Abstract
Purpose
To compare the results of silicone and polypropylene Ahmed glaucoma valves (AGV) implanted during the first 10 years of life.
Methods
A prospective study was performed on 50 eyes of 33 patients with paediatric glaucoma. Eyes were matched to either polypropylene or silicone AGV. In eyes with bilateral glaucoma, one eye was implanted with polypropylene and the other eye was implanted with silicone AGV.
Results
Fifty eyes of 33 children were reviewed. Twenty five eyes received a polypropylene valve, and 25 eyes received a silicone valve. Eyes implanted with silicone valves achieved a significantly lower intraocular pressure (IOP) compared with the polypropylene group at 6 months, 1 year, and 2 years postoperatively. The average survival time was significantly longer (P=0.001 by the log-rank test) for the silicone group than for the polypropylene group and the cumulative probability of survival by the log-rank test at the end of the second year was 80% (SE: 8.0, 95% confidence interval (CI): 64–96%) in the silicone group and 56% (SE: 9.8, 95% CI: 40–90%) in the polypropylene group. The difference in the number of postoperative interventions and complications between both groups was statistically insignificant.
Conclusion
Silicone AGVs can achieve better IOP control, and longer survival with less antiglaucoma drops compared with polypropylene valves in children younger than 10 years.
Keywords: paediatric glaucoma, Ahmed valves, buphthalmos, silicone, polypropylene
Introduction
The management of glaucoma in paediatric eyes that have failed or are unsuitable for angle surgery is a challenge. Trabeculectomy has shown variable results in paediatric glaucoma,1, 2, 3, 4, 5 but its drawbacks in children, especially the long-term risk of bleb-related complications,4, 5 has directed the attention towards glaucoma drainage implant surgery.
The Ahmed glaucoma valve (AGV, New World Medical, Inc., Rancho Cucamonga, CA, USA) is an aqueous drainage device that consists of a lumened silicone rubber tube connected to an explant.6 The explant is made of polypropylene or silicone. In experimental models of glaucoma drainage devices, silicone was associated with less inflammation and fibrosis compared with polypropylene.7, 8 It is postulated that the less the encapsulation around the explant, the less the resistance will be to aqueous flow, thus yielding better intraocular pressure (IOP) control and a longer term success of the device.6 Most clinical studies comparing silicone to polypropylene AGVs have been retrospective studies on adult eyes and showed better or similar results with the silicone AGV.9, 10, 11, 12, 13 In this prospective controlled study, we compared the efficacy and safety of flexible silicone plate and rigid polypropylene plate AGVs in paediatric glaucoma patients with previously failed conventional glaucoma surgeries.
Materials and methods
The study was approved by Cairo University research ethics committee and followed the tenets of the Declaration of Helsinki. A prospective interventional matched study was conducted on patients younger than 10 years whose glaucoma was uncontrolled by previous glaucoma surgeries. Exclusion criteria were eyes requiring combined procedures or pars plana implantation of the valve, eyes that received a previous glaucoma drainage device or silicone oil injection and eyes in which the superotemporal quadrant was unsuitable for valve implantation.
Sample size calculation
Previous studies reported a 4–5-mm Hg lower IOP with silicone valve compared with polypropylene valve.10, 13 This study was powered to detect a true difference in success rates of this size. An estimation of sample size was performed considering a study power of at 0.8 with an α error of 0.05 aiming to detect a difference of 4 mm Hg in mean IOP in the 12 postoperative months assuming SD of 6 mm Hg between the two groups. Based on this estimation, a total of 20 study subjects in each group were found to be adequate. Assuming a 25% dropout and failure rate during the follow-up, recruitment of at least 50 study subjects was targeted.
Preoperative and postoperative data included number of glaucoma medications, IOP measured by Perkins or Goldmann tonometer, uncorrected and best-corrected visual acuity when possible, corneal diameter, slit lamp, and fundus examination. Ocular ultrasonography was performed when needed. In younger children in whom examination was difficult, chloral hydrate sedation was used.
