Key Points
Survival of patients with BL improved substantially in the United States during the past decade, mainly among young adults.
Survival of patients with BL remains relatively low, particularly for older and black patients, identifying an unmet need.
Abstract
It is unknown whether the high rates of cure reported for Burkitt lymphoma/leukemia (BL) patients treated with chemoimmunotherapy can be verified outside published series and clinical trials. We used the Surveillance Epidemiology and End Results (SEER) database to describe time trends in outcomes of BL in the United States. Cases were divided into 2 eras based on year of diagnosis, reflecting improvements in HIV management, BL treatment, and supportive care. There was a marked improvement in survival among BL cases diagnosed in the 2002-2008 era (n = 1922) relative to 1973-2001 (n = 1769) with 5-year relative survival estimates of 56% and 43%, respectively (P < .001). Five-year relative survival improved from 71% to 87% for ages 0 to 19 (n = 970), 35% to 60% for ages 20 to 39 (n = 897), 28% to 48% for ages 40 to 59 (n = 1047), and from 25% to 33% for ages ≥60 (n = 777). In multivariable analysis, the 2002-2008 era (HR = 0.76, P < .001) was associated with lower mortality. Conversely, older age, black race, and advanced stage were associated with higher mortality. More effective therapies are needed for older patients with BL, along with improved access to modern therapy for younger patients.
Introduction
Burkitt lymphoma/leukemia (BL) is one of the most aggressive types of cancer and yet one of the most curable. Treatment success resulted from the introduction of dose-intense multidrug chemotherapy, prophylaxis of central nervous system disease, and improvements in supportive care.1-4 More recent series and clinical trials report success rates in excess of 90% in pediatric4,5 and 80% in adult populations.6-9
The anti-CD20 monoclonal antibody rituximab has transformed the management of other mature B-cell malignancies. Because BL cells express abundant surface CD20, rituximab has been incorporated in multiple recent regimens reporting high success rates.7,8,10 However, its impact on the management of BL has not been properly evaluated.
Since the mid-1980s, the epidemiology of BL in the United States has been vastly affected by the epidemics of HIV infection. Patients living with HIV are 57 times more likely to develop BL than HIV-negative individuals. In the United States, approximately 20% of the cases of BL are in individuals with HIV.11 It is unknown to what extent the recent availability of adequate antiretroviral therapy (ART) has impacted the survival expectancy of patients diagnosed with BL in the United States.
Using data from the Surveillance Epidemiology and End Results (SEER) program, we described overall survival for patients with BL in 2 distinct eras based on year of diagnosis, 1973-2001 and 2002-2008, reflecting the development of intense chemotherapy regimens, availability of rituximab, and adequate ART for HIV+ patients. Our objectives were to determine if there have been population-level improvements in BL survival, identify which patient groups have been most affected, and determine whether the contemporary population-based outcomes parallel those displayed in clinical trials and published patient series.
Methods
Incidence
We assessed the changes in incidence of BL among residents of the original 9 population-based cancer registry areas of the SEER program (SEER-9) between 1973 and 2008. The SEER-9 registry includes Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah, corresponding to approximately 9.5% of the US. population.12 Even though other cancer registries were subsequently added to SEER, our incidence analysis used SEER-9 to include the same population base over time.
We calculated age-adjusted incidence rates per 1 000 000 persons and 95% confidence intervals (CIs) using the Rate Session in SEER*Stat Version 7.1.0 (www.seer.cancer.gov). All incidence rates were adjusted for the 2000 US standard population. Changes in incidence rates for BL during the period covered by the study were reported separately for males and females. Case inclusion required the diagnosis of BL (International Classification of Diseases-Oncology, Third Edition, ICD-O-3 codes 9687 and 9826) as first malignant neoplasm for a given individual, with known gender and age. Individuals of all races were included. We excluded cases reported from death certificates or autopsies only.
Outcomes
Analysis of outcomes was based on the SEER-17 database, consisting of the registries included in SEER-9 plus Los Angeles, San Jose-Monterey, rural Georgia, Alaska Native Tumor Registry, greater California, Kentucky, Louisiana, and New Jersey, which corresponded to approximately 26% of the US population.13 We used the case listing session function of SEER*Stat Version 7.1.0 to access demographic and outcome information of cases meeting the same inclusion criteria applied to the incidence analysis.
