Abstract
CONTEXT
The original mild cognitive impairment (MCI) criteria exclude substantial functional deficits, but recent reports suggest otherwise. Identifying the extent, severity, type, and correlates of functional deficits that occur in MCI and mild Alzheimer’s disease (AD) can aid in early detection of incipient dementia and identify potential mechanistic pathways to disrupted instrumental activities of daily living (IADLs).
OBJECTIVES
To examine the number, type, and severity of functional impairments and identify the clinical characteristics associated with functional impairment across individuals with amnestic MCI (aMCI) and those with mild AD.
DESIGN
The study uses baseline data from the Alzheimer’s Disease Neuroimaging Initiative.
SETTING
Data from the Alzheimer’s Disease Neuroimaging Initiative was collected at multiple research sites in the US and Canada.
PATIENTS
The samples included 229 controls, 394 aMCI, and 193 AD patients.
MAIN OUTCOME MEASURE
The 10-item Pfeffer Functional Activities Questionnaire (FAQ) assessed function.
RESULTS
Informant-reported FAQ deficits were common in patients with aMCI (72.3%) and AD (97.4%) but were rarely self-reported by controls (7.9%). The average severity per FAQ deficit did not differ between patients with aMCI and controls; both were less impaired than patients with AD (P < .001). Two FAQ items (remembering appointments, family occasions, holidays, and medications; assembling tax records, business affairs, or other papers) were specific (0.95) in differentiating controls from the combined aMCI and AD groups (only 34.0% of patients with aMCI and 3.6% of patients with AD had no difficulty with these 2 items). The severity of FAQ deficits in the combined aMCI and AD group was associated with worse Trailmaking Test A scores and smaller hippocampal volumes (P < .001). Within the aMCI group, functionally intact individuals had greater hippocampal volumes and better Auditory Verbal Learning Test 30-minute delay and Trailmaking Test A (P < .001) scores compared with those with moderate or severe FAQ deficits. Patients with a high number of deficits were more likely to express the APOE ε4 allele (63.8%) compared with patients with no (46.8%) or few (48.4%) functional deficits.
CONCLUSIONS
Mild IADL deficits are common in individuals with aMCI and should be considered in MCI criteria. Two IADLs, remembering appointments, family occasions, holidays, and medications and assembling tax records, business affairs, or other papers, appear to be characteristic of clinically significant cognitive impairment. In patients with aMCI, impairment in memory and processing speed and greater medial temporal atrophy were associated with greater IADL deficits
Functional impairment is a required criterion for the diagnosis of most major neuropsychiatric disorders, including dementia.1,2 Decreases in functional ability in elderly individuals can adversely affect patients and caregivers and are associated with institutionalization.3–8 Functional decline can occur as a result of several factors including medical illness,9,10 mood disorders,11–14 and cognitive impairment.15–17 Identifying the extent and severity of functional deficits that typically occur in each disorder can aid in early diagnosis, help in estimating prognosis, and improve treatment strategies.18
The term mild cognitive impairment (MCI)19,20 is used to identify a stage of impairment that demonstrates considerable heterogeneity and comprises individuals who are at high risk for conversion to dementia.19 The MCI criteria require subjective memory complaints and a score 1.5 SDs below age-adjusted norms on a memory test (amnestic MCI [aMCI]) and require no “substantial interference with work, usual social activities, or other activities of daily living”.20 Research has shown, however, that individuals with aMCI commonly have deficits in instrumental activities of daily living (IADLs).21–28 Our group reported that in participants with MCI, baseline informant-reported functional deficits on the Pfeffer Functional Activities Questionnaire (FAQ)25 were associated with a fourfold increase in conversion to dementia during long-term follow-up.29
This study had 3 goals: to examine the number, type, and severity of functional impairments across patients with aMCI and those with mild AD, comparing them with healthy cognitively intact control individuals; to identify the clinical characteristics that explain functional impairment in individuals with aMCI and mild AD; and to explore the neuropsychological and neuroanatomical profiles in relation to functional deficits in individuals with aMCI. Baseline data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI)30 were used to address these goals.
METHODS
ALZHEIMER’S DISEASE NEUROIMAGING INITIATIVE
ADNI
Data used for article preparation were obtained from the ADNI database (http://adni.loni.ucla.edu/), a project launched in 2003 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies and non-profit organizations as a $60 million, 5-year public-private partnership. The primary goal of ADNI is to test whether serial magnetic resonance imaging, positron emission tomography, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD.
STUDY PARTICIPANTS
Data obtained from ADNI in October 2009 were selected from screening or baseline visits of all participants who completed evaluations with the key variables of interest to this report. The sample comprised 229 cognitively intact older adults, 394 individuals with aMCI, and 193 individuals with mild AD. The demographic, neuropsychological, and functional characteristics for these 3 groups are listed in Table 1.
Table 1.
Baseline characteristics for the control, aMCI, and AD groupsa
| Characteristic | Control Group (n = 229) |
aMCI Group (n = 394) |
AD Group (n = 193) |
|---|---|---|---|
| Demographic | Mean (SD) | Mean (SD) | Mean (SD) |
| Age, y | 75.90 (5.00) | 74.86 (7.40) | 75.33 (7.48) |
| Educational level, y | 16.04 (2.90) | 15.65 (3.04) | 14.71 (3.13)c,d |
| Sex, No. M/F (% F) | 119/110 (48.0%) | 256/138 (35.0%) b | 102/91 (47.2%)d |
| Neuropsychological scores | |||
| MMSE | 29.11 (1.00) | 27.04 (1.78)b | 23.34 (2.06)c,d |
| Logical Memory II immediate recall | 13.78 (3.47) | 7.11 (3.16)b | 4.07 (2.91)c,d |
| Logical Memory II delayed recall | 12.97 (3.57) | 3.81 (2.66)b | 1.27 (1.90)c,d |
| Trailmaking Test A, s | 36.45 (13.19) | 44.85 (22.83)b | 67.50 (36.43)c,d |
| Trailmaking Test B, s | 89.21 (44.26) | 130.85 (73.77)b | 197.95 (87.09)c,d |
| Digit Symbol | 45.75 (10.20) | 36.84 (11.12)b | 26.94 (12.81)c,d |
| AVLT 30-min Delay | 7.39 (3.72) | 2.84 (3.30)b | 0.74 (1.62)c,d |
| Brain volumes, cm3 | |||
| Hippocampal | 7.22 (0.89) | 6.35 (1.07)b | 5.60 (1.05)c,d |
| Entorhinal cortex | 3.80 (0.65) | 3.29 (0.75)b | 2.73 (0.71)c,d |
| CDR Scores | |||
| CDR sum of boxes | 0.03 (0.12) | 1.60 (0.89)b | 4.30 (1.64)c,d |
| Median (range) | 0.00 (0.00–0.50) | 1.50 (0.00–5.00) | 4.00 (1.00–9.00) |
| Function measure: FAQ | |||
| Severity of deficits | |||
| Mean | 0.14 (0.60) | 3.84 (4.47)b | 12.99 (6.84)c,d |
| Median (range) | 0.00 (0–6.00) | 2.00 (0–21.00) | 12.00 (0–30.00) |
| No. of deficits | |||
| Mean | 0.10 (0.38) | 2.70 (2.69)b | 6.97 (2.53)c,d |
| Median (range) | 0.00 (0–2.00) | 2.00 (0–10.00) | 7.00 (0–10.00) |
| Severity per deficit | |||
| Mean | 1.25 (0.52) (n = 18) | 1.34 (0.43) (n = 285) | 1.78 (0.50)c,d (n = 188) |
| Median (range) | 1.00 (1.00–3.00) | 1.17 (1.00–3.00) | 1.75 (1.00–3.00) |
Abbreviations: AD = Alzheimer’s disease; aMCI = amnestic mild cognitive impairment; AVLT = Auditory Verbal Learning Test; CDR = Clinical Dementia Rating Scale; MMSE = Mini Mental State Exam; FAQ = Pfeffer Functional Activities Questionnaire.