An informed consent was obtained from the patients' parents. Eyes with axial length <23 mm received paediatric-sized valves (S3 or FP8); otherwise an adult-sized valve was used (S2 or FP7). Patients were matched to receive either a silicone plate valve or polypropylene plate valve. In those with bilateral glaucoma, a paired-eye study design was used so that one eye always received a polypropylene AGV while the fellow eye received a silicone AGV. In those with unilateral glaucoma, patients were matched according to the aetiology of glaucoma. Efforts were made to ensure that in unilateral cases, each matched couple have similar preoperative IOP (difference in IOP between both groups ≤4 mm Hg) and similar number of prior surgeries (difference in number between each matched pair ≤1). In each matched pair, the decision of which eye to have the silicone plate valve and which eye to have the polypropylene plate valve was determined using a random table.
The surgical procedure was similar for both implant types. A fornix-based conjunctival flap was created in the superotemporal quadrant. Mitomycin C was applied on the sclera over the intended area for the plate in a concentration of 0.4 mg/ml for 3 min using a polyvinyl alcohol sponge (Merocel, Mystic, CT, USA) held from the tip of the applicator. The area was then irrigated with balanced saline solution (BSS). The tube was primed with BSS, inserted into the pocket between the superior and lateral recti, and sutured to the sclera 8–10 mm from the limbus using 9–0 nylon (Ethilon, Ethicon, Somerville, NJ, USA) sutures. The drainage tube was cut with bevel up to the appropriate length and a 23-gauge needle was inserted 0.5 mm from the limbus to create a track into the anterior chamber through which the tube was inserted. The tube was loosely secured to the sclera using a 9–0 nylon suture. A patch graft of donor sclera was used to cover the scleral entry site and at least the anterior 6 mm of the tube and was loosely anchored to the sclera using 9–0 nylon. The conjunctiva was approximated and sutured to the limbus using 7–0 polyglactin (Vicryl, Ethicon) sutures. Viscoelastic was used if anterior chamber shallowing was noted or to facilitate tube insertion into the anterior chamber. All surgeries were performed by one surgeon (YE).
Postoperatively patients were given antibiotic drops (Tobramycin) for 1 month and steroid drops (Prednisolone 1%) tapered gradually over the following 3 months. Antiglaucoma medications were added when required. Patients were generally seen on day 1, weekly for a month, monthly for 6 months, then every 3 months. More frequent visits were required in cases with complications or inadequate IOP control.
Complete success was defined as IOP of <22 mm Hg at the last follow-up visit with no other signs of glaucoma progression (increasing corneal diameter, axial length, or cup to disc ratio). The use of glaucoma medications or surgical valve revision (capsulectomy) to achieve such a pressure was considered as qualified success and failure was considered if no such pressure could be achieved despite maximum tolerated medical treatment, or if a subsequent glaucoma procedure was needed to control the IOP or if a devastating complication occurred (eg, retinal detachment, endophthalmitis, or suprachoroidal haemorrhage).
Encapsulation of the valve was defined as the development of an elevated, tense, dome-shaped thickening of the tissue overlying the plate. Eyes that developed encapsulation resulting in an IOP that required more than one antiglaucoma drop to control it had excision of the encapsulation tissue. Subconjunctival saline was injected to separate the conjunctiva from the encapsulated tissue and a radial incision was made temporal to the plate. The conjunctiva was dissected and the scarred tissue was separated from the underlying plate and excised, then the conjunctiva was closed.
Statistical analysis
Statistical analysis was performed with SPSS for Windows version 15.0.1 (SPSS Inc., Chicago III, IL, USA). Demographic data and preoperative data for the polypropylene and silicone AGV groups were analysed with a paired two-sample t-test or McNemar test. IOP comparisons between the two groups were analysed with a paired two-sample t-test. Rates of surgical success, postoperative interventions, and postoperative complications were analysed by the McNemar test. Kaplan–Meier survival analysis for success (complete and qualified) was calculated with the log-rank test. Mean survival times with 95% confidence intervals (CIs) were reported. Univariate and multivariate analysis was done to detect risk factors for failure of the implant. Postoperative results were compared at 1, 3, 6, 12, and 24 months of follow-up.
