Abstract
Peripheral myelination is a dynamic process orchestrated by axons and Schwann cells. Although the signaling mechanisms governing myelination are not fully understood, NF-κB activation in Schwann cells has been implicated as a key regulator in vitro. Using a mouse model, we show that nuclear factor κB activation in Schwann cells is not required for myelination in vivo.
Introduction
The nuclear factor κB (NF-κB) transcription factor regulates many physiological processes and requires IκB kinase β (IKKβ) for activation (Li et al., 1999). Commencing at birth, large-diameter axons within peripheral nerves are myelinated by Schwann cells (SCs); SC precursors arise during gestation (embryonic day 12–13) and can be identified by their expression of myelin protein zero (MPZ) (Jessen and Mirsky, 2005). Previous studies have shown that NF-κB signaling in SCs is required for SC differentiation and myelin induction in vitro; however, these findings have not been verified in vivo (Nickols et al., 2003; Yoon et al., 2008; Limpert and Carter, 2010; Chen et al., 2011; Limpert et al., 2013). Here, we show that NF-κB activation in SCs is dispensable for myelination in vivo.
Materials and Methods
Animals.
All animals were generated and housed in a virus/antigen-free facility with a 12 h light/dark cycle; food and water ad libitum. Genotyping of tail DNA was performed as previously described (Li et al., 2003). The following primers were used: 5′-GTC ATT TCC ACA GCC CTG TGA-3′ and 5′-CCT TGT CCT ATA GAA GCA CAA C-3′ to amplify both IKKβf and IKKβ+ alleles; 5′-ACG AAC CTG GTC GAA ATC AGT GCG-3′ and 5′-CGG TCG ATG CAA CGA GTG ATG AG-3′ to amplify Cre.
Sciatic nerve crush injury.
Adult, male mice were anesthetized with ketamine/xylazine (University of Miami, Division of Veterinary Resources, Miami, FL). The right sciatic nerve was exposed and crushed at the level of the midthigh until transparent, using uniformly ground microforceps. Animals were killed 1 d following injury.
Schwann cell isolation.
Adult Schwann cells were isolated from sciatic nerves of adult, male mice as previously described (Manent et al., 2003). Briefly, both sciatic nerves were removed from 2–4-month-old mice in sterile conditions. Twelve nerves were pooled per experiment and incubated in a pretreatment medium, replaced every 2 d, for 7 d to enable Wallerian degeneration. Nerves were then incubated in an enzymatic medium and mechanically dissociated through a narrowed Pasteur pipette. Following centrifugation, cells were resuspended in N2HRG medium and plated on wells coated with poly-l-lysine and laminin. Nonadhesive cells were replated 24 h later. Cells were kept in culture at 37°C, 7% CO2 until confluent. Perinatal Schwann cells were isolated from sciatic nerves of mice at 8 or 12 d of age, as previously described (Honkanen et al., 2007). Briefly, both sciatic nerves were dissected out from each animal and pooled together. Connective tissue was removed and the clean nerves were shredded with microforceps. Nerves were enzymatically digested in trypsin and collagenase for 30 min at 37°C. Cells were plated on poly-l-lysine-coated culture dishes in Schwann cell growth medium (high-glucose DMEM with 10% inactivated horse serum, 4 mm l-glutamine, 1× penicillin/streptomycin (Invitrogen), 2 ng/ml human heregulin-β1, 0.5 μm forskolin, 10 ng/ml human basic fibroblast growth factor, and 20 μg/ml bovine pituitary extract) at 37°C, 7% CO2 for 4 d.
Schwann cell quantification.
Following 4 d of incubation, Schwann cell cultures were fixed with 4% PFA in PBS for 20 min and stained with a rabbit antibody against S100β (1:5000; DAKO) along with a tyramide signal amplification kit (TSA), with HRP-goat anti-rabbit IgG (Invitrogen). Cells were then stained with a rabbit antibody against p65, phosphoSer276 (1:400; Millipore) followed by anti-rabbit Alexa Fluor 594 (1:750; Invitrogen). Images were obtained at 10×. The total number of S100β+ and S100β+(p)-p65+ cells were counted from three random fields per animal per group.
Generation of MPZ-CreIKKβ−/− mice.