Cases were divided in 2 eras based on year of diagnosis, 1973-2001 and 2002-2008. The latter was expected to reflect the implementation of multiagent intense chemotherapy regimens, availability of rituximab, potential improvements in supportive care, and broad use of modern ART. The impact of era on survival was described for different age groups, races, gender, and disease stages defined as limited (stages 1 or 2) or advanced (stages 3, 4, and BL). Before 1998, stage was not captured properly in the SEER database for any BL patient; therefore, the impact of era on survival according to stage is restricted to 2 time periods: 1998-2001 and 2002-2008. Last, univariate and multivariable analyses of the overall survival end point were performed assessing the role of age, race, gender, stage, and era of the diagnosis on the risk of death after diagnosis of BL. Only subjects with a complete dataset were included in the multivariable analysis. In all analyses, the relatively small race categories American Indian/Alaska Native, Asian or Pacific Islander, and other unspecified were grouped under the category “other.”
Statistics
Descriptive statistics including median and interquartile range (for continuous variables) and frequencies and percentages (for categorical variables) were used to describe the population. Associations between patient clinical/demographic variables and era were examined using Wilcoxon rank-sum test for continuous variables and χ-square tests for categorical variables. Kaplan-Meier survival curves were constructed and used to estimate survival rates at specific time points following diagnosis. Greenwood variance estimates were used to construct corresponding 95% CIs. Comparisons between survival curves were performed using log-rank test. For survival analysis, all deaths were considered events irrespective of cause and subjects were censored at the last time they were reported alive. Relative survival estimates were obtained using the survival function of SEER*Stat. To determine the effects of age, race, stage, gender, and era on overall survival, both univariate and multivariable Cox proportional hazard regression models were used. The multivariable model included all stated covariates. Fifteen percent (n = 551) of subjects died within 1 month of diagnosis, resulting in a violation of the proportional hazards assumption. To mitigate this effect, we used stratified Cox models for all univariate and multivariable hazard regression analyses, with strata defined by whether a patient died early (≤1 month vs ≥1 month).14 This approach facilitates fitting separate models within strata, each with a different baseline hazard function (reflecting differences in the underlying frailties for patients who died within 1 month of diagnoses and those who did not), but with common coefficients across strata. All statistical analyses were performed using R version 2.15.0. Statistical tests were 2-sided and considered significant when P < .05.
Results
Incidence
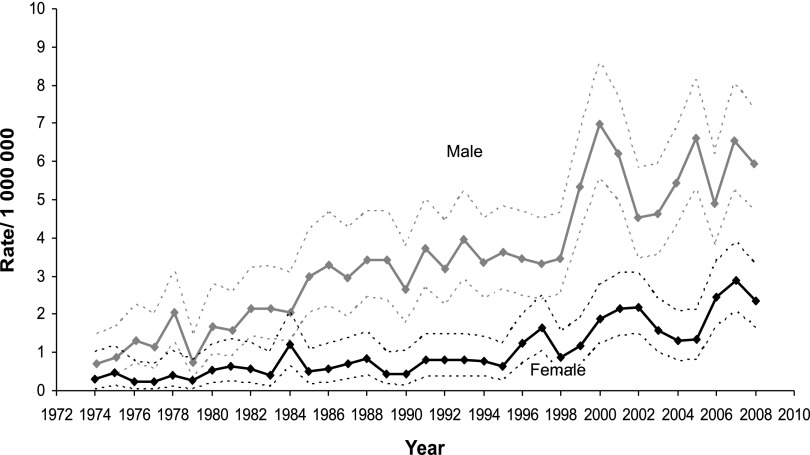
Even though this is not primarily an incidence study, knowledge of the pattern of incidence of BL is helpful in interpreting the findings of the survival analysis. As displayed in Figure 1, there was a marked increase in BL incidence starting in the late 1980s, and predominantly affecting men for whom, in 2008, it reached a rate of 5.95 per 1 000 000 (95% CI = 4.74-7.39). This increase is believed to be linked, at least in part, to the AIDS epidemic, a disease that disproportionately affects men. In fact, the incidence of BL among women appears only to increase substantially in the mid-1990s and, in 2008, reached a rate of 2.36 per 1 000 000 (95% CI = 1.64-3.28).