Data are presented as mean (SD) unless otherwise indicated. Neuropsychological scores are raw scores.
Significant difference in post hoc comparisons between control individuals and patients with aMCI (P < .01).
Denotes a Significant difference in post hoc comparisons between controls and patients with AD at (P < .01).
Denotes a Significant difference in post hoc comparisons between patients with aMCI and patients with AD (P < .01).
Participants were enrolled if they were aged 55 to 90 years, had at least 6 years of educational attainment, spoke English or Spanish as their primary language, agreed to undergo longitudinal follow-up and neuroimaging tests, and had a study partner. Cognitively intact participants had Mini Mental State Examination (MMSE)31 scores between 24 and 30, Clinical Dementia Rating Scale (CDR)32 scores of 0 (no dementia), and no significant memory complaints. The aMCI participants were classified as having aMCI single-domain or multi-domain aMCI according to the Petersen criteria:19 a CDR score of 0.5, MMSE scores between 24 and 30, a memory complaint verified by an informant, an abnormal memory score (1.5 SDs below the age-adjusted cutoff) on the Logical Memory II subscale (delayed paragraph recall) from the Wechsler Memory Scale-Revised,33 and absence of a diagnosis of dementia as made by site physicians. Mild AD participants had a CDR score of 0.5 or 1.0, had MMSE scores between 20 and 26, and met criteria for probable AD.2 All participants had a Geriatric Depression Scale34 score of less than 6 (no significant depression), and a modified Hachinski score35 of 4 or less (no significant vascular impairment, including hypertension, stroke, and/or neurologic signs/symptoms). For a more detailed account of the inclusion/exclusion criteria, please see http://adni.loni.ucla.edu/about/about-the-study/.
NEUROPSYCHOLOGICAL ASSESSMENT
At baseline participants underwent an extensive neuropsychological battery. We selected specific cognitive measures a priori because they assess cognitive functions shown in prior research15–17 to correlate with functional impairment; these measures include the Trailmaking Test A and B,36 the Digit Symbol Substitution Test of the Wechsler Adult Intelligence Scale-Revised,37 and the Auditory Verbal Learning Test (AVLT).38
FUNCTIONAL ASSESSMENT
The FAQ25 is a 10-item IADL measure (Table 2). Self-reports of functional deficits were collected for controls, but informant-reports were collected for the aMCI and AD groups. Each item is rated from 0 (no difficulty or independent) to 3 (dependent). Analyses classified functional impairment in 1 of 3 ways: by total severity (total sum score from all 10 items; range, 0–30), total number of deficits (total sum score of dichotomized items, with 0 indicating no difficulty and 1 indicating any difficulty; range 0–10)26; and average severity per deficit (total severity divided by total number of deficits). The means and standard deviations for each and selected medians (with ranges) are listed in Table 1.
Table 2.
Functional deficits per item for the controls, aMCI, and AD groups with Cochran-Armitage Linear Trend Test results.a
| FAQ item | Control Group % (n = 229) |
aMCI Group % (n = 394) |
AD Group % (n = 193) |
Trend Test z Score |
|---|---|---|---|---|
| 1. Writing checks, paying bills, or balancing checkbook. | 2.2% (5) | 33.8% (133) | 88.1% (170) | 17.97 |
| 2. Assembling tax records, business affairs, or other papers.b,c | 1.7% (4) | 42.9% (169) | 91.2% (176) | 18.47 |
| 3. Shopping alone for clothes, household necessities, or groceries. | 0.4% (1) | 18.5% (73) | 71.0% (137) | 16.19 |
| 4. Playing a game of skill such as bridge or chess, or working on a hobby.c | 0.4% (1) | 21.8% (86) | 61.7% (119) | 14.27 |
| 5. Heating water, making a cup of coffee, turning off the stove. | 0.0% (0) | 7.6% (30) | 27.5% (53) | 9.14 |
| 6. Preparing a balanced meal. | 0.4% (1) | 19.8% (78) | 64.8% (125) | 14.98 |
| 7. Keeping track of current events.c | 0.4% (1) | 22.8% (90) | 67.4% (130) | 15.23 |
| 8. Paying attention to and understanding a television program, book, or magazine.c | 0.4% (1) | 21.3% (84) | 59.1% (114) | 13.83 |
| 9. Remembering appointments, family occasions, holidays, and medications.b,c | 3.5% (8) | 54.8% (216) | 90.7% (15) | 18.01 |
| 10. Travelling outside the neighborhood, driving, or arranging to take public transportation.c | 0.9% (2) | 26.1% (103) | 75.6% (146) | 16.39 |
Abbreviations: AD = Alzheimer’s disease; aMCI = Amnestic Mild Cognitive Impairment; FAQ = Pfeffer Functional Activities Questionnaire.
P < .001 for all items.
Identified as a subset of functional items that reliably differentiates control individuals from a combined aMCI and AD group.
Identified through bootstrapping techniques as the most reliable subset of functional items that differentiate controls from aMCI.