Results
Patient population
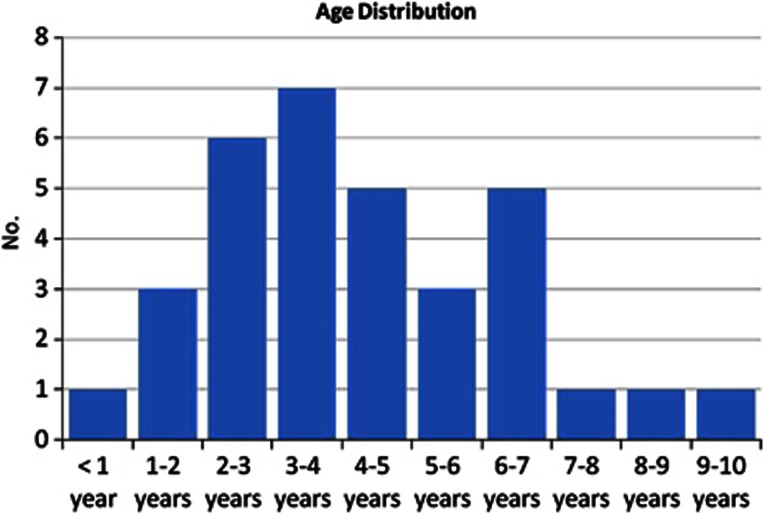
Thirty three patients were enroled in the study (online supplementary). The mean age of patients (Figure 1) was 34.6±65.7 months (range, 7–117). Eighteen patients were females (55.5%). 17 patients had bilateral glaucoma valve implantation (with a silicone valve in one eye and polypropylene valve in the other eye). The remaining 16 patients received glaucoma valve in only one eye. There was no statistically significant difference between the two groups with respect to age (P=0.94), gender (P=0.96), lens status (P=0.88), diagnosis (P=1.00), or prior glaucoma surgeries (P=0.98).
Figure 1.
Histogram showing the age distribution of the included patients in the study.
Types of AGVs
Sixteen out of the 25 eyes implanted with silicone valves (64%) had an axial length <23 mm Hg and so received paediatric-sized, FP8 valves. In the polypropylene group, 8 eyes (32%) received paediatric-sized S3 valves.
IOP reduction
There was a statistically significant decrease of IOP both after polypropylene AGV implantation, and after silicone AGV implantation during all postoperative visits (P<0.0001). The mean postoperative IOP was lower in the silicone AGV compared with the polypropylene AGV at all postoperative visits (Table 1). However, the difference started to become statistically significant after 6 months. (P=0.03) and remained statistically significant till the end of the second year (P=0.04).
Table 1. Changes in IOP and number of medications used in both groups.
| Silicone mean±SD | Polypropylene mean±SD | P-value | |
|---|---|---|---|
| Preoperative | |||
| IOP (mm Hg) | 33.8±5.6 | 34.1±5.9 | 0.86 |
| Medications (no) | 2.93±0.69 | 2.81±0.73 | 0.81 |
| Postoperatively | |||
| 1 Month | |||
| IOP (mm Hg) | 14.1±5.2 | 15.2±5.7 | 0.54 |
| IOP percentage reduction (%) | 58±26 | 55±27 | 0.66 |
| Medications (no) | 0 | 0 | 1.00 |
| Three months | |||
| IOP (mm Hg) | 14.9±5.9 | 17.2±6.2 | 0.27 |
| IOP percentage reduction (%) | 56±27 | 50±29 | 0.43 |
| Medications (no) | 0.22±0.54 | 0.53±0.64 | 0.03 |
| Sixth months | |||
| IOP (mm Hg) | 15.1±5.8 | 19.2±6.7 | 0.03 |
| IOP percentage reduction (%) | 55±27 | 44±32 | 0.11 |
| Medications (no) | 0.76±1.32 | 1.12±1.16 | 0.04 |
| 12 months | |||
| IOP (mm Hg) | 15.8±5.9 | 19.8±6.2 | 0.04 |
| IOP percentage reduction (%) | 53±27 | 42±31 | 0.12 |
| Medications (no) | 1.12±1.04 | 1.56±1.19 | 0.06 |
| 24 months | |||
| IOP (mm Hg) | 17.6±5.8 | 20.2±6.6 | 0.04 |
| IOP percentage reduction (%) | 48±27 | 41±33 | 0.35 |
| Medications (no) | 1.34±1.11 | 1.67±1.15 | 0.07 |
Abbreviation: IOP, intraocular pressure.
