Abstract
Introduction
Carboxylesterase 1 (CES1) is the primary enzyme responsible for converting clopidogrel into biologically inactive carboxylic acid metabolites.
Methods
We genotyped a functional variant in CES1, G143E, in participants of the Pharmacogenomics of Anti-Platelet Intervention (PAPI) study (n=566) and in 350 patients with coronary heart disease treated with clopidogrel, and carried out an association analysis of bioactive metabolite levels, on-clopidogrel ADP-stimulated platelet aggregation, and cardiovascular outcomes.
Results
The levels of clopidogrel active metabolite were significantly greater in CES1 143E-allele carriers (P = 0.001). Consistent with these findings, individuals who carried the CES1 143E-allele showed a better clopidogrel response as measured by ADP-stimulated platelet aggregation in both participants of the PAPI study (P = 0.003) and clopidogrel-treated coronary heart disease patients (P = 0.03). No association was found between this single nucleotide polymorphism and baseline measures of platelet aggregation in either cohort.
Conclusion
Taken together, these findings suggest, for the first time, that genetic variation in CES1 may be an important determinant of the efficacy of clopidogrel.
Keywords: carboxylesterase 1, CES1, clopidogrel, percutaneous coronary intervention, pharmacogenetics
Introduction
Clopidogrel therapy, as part of a dual antiplatelet regimen with aspirin, is the standard care for preventing recurrent cardiovascular events in patients with coronary heart disease (CHD) undergoing a percutaneous coronary intervention (PCI) and in patients with acute coronary syndromes. Bioactivation of clopidogrel, a thienopyridine prodrug, requires a two-step conversion by hepatic cytochrome P450 (CYP) enzymes into a biologically active thiol metabolite. This metabolite inhibits ADP-induced platelet activation and aggregation by irreversibly binding to the P2Y12 receptor on the surface of platelets [1]. Genetic polymorphisms identified by our group and others [e.g. CYP2C19*2 (rs4244285)] influence clopidogrel active metabolite formation, on-clopidogrel ADP-induced platelet aggregation, and cardiovascular event rates in PCI patients on-clopidogrel therapy and explain, in part, the wide interindividual variability observed in clopidogrel response [2–5].
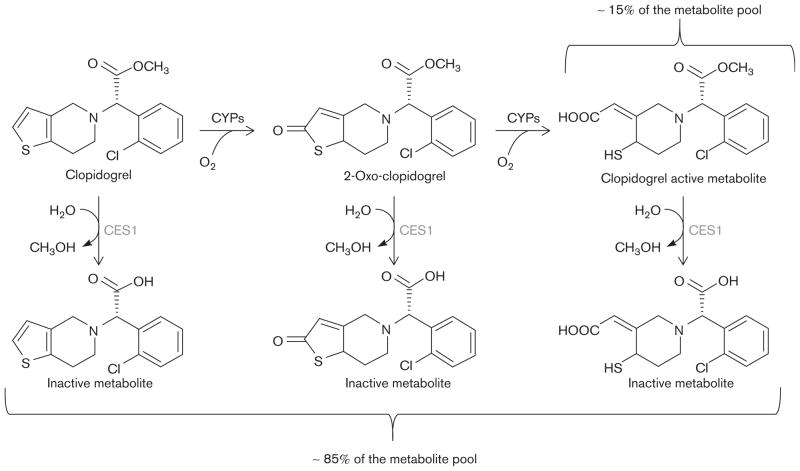
Although currently identified common polymorphisms account for a significant proportion of the variability in clopidogrel response, heritability estimates suggest that other genetic factors are likely to exist [3]. Carboxylesterase 1 (CES1) is a widely expressed serine esterase that is involved in the hydrolysis of multiple amide-containing and ester-containing endogenous and xenobiotic compounds including therapeutic agents such as methylphenidate [6], oseltamivir [7], angiotensin-converting enzyme inhibitors (e.g. trandolapril and temocapril) [8,9], and anticancer drugs (e.g. capecitabin) [10]. In addition, CES1 is the primary enzyme responsible for metabolizing clopidogrel, its intermediate metabolite (2-oxo-clopidogrel), and the final bioactive thiol metabolite into biologically inactive carboxylic acid derivatives (Fig. 1) [11]. Given that only ~15% of the prodrug is transformed into the active clopidogrel metabolite, genetic variation affecting CES1 expression and/or activity may be an important determinant of clopidogrel response [12,13].
Fig. 1.
Schematic of the major enzymatic steps in clopidogrel metabolism. CES1, carboxylesterase 1; CYP, cytochrome P450.