IMAGING VOLUME DERIVATIONS
Hippocampal (derived by adding right and left hippocampal volumes), entorhinal, and intracranial volumes were downloaded from post-processed image analysis using FreeSurfer, version 4.3.0 by researchers at the University of California, San Francisco; the data are available at http://adni.loni.ucla.edu/. We used the cross-sectional baseline data recommended for use by the ADNI investigators. A detailed account of the volume derivation process is located at: http://www.loni.ucla.edu/twiki/bin/view/ADNI/ADNIPostProc.
STATISTICAL ANALYSES
Analysis of variance or χ2 tests were used to detect group differences for continuous and categorical variables. Analysis of covariance was used for group comparison on all brain volumetric measures with intracranial volume as the covariate. A stepwise selection procedure for item selection from a unidimensional scale was used to identify a subset of FAQ items that best differentiated controls from patients with aMCI.39 To obtain a reliable subset of items classifying the 2 groups with accuracy similar to that of the full scale, we applied the procedure with a significance criterion of .05 for item contribution to the subset classification accuracy to 500 bootstrap samples, including the item response data of the controls and patients with aMCI randomly sampled with replacement from the study sample, choosing the items most frequently selected (included in > 50% of bootstrap samples). The area under the receiver operating characteristic curve was used to compare the usefulness of the identified FAQ subset with the full 10-item FAQ in differentiating controls and patients with aMCI. In addition, for potential clinical applicability, the sensitivity and specificity of a 2-item subset was assessed. Linear regression models were used to examine the relationship between the FAQ and demographic, physical, depression, neuropsychological, and neuroimaging characteristics in a combined aMCI and AD group and in the aMCI group alone. Within the aMCI group, linear models were used to calculate the covariate-adjusted means of the neuropsychological and neuroimaging variables identified in the linear regression analysis across the 3 categorized ordinal classes of number of FAQ deficits and the severity of FAQ deficits (a functionally intact group and the functionally impaired group split into categories based on number and severity of functional deficits). In post hoc group comparisons, Bonferroni correction on the false positive error rate was used to account for multiple comparisons. Multinomial logistic regression models for the trichotomized functional severity and number of functional deficits scores were used to assess the simultaneous effect of the neuropsychological, genetic, and neuroimaging variables identified in the linear regression analysis.
RESULTS
EXTENT AND SEVERITY OF FUNCTIONAL DEFICITS ACROSS COGNITIVELY IMPAIRED GROUPS
Demographic, neuropsychological, and functional variables (Table 1) differed significantly by group with the exception of age. Item distributions among the 10-item FAQ showed an increased number of functional deficits across study groups (F2, 813 = 494.99, P < .001). 7.9% of controls, 72.3% of informants for the aMCI group, and 97.4% of informants for the mild AD group reported 1 or more functional deficits. In the AD group, 87.0% of informants reported 4 or more total deficits. As indicated in Table 1, total severity scores of functional impairment significantly increased across groups (F2, 813 = 438.60, P < .001). The average level of severity per deficit did not differ, however, between the control (mean [SD], 1.25 [0.52]) and aMCI (mean [SD], 1.33 [0.43]) groups, but the mild AD group (mean [SD], 1.78 [0.50]) had greater average severity per functional deficit than both of the other groups (F2, 488 = 56.27, P < .001). Informant-reported deficits in the aMCI sample were fewer in number and milder in severity than those reported for the mild AD group. In fact, 78.7% of informants for the aMCI group endorsed 0 (no difficulty) or 1 (patient has difficulty but still does the task by himself/herself) on each of the 10 FAQ items; this finding contrasts with that of 25.4% to 66.3% of informants of the mild AD group who reported 2 (requires assistance) or 3 (dependent) on 8 of the 10 FAQ items (only 12.4% and 15.5% of informants from the mild AD group reported 2 or 3 on items 5, [heating water, making a cup of coffee, turning off the stove] and item 8 [paying attention to and understanding a television program, book, or magazine], respectively).
Table 2 lists the percentage of deficits by item within each diagnostic group. Although few controls self-reported any functional deficits on the individual FAQ items, informants for the aMCI group most commonly reported deficits on items 1 (writing checks, paying bills, or balancing checkbook; 33.8%), 2 (assembling tax records, business affairs, or other papers; 42.9%), and 9 (remembering appointments, family occasions, holidays, and medications; 54.8%). These 3 items increased markedly in frequency on the Cochran Armitage linear trend tests from controls to aMCI to AD, but the frequency of other items such as item 5 (heating water, making a cup of coffee, turning off the stove) increased less so across groups (Table 2). To identify those items that most commonly differentiated the control and aMCI groups, an item selection process was used. The subset selected had a median size of 6; the 6-item subset (items 2, 4, 7, 8, 9, and 10) was the most frequently selected in the 500 bootstrap samples and differentiated the control and aMCI groups with classification accuracy (0.6998) similar to the full 10-item scale (0.7067).
The evaluation of 6 IADLs (including the ability to assemble tax records, business affairs, or other papers; to play a game of skill such as bridge or chess, or work on a hobby; to keep track of current events; to pay attention to and understand a television program, book, or magazine; to remember appointments, family occasions, holidays, and medications; and to travel outside of the neighborhood, drive, or arrange to take public transportation) does not represent a marked time-savings compared with the 10-item FAQ for use in clinical practice. Two of these 6 items however were selected in each of the 500 bootstrap samples. These 2 items (assembling tax records, business affairs, or other papers; remembering appointments, family occasions, holidays, and medications) were highly effective in discriminating controls from a combined aMCI and mild AD group. Although only 3.5% (n = 12) of healthy controls reported deficits on one of these two items (no controls reported deficits in both), 66.0% of informants for the aMCI group and 96.4% of informants of the mild AD group reported deficits on 1 of these 2 items; 85.5% of the mild AD group had informant-reported deficits on both of these items. Comparing controls with the combined aMCI and AD groups, these numbers are reflected in the sensitivity (.76 vs. .81) and specificity (.95 vs. .92) estimates comparing the 2-item FAQ and 10-item FAQ, respectively, with a cut-point of 1 functional deficit or more.
The control group consisted of cognitively intact older adults who used self-report assessments, thereby making it difficult to compare these rates with those of the informant-reported methods used for the aMCI and AD groups. Therefore, healthy controls were excluded from subsequent analyses.
