Abstract
Secondary lymphedema is a debilitating condition, and genetic factors predisposing to its development remain largely unknown. Adrenomedullin (AM) is peptide encoded, together with proadrenomedullin N-terminal peptide (PAMP), by the Adm gene (adrenomedullin gene). AM and its putative receptor calcitonin receptor–like receptor (CLR) are implicated in angiogenesis and lymphangiogenesis during embryogenesis and wound healing, suggesting their possible involvement in secondary lymphedema. To investigate whether AM deficiency predisposes to secondary lymphedema, we used heterozygous adult mice with Adm gene-knockin stop mutation, which selectively abrogated AM, but preserved PAMP, expression (AdmAM+/Δ animals). After hind limb skin incision, Adm messenger RNA expression was upregulated in wounded tissue of both AdmAM+/+ and AdmAM+/Δ mice. However, only AdmAM+/Δ animals developed limb swelling and histopathological lymphedematous changes, including epidermal thickening, elevated collagen fiber density, and increased microvessel diameter. Secondary lymphedema was prevented when circulating AM levels in AdmAM+/Δ mice were restored by systemic peptide delivery. In human skin, CLR was expressed in tissue components affected by lymphedema, including epidermis, lymphatics, and blood vessels. Our study identified a previously unrecognized role for endogenous AM as a key factor in secondary lymphedema pathogenesis and provided experimental in vivo evidence of an underlying germ-line genetic predisposition to developing this disorder.
Introduction
Lymphedema is a debilitating condition and a major cause of morbidity following radiotherapy, cancer, surgery, and other injuries (Rockson, 2008a; Tammela and Alitalo, 2010). Lymphedema develops as a result of localized fluid retention and subsequent swelling (edema). This is owing to insufficiency of the lymphatic system, which occurs as a consequence of abnormal structure and/or compromised function of lymphatic vessels (Rockson, 2008a; Tammela and Alitalo, 2010). It was proposed that the first abnormality during the development of breast cancer–related lymphedema is not lymphatic obstruction, but high fluid filtration that overwhelms vulnerable lymphatics (Stanton et al., 2009a). Blood vessels are therefore also implicated in the pathogenesis of lymphedema, and the edema arising from venous insufficiency, when untreated, can progress into a combined venous/lymphatic disorder (Rockson, 2008a).
Lymphedema often affects the skin. The structural and functional abnormalities in lymphedematous skin reflect a multicellular response to impaired extracellular fluid mobilization and can be distinguished from other mechanisms that lead to interstitial edema (Rockson, 2008a). The presence of epidermal thickening is pathognomonic of lymphedema (Wilson et al., 2004; Tabibiazar et al., 2006; Rockson, 2008a). Lymphedema predisposes to collagen and lipid deposition, cutaneous hypercellularity, progressive fibrosis, and susceptibility to infections (Rockson, 2001; Rutkowski et al., 2010; Wu et al., 2011).
Lymphedema is a heterogeneous condition that is either hereditary or acquired (primary or secondary disorder, respectively) (Rockson, 2008a). Although many genes having a role in lymphatic development have been identified, they are mostly associated with primary lymphedema pathogenesis. For example, mutations in VEGFR3, GATA2, or FOXC2 are the underlying causes of either congenital (Milroy disease or Emberger syndrome) or late-onset (distichiasis syndrome) lymphedema (Tammela and Alitalo, 2010; Wang and Oliver, 2010; Ostergaard et al., 2011). In contrast, little is known about the genetic susceptibility to the risk of developing secondary lymphedema following trauma, malignancy, radiation, or other tissue insults (Rockson, 2008a, 2008b). It remains unknown why lymphedema develops in some individuals following sentinel node removal, yet not in others after axillary clearance for breast cancer (Stanton et al., 2009b). Genetic variation could confer relative risk for or, conversely, protection from the development of secondary lymphedema (Stanton et al., 2009a). Recent association studies support the hypothesis that genetic susceptibility could be an important risk factor for developing secondary lymphedema (Finegold et al., 2008, 2012). However, there is no experimental evidence demonstrating an in vivo role for any gene in the pathogenesis of this disorder, and the underlying mechanisms remain to be delineated. In particular, the molecular and physiological relationships between lymphatic and blood vessels and the surrounding tissue microenvironment, which underlie this complex disorder, remain largely uncharacterized (Rockson, 2009). Therefore, therapeutic strategies to prevent or treat lymphedema are limited (Rockson, 2008a; Tammela and Alitalo, 2010).
Adrenomedullin (AM) is a 52-amino-acid vasoactive peptide (Kitamura et al., 1993a; Nikitenko et al., 2002; Brain and Grant, 2004). Together with proadrenomedullin N-terminal peptide (PAMP), AM is encoded by the ADM gene (adrenomedullin gene) (Kitamura et al., 1993b). In vitro, AM acts through the calcitonin receptor–like receptor (CLR), which is encoded by the CALCRL (gene encoding CLR) gene (Poyner et al., 2002). CLR forms heterodimeric receptors with one of the receptor activity–modifying proteins (receptor activity–modifying proteins (RAMPs) 1, 2, or 3). Co-expression of RAMP2 or RAMP3 with CLR in cultured cells leads to AM receptor formation (Poyner et al., 2002).
AM and its receptors are implicated in angiogenesis and lymphangiogenesis during embryonic development, wound healing, and cancer. The role for Adm, Calcrl, and Ramp2 during embryogenesis has been defined in knockout mice studies. These revealed close correlation among phenotypes for these genes, supporting the view that in vivo AM effects are mediated through CLR/RAMP heterodimers (Brain and Grant, 2004; Nikitenko et al., 2006c). The complete loss of Adm expression (that affects both AM and PAMP) in homozygous knockout mice leads to embryonic death at E13.5–14.5 owing to generalized edema or hemorrhage (Caron and Smithies, 2001; Shindo et al., 2001). This is phenocopied in homozygous Calcrl- and Ramp2-knockout mice with embryonic lethality and edema occurring at E12.5–13.5 (Fritz-Six et al., 2008; Ichikawa-Shindo et al., 2008). The development of edema in these embryos is attributed to blood vessel fragility and leakage, or to jugular lymphatic sac hypoplasia accompanied by structurally unaffected regional lymphatic vessels (Kahn, 2008). AM also has a role in tumor neovascularization (Nikitenko et al., 2006c). Its exogenous administration in wild-type mice ameliorates severe injury-induced swelling (Jin et al., 2008), stimulates wound healing of pressure ulcers (Harada et al., 2011), and induces angiogenesis and lymphangiogenesis. In human endometrial tissue, CLR localizes to blood and lymphatic microvessels (Maybin et al., 2011). In cultured microvascular endothelial cells (ECs), CLR interacts with AM (Nikitenko et al., 2006a). Primary human blood and lymphatic ECs express CALCRL and RAMPs messenger RNAs (mRNAs), supplemented AM promotes their proliferation in vitro and reduces endothelium monolayer hyperpermeability induced by inflammatory mediators (Hippenstiel et al., 2002; Vart et al., 2007; Fritz-Six et al., 2008). These findings suggest that in developing embryos and reproductive tissues endogenous AM signaling through CLR might be essential in both blood vessels and lymphatics. However, whether it is relevant to mature animals and human pathology, including secondary lymphedema, remains unclear (Kahn, 2008).