Recently, Zhu et al. [6] have identified a missense single nucleotide polymorphism (SNP) in exon 4 of CES1 (rs71647871) that results in a catalytic site glycine (G)-to-glutamic acid (E) amino acid change at position 143 (G143E). Follow-up in-vitro expression studies have shown that the G143E mutation severely inhibited CES1 catalytic function, resulting in a complete loss of hydrolytic activity toward methylphenidate and only 21% catalytic efficiency when p-nitrophenol acetate was used as a substrate. However, the effect of this variant on clopidogrel metabolism has not been investigated.
We hypothesized that after the administration of clopidogrel, individuals who carry the decreased function CES1 143E-allele would have increased levels of plasma clopidogrel active metabolite and decreased measures of on-treatment ADP-induced ex-vivo platelet aggregation compared with 143G-allele homozygotes. In this investigation, we examined the effect of the CES1 G143E variant on the formation of clopidogrel active metabolite and ADP-induced platelet aggregation before and after clopidogrel treatment in 566 members of the Amish Pharmacogenomics of Anti-Platelet Intervention (PAPI) study. The findings in this population were extended by examining the relationship between genotype, platelet function, and cardiovascular outcomes in an independent group of 350 clopidogrel-treated patients recruited from a cardiac catheterization laboratory at Sinai Hospital (Baltimore, Maryland, USA).
Materials and methods
Study populations
The Amish PAPI study recruited 566 apparently healthy White individuals 20 years old or older between August 2006 and July 2010. Population characteristics, recruitment, and study details have been described previously [3,14]. Briefly, participants discontinued the use of all prescription medications, supplements, and vitamins 1 week before the initiation of the study. Following an overnight fast, physical examinations, information on medical and family histories, anthropometric measures, blood samples, and other phenotype data were collected. Complete blood count with platelet numbers and levels of serum lipids (total cholesterol, high-density lipoprotein cholesterol, and triglycerides) were assayed by Quest Diagnostics (Horsham, Pennsylvania, USA) and low-density lipoprotein cholesterol levels were calculated using the Friedewald equation. Participants with low-density lipoprotein cholesterol levels greater than 160 mg/dl and/or those using prescription cholesterol-lowering medications were designated as having hyperlipidemia. Patients were designated as hypertensive if their systolic blood pressure was 140mmHg or more, and/or their diastolic blood pressure was 90mmHg or more, and/or with the use of prescription blood pressure-lowering medications. The presence of diabetes and current smoking status (cigarette, cigar, or pipe) were determined by self-report.
Baseline platelet aggregation was evaluated in platelet-rich plasma (200 000 platelets/μl) isolated from blood samples by optical aggregometry using a PAP8E Aggregometer (Bio/Data Corporation, Horsham, Pennsylvania, USA) after stimulation with ADP (20 μmol/l) and was expressed as the maximal percentage change in light transmittance using platelet-poor plasma as a referent [3]. All participants were then administered a 300 mg oral loading dose of clopidogrel and instructed to take 75mg/day for the following 6 days. Blood was drawn 1 h after the last dose of clopidogrel for platelet aggregation and assessment of clopidogrel metabolite levels. A second follow-up platelet aggregation measurement was carried out later the same day, 1 h after the administration of 324mg of aspirin.
An independent cohort of CHD patients older than 18 years (n=350) undergoing a coronary intervention were recruited at Sinai Hospital between January 2004 and November 2011. The recruitment and characterization of these patients has been described previously [3,14]. Briefly, 63.4% of these patients were White (n=222), 33.4% were African American (n=117), and 3.1% were of other race/ethnicity (n=11). One hundred and forty-six patients were receiving clopidogrel maintenance therapy (75 mg daily dose) at the time of PCI and received no loading dose. The remaining patients received either a 600 mg (n=173) or a 300 mg (n=31) loading dose of clopidogrel before the procedure. All patients received aspirin (81–325 mg/day) for at least 1 week before PCI and 325 mg on the day of the procedure.
In patients treated with bivalirudin or heparin anti-coagulant therapy (n=238), these medications were discontinued at the completion of the coronary intervention procedure. Platelet function was measured on the day of hospital discharge in patients not treated with a glycoprotein IIb/IIIa (GPIIbIIIa) inhibitor or 5 days or more after discharge in patients treated with a GPIIbIIIa inhibitor (n=112). Stratification by clopidogrel dosing or GPIIbIIIa inhibitor treatment did not show significant differences in the baseline characteristics or cardiovascular event rates at 1 year and thus these groups were combined for further analyses. At the time of hospital discharge, all patients were prescribed clopidogrel (75 mg/day) and aspirin (325 mg/day) as suggested by American College of Cardiology and American Heart Association guidelines [15]. Medication adherence was obtained by self-report and by a review of the source documents.