FACTORS ASSOCIATED WITH FUNCTIONAL DEFICITS IN AMCI AND MILD AD GROUPS
The bivariate relationships in the combined aMCI and AD groups between functional impairment and demographics (age, sex, and educational level), physical health (Hachinski score, which assesses history of hypertension, stroke, and neurologic signs and symptoms), depression (Geriatric Depression Scale), brain volumes (intracranial, hippocampal, and entorhinal cortex volumes), APOE ε4 allele status (present or absent), and neuropsychological variables (Trailmaking Test A and B, AVLT 30-minute delay, and Digit Symbol) showed significant associations between functional impairment and brain volumes, APOE ε4 status, and the neuropsychological variables (Ps < .01). Linear regression analyses in the combined aMCI and AD group were used to identify variables associated with functional impairment after controlling for all other independent variables. Three aspects of functional impairment served as outcome variables in separate analyses: total severity score, total number of deficits, and average severity per deficit score. In each regression analysis the demographic, physical health, depression, brain volume, genetic, and neuropsychological variables were entered simultaneously into the model. The independent variables explained 30.8% of the variance in total severity, F13, 545 = 18.65, P < .001, 29.4% of the variance in total number of deficits, F13, 545 = 17.44, P < .001, and 17.8% of the variance in average severity per deficit, F13, 438 = 7.30, P < .001. Two independent variables were significant in each of the analyses: hippocampal volume (Ptotal severity < .001; Ptotal number < .001; Pseverity per deficit = .001), and Trailmaking Test A (Ptotal severity < .001; Ptotal number < .001; Pseverity per deficit = .004). Three other independent variables were associated with total severity and total number of deficits: Age (Ptotal severity = .044; Ptotal number = .008), AVLT 30-minute delay (Ptotal severity = .004; Ptotal number < .001) and entorhinal cortex volume (Ptotal severity = .029; Ptotal number = .048). The effect of the above independent variables remained significant and the R2 virtually unchanged when the independent variables that did not contribute to the models were excluded.
Profiles of Informant-reported Functional Impairment in the aMCI Group
To identify variables uniquely associated with functional deficits in the aMCI stage, linear regression analyses were conducted with the aMCI group with total severity and total number of functional deficit scores serving as the outcome variables. As in the combined aMCI and AD analysis, the demographic, physical health, depression, brain volume, genetic, and neuropsychological variables were entered simultaneously into the model. The independent variables explained 13.3% of the variance in total severity, F13, 365 = 4.29, P < .001, and 13.2% of the variance in total number of deficits, F13, 365 = 4.28, P < .001. Hippocampal volume (Ptotal severity = .013; Ptotal number = .030), AVLT 30-minute delay (Ptotal severity = .037; Ptotal number = .010), and Trailmaking Test A (Ptotal severity = .009; Ptotal number = .005) predicted each of the functional outcomes. Digit Symbol predicted total severity (P = .03) but not total number of deficits (P = .28). APOE ε4 status predicted total number of deficits (P = .05) but not total severity (P = .09). The effect of these independent variables remained significant and the R2 virtually unchanged when the independent variables that did not contribute to the models were excluded.
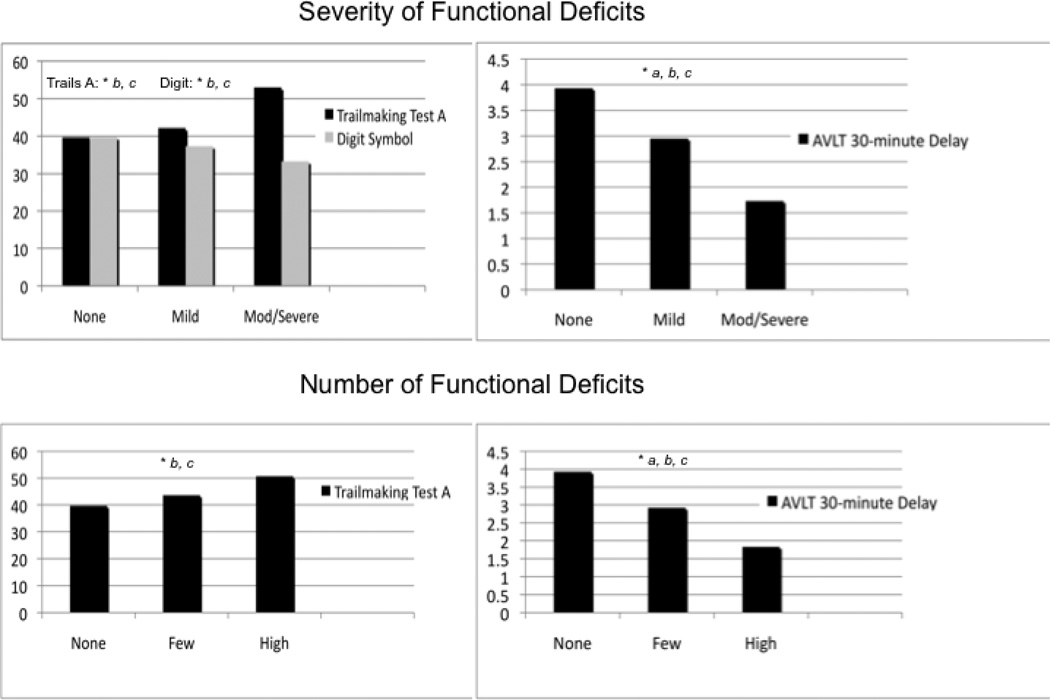
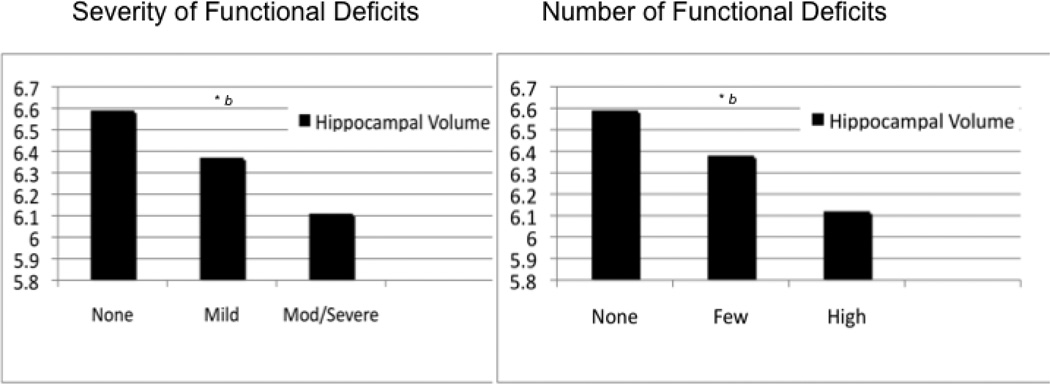
To investigate these relationships further comparing those individuals with aMCI who have and those who do not have functional impairment, analyses of covariance and post hoc comparisons adjusting for age, sex, and educational level (and intracranial volume in the hippocampal volume analysis) were conducted for the 6 independent variables (including age) identified as significant in the linear models (Figures 1 and 2). Total functional severity scores were categorized into 3 groups: functionally intact (total severity scores = 0; n = 109), mild (total severity scores ≥ 1 and ≤ 4; n = 162), and moderate to severe (total severity scores ≥ 5; n = 123). The functionally intact and mild severity groups performed better on Trailmaking Test A (P < .001), Digit Symbol (P < .001), and AVLT 30-minute delay (P < .001 and P = .001, respectively) than the moderate to severe group; the adjusted means for the functionally intact group did not differ from the mild group on Trailmaking Test A (P = .36) and Digit Symbol (P = .07), but did differ on AVLT 30-minute delay (P = .014; Figure 1A and 1B). The adjusted mean hippocampal volumes in the functionally intact group were larger than those in the moderate to severe group (P < .001) and the mild group, although the latter did not reach significance (P = .06); the adjusted mean hippocampal volume for the mild group was larger than the moderate to severe group (P = .018; Figure 2A) although the difference did not reach the Bonferroni corrected significance level.