MPZ-CreIKKβ−/− mice were generated by crossing MPZ-Cre females (a kind gift from Dr. Jeffrey Milbrandt, Washington University, St. Louis, MO) (Ryu et al., 2007) with IKKβF/F males (generously donated by Dr. Michael Karin, University of California San Diego, La Jolla, CA), which have a floxed 1.65 kb fragment of IKKβ flanking exon 3, on a C57BL/6 background (Li et al., 2003). MPZ-CreIKKβ+/− and IKKβF/F progeny were bred to generate MPZ-CreIKKβ−/− mice lacking the third exon of IKKβ, which codes for the ATP binding site of the kinase domain. No overt behavioral phenotypes were observed in homozygotes throughout adulthood (≤2 years of age). Animals were housed on a 12 h light/dark cycle in a virus/antigen-free facility with food and water ad libitum.
ROSAtm9 reporter mice.
B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J (ROSAtm9) homozygous mice were obtained from The Jackson Laboratory and crossed with WT or MPZ-CreIKKβ−/− mice to determine promoter specificity and Cre efficacy. ROSAtm9 mice possess a loxP-flanked STOP cassette upstream of tdTomato (a red fluorescent protein variant). Upon Cre-recombination, tdTomato is expressed in the cre-expressing tissue.
Immunohistochemistry.
Following transcardial perfusion with 4% p-formaldehyde in 0.1 mol/l PBS, sciatic nerves were removed and postfixed for 1 h. Nerves were cryoprotected in 0.1 mol/l PBS containing 20% sucrose. Longitudinal sections were cut at 16 μm, incubated overnight at 4°C with a chicken antibody against MPZ (1:100; Abcam) followed by anti-chicken Alexa Fluor 488 (1:750; Invitrogen) for 1 h at room temperature. For phospho-p65 (p-p65) stains, a TSA kit, with HRP-goat anti-rabbit IgG (Invitrogen) was used, according to manufacturer's instructions, along with a rabbit antibody against p65, phosphoSer276 (1:400; Millipore). Confocal images were acquired on an Olympus FluoView 1000 confocal microscope with a 20× objective or 63× oil objective.
Western blot analysis.
Mice were anesthetized and sciatic nerves were removed and immediately frozen in liquid nitrogen. Nerve samples were then homogenized in 200 μl of lysis buffer (20 mmol/l Tris-HCl, pH 7.4, 150 mmol/l NaCl, 1% Triton X-100, 1 mmol/l EDTA, and 5 mmol/l dithiothreitol) containing complete protease inhibitor (Roche) and phosphatase inhibitors (Sigma-Aldrich). Following centrifugation (13,000 rpm for 20 min at 4°C), the supernatant was removed and protein concentration was determined with a DC Protein Assay kit (Bio-Rad). Ten micrograms of each protein sample were resolved on a 12% SDS-polyacrylamide gel by electrophoresis and transferred to a nitrocellulose membrane (Bio-Rad). Membranes were blocked in 5% bovine serum albumin for 1 h at room temperature and probed with antibodies against MPZ (chicken, 1:1000; Abcam), MBP (rat, 1:1000; Millipore), and β-actin (mouse, 1:1000; Sigma-Aldrich). Following overnight incubation at 4°C with primary antibodies, membranes were rinsed with TBS-T and incubated with horseradish peroxidase-conjugated anti-chicken (Jackson ImmunoResearch Laboratories), anti-rat (Sigma-Aldrich), or anti-mouse (GE Healthcare) antibodies (1:2000) in 3% bovine serum albumin at room temperature for 1 h. Proteins were visualized with ECL (GE Healthcare/GE Healthcare) and quantified with Quantity One software (Bio-Rad). Data were normalized against β-actin and expressed as a percentage of IKKβf/f postnatal day 4. A total of 4 animals/group per time point were used.
p-Phenylenediamine and toluidine blue staining.
Following transcardial perfusion, sciatic nerves were removed and fixed in 2% glutaraldehyde/0.05 m PO4/100 mm sucrose. Nerves were then rinsed with 0.15 m phosphate buffer and postfixed in 2% OsO4/0.1 m PO4 for 1 h at room temperature at the EM core facility in the Miami Project to Cure Paralysis. Tissues were embedded in fresh epoxy resin (Embed, Electron Microscopy Sciences) molds, following dehydration in graded ethanol solutions, and incubated overnight at 64°C. Semithin (1 μm) sections were cut transversely from the proximal portion of the sciatic nerve using a Leica Ultracut E microtome and stained with p-phenylenediamine (PPD) and counterstained with toluidine blue (TB) to visualize myelin. Images were acquired on a Zeiss Axiovert 200M microscope using a 63× oil objective and Neurolucida Imaging software.
Morphometric analysis.