Platelet function in patients from Sinai Hospital was assessed in platelet-rich plasma obtained from blood samples by optical aggregometry after stimulation with ADP (20 μmol/l) using a Chronolog Lumi-Aggregometer (Model 490-4D; Chronolog, Havertown, Pennsylvania, USA). Platelet aggregation was expressed as the maximum percentage change in light transmittance using platelet-poor plasma as a referent as described previously [2].
Postdischarge cardiovascular events were evaluated in patients at 1 year of follow-up by the CES1 G143E genotype. A physician, blinded to the study results of the patient, adjudicated all end points through a review of source documents obtained from the medical records. Cardiovascular events were defined as myocardial infarction (the occurrence of ischemic symptoms and a troponin I value greater than the upper limit of normal), ischemic stroke, stent thrombosis (definite stent thrombosis according to the Academic Research Consortium [16]), unplanned target vessel revascularization (revascularization of vessel treated at the time of enrollment), hospitalization for coronary ischemia without revascularization (hospitalization for chest pain with evidence of ischemia on ECG and no evidence of myocardial infarction as measured by troponin I value), and death secondary to any cardiovascular cause.
All study protocols were approved by the respective institutional review boards at the University of Maryland and Sinai Hospital of Baltimore and adhered to the principles of the Declaration of Helsinki. Written informed consent was obtained from each participant. Patients were compensated for their participation.
Genotyping
CES1 G143E (rs71647871) SNP genotyping was performed using a TaqMan SNP genotyping assay (Applied Biosystems, Foster City, California, USA). The mean genotype concordance rate for this polymorphism in a subset of duplicate samples was 100% and the genotype call rate was 98.4%.
Parent clopidogrel and active metabolite quantification
Parent clopidogrel compound and its active metabolite concentrations were assessed in a subset of 506 PAPI study participants (499 CES1 143G homozygotes and seven 143E-allele carriers). The phenotypic characteristics of this subset were the same as those of the full PAPI cohort (data not shown). Blood samples from each participant were collected within 1 h after the last dose of clopidogrel (see above) into EDTA tubes containing 2mmol/l (E)-2-bromo-3′-methoxyacetophenone (MPB; Sigma Aldrich, St Louis, Missouri, USA) to derivatize the clopidogrel active metabolite.
Plasma levels of clopidogrel and its MPB-derivatized active metabolite were assessed simultaneously using an ultrahigh performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) assay with a clopidogrel calibration range of 0.01–50 ng/ml and an active metabolite calibration range of 0.1–150 ng/ml as described previously [17]. Briefly, 500 μl of ice-cold acetonitrile (Fisher Scientific, Fairlawn, New Jersey, USA) containing 15 ng/ml of the internal standard (IS) ticlopidine (Sigma Aldrich) was added to 50 μl of plasma to precipitate plasma proteins. After vortex mixing (30 s) and centrifugation (11 700g for 10 min), 5.0 μl of the resulting supernatant was injected into a Waters Acquity UPLC system (Waters Corporation, Milford, Massachusetts, USA) for chromatographic separation before detection by tandem mass spectrometry (MS/MS) using an AB Sciex Qtrap 5500 (AB Sciex, Foster City, California, USA). Chromatographic separation of the analytes, parent clopidogrel, and active metabolite from IS and matrix interferences was carried out using a gradient with 0.1% formic acid (aqueous) and 0.1% formic acid in acetonitrile. Three multiple reaction monitoring transitions were performed simultaneously with dwell times of 200 ms each. Parent clopidogrel was identified selectively by the transition of its parent to product ion at m/z 322>212, active metabolite at m/z 504>155, and ticlopidine (IS) at m/z 264>154. Multiple reaction monitoring peak integrations and data analysis were carried out using the MultiQuant algorithm from MultiQuant 4.0 (Analyst; AB Sciex). The total run time was 1.5 min.