Medical therapy
The number of glaucoma medications was statistically significantly reduced in both groups during follow-up. Patients who underwent additional glaucoma surgery were excluded from analysis after the time of reoperation. The mean number of glaucoma medications decreased from 2.93±0.69 at baseline to 1.34±1.11 at the 2-year follow-up visit (P<0.001, paired t-test) and from 2.81±0.73 at baseline to 1.67±1.15 at the 2-year follow-up visit (P<0.001, paired t-test). There was a tendency toward greater use of glaucoma medical therapy in the polypropylene group compared with the silicone group that was statistically significant at 3 and 6 months, but after 1 year (Table 1) level of statistical significance decreased (P=0.06 at 1 year and P=0.07 at 2 years, paired t-test).
Treatment failure
At the end of the first year, treatment failure occurred in 40% of patients in the polypropylene group and 16% of patients in the silicone group. The difference in failure rates at 1 year between the two treatment groups was statistically insignificant (P=0.11). At the end of the second year, treatment failure occurred in 20% of patients in the silicone group and 44% of patients in the polypropylene group. The difference in failure rate at 2 years between the two treatment groups remained statistically insignificant (P=0.11).
The number of patients who fulfilled the criteria of complete success was higher in the silicone group compared with those in the polypropylene group at both 1 and 2 years. However, the difference was statistically insignificant at both 1 and 2 years (P=0.07 at 1 year and 0.25 at 2 years). By the end of the second year, 64% of eyes in the silicone group required drops to control their pressures compared with 52% in the polypropylene group (P=0.11).
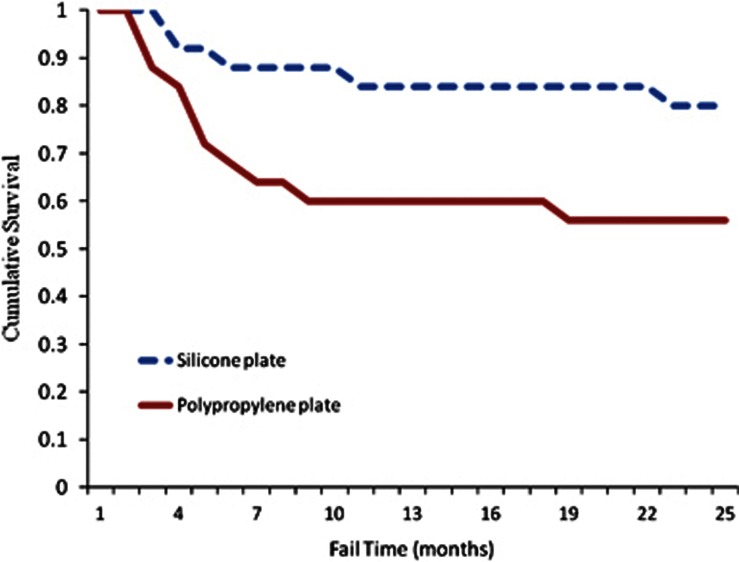
Kaplan–Meier survival analysis was done to compare the survival rates between the two treatment groups (Figure 2). The cumulative probability of survival by the log-rank test at the end of the second year was 80% (SE, 8.0, 95% CI 64–96%) in the silicone AGV group and 56% (SE, 9.8, 95% CI 40–90%) in the polypropylene AGV group.
Figure 2.
Survival curves for the polypropylene and silicone AGV show a longer survival for silicone AGV after implantation.
The mean survival time was significantly longer (P=0.001, stratified log-rank test) for the silicone AGVs (22.84 months (SE 1.73), 95% CI 19.88–24.00 months) than for the polypropylene AGVs (18.36 months (SE 1.94), 95% CI 14.8–21.76 months).
Univariate and multivariate analyses was done to evaluate possible predictors for treatment failure. In univariate analysis, a polypropylene AGV, a higher baseline IOP, and more than two prior glaucoma surgeries were predictors for treatment failure. In multivariate analysis, only larger number of prior glaucoma surgeries remained as a predictor for treatment failure.
Reoperation for glaucoma
As the surgeons were not masked to the treatment assignment, a potential bias existed in the decision to reoperate for IOP control. Therefore, it was decided that all patients with complete failure of IOP control were treated with diode laser cyclophotocoagulation to minimise bias in the selection. To evaluate for reoperation bias, the IOP levels before reoperation were compared between both treatment groups. The mean IOP before reoperation was 29.23±4.7 mm Hg in the silicone group and 30.12±5.1 in the polypropylene group. There were no significant difference between both groups (P=0.56).