Using StereoInvestigator software, the following were quantified: average diameter of the sciatic nerve, number of Schwann cell nuclei, number of large-diameter axons, and number of compact myelin rings. A 60× oil objective, fractionater probe, and nucleator probe were used and total population projections of each identifier were compared between IKKβf/f and IKKβ−/− littermates.
Statistical analysis.
A two-tailed, unpaired Student's t test was used for single comparisons; p < 0.05 determined significance.
Results
Characterization of IKKβ−/− mice
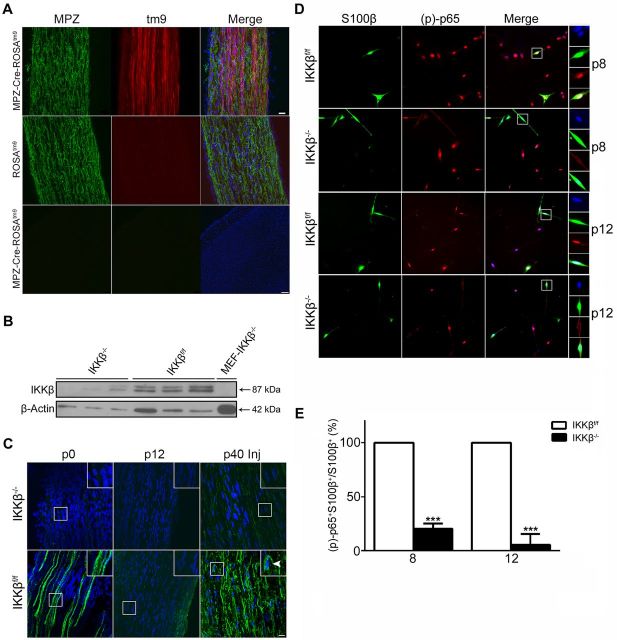
To assess the role of NF-κB activation in SC myelination, we crossed MPZ-Cre and IKKβf/f mice to ablate IKKβ in SCs. To test the specificity of the MPZ promoter, MPZ-Cre mice were crossed with ROSAtm9 reporter mice to visualize Cre-mediated recombination in SCs within the sciatic nerve (Fig. 1). Cre-recombination occurred in SCs, but not within the brain, of MPZ-Cre-ROSAtm9 heterozygotes at postnatal day 15 (p15); no tm9 was visible in ROSAtm9 controls (Fig. 1A).
Figure 1.
Characterization of IKKβ−/− mice. A, MPZ-Cre-ROSAtm9 heterozygotes and ROSAtm9 reporter mice. MPZ immunofluorescence (green) in longitudinal sections of sciatic nerves (top, middle) and transverse sections of cortex (bottom) at p15. Cre recombination, driven by the MPZ promoter, can be seen in the sciatic nerve but not the cortex. DAPI (blue) illuminates cell nuclei. tm6, tomato 6. Scale bars: top, 20 μm; bottom, 100 μm. n = 3 animals per group. B, Immunoblot for IKKβ and loading control (β-actin) on Schwann cell cultures isolated from adult sciatic nerves. MEF-IKKβ−/− cells serve as a negative control. n = 3 groups (pooled nerves from 6 animals) per well. MEF, mouse embryonic fibroblast. C, Immunostains of p-p65 (green) and DAPI (blue) in naive sciatic nerves at p0 and p12), and injured (Inj) adult (p40) sciatic nerves, 1 d following injury (1 dpi). Robust p-p65 expression can be seen in naive p0 and injured (1dpi) adult IKKβf/f (white arrowheads) but not IKKβ−/− sciatic nerves. p-p65 Immunoreactivity appears to be absent in p12 sciatic nerves in both groups. Scale bar, 20 μm. D, Immunostains of S100β (green) and p-p65 (red) in Schwann cell cultures isolated from perinatal sciatic nerves at p8 and p12. Insets show nuclear colocalization (DAPI, blue) of activated p65 in S100β+ Schwann cells. E, Images were obtained on the fourth day in vitro. Quantification of S100β+ p-p65+ double-positive cells (D), harvested from p8 and p12 sciatic nerves, expressed as a percentage of the total number of S100β+ Schwann cells. All Schwann cells from IKKβf/f nerves expressed activated p65. Data are expressed as the mean ± SEM of 3 animals/group. ***p < 0.0001, unpaired Student's t test (IKKβ−/− vs IKKβf/f).
