Abstract
The cell-surface receptor for the gonadotropin follicle-stimulating hormone (FSH) is expressed exclusively on Sertoli cells of the testis and granulosa cells of the ovary. FSH signal transduction through its receptor (Fshr) is critical for the timing and maintenance of normal gametogenesis in the mammalian gonad. In the 13 years since the gene encoding Fshr was first cloned, the mechanisms controlling its transcription have been extensively examined, but a clear understanding of what drives its unique cell-specificity remains elusive. Current knowledge of basal Fshr transcription highlights the role of an E-box in the proximal promoter which is bound by the basic helix-loop-helix transcription factors upstream stimulatory factor 1 (Usf1) and Usf2. Recent studies utilizing knockout mice and chromatin immunoprecipitation validated the importance of Usf to Fshr transcription and demonstrated a sexually dimorphic requirement for the Usf proteins to maintain normal Fshr expression. Studies have also shown that the promoter region itself is insufficient for appropriate Fshr expression in transgenic mice, indicating Fshr transcription depends on regulatory elements that lie outside of the promoter. Identification of such elements has been propelled by recent availability of genome sequence data, which facilitated studies using comparative genomics, DNase I hypersensitivity mapping, and transgenic analysis with large fragments of DNA. This review will focus on the current understanding of transcriptional regulatory processes that control expression of rat Fshr, including recent advances from our laboratory.
Keywords: Fshr, Sertoli cell, granulosa cell
I. Introduction
FSH and its relative, luteinizing hormone (LH), are major components of the hypothalamic-pituitary-gonad axis, which regulates reproductive function and ultimately the production of gametes and fertility. Hormone levels for LH and FSH are controlled by hypothalamic gonadotropin releasing hormone, which induces their synthesis and secretion from anterior pituitary gonadotrope cells. Once in circulation, hormonal activity is directed through specific cell surface receptors, whose expression ultimately determines the site of action of these hormones. In the case of FSH, its receptor is expressed only in a subpopulation of gonadal somatic cells, Sertoli cells of the testis and granulosa cells of the ovary (Camp et al., 1991; Heckert and Griswold, 1991; Kliesch et al., 1992; Bockers et al., 1994; Houde et al., 1994; Sites et al., 1994; Dankbar et al., 1995; Rannikki et al., 1995; Tisdall et al., 1995; Xu et al., 1995; Yuan et al., 1996; Oktay et al., 1997; Yamamura et al., 2001; Mattiske et al., 2002; Bluhm et al., 2004; Kwok et al., 2005). Therefore, as the basis for directing FSH biological action to Sertoli and granulosa cells, the mechanisms regulating expression of Fshr are key determinants of endocrine control of gamete production and fertility.
II. The Fshr gene
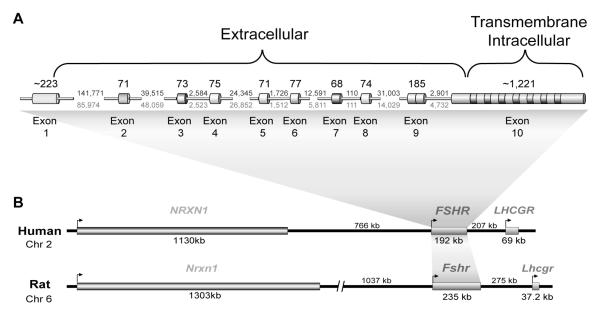
The quest to understand the mechanisms that direct Fshr expression to these two cell types has led to the evaluation of its gene structure and examination of regions that may contain key elements that drive Fshr transcription. The structure of Fshr has been determined in a number of species, including rat (Heckert et al., 1992), mouse (Huhtaniemi et al., 1992; Tena-Sempere et al., 1999), human (Gromoll et al., 1994; Gromoll et al., 1996), sheep (Sairam and Subbarayan, 1997), tammar wallaby (Mattiske et al., 2002), and computationally predicted in the chimpanzee, bovine, dog, chicken and zebrafish (Table 1). In each, there are 10 exons, with exons 1-9 encoding the extracellular domain and exon 10 the transmembrane and intracellular domains (Fig. 1A). Although the basic structure for Fshr has been known for more than a decade, not until recently, with completion of various genome sequences, have we had an accurate understanding of the size of Fshr or any appreciation of its surrounding genomic environment. The coding sequence spans a substantial distance, ranging in mammals from roughly 80kb in the bovine to more than 235kb in the rat (Fig. 1B, Table 1). The genomic environment surrounding Fshr is relatively devoid of other coding genes, with its closest neighbor Lhcgr over 200 kb away, placing it in a class of genes that reside in gene deserts (Nobrega et al., 2003; Ovcharenko et al., 2005). In addition to it’s gene-poor chromosomal environment, Fshr shares other similarities with gene deserts with respect to its guanine-cytosine content, evolutionary conservation, repetitive sequence content, and density of single nucleotide polymorphisms (Ovcharenko et al., 2005). Notably, gene deserts are thought to house regulatory elements that act over great genomic distances to affect expression of resident genes (Lettice et al., 2002; Nobrega et al., 2003; Ovcharenko et al., 2005). Insights on Fshr structure and its surrounding environment have facilitated studies to identify specific sequences responsible for controlling its transcription.