Statistical analysis
SAS version 9.1 (SAS Institute Inc., Cary, North Carolina, USA) was used to calculate the summary statistics, distributions, and frequencies in both the PAPI study and the cohort of patients from Sinai Hospital. All statistical tests were two-sided. In the PAPI study participants, the effect of the CES1 G143E variant on ADP-stimulated platelet aggregation before and after clopidogrel administration, parent clopidogrel level, and clopidogrel active metabolite concentration was determined using a variance component method under an additive model that simultaneously adjusted for age, sex, BMI, diabetes, smoking, renal function, proton pump inhibitor use, preclopidogrel platelet aggregation (for postclopidogrel analyses of platelet aggregation only), and relatedness among study participants. Relatedness among participants was accounted for by including a polygenic component as a random effect as described previously [3]. Briefly, a model for the polygenic component was established by constructing a relationship matrix derived from the complete Amish pedigree structure available through published genealogical records maintained by the church [18]. In the cohort of patients from Sinai Hospital, association analyses between CES1 G143E and ADP-stimulated platelet aggregation were carried out under an additive genetic model using analysis of variance with adjustment for age, sex, BMI, diabetes, smoking, proton pump inhibitor use, race, study group [Peri-Procedural Myocardial Infarction, Platelet Reactivity, Thrombin Generation, and Clot Strength: Differential Effects of Eptifibatide+Bivalirudin vs. Bivalirudin study (CLEAR PLATELETS-1); Clopidogrel Loading With Eptifibatide to Arrest the Reactivity of Platelets study (CLEAR PLATELETS-2)], and treatment (clopidogrel dose and use of eptifibatide). Cardiovascular event-free survival at 1 year of follow-up was evaluated between participants with and without the CES1 143E-allele using a proportional hazards model while simultaneously adjusting for age and sex.
Results
The characteristics of the Amish PAPI study participants (see Table, Supplemental digital content 1, http://links.lww.com/FPC/A533) have been reported previously [14]. Briefly, these participants were generally healthy, middle-aged (mean age=45.5 years), drug-naive, and had a low prevalence of disease [e.g. diabetes (<1%), hypertension (5.0%), hypercholesterolemia (25.6%)], obesity (mean BMI=27.0), and smoking (9.8%). We identified seven individuals in this cohort who were heterozygous for the decreased CES1 143E-allele, resulting in an allele frequency of 0.6%, slightly less than that reported by Zhu et al. [6].
The concentration of active clopidogrel metabolite differed significantly between the seven CES1 143E-allele carriers and the 499 143G homozygotes. Individuals who were heterozygous for the CES1 143E-allele had significantly higher levels of clopidogrel active metabolite compared with 143G-allele homozygotes (30.3±6.1 vs.19.0±0.4 ng/ml, respectively; P=0.001). No significant difference in the clopidogrel parent compound concentration was observed between genotype groups (1.02±0.08 ng/ml for CES1 143G homozygotes vs. 1.46±0.64 ng/ml for CES1 143E-allele carriers; P=0.57).
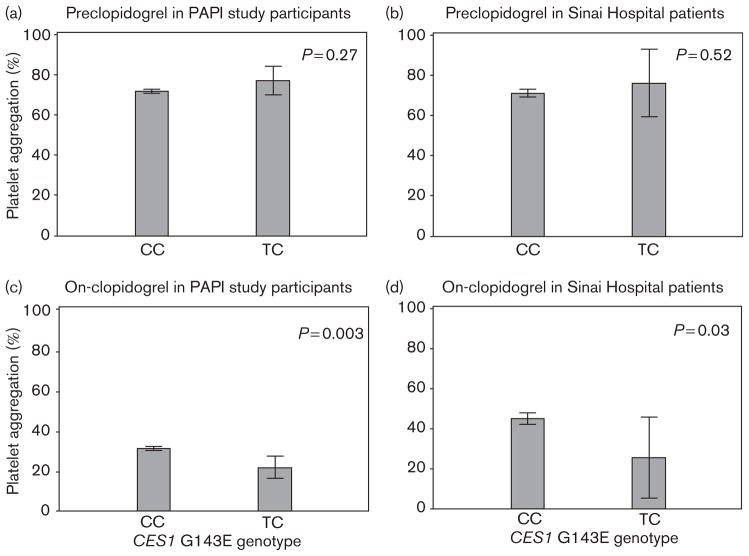
Before the administration of clopidogrel, we observed no evidence of an association between the CES1 G143E variant and ADP-induced platelet aggregation (P=0.27). However, this SNP was associated significantly with ADP-induced platelet aggregation after clopidogrel treatment (Fig. 2). ADP-induced platelet aggregation was reduced to 29% of the baseline in response to clopidogrel in individuals who carried the CES1 143E-allele compared with 43% in individuals who were homozygous for the CES1 143G-allele (P=0.003).
Fig. 2.
Association of the carboxylesterase 1 (CES1) G143E variant (rs71647871) with ADP-stimulated platelet aggregation before (a, b) and after (c, d) clopidogrel administration in participants of the Pharmacogenomics of Anti-Platelet Intervention (PAPI) study (a, c) and coronary intervention patients at Sinai Hospital of Baltimore (b, d).