Figure 1. Post hoc adjusted mean comparisons of neuropsychological predictors of functional deficits in the amnestic mild cognitive impairment sample.
Means adjusted for age, sex, and educational level. A and B, Severity of functional deficits. C and D, Number of functional deficits.
A significant difference was found in post hoc comparisons between no deficits and mild or few for the severity of functional deficits and the number of functional deficits as measured by the Auditory Verbal Learning Test (AVLT) 30-minute delay.
A significant difference was found in post hoc comparisons between no deficits and moderate to severe or high deficits for all measures.
A significant difference was found in post hoc comparisons between mild or few and moderate to severe or high deficits for all measures.
Severity groups were defined as follows: no deficits, 0; mild severity, 1 or more and 4 or less; and moderate to severe, 5 or more.
Number of deficit groups were defined as follows: no deficits, 0; few deficits, 1 or more and 3 or less; and high number deficits, 4 or more. (P < .0167, Bonferroni corrected).
Figure 2. Post hoc adjusted mean comparisons of neuroanatomical predictors of functional deficits in the amnestic mild cognitive impairment sample.
Means adjusted for age, sex, and educational level, (and intracranial volume in the hippocampal volume analysis).
A, Severity of functional deficits. B, Number of functional deficits.
A significant difference was found in post hoc comparisons between no deficits and moderate to severe or high deficits.
Severity groups were defined as follows: no deficits, = 0; mild severity, 1 or more and 4 or less; moderate to severe, 5 or more.
Number of deficit groups were defined as follows: no deficits, 0; few deficits, 1 or more and 3 or less; and high number deficits, 4. or more (P <.0167, Bonferroni corrected).
Similar analyses of covariance and post hoc comparisons (Figures 1 and 2) were conducted for total number of functional deficits categorized into 3 groups: functionally intact (total number of deficits = 0; n = 109), few deficits (total deficits ≥ 1 and ≤ 3; n = 155), and high number of deficits (total deficits ≥ 4; n = 130). Again, the functionally intact group differed from the high deficit group on Trailmaking Test A (P < .001), AVLT 30-minute delay (P < .001), and hippocampal volumes (P < .001; Figure 1C and 1D and Figure 2B). Higher percentages of individuals had positive expression for the APOE ε4 allele in the high deficit group (63.8%) compared with the functionally intact (46.8%) or few deficit (48.4%) groups.
Finally, multinomial logistic regression analyses assessed the simultaneous effect of these significant independent variables on the trichotomized functional severity and the number of functional deficit groups, with the functionally intact group serving as the reference category in each analysis with age, sex, educational level, and intracranial volume entered as covariates into each model. Table 3 lists the odds ratios for the group comparisons in the models. These comparisons mirror the post hoc analyses: the functionally intact group performed better on Trailmaking Test A (P = .006), and AVLT 30-minute delay (P < .001) than the moderate to severe group, as well as the high deficit group (P < .001 for both Trailmaking Test A and AVLT 30-minute delay) in each logistic model; the functionally intact group also had greater hippocampal volume than the moderate to severe (P = .024) and high deficit groups (P = .044). None of the functionally intact vs few deficits group or functionally intact vs mild severity group-comparisons were significant (Table 3).
Table 3.
Covariate-adjusted odds ratios (95% confidence intervals) for FAQ deficit class comparisons within the aMCI group.
| Number of FAQ Deficits | ||
|---|---|---|
| Predictors | Few deficits vs. None OR (95% CI) |
High deficits vs. None OR (95% CI) |
| Hippocampal volume per 1000 cc3 | 0.828 (0.610, 1.124) | 0.711 (0.510, 0.991)* |
| Trailmaking Test A | 1.009 (0.994, 1.024) | 1.027 (1.012, 1.042)*** |
| AVLT 30-min delay | 0.946 (0.873, 1.024) | 0.832 (0.750, 0.924)*** |
| APOE ε4 | 0.944 (0.556, 1.605) | 1.706 (0.957, 3.042) |
| Severity of FAQ Deficits |
||
| Mild vs. None OR (95% CI) |
Moderate/Severe vs. None OR (95% CI) |
|
| Hippocampal volume/1000 | 0.828 (0.614, 1.118) | 0.679 (0.485, 0.950)* |
| Trailmaking Test A | 1.003 (0.986, 1.022) | 1.025 (1.007, 1.044)** |
| AVLT 30-minute delay | 0.951 (0.878, 1.030) | 0.809 (0.723, 0.905)*** |
| Digit symbol | 0.993 (0.964, 1.023) | 0.984 (0.951, 1.018) |
Abbreviations: aMCI, amnestic mild cognitive impairment; AVLT, Auditory Verbal Learning Test; ellipses, not applicable; PFAQ, Pfeffer Functional Activities Questionnaire.
Covariates adjusted for age, sex, educational level, and intracranial volume.
P < .05.
P < .01.
P < .001.