Figure 1.
Changes in estimated incidence of BL over the time span covered in the study.
Survival analysis
Using the methodology described, we were able to identify 3691 cases of BL diagnosed between 1973 and 2008. Of those, 1769 were diagnosed between 1973 and 2001 and the remaining 1922 cases were diagnosed between 2002 and 2008. Characteristics of the cases included in the analysis are displayed in Table 1. Patients in the 2002-2008 cohort were significantly older than 1973-2001 patients at the time of diagnosis (median 43 vs 35 years). Staging information was only available only for patients diagnosed after 1998, after which the rate of missing stage is quite low (4%).
Table 1.
Characteristics of the subjects included in the study
| Era | ||||
|---|---|---|---|---|
| Variable | All (N = 3691) | 1973-2001 (n = 1769; 48%) | 2002-2008 (n = 1922; 52%) | P |
| Gender | .07 | |||
| Female | 935 (25%) | 424 (24%) | 511 (27%) | |
| Male | 2756 (75%) | 1345 (76%) | 1411 (73%) | |
| Age* | 39 (18-56) | 35 (14-52) | 43 (22-59) | <.001 |
| Age group | <.001 | |||
| 0-19 y | 970 (26%) | 545 (31%) | 425 (22%) | |
| 20-39 y | 897 (24%) | 473 (27%) | 424 (22%) | |
| 40-59 y | 1047 (28%) | 432 (24%) | 615 (32%) | |
| 60+ y | 777 (21%) | 319 (18%) | 458 (24%) | |
| Stage | .2# | |||
| Limited | 908 (36%)† | 246 (35%)† | 662 (36%)† | |
| Advanced | 1647 (64%)† | 460 (65%)† | 1187 (64%)† | |
| Unknown | 1136 (31%) | 1063 (60%) | 73 (4%) | |
| Race | .3# | |||
| American Indian/Alaska Native | 15 (<1%) | 8 (<1%) | 7 (<1%) | |
| Asian or Pacific Islander | 278 (8%) | 125 (7%) | 153 (8%) | |
| Black | 304 (8%) | 137 (8%) | 167 (9%) | |
| White | 3074 (83%) | 1492 (84%) | 1582 (82%) | |
| Other unspecified | 2 (<1%) | 0 (0%) | 2 (<1%) | |
| Unknown | 18 (<1%) | 11 (<1%) | 7 (<1%) | |
Median (interquartile range).
Percentage of cases with known stage.
Analysis excludes unknown or missing values.
Overall, there was a significant improvement in relative survival at 5 years after diagnosis of BL from 43% (95% CI = 40-45) in the 1973-2001 era to 56% (95% CI = 53-58) in the 2002-2008 era (supplemental Table 1). Absolute 5-year survival also improved from 41% (95% CI = 39-44) in the 1973-2001 era to 54% (95% CI = 51-56) in the 2002-2008 era (Figures 2-4).
Figure 2.
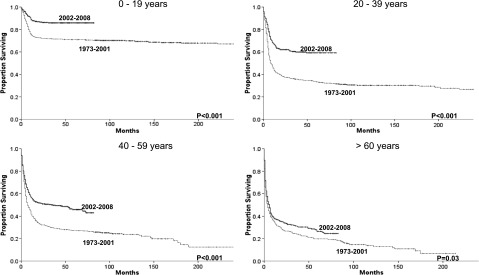
Changes in overall survival for BL across the 2 eras for each specific age group.
Figure 4.
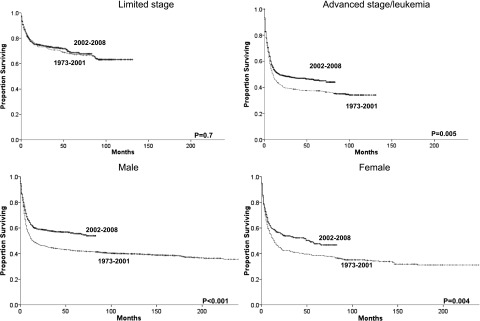
Changes in overall survival for BL patients across the 2 eras and grouped by gender or summary stage.