The findings in the PAPI study were extended by examining the relationship between the CES1 G143E genotype and platelet aggregation as well as cardiovascular outcomes in a group of 350 patients with a clinical indication for clopidogrel recruited from a cardiac catheterization laboratory in Baltimore (Maryland, USA). The characteristics of these patients (see Table, Supplemental digital content 1, http://links.lww.com/FPC/A533) have been reported previously [14]. In this cohort, six of 350 patients carried the decreased function CES1 143Eallele (allele frequency=0.85%), five of whom had ADP-induced platelet aggregation measured before and during clopidogrel treatment. Before clopidogrel treatment, no difference in ADP-induced platelet aggregation was observed by the CES1 G143E genotype in these patients (maximal platelet aggregation=76.3% for 143E-allele carriers and 71.3% for 143G homozygotes; P=0.52). Consistent with the findings of the PAPI study, patients who carried the CES1 143E-allele had a significantly greater clopidogrel response compared with 143G-allele homozygotes (on-clopidogrel maximal platelet aggregation= 25 and 45%, respectively; P=0.03) (Fig. 2). After 1 year of follow-up, we observed that 13.7% of patients who were homozygous for the CES1 143G-allele and 0% of 143E-allele carriers experienced a cardiovascular event; however, this relatively low-powered comparison was not statistically significant (P=0.44).
Discussion
Increasing evidence strongly suggests that genetic variation is an important determinant of the wide interindividual variability in the response to clopidogrel therapy. Previous investigation in our laboratory indicated that ~70% of the variability in on-clopidogrel ADP-induced platelet aggregation, a widely accepted surrogate of clopidogrel response, can be attributed to heritable factors [3]. Indeed, a genome-wide association study and several candidate gene studies have been carried out in both healthy individuals and patients with cardiovascular disease who have reported common polymorphisms significantly associated with active metabolite levels, platelet aggregation, and/or rates of adverse cardiovascular events [3,4,11,19–21]. From these investigations and others, the most consistently replicated and perhaps clinically actionable genetic variant identified is the common loss-of-function cytochrome P450 2C19 (CYP2C19)*2 variant (rs4244285), which accounts for ~12% of the interindividual variability in clopidogrel response [3]. Other CYP2C19 loss-of-function variants (*3–*8) are much less common in the population, but would be expected to have a similar effect on clopidogrel response as CYP2C19*2 [22]. The effect of the common gain-of-function CYP2C19*17 variant and variants in other candidate genes (e.g. ABCB1, PON1, ITGB3, CYP2B6, and P2RY12) on-clopidogrel response have been evaluated, with mixed results [11,19–21,23–25]. Despite these efforts, heritability estimates suggest that a large proportion of the variability in clopidogrel response explained by genetic factors remains unknown.
The oxidation of clopidogrel prodrug into 2-oxo-clopidogrel, followed by subsequent hydrolysis into its active thiol metabolite, is a two-step reaction catalyzed by cytochrome P450 enzymes. CES1 hydrolyzes a large number of endogenous and therapeutic compounds that contain functional thioester, carboxylic acid ester, and amide functional groups [26]. Recent investigations have shown that CES1 is the primary enzyme responsible for hydrolyzing the methyl ester of clopidogrel, 2-oxo-clopidogrel, and the active metabolite, yielding carboxylic acid derivatives that are biologically inactive [11,27]. Furthermore, the production of these inactive carboxylic acid derivatives occurs at a considerably higher rate compared with the formation of the clopidogrel active metabolite [11]. Therefore, genetic variation affecting the activity or expression of CES1 may have important clinical relevance.
The CES1 variant at position 143 results in a glycine-to-glutamic acid amino acid substitution that, on the basis of its location within the enzyme, would be predicted to considerably affect the catalytic function of CES1. X-ray crystallography data show that glycine 143 of CES1 constitutes, in part, an oxyanion hole near the active site catalytic triad (serine 221, histidine 468, and glutamic acid 354) [28]. Oxyanion holes are used to stabilize substrate–enzyme intermediates and several investigations of other esterases show that abrogation of these moieties alters the catalytic function significantly [29–31]. Consistent with these findings, previous in-vitro expression studies have shown that the 143E substitution resulted in a complete loss of catalytic function when methylphenidate was used as a substrate [6]. Furthermore, the same study showed that when p-nitrophenol acetate was used, the catalytic function (Vmax/Km) of CES1 143E was reduced to 21.4% compared with wild-type CES1 (143G). Clopidogrel or its active metabolites were not tested as substrates in this study.