Postoperative complications
Postoperative complications are listed in Table 2. The most commonly encountered complications were hypotony and choroidal effusion and tube-related complications. The difference in the rate of complications between both groups was statistically insignificant. Silicone AGVs were more likely to be associated with hypotony and hypotony-related complications (such as shallowing of the anterior chamber and choroidal effusion), whereas the polypropylene AGVs were more likely to be complicated by fibrosis and encapsulation. None of the patients in either group developed persistent hypotony. Three eyes in the silicone group developed anterior chamber shallowing and choroidal detachment in the immediate postoperative period, which required reformation of the anterior chamber.
Table 2. Postoperative complications and interventions in both groups during follow-up period.
| Silicone AGV | Polypropylene AGV | P-value | |
|---|---|---|---|
| Complications | |||
| Tube related | |||
| Obstruction | 1 (4%) | 2 (8%) | 1.00 |
| Tube exposure | 4 (16%) | 1 (4%) | 1.00 |
| Tube migration | 1 (4%) | 0 | 1.00 |
| Choroidal effusion | 4 (16%) | 2 (8%) | 0.68 |
| Shallow AC | 3 (12%) | 1 (4%) | 0.62 |
| Encapsulation | 0 | 5 (20%) | 0.07 |
| Endophthalmitis | 1 (4%) | 0 | 1.00 |
| Vitreous haemorrhage | 1 (4%) | 1 (4%) | 1.00 |
| Eye motility disorder | 0 | 1 (4%) | 1.00 |
| Corneal oedema | 1 (4%) | 1 (4%) | 1.00 |
| Recurrent or persistent iritis | 0 | 1 (4%) | 1.00 |
| Pupillary membrane | 0 | 1 (4%) | 1.00 |
| Retinal detachment | 2 (8%) | 1 (4%) | 1.00 |
| Interventions | |||
| Tube repositioning (extension or trimming) | 3 (12%) | 1 (4%) | 0.62 |
| Tube clearing from occlusion | 1 (4%) | 2 (8%) | 1.00 |
| Reformation of AC for hypotony | 3 (12%) | 2 (8%) | 1.00 |
| Excision of encapsulation | 0 | 3 (12%) | 0.25 |
| Scleral graft for exposed tube | 4 (16%) | 1 (4%) | 0.37 |
| Total number of interventions | 11 (44%) | 9 (36%) | 0.82 |
Abbreviation: AGV, Ahmed glaucoma valve.
Five eyes in the polypropylene group developed encapsulation around the plate. Of these three had an IOP that was uncontrolled on one topical antiglaucoma medication and went on to have excision of the encapsulation tissue. Capsulectomy succeeded in controlling the IOP in one eye on a single antiglaucoma drop until the last follow-up (5 months after the revision), while the other two eyes failed because of poor pressure control and eventually required diode laser cyclophotocoagulation (6 and 8 months after the revision).
Postoperative interventions
The number and frequency of patients who required postoperative interventions during the follow-up period are listed in Table 2. The most commonly performed intervention was tube repositioning, reformation of the anterior chamber for hypotony, and revision of an exposed tube. The total number of interventions was higher in the silicone AGV group, but the difference was not statistically significant (P=0.82). Reoperations for complications were performed in 44% of patients in the silicone AGV group and in 36% of patients in the polypropylene AGV group.
Discussion
Bleb failure following glaucoma drainage device implantation is likely to be related to the biomaterial-associated inflammation. Experimental studies in rabbits have shown that polypropylene and other rigid biomaterials were associated with significantly more inflammation than silicone.6, 8 Law et al9 compared the results of 50 silicone and 49 polypropylene AGVs in refractory glaucoma over a period of 12 months and showed that silicone AGVs resulted in lower IOPs with the difference between both groups being statistically significant at 3 months. However, more non-tube-related complications were encountered in the silicone group. This was mostly attributed to overfiltration in the immediate postoperative period. Ishida et al10 showed that the mean IOP was significantly lower in silicone valves at 3, 6, and 12 months postoperatively. Conversely, other authors11, 12 could not detect any statistically significant difference in IOP decrease, postoperative antiglaucoma medications, rate of complications, or changes in visual acuity between silicone and polypropylene valves at any follow-up visit.