Here, we tested the hypothesis that endogenously produced AM affects the development of secondary lymphedema. To our knowledge, the present study identified a previously unrecognized role for Adm in the pathogenesis of this disorder and provided previously unreported experimental in vivo evidence of a genetic susceptibility or predisposition to the risk of developing secondary lymphedema.
Results
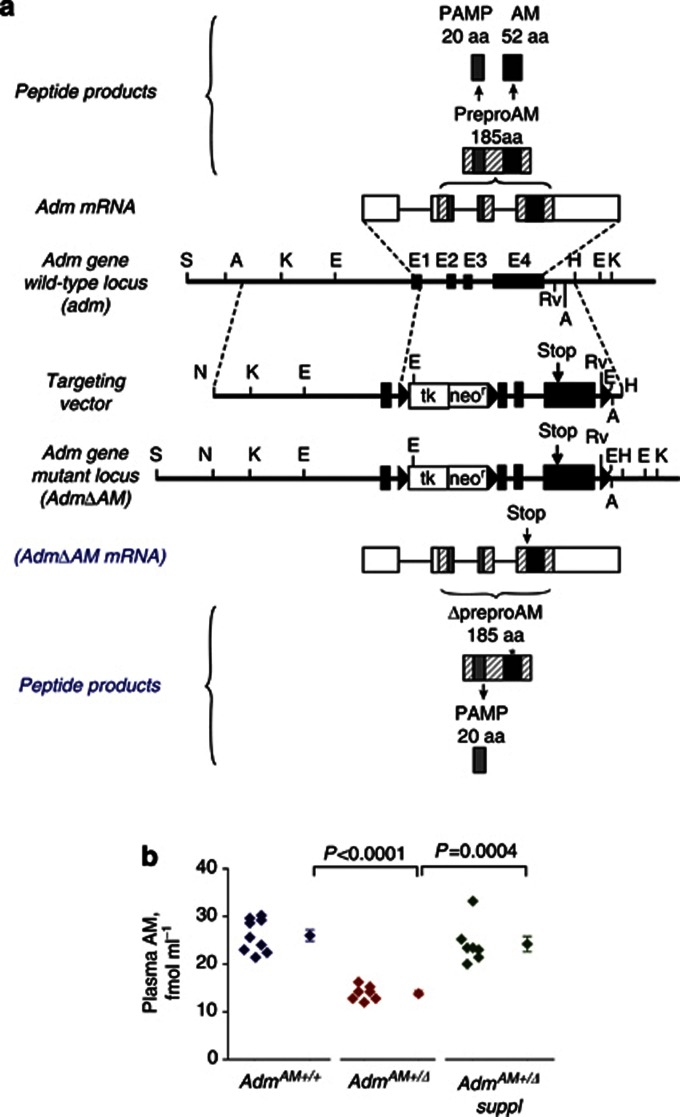
We used mice with Adm-knockin stop mutation that selectively abrogates AM, but not PAMP, expression (AdmAM-knockin animals) (Figure 1a; (Shimosawa et al., 2002)). Although the homozygous mutation (AdmAMΔ/Δ) in mice was lethal, heterozygous (AdmAM+/Δ) fetuses were viable and adult mice (also termed here as AdmAM haploinsufficient animals) were fertile, with their plasma AM levels approximately half (∼53%) of those of wild-type animals, whereas PAMP levels remained unaffected (Supplementary Table S1 online).
Figure 1.
Scheme of targeted knockin stop mutation in Adm gene (modified from Shimosawa et al., 2002). (a) Wild-type Adm locus, resulting Adm messenger RNA (mRNA), preproAM, AM, and PAMP peptide products (top). Targeting vector, mutation-containing locus AdmΔAM, resulting knockin stop mutant AdmΔAM mRNA, preproAM (ΔpreproAM), and PAMP peptide products (bottom). Solid boxes, exons; solid triangles, lox-P sequences. Knockin stop mutation (Stop) at the beginning of the AM-coding region (exon 4) preserves PAMP but not AM translation from the ΔAdm mRNA. (b) AM was measured (7–9 mice in each experimental group) and compared (Welch two-sample t-test) in plasma from wild-type (AdmAM+/+) and AdmAM haploinsufficient mice without (AdmAM+/Δ) or with exogenous AM supplement (AdmAM+/Δ suppl). aa, amino acid.
Primary congenital or adult-onset lymphedema is absent in adult Adm AM haploinsufficient mice
Under physiological, i.e., unchallenged, conditions, adult AdmAM+/+and AdmAM+/Δ mice showed no apparent abnormality in the skin, dermal microvessels, and function of lymphatic vessels (Supplementary Material online, Additional Results and Supplementary Figure S1 online). On the basis of these findings, we concluded that AdmAM haploinsufficiency by itself did not significantly affect (lymph)angiogenesis during embryonic development and caused no symptoms or signs of congenital or adulthood-onset primary lymphedema.
Secondary lymphedema is a phenotypic feature of adult Adm AM haploinsufficient mice, and it is reversible upon exogenous AM supplementation
To evaluate a role for endogenously produced AM in secondary lymphedema development, we used an established experimental model of hind limb skin incision surgery (Kanter et al., 1990). Adm mRNA expression in the skin was increased after surgery in both AdmAM+/+ and AdmAM+/Δ mice (Supplementary Figure S2 online), suggesting a functional role for AM during tissue injury. We used osmotic minipump (effective in the range 10−8 to 10−6 mol/l) (Shimosawa et al., 2003) to restore circulating AM levels in AdmAM+/Δ mice (Figure 1b).