To our knowledge, this is the first investigation to examine the effect of the CES1 G143E variant on-clopidogrel metabolism, ADP-induced platelet aggregation, and cardiovascular outcomes. In this study, we observed that PAPI participants carrying the decreased function CES1 143E-allele had significantly higher plasma levels of the clopidogrel active metabolite compared with 143G-allele homozygotes. All individuals received the same dose of parent clopidogrel and no difference in the prodrug level was observed between genotype groups. Consistent with metabolite data, we observed in two independent studies that CES1 143E-allele carriers had lower on-treatment ADP-induced platelet aggregation in response to clopidogrel treatment but not at baseline. Interestingly, we observed a moderate difference in the cardiovascular event rates between genotype groups (13.7% in CES1 143G-allele homozygotes vs. 0% in 143E-allele carriers) that did not achieve statistical significance in our sample size. Given that the frequency of the 143E carriers in the population is relatively rare (~1–2%), larger studies will be required to further elucidate whether they are more responsive to the antiplatelet actions of clopidogrel.
Although the CES1 G143E variant is relatively uncommon, it appears to exert a large effect on both clopidogrel metabolism and ADP-induced platelet aggregation even in its heterozygous state. In fact, its magnitude of effect on these traits is comparable, if not larger than that of the CYP2C19*2 variant. As reported previously in the same PAPI study, the absolute β value for the effect of CYP2C19*2 on ADP-induced platelet aggregation was 7.4, compared with 11.6 for CES1 143E (Table 1).
Table 1.
Effect of CES1 143E, CYP2C19*2, and CYP2C19*17 variants on postclopidogrel ADP-stimulated platelet aggregation in participants of the Amish Pharmacogenomics of Anti-Platelet Intervention study
| Variants | Mean preclopidogrel maximal platelet aggregation value | Mean postclopidogrel maximal platelet aggregation value | Percent decrease in maximal platelet aggregation | β coefficienta | Association P valuea |
|---|---|---|---|---|---|
| CES1 143Eb | 77.9 | 22.9 | 70.6 | 11.6 | 0.003 |
| CYP2C19*2c | 71.9 | 48.1 | 33.1 | −7.4 | 1.53 × 10−17 |
| CYP2C19*17c | 71.8 | 30.8 | 57.1 | 2.1 | 0.007 |
CES1, carboxylesterase 1; CYP2C19*2, cytochrome P450 2C19*2 variant; CYP2C19*17, cytochrome P450 2C19*17 variant.
β Coefficients and associated P values were calculated during clopidogrel exposure.
Mean preclopidogrel and postclopidogrel maximal platelet aggregation values and percent difference in maximal platelet aggregation were based on participants who were heterozygous for the 143E-allele as no 143E homozygotes were identified in the Pharmacogenomics of Anti-Platelet Intervention study.
Mean preclopidogrel and postclopidogrel maximal platelet aggregation values and percent reduction in maximal platelet aggregation were based on participants who were homozygous for the minor/effect allele.
Importantly, a recent meta-analysis has shown that carriers of the gain-of-function CYP2C19*17 variant had an increased risk of experiencing an adverse bleeding event compared with noncarriers (odds ratio=1.25, 95% CI 1.07–1.47; P=0.006) [21]. In the PAPI study, the CES1 143E-allele has a much larger effect on on-clopidogrel ADP-induced aggregation than CYP2C19*17, thus leading us to hypothesize that CES1 143E carriers may also be at a greater risk for bleeding events (Table 1). However, it is important to keep in mind that given the small number of CES1 143E-allele carriers in this study, it is also possible that the effect size of this variant has been overestimated. Given that prasugrel is inactivated pre-dominantly by CES2 and thus its activity would not be expected to be significantly affected by the 143E CES1 variant, additional studies will be required to investigate these hypotheses further [32].
Conclusion
We found that a functional genetic variant (G143E) in CES1, a gene critical in clopidogrel inactivation, significantly affects both the formation of clopidogrel active metabolite and ADP-induced platelet aggregation in healthy clopidogrel-treated individuals. The association with ADP-induced platelet aggregation was replicated in an independent cohort of CHD patients undergoing a percutaneous coronary intervention. Given the relatively low frequency of the CES1 143E-allele, we could not adequately test the effect of this polymorphism on adverse cardiovascular or bleeding events. However, given the large effect size, we predict that this variant may have clinical utility to optimize antiplatelet therapy. Future studies examining the role of this variant on clinical outcomes will provide important insights into the use of CES1 genotype information in prescribing the most effective antiplatelet therapy.
Supplementary Material
Supplementary Table 1. Characteristics of PAPI Study and Sinai Hospital of Baltimore Participants
Acknowledgments
The authors gratefully acknowledge Amish liaisons, field workers, the Amish community, and patients at Sinai Hospital of Baltimore, without whom these studies would not have been possible.
This investigation was supported by National Institutes of Health grants U01 GM074518, U01 GM074518-05S1, and U01 HL105198, the University of Maryland General Clinical Research Center, Grant M01 RR 16500, General Clinical Research Centers Program, National Center for Research Resources (NCRR), NIH, the Baltimore Veterans Administration Geriatric Research and Education Clinical Center (GRECC), and Sinai Hospital of Baltimore.