We are aware of only one published study that compared silicone and polypropylene AGVs in the paediatric age group. In that study, Khan and Al-Mobarak13 found that 2 years after AGV implantation the success rates were higher with silicone (90.9%) compared with polypropylene AGVs (54.8%). The mean survival time was significantly longer for the silicone group compared with the polypropylene group. However, all eyes receiving silicone AGVs in their study had primary congenital glaucoma compared with only around half the eyes implanted with polypropylene AGVs, which may have contributed to the superior results of silicone. In addition, their study was retrospective, non matched, and included only infants younger than 2 years.
In our prospective study on children aged <10 years, patients in both groups were matched for the number of previous glaucoma surgeries and the type of glaucoma. Seventeen of our 33 patients had bilateral valves implanted. In these patients, one eye received a silicone or a polypropylene AGV and the other eye received the opposite type of implant. Such design allows better statistical evaluation, as each patient serves as his own control and reduces the inter-individual variability.
Most of our patients required one or more antiglaucoma medications by the end of the study period to control their pressure, so that the rate of complete success dropped from 100% in the first month to 16% by the second year in the silicone group and to 4% in the polypropylene group. Such findings show that while the pressure in paediatric glaucoma can initially be controlled with valve implantation alone, AGV is still not by any means a life-long cure for paediatric glaucoma and further medical or surgical therapy will eventually be needed for most patients over the long term. Nevertheless, eyes implanted with silicone AGVs required a fewer mean number of medications at all postoperative visits as well as showing a longer mean survival time compared with the polypropylene AGVs.
Studies have shown that surgeon's experience may influence the results of glaucoma valve surgery.14, 15 In a prior study,15 it was shown that failure was perhaps 20% less likely (95% CI, 0.6–1.0) in AGV if the surgeon had placed 20≤ AGVs before. In the present study, to minimise the effect of surgeon's experience on the outcome, all surgeries were performed by one surgeon who had placed over 20 AGVs before the study.
Encapsulation around the plate occurred only in polypropylene group (20%) and excision of the fibrous capsule was done in those who developed uncontrolled IOP as a result of the encapsulation. We did not attempt needling as these eyes had rather fibrosed thickened tissue surrounding the plate and there is evidence that the effect of needling such cases is very limited and they eventually end up requiring capsulectomy.16
There were some limitations in this study that may have influenced the results: physicians were not masked to the treatment groups during follow-ups. Some differences in the plates other than their material, such as the presence of fenestration holes in the silicone plate and the differently sized paediatric and adult plates may have contributed to the difference in results. We did not attempt to match our cases to the implant size as we were guided by the axial length in choosing the size of the valve. Nine (36%) out of the 25 eyes implanted with silicone AGVs had an adult-sized valve (FP7) compared with 68% of eyes receiving polypropylene AGVs (S2). Larger surface implants were previously shown to be associated with better filtration and less resistance to flow17 and had the two groups been matched according to the size of the plates, the difference in survival and IOP outcomes may have been more pronounced in favour of the silicone group.