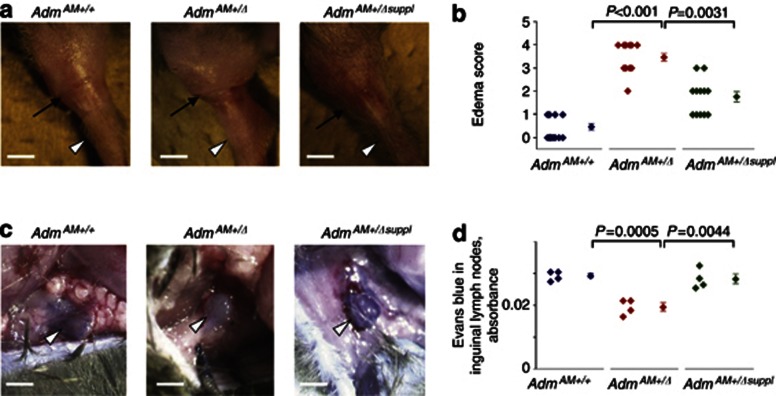
Following hind limb skin incision, adult AdmAM+/Δ but not AdmAM+/+mice developed symptoms and functional changes associated with lymphedema. First, AdmAM+/Δ mice showed impaired ability to move, swelling of the limb, and pitting (or non-pitting in severe cases) edema (Figure 2a and b; Supplementary Figure S3a and b online and Supplementary Table S2 online). These changes in the AdmAM+/Δ mice were accompanied by decreased accumulation of Evans blue in inguinal lymph nodes after intradermal injection of the dye into the hindlimb footpads (Figure 2c and d), suggesting that edema was accompanied by aberrant lymph flow and fluid uptake by regional lymphatics. All acquired changes were partially, but significantly, prevented upon AM supplementation (Figure 2).
Figure 2.
Edema and aberrant fluid uptake in the skin of the adult AdmAM haploinsufficient mice after hind limb skin incision wounding surgery. Wild-type (AdmAM+/+) and AdmAM haploinsufficient mice without (AdmAM+/Δ) or with exogenous AM supplement (AdmAM+/Δ suppl) were used for surgery. (a) The incision site (black arrow) and the ankle (white arrowhead) are indicated (bar=5 mm). For comparison, the images of the sham-operated limbs are presented in Supplementary Figure S3a online. (b) Hind limb swelling degree was assessed and compared (Wilcoxon rank test) between groups. (c, d) Evans blue uptake by inguinal lymph nodes was assessed (four mice in each experimental group) and compared (t-test). Representative images of the inguinal lymph nodes (white arrowheads) after dye uptake are shown (bar=1.5 mm).
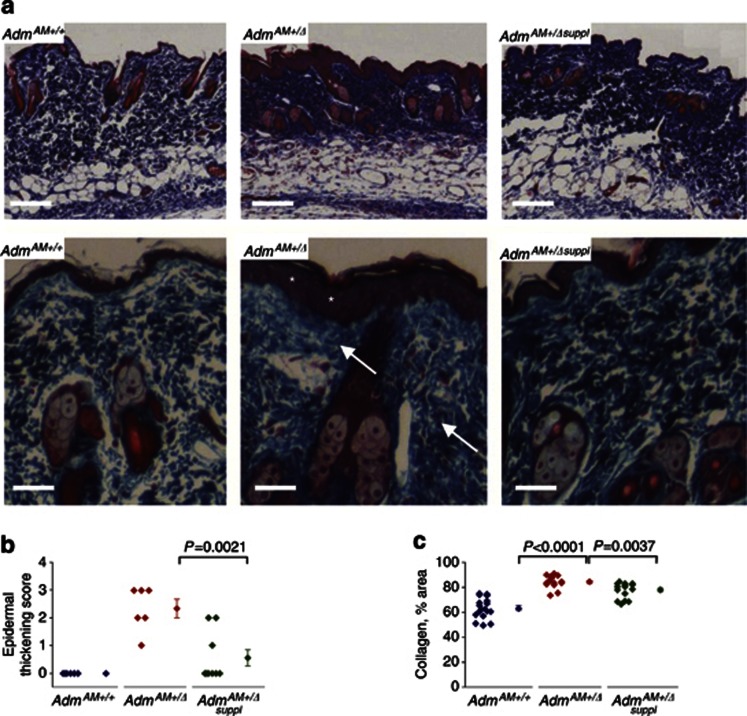
Second, histopathological features characteristic of lymphedema (thickening and hyperplasia of the epidermis, dermal hypercellularity, and increased collagen fiber density) were found in the hind limb skin of AdmAM+/Δ mice after injury (Figure 3a and b; Supplementary Figure S3c and d online). After injury, we also detected an increased deposition of fibrin, used as a marker of plasma filtration and vascular leakage (Chen et al., 2005) in AdmAM+/+and AdmAM+/Δ mice (Supplementary Figure S4a online), compared with that before surgery (Supplementary Figure S1h online). Fibrin accumulation was more pronounced in the skin of AdmAM+/+ animals compared with AdmAM+/Δ animals (Supplementary Figure S4a online). Furthermore, Evans blue accumulation in the dermis after its intravenous injection was elevated in AdmAM+/+ compared with AdmAM+/Δ mice (Supplementary Figure S4b online). These findings were in accordance with our observation of changes in skin collagen density in AdmAM+/Δ animals (Figure 3a and c). All histopathological alterations in the skin of the adult AdmAM+/Δ mice were partially, but significantly, prevented by AM supplementation (Figure 3; Supplementary Figures S3 and S4 online).
Figure 3.
Histopathological features characteristic of lymphedema in the skin of the adult AdmAM haploinsufficient mice after hind limb skin incision wounding surgery. (a) Azan staining. The representative images from six to eight mice in each group are shown. Lower panels (bar=50 μm) are magnified images from the same group as on the upper panel (bar=100 μm). Note thickened epidermis (white asterisks) and increase in collagen fiber (blue color, white arrows) density in AdmAM+/Δ in comparison with wild-type mice. (b) Epidermal thickness was measured (six to eight mice in each group) and compared (Wilcoxon rank test). (c) The areas occupied by collagen fibers are presented as the percentage of “signal/area” ratio and compared (Welch two-sample t-test).