Since IKKβ is ubiquitously expressed and fibroblasts constitute a large portion of the sciatic nerve, we isolated SCs from adult sciatic nerves to verify IKKβ deletion. Immunoblots for IKKβ from cultured SCs revealed a substantial reduction in IKKβ immunoreactivity in IKKβ−/− SCs when compared with IKKβf/f SCs (Fig. 1B). Although some IKKβ was present in IKKβ−/− SCs, it is likely due to residual fibroblast contamination. Since IKKβ is required for NF-κB activation, we stained postnatal (p0 and p12) sciatic nerves with p-p65 (activated NF-κB) to verify the efficacy of our knock-out (Fig. 1C). We were able to detect high p-p65 expression levels in the sciatic nerves of p0 IKKβf/f mice, a finding in agreement with previous studies (Nickols et al., 2003). No NF- κB activation was detected in p0 IKKβ−/− nerves, indicating functional knockdown of IKKβ. We were unable to detect p-p65 in p12 mouse sciatic nerves in either group. However, we were able to detect a robust NF-κB response 1 d following sciatic nerve crush injury in the distal segment of sciatic nerves from IKKβf/f but not IKKβ−/− adult mice (Fig. 1C).
To further verify the efficacy of IKKβ deletion on the inhibition of NF-κB activation, we isolated and cultured SCs from mice at p8 and p12 (Fig. 1D). SCs were clearly labeled with S100β on the fourth day in vitro. We saw a significant decrease in p-p65 immunoreactivity in SCs from IKKβ−/− mice (IKKβ−/− p8: 0.203 ± 0.028; IKKβf/f p8: 1.000 ± 0.000; IKKβ−/− p12: 0.057 ± 0.057; IKKβf/f p12: 1.000 ± 0.000) (Fig. 1E). All SCs in IKKβf/f mice expressed activated NF-κB (Fig. 1D), and there was no significant difference in the total number of Schwann cells between groups (data not shown). Additionally, all other cell types (i.e., fibroblasts) expressed p-p65 in both groups. Together, these data suggest that IKKβ protein function is absent in IKKβ−/− SCs, which nearly completely ablates NF-κB activation.
Inhibition of NF-κB activation in Schwann cells transiently delays myelination
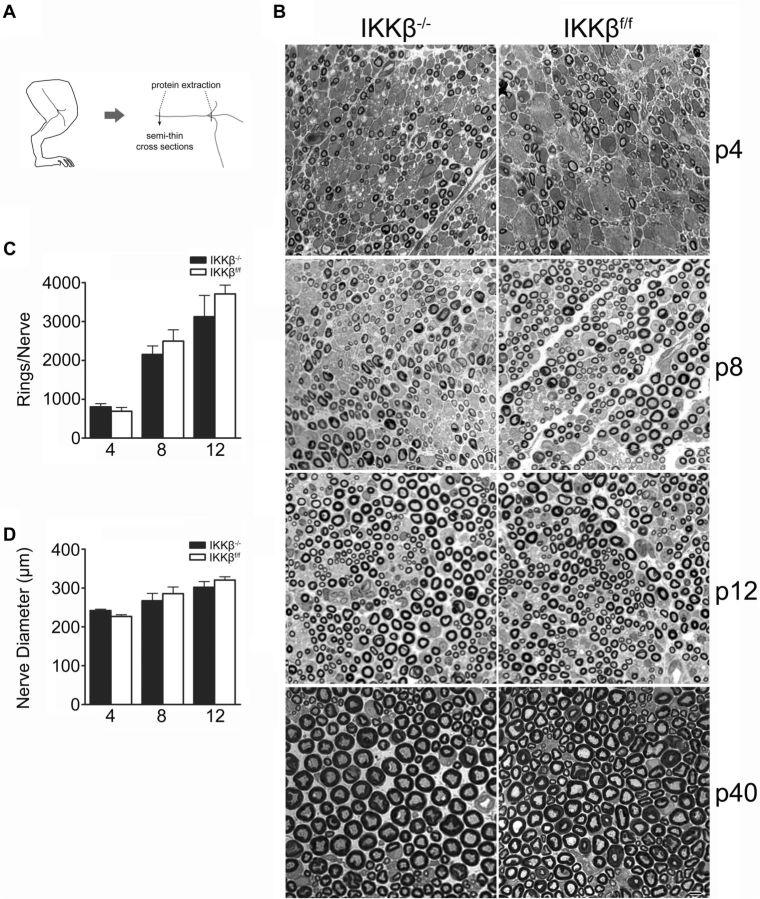
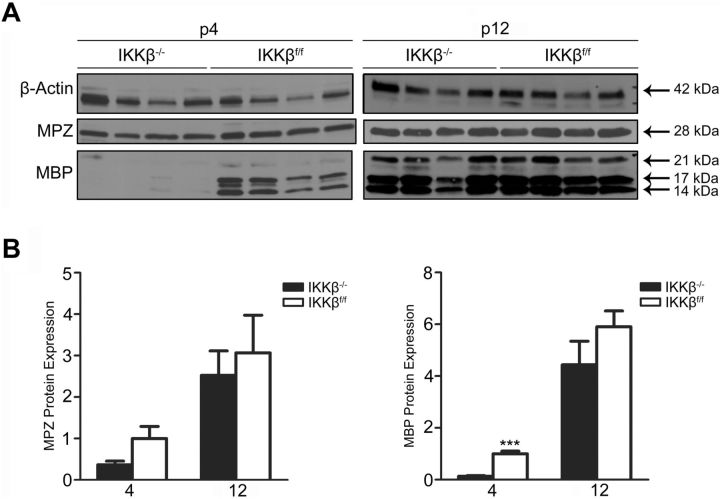
To determine whether NF-κB activation in SCs is required for myelinogenesis, sciatic nerves were harvested from mice at p4, p8, p12, and p40 (Fig. 2A). Semithin, transverse, PPD-stained, and TB-stained sections revealed no significant, morphological differences in myelination between IKKβ−/− and control littermates (Fig. 2B). Both groups displayed similar nerve-trunk diameters (Fig. 2D) (IKKβ−/− p4: 242.2 ± 3.379 μm; IKKβf/f p4: 227.0 ± 4.479 μm; IKKβ−/− p8: 267.4 ± 19.01 μm; IKKβf/f p8: 285.7 ± 17.02 μm; IKKβ−/− p12: 302.2 ± 14.57 μm; IKKβf/f p12: 320.6 ± 8.442 μm). There were no differences in the total number of myelin rings (Fig. 2C) between groups (IKKβ−/− p4: 806.4 ± 80.18; IKKβf/f p4: 689.4 ± 98.49; IKKβ−/− p8: 1893 ± 358.4; IKKβf/f p8: 1831 ± 384.6; IKKβ−/− p12: 2968 ± 527.8; IKKβf/f p12: 3616 ± 243.8). Immunoblots showed a significant reduction in myelin basic protein (MBP) protein expression levels within the sciatic nerves of IKKβ−/− mice at p4, which was attenuated by p12, when compared with IKKβf/f littermates (Figs. 2A, 3). There was no significant difference in MPZ protein expression levels within the sciatic nerves of IKKβ−/− and IKKβf/f mice at p4 or p12. Myelin protein expression increased between p4 and p12 in both groups as myelination ensued (Fig. 3). Hence, NF-κB activation is dispensable for myelination and only transiently required for myelin-specific protein expression in vivo.
Figure 2.
Morphometric assessment of perinatal, sciatic nerve development. A, Schematic of sciatic nerve dissection. B, Transverse, semithin sections of perinatal sciatic nerves stained with PPD and TB at p4, p8, p12, and p40. Scale bar, 5 μm. C, D, Quantification of sciatic nerve diameter (C) and total number of myelin rings at p4, p8, and p12 (D). Data were obtained from PPD-stained sections and are expressed as the mean ± SEM of 3–5 animals/group. *p < 0.05, unpaired Student's t test (IKKβ−/− vs IKKβf/f).
Figure 3.
Myelin basic protein expression is transiently reduced in mice lacking IKKβ expression in Schwann cells. A, Immunoblots of MPZ, MBP, and β-actin (loading control) on protein isolated from sciatic nerves at p4 and p12. n = 4 animals per group; each well represents one animal. B, Densitometric quantification of MPZ and MBP protein expression (seen in A). All three isoforms of MBP were quantified. Data expressed as the mean ± SEM of 4 animals/group. ***p < 0.001, unpaired Student's t test (IKKβ−/− vs IKKβf/f).
Discussion
Here, we show for the first time that NF-κB activation in SCs is not required for myelination in vivo. IKKβ deletion resulted in a strong suppression of NF-κB activation at birth and during the developmental time window of ongoing myelinogenesis (Fig. 1). Although we observed a significant decrease in MBP protein expression at p4 in the absence of IKKβ (Fig. 2), this effect was quickly attenuated. We were unable to assess NF-κB activation at p4 in vitro, due to poor cell viability in both groups. Since NF-κB activation was greatly ablated in IKKβ−/− sciatic nerves at birth and in SC cultures isolated at p8 and p12, NF-κB activation is likely absent at p4 in knock-out sciatic nerves. Therefore, during myelinogenesis, NF-κB is highly active at birth and minimally active by p12 in mouse sciatic nerves; however, NF-κB activation is robust in cultured SCs isolated from postnatal nerves.