Footnotes
Conflicts of interest
Dr Shuldiner receives grant support from NIH to study the pharmacogenomics of antiplatelet therapy. He is also a consultant for United States Diagnostic Standards Inc. Dr Gurbel receives grant support from Astra Zeneca, Daiichi-Sankyo, Bayer Healthcare, Eli Lilly, Haemonetics, Pozen, and Sanofi-Aventis; and Honoraria/Consulting income from Accumetrics, Astra Zeneca, Bayer Healthcare, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Merck, Pozen, Novartis, CSL Limited, and Sanofi-Aventis. For the remaining authors there are no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Website (www.pharmacogeneticsandgenomics.com).
References
- 1.Dansette PM, Rosi J, Bertho G, Mansuy D. Cytochromes P450 catalyze both steps of the major pathway of clopidogrel bioactivation, whereas paraoxonase catalyzes the formation of a minor thiol metabolite isomer. Chem Res Toxicol. 2012;25:348–356. doi: 10.1021/tx2004085. [DOI] [PubMed] [Google Scholar]
- 2.Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 3.Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie HG, Zou JJ, Hu ZY, Zhang JJ, Ye F, Chen SL. Individual variability in the disposition of and response to clopidogrel: pharmacogenomics and beyond. Pharmacol Ther. 2011;129:267–289. doi: 10.1016/j.pharmthera.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Zhu HJ, Patrick KS, Yuan HJ, Wang JS, Donovan JL, DeVane CL, et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82:1241–1248. doi: 10.1016/j.ajhg.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi D, Yang J, Yang D, LeCluyse EL, Black C, You L, et al. Anti-influenza prodrug oseltamivir is activated by carboxylesterase human carboxylesterase 1, and the activation is inhibited by antiplatelet agent clopidogrel. J Pharmacol Exp Ther. 2006;319:1477–1484. doi: 10.1124/jpet.106.111807. [DOI] [PubMed] [Google Scholar]
- 8.Vistoli G, Pedretti A, Mazzolari A, Bolchi C, Testa B. Influence of ionization state on the activation of temocapril by hCES1: a molecular-dynamics study. Chem Biodivers. 2009;6:2092–2100. doi: 10.1002/cbdv.200900174. [DOI] [PubMed] [Google Scholar]
- 9.Zhu HJ, Appel DI, Johnson JA, Chavin KD, Markowitz JS. Role of carboxylesterase 1 and impact of natural genetic variants on the hydrolysis of trandolapril. Biochem Pharmacol. 2009;77:1266–1272. doi: 10.1016/j.bcp.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Tabata T, Katoh M, Tokudome S, Nakajima M, Yokoi T. Identification of the cytosolic carboxylesterase catalyzing the 5′-deoxy-5-fluorocytidine formation from capecitabine in human liver. Drug Metab Dispos. 2004;32:1103–1110. doi: 10.1124/dmd.104.000554. [DOI] [PubMed] [Google Scholar]
- 11.Bouman HJ, Schomig E, van Werkum JW, Velder J, Hackeng CM, Hirschhauser C, et al. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med. 2011;17:110–116. doi: 10.1038/nm.2281. [DOI] [PubMed] [Google Scholar]
- 12.Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, et al. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost. 2000;84:891–896. [PubMed] [Google Scholar]
- 13.Scott SA, Sangkuhl K, Gardner EE, Stein CM, Hulot JS, Johnson JA, et al. Clinical pharmacogenetics implementation consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clin Pharmacol Ther. 2011;90:328–332. doi: 10.1038/clpt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis JP, Fisch AS, Ryan K, O’Connell JR, Gibson Q, Mitchell BD, et al. Paraoxonase 1 (PON1) gene variants are not associated with clopidogrel response. Clin Pharmacol Ther. 2011;90:568–574. doi: 10.1038/clpt.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, et al. 2007 Focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines: developed in collaboration with the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to review new evidence and update the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction, writing on behalf of the 2004 Writing Committee. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- 16.Applegate SL, Rice MS, Stein F, Maitra KK. Knowledge of results and learning to tell the time in an adult male with an intellectual disability: a single-subject research design. Occup Ther Int. 2008;15:32–44. doi: 10.1002/oti.242. [DOI] [PubMed] [Google Scholar]