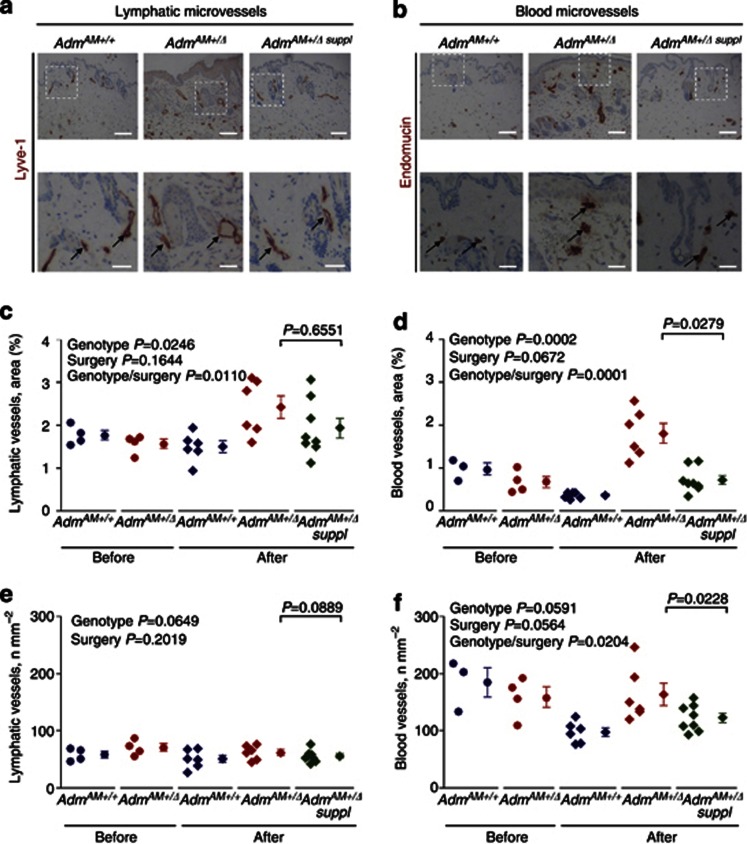
Third, these lymphedematous changes were accompanied by a significant increase in average cross-sectional areas of both lymphatic and blood dermal microvessels in AdmAM+/Δ mice (Figure 4). The number of blood, but not lymphatic, microvessels was decreased in AdmAM+/+, but not in AdmAM+/Δ, mice. Upon AM supplementation, the observed changes were partially prevented in the blood and to a lesser extent in lymphatic microvessels (Figure 4). Epidermal thickening and hyperplasia in lymphedema are frequently accompanied by lymphatic vessel dilation, and this frequently occurs as a result of lymphatic endothelium hyperproliferation, especially when infection is an underlying cause or when inflammatory tissue response is involved (Baluk et al., 2005; Tabibiazar et al., 2006; Kajiya et al., 2009). Dilation of the microlymphatics in AdmAM+/Δ mice after surgery was not accompanied by alterations in their numbers (Figure 4e), their hyperplasia, and increased proliferation or number of lymphatic ECs (Supplementary Figure S5 online), suggesting that lymphangiogenesis was grossly unaffected. Incision wounding–onset lymphedema in AdmAM+/Δ mice was associated with elevated dermal blood/lymphatic microvessel number ratio, whereas in AdmAM+/+mice this parameter was markedly decreased, when compared with animals before the surgery (Supplementary Figure S6 online).
Figure 4.
Microvessel morphology and numbers in the skin of the wild-type and adult AdmAM haploinsufficient mice after incision wounding. Immunohistochemistry was performed on sections from wild-type (AdmAM+/+) and AdmAM haploinsufficient mice without (AdmAM+/Δ) or with exogenous AM supplement (AdmAM+/Δ suppl) (six to eight mice in each group). Lower panels (bar=50 μm) are magnified boxed areas from the upper panel (bar=100 μm) of images. (a) Lyve-1 (lymphatic vessel endothelium marker; black arrows) and (b) endomucin (blood vessel endothelium marker; black arrows) immunostaining. Lymphatic and blood microvessel (c, d) average cross-sectional area and (e, f) numbers. The values for the experimental groups before the surgery are shown for direct comparison with post-incision wounding conditions. The comparisons among the first four groups were done using two-way ANOVA testing for effects of genotype (genotype, i.e., AdmAM+/+or AdmAM+/Δ) and incision wounding (surgery) independently; where it was suggested by the plots and data, the possible interactions between these factors (i.e., whether the effect of genotype was different after surgery to before; genotype/surgery) were also tested (P-values are shown in upper left corner), followed by Welch two-sample t-test for the groups 4 and 5 (AdmAM haploinsufficient mice with or without AM; P-value is shown in the upper right corner).
A summary of the impact of endogenous and exogenous/supplemented AM on acute secondary lymphedema development (3 days after surgery) is presented in Supplementary Table S2 online and Supplementary Figure S7 online.
CLR is expressed in lymphatic and blood vessels in human skin tissue
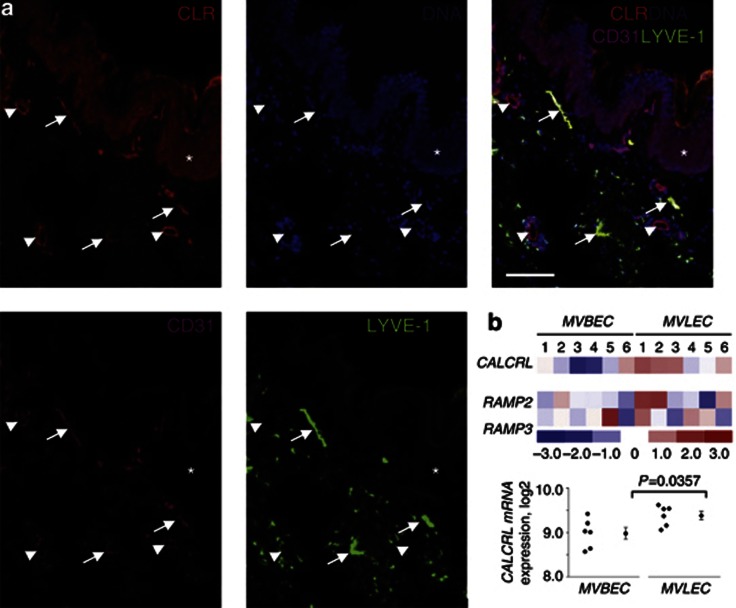
Finally, to investigate the potential targets of endogenously produced AM in the skin, we analyzed CALCRL/CLR expression in tissue samples and primary cells by using immunostaining, GeneChip expression array analysis, immunoblotting, and deglycosylation (Figure 5; Supplementary Figures S8–S10 online). In human skin, CLR was expressed in microvascular lymphatic and blood vessel ECs (MVLEC and MVBEC, respectively), as well as in the epidermis and vascular smooth muscle cells (Figure 5a; Supplementary Figure S8a online). In other human tissues, e.g., myometrium, CLR was also localized in both lymphatic and blood endothelium (Supplementary Figure S8b online). Primary cultured human dermal MVLECs and MVBECs both expressed CALCRL and RAMPs 2 and 3 mRNAs (Figure 5b; Supplementary Figure S9 online), which is in accordance with previous reports on the predominant expression of CALCRL/CLR in ECs (Nikitenko et al., 2003, 2006a). In cultured MVLECs, CLR generated functional AM receptors (Supplementary Material online; Additional Results and Supplementary Figure S10 online).