Age had a profound impact on the survival of patients diagnosed with BL irrespective of era. Although 5-year relative survival was 87% for patients younger than 20, it was only 33% for patients 60 and older in the more recent era. Improvements in relative survival across the 2 eras were also highly dependent on age. The 5-year relative survival rate improved from 71% to 87% for patients younger than 20, from 35% to 60% for patients 20 to 39, from 28% to 48% for patients 40 to 59, but only from 25% to 33% for patients 60 and older (Table 2 and Figure 2).
Table 2.
Cox proportional hazards univariate and multivariable regression analyses summarizing associations with BL overall survival
| Univariate | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Factor | Reference | HR | 95% CI | P | HR | 95% CI | P |
| Era | 2002-2008 | 1973-2001* | 0.64 | (0.59-0.71) | <.001 | 0.76 | (0.67-0.86) | <.001 |
| 1998-2001† | ||||||||
| Age | 20-39 y | 0-19 y | 3.05 | (2.60-3.58) | <.001 | 3.69 | (2.85-4.79) | <.001 |
| 40-59 y | 0-19 y | 3.52 | (3.01-4.11) | <.001 | 4.77 | (3.73-6.11) | <.001 | |
| 60+ y | 0-19 y | 4.24 | (3.60-4.99) | <.001 | 6.49 | (5.04-8.35) | <.001 | |
| Gender | Male | Female | 0.94 | (0.85-1.04) | .22 | 1.00 | (0.88-1.14) | .98 |
| Race | White | Black | 0.86 | (0.74-1.00) | .05 | 0.78 | (0.64-0.95) | .01 |
| Other‡ | Black | 0.72 | (0.57-0.89) | .003 | 0.79 | (0.60-1.03) | .08 | |
| Stage | Advanced | Limited | 1.99 | (1.73-2.30) | <.001 | 1.90 | (1.65-2.19) | <.001 |
Reference for univariate analysis.
Reference for multivariable analysis.
American Indian/Alaska Native, Asian or Pacific Islander, and other unspecified.
Improvements in survival across eras occurred in all races (Figure 3), but outcomes for black patients remain significantly inferior to outcomes for whites, even within the most recent era (HR= 1.38, 95% CI 1.10-1.74, P = .005). There were also survival improvements for both genders and in patients with advanced disease. However, no overall survival improvement was detected among patients with limited disease, in which the 5-year relative survival rate stayed at 71% across the 2 eras (Table 2, Figure 4). Nevertheless, stage remains intrinsically associated with survival even within the most recent era when individuals with limited stage disease had a lower risk of death than patients with advanced stage (HR = 0.44, 95% CI 0.37-0.52, P < .001). The same can be said about age with patients age 20 to 39 (HR = 3.43, 95% CI 2.49-4.73, P < .001), 40 to 59 (HR = 5.17, 95% CI 3.83-6.98, P < .001), and 60+ (HR = 8.89, 95% C.I 6.59-12.00, P < .001) having higher risk of death than individuals younger than 20 in the more recent era. We observed no significant relationship between gender and risk of death among individuals with BL in 2002-2008.
Figure 3.

Changes in overall survival for BL across the 2 eras for each specific race. API, Asian or Pacific Islander; IA/AN, Indian American/Alaska Native; Other, other unspecified.
Univariate and multivariable analysis
Univariate analysis identified better survival associated with diagnosis in the later era (2002-2008), with younger age, limited stage disease, and worse survival associated with black race (Table 2). The multivariable analysis confirmed that diagnosis in the most recent era was independently associated with a 24% reduction in the risk of death after adjusting for race, gender, age, and stage (HR = 0.76, 95% CI = 0.67-0.86; P < .001). Longer survival was also influenced by younger age, race other than black, and limited stage. Gender was not independently associated with survival (Table 2).
As previously noted, stage was not reported for any BL patient before 1998. Because our multivariable modeling was based on a complete case analysis (records missing a value for any variable are dropped from the analysis), subsequent inference is restricted to the subset of BL patients diagnosed after 1998. The observed differences in the estimated univariate and multivariable HRs are explained in part by changes in the associations between patient factors and survival over time (eg, improved outcomes over time for the youngest BL patients).