Although we included patients with different causes of paediatric glaucoma, the number of patients with paediatric glaucoma from causes other than congenital and aphakic ones are still too small to allow adequate statistical analysis. In addition, 2 years of follow-up is considered a short period in patients with life-long diseases like paediatric glaucoma; a longer follow-up is needed to obtain more reliable long-term results.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Eye website (http://www.nature.com/eye)
Supplementary Material
References
- Low S, Hamada S, Nischal KK. Antimetabolite and releasable suture augmented filtration surgery in refractory pediatric glaucomas. J AAPOS. 2008;12 (2:166–172. doi: 10.1016/j.jaapos.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Sidoti PA, Belmonte SJ, Liebmann JM, Ritch R. Trabeculectomy with mitomycin-C in the treatment of pediatric glaucomas. Ophthalmology. 2000;107 (3:422–429. doi: 10.1016/s0161-6420(99)00130-x. [DOI] [PubMed] [Google Scholar]
- Freedman SF, McCormick K, Cox TA. Mitomycin C-augumented trabeculectomy with postoperative wound modulation in pediatric glaucoma. J AAPOS. 1999;3 (2:117–124. doi: 10.1016/s1091-8531(99)70082-0. [DOI] [PubMed] [Google Scholar]
- Beck AD, Wilson WR, Lynch MG, Lynn MJ, Noe R. Trabeculectomy wit adjunctive mitomycin C in pediatric glaucoma. Am J Ophthalmol. 1998;126 (5:648–657. doi: 10.1016/s0002-9394(98)00227-x. [DOI] [PubMed] [Google Scholar]
- Al-Hazmi A, Zwaan J, Awad A, al-Mesfer S, Mullaney PB, Wheeler DT. Effectiveness and complications of mitomycin C use during pediatric glaucoma surgery. Ophthalmology. 1998;105 (10:1915–1920. doi: 10.1016/S0161-6420(98)91041-7. [DOI] [PubMed] [Google Scholar]
- Hong CH, Arosemena A, Zurakowski D, Ayyala RS. Glaucoma drainage devices: a systematic literature review and current controversies. Surv Ophthalmol. 2005;50 (1:48–60. doi: 10.1016/j.survophthal.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Ayyala RS, Harman LE, Michelini-Norris B, Ondrovic LE, Haller E, Margo CE, et al. Comparison of different biomaterials for glaucoma drainage devices. Arch Ophthalmol. 1999;117 (2:233–236. doi: 10.1001/archopht.117.2.233. [DOI] [PubMed] [Google Scholar]
- Ayyala RS, Michelini-Norris B, Flores A, Haller E, Margo CE. Comparison of different biomaterials for glaucoma drainage devices: part 2. Arch Ophthalmol. 2000;118 (8:1081–1084. doi: 10.1001/archopht.118.8.1081. [DOI] [PubMed] [Google Scholar]
- Law SK, Nguyen A, Coleman AL, Caprioli J. Comparison of safety and efficacy between silicone and polypropylene Ahmed glaucoma valves in refractory glaucoma. Ophthalmology. 2005;112 (9:1514–1520. doi: 10.1016/j.ophtha.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Ishida K, Netland PA, Costa VP, Shiroma L, Khan B, Ahmed II. Comparison of polypropylene and silicone Ahmed Glaucoma Valves. Ophthalmology. 2006;113 (8:1320–1326. doi: 10.1016/j.ophtha.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Brasil MV, Rockwood EJ, Smith SD. Comparison of silicone and polypropylene Ahmed Glaucoma valve implants. J Glaucoma. 2007;16 (1:36–41. doi: 10.1097/01.ijg.0000243477.82779.31. [DOI] [PubMed] [Google Scholar]
- Mackenzie PJ, Schertzer RM, Isbister CM. Comparison of silicone and polypropylene Ahmed glaucoma valves: two-year follow-up. Can J Ophthalmol. 2007;42 (2:227–232. [PubMed] [Google Scholar]
- Khan AO, Al-Mobarak F. Comparison of polypropylene and silicone Ahmed valve survival 2 years following implantation in the first 2 years of life. Br J Ophthalmol. 2009;93 (6:791–794. doi: 10.1136/bjo.2008.151258. [DOI] [PubMed] [Google Scholar]
- Barton K, Gedde SJ, Budenz DL, Feuer WJ, Schiffman J, Ahmed Baerveldt Comparison Study Group The Ahmed Baerveldt Comparison Study: methodology, baseline patient characteristics, and intraoperative complications. Ophthalmology. 2011;118 (3:435–442. doi: 10.1016/j.ophtha.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budenz DL, Barton K, Feuer WJ, Schiffman J, Costa VP, Godfrey DG, Ahmed Baerveldt Comparison Study Group et al. Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology. 2011;118 (3:443–452. doi: 10.1016/j.ophtha.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibschitz-Tsimhoni M, Schertzer RM, Musch DC, Moroi SE. Incidence and management of encapsulated cysts following Ahmed glaucoma valve insertion. J Glaucoma. 2005;14 (4:276–279. doi: 10.1097/01.ijg.0000169391.94555.c1. [DOI] [PubMed] [Google Scholar]
- Prata JA, Jr, Santos RC, Labree L, Minckler DS. Surface area of glaucoma implants and perfusion flow rates in rabbit eyes. J Glaucoma. 1995;4 (4:274–280. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.