Figure 5.
CLR protein and CALCRL messenger RNA (mRNA) expression in human skin and primary dermal endothelium. (a) CLR, LYVE-1, and CD31 immunofluorescence and DAPI (DNA) staining (bar=75 μm). Lymphatic (CD31-positive/LYVE-1-positive; white arrows) and blood (CD31-positive/LYVE-1-negative; white arrowheads) vessel endothelium, and epidermis (white asterisk). (b) CALCRL, RAMP2, and RAMP3 mRNA expression in primary human dermal microvascular blood and lymphatic ECs (MVBEC and MVLEC) was determined using GeneChip Human Genome array data (E-MEXP-66) from 12 isolates as described in detail in Supplementary Methods online. Heatmap reflects relative changes in gene expression. Color scale—units of SD from the mean values of “rma” log2 expression units across all samples. Red, gene expression is significantly upregulated; blue, significantly downregulated; white, no significant change. A difference in CALCRL expression between MVBEC and MVLEC is also presented as dot plots (left) alongside the mean and SEM (right; Welch two-sample t-test).
Discussion
Secondary lymphedema occurs after surgery or trauma, but risk factors and molecular mechanisms underlying resistance or predisposition to its development remain largely unknown. Therefore, pharmacological strategies to prevent or treat this disorder are limited. Here, we show that endogenously produced AM prevents, and that its deficiency predisposes to, the development of secondary lymphedema in adult mice. Importantly, such genetic predisposition could be corrected through AM supplement therapy. Therefore, to our knowledge, this study provides previously unreported experimental in vivo evidence of a genetic susceptibility to the risk of developing secondary lymphedema.
The mechanisms of lymphedema development after wound infliction are largely unknown and yet to be delineated in our, as well as in other, in vivo models (Tabibiazar et al., 2006; Tammela et al., 2007; Rutkowski et al., 2010). It is proposed that genetic features, which are not accompanied by easily detectable morphological abnormalities, can predispose lymphatic and blood vessels to dysfunction/insufficiency that underlies secondary lymphedema only when coupled with a sufficient initiating stimulus (Rockson, 2008a). However, such genetic factors have not yet been identified. The Adm locus has an essential role in regulating vascular integrity and permeability during embryogenesis. Either the deletion of the protein-coding region, resulting in abrogated expression of both AM and PAMP (Caron and Smithies, 2001; Shindo et al., 2001), or the introduction of a knockin stop mutation resulting in functional inactivation of AM expression only (Shimosawa et al., 2002) led to embryonic lethality in homozygous mice, and this was attributed to generalized edema or hemorrhage in the first two models. Previous studies showed that upon exogenous administration AM has a role in lymphatic EC biology in vitro and in wound healing in vivo. However, the role for endogenous AM in the pathogenesis of secondary lymphedema remained unknown. We found that AdmAM haploinsufficiency did not cause primary lymphedema, but that Adm expression was upregulated upon skin surgery. We hypothesized that lymphatic and/or blood microvessels in AdmAM+/Δ mice could be affected by interventions that cause secondary lymphedema. After skin incision, we detected macroscopic (tissue swelling), functional (aberrant fluid uptake), and histopathological (epidermal thickening, increased collagen deposition, and dilated blood/lymphatic vessels) features, which are pathognomonic of secondary lymphedema, in AdmAM+/Δ but not in AdmAM+/+ mice. These findings support the view that both lymphatic and blood vessels are involved in the pathogenesis of secondary lymphedema. It is possible that upon tissue wounding the high fluid filtration overwhelmed fragile blood vessels and/or vulnerable lymphatics in AdmAM+/Δmice, subsequently leading to impaired fluid uptake and edema, as suggested in breast cancer patients (Stanton et al., 2009a). Such alterations within skin tissue could have ultimately led to lymph stagnation and elevated collagen fiber density, subsequently exerting further effects on grossly morphologically intact but possibly functionally impaired lymphatics and/or blood vessels and contributed to their constitutive dilation/dysfunction. How vascular and lymphatic insufficiencies are coordinated to predispose to secondary lymphedema and whether lymphedematous changes in AM-deficient mice develop in part owing to non-lymphatic vessel–dependent mechanisms remain to be defined. Nevertheless, our data reveal that aberrant expression of a single gene is sufficient to affect both lymphatic and blood vessels and predispose to the development of secondary lymphedema, thus suggesting a crucial, possibly even “gatekeeping”, role for AM in this disorder, at least upon wounding. In addition, our study uncovers a previously unrecognized spectrum of potential AM targets in human skin by demonstrating that CLR is expressed in both lymphatic and blood vessel endothelium, as well as in the epidermis, which are all affected in lymphedematous organs (Wilson et al., 2004; Tabibiazar et al., 2006; Rockson, 2008a; Nakamura et al., 2009).