Although previous findings implicate a role for NF-κB signaling in SC myelin induction in vitro, our results show that NF-κB activation is unnecessary to achieve myelination in vivo. One possible explanation for the discrepancy between these findings is that SCs behave differently in vivo than in vitro. It was recently shown that NF-κB activation in SCs is required for timely remyelination following peripheral nerve injury (Morton et al., 2012). Perhaps dissociation of nerve tissue and SC isolation techniques, commonly used to study SCs in vitro, impose/reflect an injury event that alters the typical SC signaling ensued during development. In the context of NF-κB regulation of myelination, in vitro, immature SCs behave as in vivo, denervated SCs, and do not accurately reflect the signaling mechanisms used by immature SCs in vivo. Hence, in vitro SC myelination does not appear to hinge on the same signaling cascades as SC myelination during perinatal nerve development. SC myelination during development and following injury likely use different transcriptional programs.
Footnotes
This work was supported by the National Institutes of Health (NS051709, NS065479 to J.R.B.) and The Miami Project to Cure Paralysis. We thank M. Karin (University of California, San Diego) for providing the IKKβf/f mice and J. Milbrandt (Washington University, School of Medicine) for providing the MPZ-Cre mice. We also thank M. Bates for EM sample preparation at the University of Miami EM core facility.
The authors declare no competing financial interests.
References
- Chen Y, Wang H, Yoon SO, Xu X, Hottiger MO, Svaren J, Nave KA, Kim HA, Olson EN, Lu QR. HDAC-mediated deacetylation of NF-kappaB is critical for Schwann cell myelination. Nat Neurosci. 2011;14:437–441. doi: 10.1038/nn.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkanen H, Lahti O, Nissinen M, Myllylä RM, Kangas S, Päiväläinen S, Alanne MH, Peltonen S, Peltonen J, Heape AM. Isolation, purification, and expansion of myelin-competent, neonatal mouse Schwann cells. Eur J Neurosci. 2007;26:953–964. doi: 10.1111/j.1460-9568.2007.05726.x. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZW, Omori SA, Labuda T, Karin M, Rickert RC. IKK beta is required for peripheral B cell survival and proliferation. J Immunol. 2003;170:4630–4637. doi: 10.4049/jimmunol.170.9.4630. [DOI] [PubMed] [Google Scholar]
- Limpert AS, Carter BD. Axonal neuregulin 1 type III activates NF-kappaB in Schwann cells during myelin formation. J Biol Chem. 2010;285:16614–16622. doi: 10.1074/jbc.M109.098780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpert AS, Bai S, Narayan M, Wu J, Yoon SO, Carter BD, Lu QR. NF-κB forms a complex with the chromatin remodeler BRG1 to regulate Schwann cell differentiation. J Neurosci. 2013;33:2388–2397. doi: 10.1523/JNEUROSCI.3223-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manent J, Oguievetskaia K, Bayer J, Ratner N, Giovannini M. Magnetic cell sorting for enriching Schwann cells from adult mouse peripheral nerves. J Neurosci Methods. 2003;123:167–173. doi: 10.1016/S0165-0270(02)00349-7. [DOI] [PubMed] [Google Scholar]
- Morton PD, Johnstone JT, Ramos AY, Liebl DJ, Bunge MB, Bethea JR. Nuclear factor-kappaB activation in Schwann cells regulates regeneration and remyelination. Glia. 2012;60:639–650. doi: 10.1002/glia.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols JC, Valentine W, Kanwal S, Carter BD. Activation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formation. Nat Neurosci. 2003;6:161–167. doi: 10.1038/nn995. [DOI] [PubMed] [Google Scholar]
- Ryu EJ, Wang JY, Le N, Baloh RH, Gustin JA, Schmidt RE, Milbrandt J. Misexpression of Pou3f1 results in peripheral nerve hypomyelination and axonal loss. J Neurosci. 2007;27:11552–11559. doi: 10.1523/JNEUROSCI.5497-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C, Korade Z, Carter BD. Protein kinase A-induced phosphorylation of the p65 subunit of nuclear factor-κB promotes Schwann cell differentiation into a myelinating phenotype. J Neurosci. 2008;28:3738–3746. doi: 10.1523/JNEUROSCI.4439-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]