.11.4504-4509.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels I., Lindsay M., Porter C., Haynes A. P., Fletcher J., Morgan A. G. Effect of peritoneal dialysis effluent on superoxide anion production by polymorphonuclear neutrophils. Nephron. 1993;64(3):382–387. doi: 10.1159/000187358. [DOI] [PubMed] [Google Scholar]
- Duwe A. K., Vas S. I., Weatherhead J. W. Effects of the composition of peritoneal dialysis fluid on chemiluminescence, phagocytosis, and bactericidal activity in vitro. Infect Immun. 1981 Jul;33(1):130–135. doi: 10.1128/iai.33.1.130-135.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M. P., Gallin J. I. Degranulating stimuli increase the availability of receptors on human neutrophils for the chemoattractant f-met-leu-phe. J Immunol. 1980 Apr;124(4):1585–1588. [PubMed] [Google Scholar]
- Fletcher M. P., Gallin J. I. Human neutrophils contain an intracellular pool of putative receptors for the chemoattractant N-formyl-methionyl-leucyl-phenylalanine. Blood. 1983 Oct;62(4):792–799. [PubMed] [Google Scholar]
- Gallin J. I. Neutrophil specific granules: a fuse that ignites the inflammatory response. Clin Res. 1984 Sep;32(3):320–328. [PubMed] [Google Scholar]
- Gupta D. K., Ing B. L., Manahan F. J., Zhou F. Q., Yu A. W., Nawab Z. M., Rahman M. A. Superoxide generation by neutrophils after exposure to a conventional peritoneal dialysis solution for different time periods. Int J Artif Organs. 1990 Apr;13(4):228–230. [PubMed] [Google Scholar]
- Gyllenhammar H. Lucigenin chemiluminescence in the assessment of neutrophil superoxide production. J Immunol Methods. 1987 Mar 12;97(2):209–213. doi: 10.1016/0022-1759(87)90461-3. [DOI] [PubMed] [Google Scholar]
- Harvey D. M., Sheppard K. J., Morgan A. G., Fletcher J. Effect of dialysate fluids on phagocytosis and killing by normal neutrophils. J Clin Microbiol. 1987 Aug;25(8):1424–1427. doi: 10.1128/jcm.25.8.1424-1427.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey D. M., Sheppard K. J., Morgan A. G., Fletcher J. Neutrophil function in patients on continuous ambulatory peritoneal dialysis. Br J Haematol. 1988 Mar;68(3):273–278. doi: 10.1111/j.1365-2141.1988.tb04202.x. [DOI] [PubMed] [Google Scholar]
- Keane W. F., Comty C. M., Verbrugh H. A., Peterson P. K. Opsonic deficiency of peritoneal dialysis effluent in continuous ambulatory peritoneal dialysis. Kidney Int. 1984 Mar;25(3):539–543. doi: 10.1038/ki.1984.51. [DOI] [PubMed] [Google Scholar]
- Lacal P., Pulido R., Sánchez-Madrid F., Mollinedo F. Intracellular location of T200 and Mo1 glycoproteins in human neutrophils. J Biol Chem. 1988 Jul 15;263(20):9946–9951. [PubMed] [Google Scholar]
- Quinn M. T., Mullen M. L., Jesaitis A. J., Linner J. G. Subcellular distribution of the Rap1A protein in human neutrophils: colocalization and cotranslocation with cytochrome b559. Blood. 1992 Mar 15;79(6):1563–1573. [PubMed] [Google Scholar]
- Richter J., Andersson T., Olsson I. Effect of tumor necrosis factor and granulocyte/macrophage colony-stimulating factor on neutrophil degranulation. J Immunol. 1989 May 1;142(9):3199–3205. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Arnaout M. A., Rosin R. E., Crowley C. A., Peters W. A., Babior B. M. Subcellular localization of the large subunit of Mo1 (Mo1 alpha; formerly gp 110), a surface glycoprotein associated with neutrophil adhesion. J Clin Invest. 1984 Oct;74(4):1280–1290. doi: 10.1172/JCI111538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topley N., Alobaidi H. M., Davies M., Coles G. A., Williams J. D., Lloyd D. The effect of dialysate on peritoneal phagocyte oxidative metabolism. Kidney Int. 1988 Sep;34(3):404–411. doi: 10.1038/ki.1988.195. [DOI] [PubMed] [Google Scholar]
- Zabucchi G., Romeo D. The dissociation of exocytosis and respiratory stimulation in leucocytes by ionophores. Biochem J. 1976 May 15;156(2):209–213. doi: 10.1042/bj1560209. [DOI] [PMC free article] [PubMed] [Google Scholar]