Ways to prevent or treat lymphedema are limited and mainly palliative (Rockson, 2008a; Tammela and Alitalo, 2010; Wang and Oliver, 2010). The development of an experimental model for the commonest form of lymphedema will lead to further insight into the underlying molecular mechanisms, and potentially effective therapies (Shin and Rockson, 2008). AdmAM haploinsufficient mice represent an example of a new, to our knowledge previously unreported, type of an in vivo model for studying secondary lymphedema occurring as a result of an underlying germ-line genetic alteration. Certain strategies (e.g., VEGF-C or non-steroidal anti-inflammatory drugs) have been tested to treat postsurgery-onset lymphedema in experimental models (Tammela et al., 2007; Nakamura et al., 2009). Here, we report partial but significant prevention of secondary lymphedema in AdmAM+/Δ mice by postsurgical and systemic AM supplementation, which attained circulating peptide levels comparable to those in AdmAM+/+ mice. These findings suggest that circulating vasoactive factors could have a role in secondary lymphedema and that vascular treatments could potentially be used to alleviate its development. In our in vivo model, Adm expression in injured tissue was upregulated, as previously reported for VEGF-C or VEGFR-3 (Nakamura et al., 2009), suggesting that AM levels could be higher locally and that the systemic supplementation was probably not sufficient to completely prevent lymphedema development. Therapeutic approaches using AM have already been developed for the treatment of vascular disease, and this peptide could be delivered topically or systemically to reverse vascular damage associated with pulmonary hypertension or response to tissue injury (Kawai et al., 2004; Nagaya et al., 2004; Tokunaga et al., 2004; Harada et al., 2011). Therefore, similar AM topical application strategies together with systemic delivery could be explored as combinatorial therapies with VEGF-C (Tammela et al., 2007) or other factors (Kajiya et al., 2005), to prevent or treat lymphedema.
Experimental models of acute postsurgical lymphedema (including our model of genetically predisposed secondary lymphedema) are useful in studying mechanisms of human lymphedema, which represents chronic unremitting condition, because they are associated with lymphatic insufficiency and share characteristic lymphedematous changes in affected tissues/organs (Tabibiazar et al., 2006; Shin and Rockson, 2008). Studies investigating whether AdmAM haploinsufficiency also predisposes to long-lasting secondary lymphedema and whether there is a potential for AM treatment in ameliorating or reversing complex changes associated with chronic lymphedema will be necessary in the future. The detection of AM receptor CLR expression in those cell types that are affected in lymphedematous tissues prompts further studies using complex genetic and physiological approaches to dissect underlying mechanisms. For example, animal models using lymphatic/blood vessel EC–specific conditional knockdown technologies (Bazigou et al., 2011) might provide further insight into the lymphatic/vascular insufficiency underlying secondary lymphedema development upon aberrant AM signaling through CLR.
Apart from previously identified genetic factors, e.g., mutations in VEGFR3 or GATA2, it is likely that additional predisposition mutations contribute to the development of primary and secondary lymphedema (Finegold et al., 2008). Genome-wide data identified a significant association between the ADM locus and predisposition to the development of cardiovascular disease (Ehret et al., 2011). Similar studies will be required to address its potential association with secondary lymphedema in humans. Similar to AdmAM haploinsufficient mice, individuals with AM deficiency might not have overt symptoms of vascular or lymphatic insufficiency under physiological conditions, and lymphatic or blood vessel abnormalities may therefore remain undetected unless injury occurs. Whether mutations and polymorphisms in ADM, CALCRL, or RAMPs have a role in the pathogenesis of secondary lymphedema in humans remains to be investigated.
In summary, we identify AM as a key regulator in the pathogenesis of secondary lymphedema in adult mice. Our data suggest that circulating vasoactive factors, blood, and lymphatic vessels are intimately involved in this disorder. Although it remains to be shown that AM deficiency in humans is associated with this disorder, our experimental model provides previously unreported in vivo evidence that germ-line genetic factors predispose to the development of secondary lymphedema.
Materials and Methods
Please see details of the following methods in Supplementary Information online: lymphangiography, Evans blue accumulation, and deglycosylation assays; GeneChip Human Genome array analysis; histology, immunostaining, immunoblotting, and image analysis.
Animals
AM-knockin mice were generated as previously reported (Shimosawa et al., 2002). As the homozygote (AdmAMΔ/Δ) animals are embryonic lethal, in the present study we used heterozygotes (AdmAM+/Δ), which were back-crossed with C57/B6crj mice for more than 20 generations, and C57/B6crj was used as a wild-type (AdmAM+/+) control. Animals were handled in accredited facility in accordance with the institutional animal care policies, and all research protocols have been approved and conformed to the guiding principles for animal experimentation, as outlined by the Ethics Committee on Animal Research of the University of Tokyo, Faculty of Medicine.
AM measurement
AM and PAMP concentrations were measured in the plasma as described previously (Shimosawa et al., 2002).
Skin incision wounding model
Skin incision wounding model (Kanter et al., 1990) was performed in both AdmAM+/+and AdmAM+/Δ eight-week-old male mice. Mice were anesthetized by pentobarbital (1 g kg−1 body weight, intraperitoneal), and after shaving the operated area hind limb skin at an achilles tendon was incised. The following modifications were introduced: the Evans blue dye was not injected and inguinal lymph node was not excised. Next, AdmAM+/Δ mice were randomized for treatment with vehicle or synthetic murine AM (Peptid Institute, Osaka, Japan). AM was administered intraperitoneally at a dose of 300 ng kg−1 per hour by ALZET osmotic pump 1002 (Durect, Cupertino, CA) (Shimosawa et al., 2003), which was implanted straight after incision. Three days after operation, animals were anesthetized with pentobarbital and the swelling of the limbs was scored (edema score) in a blind manner from 0 to 5: 0—no edema; 1—edema at cutting edge; 2—edema 1/3 of the hind limb; 3—edema 2/3 of the hind limb; 4—edema in the whole hind limb; and 5—edema above the knee. Skin samples were fixed in phosphate-buffered saline containing 4% paraformaldehyde for 6–12 hours at 4 °C, dehydrated, and embedded in paraffin.
Human skin tissue samples
Human skin tissue samples were obtained from the Royal National Orthopaedic Hospital Musculoskeletal BioBank (Stanmore, UK), snap-frozen, and stored in liquid nitrogen. The Cambridgeshire Research Ethics Committee has approved their use for this study.