Discussion
BL is regarded as one of the greatest therapeutic successes in oncology. Current therapeutic approach includes intense multidrug chemotherapy (including fractionated cyclophosphamide, anthracyclines, and high-dose methotrexate), intrathecal chemotherapy administration for prophylaxis of leptomeningeal disease, and, more recently, the incorporation of the anti-CD20 monoclonal antibody rituximab,15 as demonstrated in a recent randomized trial.9
Long-term survival in excess of 90% has been reported for children with BL treated on the Lymphome Malins de Burkitt protocols.4,5 In a study including children and adults, Magrath et al reported 2-year survival of 89% and no difference in outcome between children and adults using only 4 cycles of therapy—alternating cycles of cyclophosphamide, vincristine, dexamethasone, doxorubicin, and high-dose methotrexate with cycles of ifosfamide, etoposide, and cytarabine.3 Similar results were obtained by investigators from the University of Texas by treating 31 adult patients with cyclophosphamide, vincristine, dexamethasone, and doxorubicin alternating with cycles of high-dose methotrexate plus high-dose cytarabine with the addition of rituximab to each cycle.7 High cure rates have been reported even for patients with HIV infection. In a series of 14 HIV+ patients with BL treated with Magrath’s program, 2-year survival was 60% even though the vast majority of patients had stage IV disease.16
We found a substantial difference between BL treatment outcomes reported in clinical trials and those found in this population-based study, with clinical trials survival rates far exceeding the rates verified in this study. In fact, among 4 series reporting the outcomes of BL patients treated with modern immunochemotherapy, the median age ranged from 46 to 52 years and 3-year survival from 82% to 89%.7-10 This is in sharp contrast with the 2002-2008 cohort reported here in which, despite lower median age (43 years), the 3-year survival was only 56%. Possible factors affecting this difference are selection bias and unequal access to tertiary medical centers able to deliver the complex treatment plan and supportive care required for effective BL management. Similar discrepancy has been reported in other hematological malignancies with complex and resource-intense management, as in the case of acute promyelocytic leukemia.17
It is important to emphasize that the gap between clinical trials and “real-world” outcomes verified in this study is not the same across age groups. In fact, the outcomes verified for patients 0 to 19 years old are not distinct from what is reported in the literature. One main contributing factor may be the well-known high rate of participation of pediatric patients in therapeutic clinical trials.18 Unfortunately, the outcomes for adult patients were far inferior, even for patients in the age group of 20 to 39 years diagnosed in the 2002-2008 era, with only 60% of patients being alive at 5 years from diagnosis. The outcomes are much inferior for older patients with only approximately one-fourth of patients age 60 or older being alive 5 years after the diagnosis.
Another concerning finding of this study is the significantly worse outcome of black patients when compared with other races. This may be partially explained by other characteristics such as more advanced stage at diagnosis (53% of black patients vs 45% of white patients, P = .02). However, the negative impact of black race on survival persists even when adjusted for stage, age, gender, and era of diagnosis. Inferior outcomes for black patients have been reported in other hematological malignancies19,20 and are likely to reflect unequal access to care.
For being retrospective and registry-based, the present study has some limitations, with the most important being the lack of treatment information and centralized pathology review. The latter is particularly relevant in the context of multiple reclassifications of hematological neoplasms that occurred during the time span covered by the study.21 For instance, some B-cell lymphomas harboring c-myc translocation (and potentially classified as BL in the past) are now classified as diffuse large B-cell lymphoma or “B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma.” Some of these lymphomas will have additional immunoglobulin H translocations involving bcl2 or bcl6 (double hit lymphomas) carrying a much worse prognosis than true BL.22
Another important limitation is the lack of information on the HIV status of the patients included in the analysis. There is a close association between HIV infection and the development of BL. This association seems independent of the immune status, and HIV+ patients are at high risk of BL even with relatively high CD4+ count.23 Currently nearly 20% of BL diagnoses in the United States are in HIV+ patients.11 Even though aggressive treatments are feasible in HIV+ patients, leading to results similar to the ones seen in the HIV− population,24,25 it is unknown if the lack of discrepancy in outcomes is due to patient selection. We believe the steep increase in the incidence of BL observed in the late 1980s (Figure 1) was greatly affected by the epidemics of HIV infection, also explaining a greater increase in incidence among men, mirroring the early epidemiological aspects of HIV infection in the United States. At least 1 other factor, the improvement in pathological and molecular techniques of diagnosis, may have contributed to the overall increase in reported incidence of BL.