Discussion
EXTENT AND SPECIFIC FUNCTIONAL IMPAIRMENTS ACROSS GROUPS
Functional impairment is necessary to make a diagnosis of dementia,2 but the Peterson criteria for MCI stipulate no “substantial” functional impairments.19,20 The present study, however, found that 72.3% of informants of individuals with aMCI reported 1 or more deficits in daily functioning compared with 97.4% with mild AD and 7.9% of self-reported healthy controls. This proportion of functional impairment in the aMCI group is consistent with previous findings identifying IADL deficits in patients with MCI.15,17,21,22,26,28,29 The severity of these impairments however was mild; that is, individuals with aMCI show difficulty in IADL functioning, but this difficulty does not require the assistance of others. Only 1.8% to 21.3% of informants reported that individuals with aMCI require assistance or were dependent on the IADLs assessed. This finding is in contrast with those individuals with mild AD, of whom 12.4% to 66.3% of informants reported that patients with mild AD required assistance or were dependent on most of the IADLs assessed. Thus, physicians should be sensitive to mild informant-reported deficits at the stage of aMCI, which is often a precursor to the diagnosis of dementia.
To aid physicians in the ability to detect impairment early in the dementia process, we identified 6 IADLs that distinguished controls from individuals with aMCI. Two of these items in particular, remembering appointments, family occasions, holidays, and medications and assembling tax records, business affairs, or other papers, may improve the ability of physicians to briefly identify aMCI functional impairment. These 2 items were highly specific in their ability to differentiate controls from the 2 combined cognitively impaired groups. Only 34.0% of individuals with aMCI (and 3.6% of individuals with AD) have intact informant-reported functioning on both of these items. These findings highlight the types of daily activities that are disrupted during different stages of cognitive impairment and specifically identified 2 daily tasks that physicians can use to differentiate controls from cognitive impaired individuals.
The results of this study support recent proposals to modify the Petersen criteria for MCI to reflect these deficits in complex instrumental functions.40,41 Although basic activities of daily living usually remain intact in aMCI, mild IADL deficits appear to often occur in aMCI. The aMCI group in this study was rigorously defined according to the Peterson criteria for aMCI,19 yet it still represents a heterogeneous stage of cognitive impairment. Other classification systems have been used to define cognitive impairment and each of these systems is likely to differ in the extent and severity of IADL deficits and identify slightly different clinical courses and outcomes.42 These findings represent a reminder that the MCI classification system denotes a continuum of impairment, and impairments based on the extent and severity of daily functioning can play an important role in defining where on this continuum a cognitively impaired individual can be classified. Greater functional deficits, associated with greater medial temporal atrophy, memory, and processing speed deficits, can aid practicing physicians and researchers in interpreting the point in the predementia stage in which the patient should be classified, and help predict the speed at which the condition of that patient will convert to dementia.26,29 Aiding in earlier identification of the stage of the disease can lead to earlier enrollment of patients in clinical trials for treatment of cognitive impairment, earlier financial and estate planning, the designation of health care proxies, and the preparation of families for the future responsibility and cost of providing care for the patient. These findings show that disruptions in daily functioning, even mild disruptions, may be an important clinical indicator of disease and represent latter phases of disease progression within the MCI classification system for cognitive impairment.43
FACTORS ASSOCIATED WITH FUNCTIONAL DEFICITS IN COGNITIVELY IMPAIRED INDIVIDUALS
Past studies have identified strong associations between functional impairment and medical illness,9,10 mood disorders,11–14 and cognitive impairment.15–17 This study found that the functional impairment (total severity, total number, and average severity per deficit) was associated with smaller hippocampal volumes and decreased processing speed in the combined aMCI and AD groups. Functional severity and total number of functional deficits were also associated with worse memory performance on the AVLT 30-minute delay and decreased entorhinal cortex volumes.
Within the aMCI group, the associations among memory deficits (AVLT 30-minute delay and decreased hippocampal volume), processing speed decrements (Trailmaking Test A), and greater functional impairment were again identified. There was distinct heterogeneity within the aMCI group illustrated in the post hoc and logistic models. Although the trends across groups for the severity and number of functional deficits analyses showed that with increasing deficits in daily activities, impairment in memory and processing speed and medial temporal atrophy worsened, the strongest difference was between the functionally intact and moderate to severe or high deficit groups. The moderate to severe and high number of functional deficits groups with aMCI had greater hippocampal atrophy and impairment in memory and speed of processing compared with the functionally intact individuals with aMCI.
These results illustrate 2 possible mechanistic pathways that contribute to functional impairment in cognitively impaired individuals. One potential pathway, memory dysfunction, is represented by neuropsychological measures (AVLT 30-minute delay) and neurobiological markers (increased medial temporal atrophy). This finding is consistent with past findings showing increased atrophy in the 2 areas of the brain consistently associated with AD, the hippocampus and entorhinal cortex.29,44,45 Speed of processing marks the second potential pathway associated with functional impairment. Processing speed declines with age.46,47 Although researchers have focused on the association between executive dysfunction and daily functional deficits,48 the present study concurs with recent findings by Wadley and colleagues,15 who showed that individuals with aMCI differed from healthy controls in speed of processing on a financial performance measure. These findings intimate that gradual decreases in processing speed that occur with normal aging may accelerate in individuals with incipient dementia. We speculate that processing speed decrements may mark the initial onset of milder deficits in daily functioning, but executive dysfunction may lead to more severe impairments in daily functioning as the disease progresses. Longitudinal data need to be examined to test this hypothesis.
The status of APOE ε4, a genetic marker shown to increase risk of developing Alzheimer’s disease,49 was associated with an increased number of informant-reported functional deficits in the aMCI group. Those individuals with aMCI with a positive expression for the APOE ε4 allele had a greater number of functional deficits, although not more severe deficits, than those without the APOE ε4 allele. Individuals with an APOE ε4 allele may be predisposed to earlier onset of impairments in daily functioning, consistent with the increased risk of incipient dementia conferred by the presence of the APOE ε4 allele49, although these disruptions in daily activities do not require overt assistance by or overall dependence on spouses, family members, or friends.
The study has some design limitations. It used carefully selected individuals who agreed to participate in a research study with intensive serial procedures during an extended period. Part of the selection criteria included the exclusion of significant depressive symptomatology or coexisting medical disorders including vascular impairment. Although the present study illustrated that functional deficits partly overlap with cognitive deficits (specifically memory impairment and processing speed deficits)15,16 and may in part be considered a consequence of these impairments, the moderate amount of explained variance between functional and cognitive deficits in this study and another50 suggests that other factors contribute to the development of these deficits. Possible contributing factors to increased functional deficits include physical10 and psychological14 comorbidities, but these comorbidities were excluded in this sample. The exclusion of moderate to severe depression, in particular, hinders generalizibility of these findings because depression is common in patients with cognitive impairment, and the bidirectional relationship between depression and functional impairment is well-established.51 The use of different assessment methods (self-report for controls, and informant-report for the aMCI and mild AD groups) makes cross-comparisons between the cognitively impaired and cognitively intact groups difficult. Previous research has shown that self-reports can underreport symptoms in part due to worsening cognitive impairment and awareness,17,21,26 but informant reports in AD may overreport symptoms perhaps due to caregiver burden.52 Performance-based measures53 may more accurately reflect the ability of the patient to perform specific behaviors, although this remains to be established; performance-based measures were not evaluated in this study.