Histology, immunostaining, and image analysis
Paraffin-embedded or frozen sections of mouse skin and human tissues were prepared as previously described (Shimosawa et al., 2002; Nikitenko et al., 2006a, 2006b) and stained either with hematoxylin and eosin or Azan or processed for immunostaining. Histopathological analysis was performed by scoring epidermal thickening in the skin. A severity scale from 0 to 4 was used (0, normal; 0.5, focal and rare; 1, focal and mild; 2, diffuse and mild; 3, diffuse and moderate; and 4, diffuse and severe). The tissue area occupied by the collagen fibers, as a measure of fibrotic changes (Chen et al., 2005), was quantified using the Scion Image Software (Scion, Frederick, MD). Immunohistochemistry and immunofluorescence were performed using previously described methods (Nikitenko et al., 2006a, 2006b).
Blood and lymphatic vessel number and density (occupied tissue area) in mouse skin tissue were analyzed as described elsewhere (Kajiya et al., 2009), based on endomucin (blood vessel endothelium marker; (Kuhn et al., 2002)) and Lyve-1 (lymphatic endothelium marker; (Tammela and Alitalo, 2010)) immunostaining and quantified using the ImageJ or Scion Image Software, according to approaches adapted by others (Kajiya et al., 2009). Lymphatic/blood microvessel number ratio was calculated based on obtained numbers for each vessel type.
Statistical analysis
Four independent sets of skin incision wounding experiments were performed in this study, and the phenotype was reproduced. All obtained values and quantitative data for each experimental group were analyzed using R and GraphPad Prism (GraphPad Prism, La Jolla, CA) software programs and presented as dot plots (left) shown alongside means±SEM (right). The comparisons among the groups were done using either Wilcoxon rank test or two-way ANOVA testing for effects of genotype and surgery independently, and where it was suggested by the plots and data, we also tested possible interactions between these factors (i.e., whether the effect of genotype was different after surgery to before), followed by Welch two-sample t-test for the AdmAM+/Δ mice with or without AM supplement, with a two-tailed significance set at the 0.05 levels and marginal significance set at the 0.1 level. We reported the actual P-value for each test.
Acknowledgments
We thank Professor Peter Mortimer (St George's Hospital, University of London, London, UK), Rajeev Gupta, and Nischalan Pillay for helpful discussions and comments, Professor Adrienne M. Flanagan for human tissue samples, and Thomas Adejumo for technical advice (all—UCL Cancer Institute, London). This work was supported in part by the Cancer Research UK (grants C575/A6125 and C575/A13100; LLN, SH, TM, and CB), Grants-in-Aid for Scientific Research from the Japan Society of the Promotion of Science (A17209034 and C18591016; TS and TF), Wellcome Trust (LLN and MCPR), The Royal Society, Cancer Research UK, UCL, International Society of Hypertension, and The Maurice and Phyllis Paykel Trust Travel Awards (LLN).
Glossary
- ADM
adrenomedullin gene
- AM
adrenomedullin
- CALCRL
gene encoding CLR
- CLR
calcitonin receptor–like receptor
- EC
endothelial cell
- PAMP
proadrenomedullin N-terminal peptide
- RAMP
receptor activity–modifying protein
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Baluk P, Tammela T, Ator E, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Lyons OT, Smith A, et al. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J Clin Invest. 2011;121:2984–2992. doi: 10.1172/JCI58050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- Caron KM, Smithies O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc Natl Acad Sci USA. 2001;98:615–619. doi: 10.1073/pnas.021548898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Somanath PR, Razorenova O, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11:1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold DN, Baty CJ, Knickelbein KZ, et al. Connexin 47 mutations increase risk for secondary lymphedema following breast cancer treatment. Clin Cancer Res. 2012;18:2382–2390. doi: 10.1158/1078-0432.CCR-11-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold DN, Schacht V, Kimak MA, et al. HGF and MET mutations in primary and secondary lymphedema. Lymphat Res Biol. 2008;6:65–68. doi: 10.1089/lrb.2008.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz-Six KL, Dunworth WP, Li M, et al. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest. 2008;118:40–50. doi: 10.1172/JCI33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K, Yamahara K, Ohnishi S, et al. Sustained-release adrenomedullin ointment accelerates wound healing of pressure ulcers. Regulatory Peptides. 2011;168:21–26. doi: 10.1016/j.regpep.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Hippenstiel S, Witzenrath M, Schmeck B, et al. Adrenomedullin reduces endothelial hyperpermeability. Circ Res. 2002;91:618–625. doi: 10.1161/01.res.0000036603.61868.f9. [DOI] [PubMed] [Google Scholar]
- Ichikawa-Shindo Y, Sakurai T, Kamiyoshi A, et al. The GPCR modulator protein RAMP2 is essential for angiogenesis and vascular integrity. J Clin Invest. 2008;118:29–39. doi: 10.1172/JCI33022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Harada K, Ohnishi S, et al. Adrenomedullin induces lymphangiogenesis and ameliorates secondary lymphoedema. Cardiovasc Res. 2008;80:339–345. doi: 10.1093/cvr/cvn228. [DOI] [PubMed] [Google Scholar]
- Kahn ML. Blood is thicker than lymph. J Clin Invest. 2008;118:23–26. doi: 10.1172/JCI34485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya K, Hirakawa S, Ma B, et al. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya K, Sawane M, Huggenberger R, et al. Activation of the VEGFR-3 pathway by VEGF-C attenuates UVB-induced edema formation and skin inflammation by promoting lymphangiogenesis. J Invest Dermatol. 2009;129:1292–1298. doi: 10.1038/jid.2008.351. [DOI] [PubMed] [Google Scholar]
- Kanter MA, Slavin SA, Kaplan W. An experimental model for chronic lymphedema. Plast Reconstr Surg. 1990;85:573–580. doi: 10.1097/00006534-199004000-00012. [DOI] [PubMed] [Google Scholar]
- Kawai J, Ando K, Tojo A, et al. Endogenous adrenomedullin protects against vascular response to injury in mice. Circulation. 2004;109:1147–1153. doi: 10.1161/01.CIR.0000117231.40057.6D. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993a;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Sakata J, Kangawa K, et al. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochem Biophys Res Commun. 1993b;194:720–725. doi: 10.1006/bbrc.1993.1881. [DOI] [PubMed] [Google Scholar]