The most meaningful finding of the present study is the verification that reported advances in BL management have translated to outcome improvement at the population level. Because the multivariable Cox proportional hazard regression analysis excluded subjects without staging information and the majority of those subjects were diagnosed before 1998, it is possible that the impact of era on survival was underestimated in the present analysis. Improvement in BL survival affected patients of all age groups, both genders, and all races, although it was less pronounced among black patients. The most likely explanation for this advance is the refinement of BL therapy as previously described. Other likely contributing factors are the availability of ART and consequent improvement in the survival of patients with HIV infection and improvements in supportive care. Interestingly, no improvement in survival was seen for patients with limited stage BL, likely because of the already very favorable outcomes obtained in the 1973-2001 era.
In summary, we verified improved survival for patients with BL diagnosed in the United States since the development of intense chemotherapy regimens, availability of rituximab, and implementation of effective ART. However, this study points to the need of better access to complex care particularly for adult and black patients and the need for differential treatment strategies appropriate for older patients with BL.
Supplementary Material
Acknowledgments
The work was supported in part by the Biostatistics Shared Resource as part of the Hollings Cancer Center at the Medical University of South Carolina, which is funded by Cancer Center Support Grant P30 CA138313.
Footnotes
Presented at the 54th meeting of the American Society of Hematology, Atlanta GA, December 10, 2012.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.J.C. designed the study, collected and analyzed the data, wrote the first draft of the manuscript and approved its final version; A.C.X. designed the study, collected and analyzed the data, significantly edited the manuscript, and approved its final version; A.E.W. analyzed the data, provided statistical support, significantly edited the manuscript, and approved its final version; E.G.H. analyzed the data, provided statistical support, significantly edited the manuscript, and approved its final version.
Conflict-of-interest disclosure: L.J.C. received research support from Genentech. The remaining authors declare no competing financial interests.
Correspondence: Luciano J. Costa, Associate Professor of Medicine, 96 Jonathan Lucas St, 903 CSB MSC 635, Charleston, SC 29425-6350; e-mail: costalj@musc.edu.
References
- 1.Mead GM, Barrans SL, Qian W, et al. UK National Cancer Research Institute Lymphoma Clinical Studies Group; Australasian Leukaemia and Lymphoma Group. A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial). Blood. 2008;112(6):2248–2260. doi: 10.1182/blood-2008-03-145128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas DA, Cortes J, O’Brien S, et al. Hyper-CVAD program in Burkitt’s-type adult acute lymphoblastic leukemia. J Clin Oncol. 1999;17(8):2461–2470. doi: 10.1200/JCO.1999.17.8.2461. [DOI] [PubMed] [Google Scholar]
- 3.Magrath I, Adde M, Shad A, et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14(3):925–934. doi: 10.1200/JCO.1996.14.3.925. [DOI] [PubMed] [Google Scholar]
- 4.Patte C, Auperin A, Michon J, et al. Société Française d’Oncologie Pédiatrique. The Société Française d’Oncologie Pédiatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97(11):3370–3379. doi: 10.1182/blood.v97.11.3370. [DOI] [PubMed] [Google Scholar]
- 5.Gerrard M, Cairo MS, Weston C, et al. FAB LMB96 International Study Committee. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin’s lymphoma: results of the FAB/LMB 96 international study. Br J Haematol. 2008;141(6):840–847. doi: 10.1111/j.1365-2141.2008.07144.x. [DOI] [PubMed] [Google Scholar]
- 6.Todeschini G, Bonifacio M, Tecchio C, et al. Intensive short-term chemotherapy regimen induces high remission rate (over 90%) and event-free survival both in children and adult patients with advanced sporadic Burkitt lymphoma/leukemia. Am J Hematol. 2012;87(1):22–25. doi: 10.1002/ajh.22189. [DOI] [PubMed] [Google Scholar]