- 17.Peer CJ, Spencer SD, Vandenberg DA, Pacanowski MA, Horenstein RB, Figg WD. A sensitive and rapid ultra HPLC-MS/MS method for the simultaneous detection of clopidogrel and its derivatized active thiol metabolite in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;880:132–139. doi: 10.1016/j.jchromb.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwala R, Biesecker LG, Hopkins KA, Francomano CA, Schaffer AA. Software for constructing and verifying pedigrees within large genealogies and an application to the Old Order Amish of Lancaster County. Genome Res. 1998;8:211–221. doi: 10.1101/gr.8.3.211. [DOI] [PubMed] [Google Scholar]
- 19.Mega JL, Close SL, Wiviott SD, Shen L, Walker JR, Simon T, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376:1312–1319. doi: 10.1016/S0140-6736(10)61273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouman HJ, van Werkum JW, Rudez G, Leebeek FW, Kruit A, Hackeng CM, et al. The influence of variation in the P2Y12 receptor gene on in vitro platelet inhibition with the direct P2Y12 antagonist cangrelor. Thromb Haemost. 2010;103:379–386. doi: 10.1160/TH09-06-0367. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Tang HL, Hu YF, Xie HG. The gain-of-function variant allele CYP2C19*17: a double-edged sword between thrombosis and bleeding in clopidogrel-treated patients. J Thromb Haemost. 2012;10:199–206. doi: 10.1111/j.1538-7836.2011.04570.x. [DOI] [PubMed] [Google Scholar]
- 22.Jeong YH, Tantry US, Kim IS, Koh JS, Kwon TJ, Park Y, et al. Effect of CYP2C19*2 and *3 loss-of-function alleles on platelet reactivity and adverse clinical events in East Asian acute myocardial infarction survivors treated with clopidogrel and aspirin. Circ Cardiovasc Interv. 2011;4:585–594. doi: 10.1161/CIRCINTERVENTIONS.111.962555. [DOI] [PubMed] [Google Scholar]
- 23.Di Castelnuovo A, de Gaetano G, Benedetta Donati M, Iacoviello L. Platelet glycoprotein IIb/IIIa polymorphism and coronary artery disease: implications for clinical practice. Am J Pharmacogenomics. 2005;5:93–99. doi: 10.2165/00129785-200505020-00002. [DOI] [PubMed] [Google Scholar]
- 24.Lev EI, Patel RT, Guthikonda S, Lopez D, Bray PF, Kleiman NS. Genetic polymorphisms of the platelet receptors P2Y(12), P2Y(1) and GP IIIa and response to aspirin and clopidogrel. Thromb Res. 2007;119:355–360. doi: 10.1016/j.thromres.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Cayla G, Hulot JS, O’Connor SA, Pathak A, Scott SA, Gruel Y, et al. Clinical, angiographic, and genetic factors associated with early coronary stent thrombosis. JAMA. 2011;306:1765–1774. doi: 10.1001/jama.2011.1529. [DOI] [PubMed] [Google Scholar]
- 26.Satoh T, Hosokawa M. Structure, function and regulation of carboxylesterases. Chem Biol Interact. 2006;162:195–211. doi: 10.1016/j.cbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Shi D, Yang D, Song X, Yan B. Interleukin-6 alters the cellular responsiveness to clopidogrel, irinotecan, and oseltamivir by suppressing the expression of carboxylesterases HCE1 and HCE2. Mol Pharmacol. 2007;72:686–694. doi: 10.1124/mol.107.036889. [DOI] [PubMed] [Google Scholar]
- 28.Fleming CD, Bencharit S, Edwards CC, Hyatt JL, Tsurkan L, Bai F, et al. Structural insights into drug processing by human carboxylesterase 1: tamoxifen, mevastatin, and inhibition by benzil. J Mol Biol. 2005;352:165–177. doi: 10.1016/j.jmb.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Legler PM, Kumaran D, Swaminathan S, Studier FW, Millard CB. Structural characterization and reversal of the natural organophosphate resistance of a D-type esterase, Saccharomyces cerevisiae S-formylglutathione hydrolase. Biochemistry. 2008;47:9592–9601. doi: 10.1021/bi8010016. [DOI] [PubMed] [Google Scholar]
- 30.Mandrich L, Menchise V, Alterio V, De Simone G, Pedone C, Rossi M, et al. Functional and structural features of the oxyanion hole in a thermophilic esterase from Alicyclobacillus acidocaldarius. Proteins. 2008;71:1721–1731. doi: 10.1002/prot.21877. [DOI] [PubMed] [Google Scholar]
- 31.Wu D, Li Y, Song G, Zhang D, Shaw N, Liu ZJ. Crystal structure of human esterase D: a potential genetic marker of retinoblastoma. FASEB J. 2009;23:1441–1446. doi: 10.1096/fj.08-125286. [DOI] [PubMed] [Google Scholar]
- 32.Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126–142. doi: 10.1177/0091270009343005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Characteristics of PAPI Study and Sinai Hospital of Baltimore Participants