- Kuhn A, Brachtendorf G, Kurth F, et al. Expression of endomucin, a novel endothelial sialomucin, in normal and diseased human skin. J Invest Dermatol. 2002;119:1388–1393. doi: 10.1046/j.1523-1747.2002.19647.x. [DOI] [PubMed] [Google Scholar]
- Maybin JA, Battersby S, Hirani N, et al. The expression and regulation of adrenomedullin in the human endometrium: a candidate for endometrial repair. Endocrinology. 2011;152:2845–2856. doi: 10.1210/en.2010-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya N, Kyotani S, Uematsu M, et al. Effects of adrenomedullin inhalation on hemodynamics and exercise capacity in patients with idiopathic pulmonary arterial hypertension. Circulation. 2004;109:351–356. doi: 10.1161/01.CIR.0000109493.05849.14. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Radhakrishnan K, Wong YM, et al. Anti-inflammatory pharmacotherapy with ketoprofen ameliorates experimental lymphatic vascular insufficiency in mice. PLoS One. 2009;4:e8380. doi: 10.1371/journal.pone.0008380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitenko LL, Blucher N, Fox SB, et al. Adrenomedullin and CGRP interact with endogenous calcitonin-receptor-like receptor in endothelial cells and induce its desensitisation by different mechanisms. J Cell Sci. 2006a;119:910–922. doi: 10.1242/jcs.02783. [DOI] [PubMed] [Google Scholar]
- Nikitenko LL, Cross T, Campo L, et al. Expression of terminally glycosylated calcitonin receptor-like receptor in uterine leiomyoma: endothelial phenotype and association with microvascular density. Clin Cancer Res. 2006b;12:5648–5658. doi: 10.1158/1078-0432.CCR-06-0852. [DOI] [PubMed] [Google Scholar]
- Nikitenko LL, Fox SB, Kehoe S, et al. Adrenomedullin and tumour angiogenesis. Br J Cancer. 2006c;94:1–7. doi: 10.1038/sj.bjc.6602832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitenko LL, Smith DM, Bicknell R, et al. Transcriptional regulation of the CRLR gene in human microvascular endothelial cells by hypoxia. FASEB J. 2003;17:1499–1501. doi: 10.1096/fj.02-0993fje. [DOI] [PubMed] [Google Scholar]
- Nikitenko LL, Smith DM, Hague S, et al. Adrenomedullin and the microvasculature. Trends Pharmacol Sci. 2002;23:101–103. doi: 10.1016/S0165-6147(00)01983-0. [DOI] [PubMed] [Google Scholar]
- Ostergaard P, Simpson MA, Connell FC, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome) Nat Genet. 2011;43:929–931. doi: 10.1038/ng.923. [DOI] [PubMed] [Google Scholar]
- Poyner DR, Sexton PM, Marshall I, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- Rockson SG. Lymphedema. Am J Med. 2001;110:288–295. doi: 10.1016/s0002-9343(00)00727-0. [DOI] [PubMed] [Google Scholar]
- Rockson SG. Diagnosis and management of lymphatic vascular disease. J Am Coll Cardiol. 2008a;52:799–806. doi: 10.1016/j.jacc.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Rockson SG. Secondary lymphedema: is it a primary disease. Lymphat Res Biol. 2008b;6:63–64. doi: 10.1089/lrb.2008.6201. [DOI] [PubMed] [Google Scholar]
- Rockson SG. The unique biology of lymphatic edema. Lymphat Res Biol. 2009;7:97–100. doi: 10.1089/lrb.2009.7202. [DOI] [PubMed] [Google Scholar]
- Rutkowski JM, Markhus CE, Gyenge CC, et al. Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am J Pathol. 2010;176:1122–1129. doi: 10.2353/ajpath.2010.090733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimosawa T, Ogihara T, Matsui H, et al. Deficiency of adrenomedullin induces insulin resistance by increasing oxidative stress. Hypertension. 2003;41:1080–1085. doi: 10.1161/01.HYP.0000066846.46422.2C. [DOI] [PubMed] [Google Scholar]
- Shimosawa T, Shibagaki Y, Ishibashi K, et al. Adrenomedullin, an endogenous peptide, counteracts cardiovascular damage. Circulation. 2002;105:106–111. doi: 10.1161/hc0102.101399. [DOI] [PubMed] [Google Scholar]
- Shin WS, Rockson SG. Animal models for the molecular and mechanistic study of lymphatic biology and disease. Ann N Y Acad Sci. 2008;1131:50–74. doi: 10.1196/annals.1413.005. [DOI] [PubMed] [Google Scholar]
- Shindo T, Kurihara Y, Nishimatsu H, et al. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation. 2001;104:1964–1971. doi: 10.1161/hc4101.097111. [DOI] [PubMed] [Google Scholar]
- Stanton AW, Modi S, Bennett Britton TM, et al. Lymphatic drainage in the muscle and subcutis of the arm after breast cancer treatment. Breast Cancer Res Treat. 2009a;117:549–557. doi: 10.1007/s10549-008-0259-z. [DOI] [PubMed] [Google Scholar]
- Stanton AW, Modi S, Mellor RH, et al. Recent advances in breast cancer-related lymphedema of the arm: lymphatic pump failure and predisposing factors. Lymphat Res Biol. 2009b;7:29–45. doi: 10.1089/lrb.2008.1026. [DOI] [PubMed] [Google Scholar]
- Tabibiazar R, Cheung L, Han J, et al. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med. 2006;3:e254. doi: 10.1371/journal.pmed.0030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Tammela T, Saaristo A, Holopainen T, et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med. 2007;13:1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- Tokunaga N, Nagaya N, Shirai M, et al. Adrenomedullin gene transfer induces therapeutic angiogenesis in a rabbit model of chronic hind limb ischemia: benefits of a novel nonviral vector, gelatin. Circulation. 2004;109:526–531. doi: 10.1161/01.CIR.0000109700.81266.32. [DOI] [PubMed] [Google Scholar]
- Vart RJ, Nikitenko LL, Lagos D, et al. Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6 and G-protein-coupled receptor regulate angiopoietin-2 expression in lymphatic endothelial cells. Cancer Res. 2007;67:4042–4051. doi: 10.1158/0008-5472.CAN-06-3321. [DOI] [PubMed] [Google Scholar]
- Wang Y, Oliver G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 2010;24:2115–2126. doi: 10.1101/gad.1955910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SF, Guarner J, Valme AL, et al. Histopathologic improvement with lymphedema management, Leogane, Haiti. Emerg Infect Dis. 2004;10:1938–1946. doi: 10.3201/eid1011.040548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhuo S, Chen J, et al. Real-time in vivo imaging collagen in lymphedematous skin using multiphoton microscopy. Scanning. 2011;33:463–467. doi: 10.1002/sca.20266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.