- 7.Thomas DA, Faderl S, O’Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106(7):1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 8.Corazzelli G, Frigeri F, Russo F, et al. RD-CODOX-M/IVAC with rituximab and intrathecal liposomal cytarabine in adult Burkitt lymphoma and ‘unclassifiable’ highly aggressive B-cell lymphoma. Br J Haematol. 2012;156(2):234–244. doi: 10.1111/j.1365-2141.2011.08947.x. [DOI] [PubMed] [Google Scholar]
- 9.Ribrag V, Koscielny S, Bouabdallah K, et al. Addition of rituximab improves outcome of HIV negative patients with Burkitt lymphoma treated with the Lmba protocol: results of the Randomized Intergroup (GRAALL-Lysa) LMBA02 protocol. (IGR sponsored LMBA02, NCT00180882). ASH Annual Meeting Abstracts. 2012;120:685. [Google Scholar]
- 10.Barnes JA, Lacasce AS, Feng Y, et al. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt’s lymphoma: a retrospective analysis. Ann Oncol. 2011;22(8):1859–1864. doi: 10.1093/annonc/mdq677. [DOI] [PubMed] [Google Scholar]
- 11.Shiels MS, Pfeiffer RM, Hall HI, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980-2007. JAMA. 2011;305(14):1450–1459. doi: 10.1001/jama.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Surveillance Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2010 Sub (1973-2008) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2009 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2011, based on the November 2010 submission.
- 13. Surveillance Epidemiology, and End Results (SEER) ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 17 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2010 Sub (1973-2008 varying) - Linked To County Attributes - Total U.S., 1969-2009 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2011, based on the November 2010 submission.
- 14.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. 2nd ed. New York: Springer; 2003. [Google Scholar]
- 15.Evens AM, Islam N, Carson KR, et al. A phase 2 clinical trial adding rituximab to CODOX-M/IVAC for untreated Burkitts lymphoma: correlative analysis of serum and CSF rituximab levels. ASH Annual Meeting Abstracts. 2012;120:1640. [Google Scholar]
- 16.Wang ES, Straus DJ, Teruya-Feldstein J, et al. Intensive chemotherapy with cyclophosphamide, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine (CODOX-M/IVAC) for human immunodeficiency virus-associated Burkitt lymphoma. Cancer. 2003;98(6):1196–1205. doi: 10.1002/cncr.11628. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Qiao B, Panageas KS, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118(5):1248–1254. doi: 10.1182/blood-2011-04-346437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burke ME, Albritton K, Marina N. Challenges in the recruitment of adolescents and young adults to cancer clinical trials. Cancer. 2007;110(11):2385–2393. doi: 10.1002/cncr.23060. [DOI] [PubMed] [Google Scholar]
- 19.Evens AM, Antillón M, Aschebrook-Kilfoy B, Chiu BC. Racial disparities in Hodgkin’s lymphoma: a comprehensive population-based analysis. Ann Oncol. 2012;23(8):2128–2137. doi: 10.1093/annonc/mdr578. [DOI] [PubMed] [Google Scholar]
- 20.Pollock BH, DeBaun MR, Camitta BM, et al. Racial differences in the survival of childhood B-precursor acute lymphoblastic leukemia: a Pediatric Oncology Group Study. J Clin Oncol. 2000;18(4):813–823. doi: 10.1200/JCO.2000.18.4.813. [DOI] [PubMed] [Google Scholar]
- 21.Turner JJ, Morton LM, Linet MS, et al. InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood. 2010;116(20):e90–e98. doi: 10.1182/blood-2010-06-289561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guech-Ongey M, Simard EP, Anderson WF, et al. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood. 2010;116(25):5600–5604. doi: 10.1182/blood-2010-03-275917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigo JA, Hicks LK, Cheung MC, et al. HIV-associated Burkitt lymphoma: good efficacy and tolerance of intensive chemotherapy including CODOX-M/IVAC with or without rituximab in the HAART era. Adv Hematol. 2012;2012:735392. doi: 10.1155/2012/735392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oriol A, Ribera JM, Bergua J, et al. High-dose chemotherapy and immunotherapy in adult Burkitt lymphoma: comparison of results in human immunodeficiency virus-infected and noninfected patients. Cancer. 2008;113(1):117–125. doi: 10.1002/cncr.23522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.