In conclusion, this study shows that mild deficits in daily activities are common in the aMCI stage of cognitive impairment and that this impairment should be considered in the MCI criteria. Functionally impaired individuals with aMCI had greater medial temporal atrophy and deficits in memory and processing speed compared with functionally intact individuals with aMCI. Future research should investigate the onset and course of functional impairment longitudinally to discern whether deficits in memory and processing speed or greater medial temporal atrophy are associated with the onset and progression of functional deficits during the disease process.
Acknowledgements
Funding/Support: Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, and the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott Laboratories, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation plc, Genentech Inc, GE Healthcare, GlaxoSmithKline plc, Innogenetics, Johnson & Johnson Services Inc, Eli Lilly and Company, Medpace Inc., Merck and Co Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche Ltd, Schering-Plough Corporation, Synarc, Inc., and Wyeth Ltd, as well as non-profit partners the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the US Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory of Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129 and K01 AG030514 and the Dana Foundation. This research was also supported by Grants R01AG17761 and T32 MH20004.
Footnotes
Author Contributions: Drs. Brown and Devanand had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosures: Dr. Devanand has received research support from Novartis AG and Eli Lilly and Company, and has served as a consultant to Bristol-Myers Squibb and Sanofi-Aventis.
Additional Information: Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.ucla.edu/). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. ADNI investigators include (complete listing available at http://www.loni.ucla.edu/ADNI/Collaboration/ADNI_Manuscript_Citations.pdf).
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 2000. text revision. [Google Scholar]
- 2.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 3.Ballard C, O’Brien JT, James I, Mynt P, Lana M, Potkins D, Reinchelt K, Lee L, Swann A, Fossey J. Quality of life for people with dementia living in residential and nursing home care: The impact of performance on activities of daily living, behavioral and psychological symptoms, language skills, and psychotropic drugs. Int Psychogeriatr. 2001;13:93–106. doi: 10.1017/s1041610201007499. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Salvador T, Lyketsos CG, Baker A, Hovanec L, Roques C, Brandt J, Steele C. Quality of life in dementia patients in long-term care. Int J Geriatr Psychiatry. 2000;15:181–189. doi: 10.1002/(sici)1099-1166(200002)15:2<181::aid-gps96>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Newsom JT, Shulz R. Social support as a mediator in the relation between functional status and quality of life in older adults. Psychol Aging. 1996;11:34–44. doi: 10.1037/0882-7974.11.1.34. [DOI] [PubMed] [Google Scholar]
- 6.Newsom JT, Shulz R. Caregiving from the recipient’s perspective: negative reactions to being helped. Health Psychol. 1998;17:172–181. doi: 10.1037//0278-6133.17.2.172. [DOI] [PubMed] [Google Scholar]
- 7.Hope T, Keene J, Gedling K, Fairburn CG, Jacoby R. Predictors of institutionalization for people with dementia living at home with a carer. Int J Geriatr Psychiatry. 1998;13:682–690. doi: 10.1002/(sici)1099-1166(1998100)13:10<682::aid-gps847>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Jorm AF. Disability in dementia: assessment, prevention, and rehabilitation. Disabil Rehabil. 1994;16:98–109. doi: 10.3109/09638289409166286. [DOI] [PubMed] [Google Scholar]
- 9.Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment – the relationship between physical and cognitive function. Neuroepidemiology. 2008;31:167–173. doi: 10.1159/000154929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence of a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 11.Ani C, Bazargan M, Hindman D, Bell D, Farooq MA, Akhanjee L, Yemofio F, Baker R, Rodriguez M. Depression symptomatology and diagnosis: discordance between patients and physicians in primary care settings. BMC Fam Pract. 2008;9:1–9. doi: 10.1186/1471-2296-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onyike CU, Sheppard JM, Tschanz JT, Norton MC, Green RC, Steinberg M, Welsh-Bohmer KA, Breitner JC, Lyketsos CG. Epidemiology of apathy in older adults: the Cache County Study. Am J Geriatr Psychiatry. 2007;15:365–375. doi: 10.1097/01.JGP.0000235689.42910.0d. [DOI] [PubMed] [Google Scholar]
- 13.Starkstein SE, Jorge R, Mizrahi R, Robinson RG. A prospective longitudinal study of apathy in Alzheimer’s disease. J Neurol, Neurosurg Psychiatry. 2006;77:8–11. doi: 10.1136/jnnp.2005.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elderkin-Thompson V, Ballmaier M, Hellemann G, Pham D, Lavretsky H, Kumar A. Daily functioning and prefrontal brain morphology in healthy and depressed community-dwelling elderly. Am J Geriatr Psychiatry. 2008;16:633–642. doi: 10.1097/JGP.0b013e3181794629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wadley VG, Okonkwo O, Crowe M, Rosee-Meadows LA. Mild cognitive impairment and everyday function: Evidence of reduced speed in performing instrumental activities of daily living. Am J Geriatr Psychiatry. 2008;16:416–424. doi: 10.1097/JGP.0b013e31816b7303. [DOI] [PubMed] [Google Scholar]
- 16.Pereira FS, Yassuda MS, Oliveira AM, Forlenza OV. Executive dysfunction correlates with impaired functional status in older adults with varying degrees of cognitive impairment. Int Psychogeriatr. 2008;20:1104–1115. doi: 10.1017/S1041610208007631. [DOI] [PubMed] [Google Scholar]
- 17.Okonkwo OC, Wadley VG, Griffith R, Belue K, Lanza S, Zamrini EY, Harrell LE, Brockington JC, Clark D, Raman R, Marson DC. Awareness of deficits in financial abilities in patients with mild cognitive impairment: Going beyond self-informant discrepancy. Am J Geriatr Psychiatry. 2008;16:650–659. doi: 10.1097/JGP.0b013e31817e8a9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson TL, Harvey P. Real-world functioning and self-evaluation of functioning: Brain structure, mood state, functional skills, and mortality. Am J Geriatr Psychiatry. 2008;16:617–620. doi: 10.1097/JGP.0b013e31817f8cb9. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 20.Knopman DS, Boeve BF, Petersen RC. Essentials of the proper diagnoses of mild cognitive impairment, dementia, and major subtypes of dementia. Mayo Clin Proc. 2003;78:1290–1308. doi: 10.4065/78.10.1290. [DOI] [PubMed] [Google Scholar]
- 21.Albert SM, Michaels K, Padilla M, Pelton GH, Bell K, Marder K, Stern Y, Devanand DP. Functional significance of mild cognitive impairment in elderly patients without a dementia diagnosis. Am J Geriatr Psychiatry. 1999;7:213–220. doi: 10.1097/00019442-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Farias ST, Mungas D, Reed BR, Harvey D, Cahn-Weiner D, DeCarli C. MCI is associated with deficits in everyday functioning. Alzheimer’s Dis Assoc Disord. 2006;20:217–223. doi: 10.1097/01.wad.0000213849.51495.d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 24.Peres K, Helmer C, Amieva H, Orgogozo JM, Rouch I, Dartigues JF, Barberger-Gateau P. Natural history of decline in instrumental activities of daily living performance over the 10 years preceding the clinical diagnosis of dementia: a prospective population-based study. J Am Geriatr Soc. 2008;56:37–44. doi: 10.1111/j.1532-5415.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- 25.Pfeffer RI, Kurosaki TT, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 26.Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton GH, Liu X, Stern Y, Devanand DP. Functional deficits in patients with mild cognitive impairment: Prediction of Alzheimer’s disease. Neurology. 2002;58:758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 27.Marson DC, Sawrie SM, Snyder S, McInturff B, Stalvey T, Boothe A, Aldridge T, Chatterjee A, Harrell LE. Assessing financial capacity in patients with Alzheimer disease: A conceptual model and prototype instrument. Arch Neurol. 2000;57:877–884. doi: 10.1001/archneur.57.6.877. [DOI] [PubMed] [Google Scholar]
- 28.Wadley VG, Okonkwo O, Crowe M, Vance DE, Elgin JM, Ball KK, Owsley C. Mild cognitive impairment and everyday function: An investigation of driving performance. J Geriatr Psychiatry Neurol. 2009;22:87–94. doi: 10.1177/0891988708328215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry. 2008;15:871–879. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller SG, Weiner MW, Thal LJ, Petersen RC, Jack C, Jagust W, Trojanowski JQ, Toga AW, Beckett L. The Alzheimer’s Disease Neuroimaging Initiative. Neuroimaging Clin N Am. 2005;15:869–877. doi: 10.1016/j.nic.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler DA. Wechsler Memory Scale–Revised manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 34.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–172. [Google Scholar]
- 35.Hachinski VC, Iliff LD, Zilhka E, Du Boulay GH, McAllister VL, Marshall J, Russell RW, Symon L. Cerebral blood flow in dementia. Arch Neurol. 1975;32:632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 36.Reitan R. The validity of the Trail-Making Test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 37.Wechsler DA. Manual for the Wechsler Adult Intelligence Scale–Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 38.Rey A. l’Examen Clinique en Psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 39.Liu X, Jin Z. Item reduction in a scale for screening. Stat Med. 2007;26:4311–4327. doi: 10.1002/sim.2853. [DOI] [PubMed] [Google Scholar]
- 40.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on mild cognitive impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 41.Portet F, Ousset PJ, Visser PJ, Frisoni GB, Nobili F, Scheltens PH, Vellas B, Touchon J. MCI Working Group of the European Consortium on Alzheimer’s Disease (EADC): Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure report of the MCI Working Group of the European Consortium on Alzheimer’s Disease. J Neurol Neurosurg Psychiatry. 2006;77:714–718. doi: 10.1136/jnnp.2005.085332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephan BC, Matthews FE, McKeith IG, Bond J, Brayne C. Early cognitive change in the general population: How do different definitions work? J Am Geriatr Soc. 2007;55:1534–1540. doi: 10.1111/j.1532-5415.2007.01386.x. [DOI] [PubMed] [Google Scholar]
- 43.Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, Walter S, Trojanowski JQ, Shaw LM, Beckett LA, Jack CR, Jagust W, Toga AW, Saykin AJ, Morris JC, Green RC, Weiner MW. Clinical core of the Alzheimer’s disease Neuroimaging initiative: Progress and plans. Alzheimers Dement. 2010;6:239–246. doi: 10.1016/j.jalz.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R, Stern Y, Tabert MH, de Leon MJ. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer’s disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 45.Stoub TR, Bulgakova M, Leurgans S, Bennett DA, Fleischman D, Turner DA, deToledo-Morrell L. MRI predictors of risk of incident Alzheimer’s disease: A longitudinal study. Neurology. 2005;64:1520–1524. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- 46.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 47.Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- 48.Vaughan L, Giovanello K. Executive function in daily life: Age-related influences of executive processes on instrumental activities of daily living. Psychol Aging. 2010;25:343–355. doi: 10.1037/a0017729. [DOI] [PubMed] [Google Scholar]
- 49.Wolk DA. Alzheimer’s Disease Neuroimaging Initiative. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proc Natl Acad Sci. 2010;107:10256–10261. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okonkwo OC, Wadley VG, Griffith HR, Ball K, Marson DC. Cognitive correlates of financial abilities in mild cognitive impairment. J Am Geriatr Soc. 2006;54:1745–1750. doi: 10.1111/j.1532-5415.2006.00916.x. [DOI] [PubMed] [Google Scholar]
- 51.Lenze EJ, Rogers JC, Martire LM, Mulsant BH, Rollman BL, Dew MA, Schulz R, Reynolds CF. The association of late-life depression and anxiety with physical disability: a review of the literature and prospectus for future research. Am J Geriatr Psychiatry. 2001;9:113–135. [PubMed] [Google Scholar]
- 52.Arguelles S, Loewenstein DA, Eisdorfer C, Arguelles T. Caregivers’ judgments of the functional abilities of the Alzheimer’s disease patient: impact of caregivers’ depression and perceived burden. J Geriatr Psychiatry Neurol. 2001;14:91–98. doi: 10.1177/089198870101400209. [DOI] [PubMed] [Google Scholar]
- 53.Goldberg TE, Koppel J, Keehlisen L, Keehlisen L, Christen E, Dreses-Werringloer U, Conejero-Goldberg C, Gordon ML, Davies P. Performance-based measures of everyday function in mild cognitive impairment. Am J Psychiatry. 2010;167:845–853. doi: 10.1176/appi.ajp.2010.09050692. [DOI] [PMC free article] [PubMed] [Google Scholar]