Table 1.
Sizes and location of known and predicted Fshr genes from several species
| Organism | Chromosome | Size (bp)# | Entrez Gene ID |
cDNA Accession‡ |
Reference |
|---|---|---|---|---|---|
| Rat (Rattus norvegicus) |
6 | 235303* | 25449 | NM_199237 | Heckert et al., 1992 |
| Mouse (Mus musculus) |
17 | 216493 | 14309 | NM_013523 |
Huhtaniemi et al., 1992; Tena-Sempere et al., 1999 |
| Human (Homo sapiens) |
2 | 191978 | 2492 | NM_000145 |
Gromoll et al., 1994; Gromoll et al., 1996 |
| Sheep (Ovis aries) |
nd | 85000- 100000 |
443299 |
NM_001009
289 |
Sairam and Subbarayan, 1997 |
| Chicken (Gallus gallus) |
3 | 77614§ | 395962 | NM_205079 | You et al., 1996 |
| Bovine (Bos taurus) |
11 | 79368§ | 281172 | NM_174061 | Houde et al., 1994 |
| Zebrafish (Danio rerio) |
2 | 31229§ | 195820 |
NM_001001
812 |
Kwok et al., 2005 |
| Dog (Canis familiaris) |
10 | 167410§ψ | N/A | N/A | N/A |
| Chimpanzee (Pan troglodytes) |
12 | 192102§† | N/A | N/A | N/A |
nd: not determined.
Size of rat Fshr is 23.5kb shorter than previous reports due to resolution of unsequenced gaps.
Determined using the Ensembl genome browser (http://www.ensembl.org).
Estimated size based on automated exon alignment from the referenced mRNA sequences.
Predicted by Ensembl automatic analysis pipeline using a GeneWise model from a dog/vertebrate protein.
Predicted from Ensembl human gene predictions via whole genome blastz alignments from UCSC.
N/A: not available.
GenBank accession numbers as assigned by National Center for Biotechnical Information at the National Library of Medicine.
Figure 1. Fshr gene structure and chromosomal location.
(A) The exon-intron structure of Fshr corresponds to the domain structure of the protein. Exons 1 through 9 code for the extracellular ligand binding domain while exon 10 codes for the transmembrane and intracellular domains. Each of the small exons 2 through 8 encode individual leucine rich repeats and exon 9 codes for 2. The size of each exon in base pairs is shown above the diagram and the intron sizes are noted for rat (above) and human (below) between each exon. (B) In addition to the gene structure, synteny in the chromosomal environment surrounding Fshr is conserved between species. The size of Fshr and neighboring genes in humans and rats is shown above each gene and intergenic distances are noted. Adapted with permission from Heckert, 2005 (Copyright 2005, Elsevier Academic Press).
III. Fshr transcriptional regulation
A. The Fshr promoter region
Studies examining Fshr transcriptional regulation have focused almost entirely on the region 5′ to the gene’s transcriptional start site, and included as much as 5000bp of sequence. Evaluation of this promoter region uncovered a number of important regulatory elements and transcription factors that establish basal promoter activity. For purposes of this review, discussion will primarily reflect data from our laboratory on rat Fshr, but notably, comparison to studies with mouse, sheep, and human 5′flanking regions revealed several key promoter features that are conserved between species (reviewed in Heckert, 2005; Huhtaniemi et al., 1992; Gromoll et al., 1994; Linder et al., 1994; Goetz et al., 1996; Sairam and Subbarayan, 1997; Heckert et al., 1998; Kim and Griswold, 2001). Transcription of rat Fshr initiates from two majors sites positioned 98bp and 80bp upstream of the translational start codon (Heckert et al., 1992). The transcriptional start sites lie within a putative initiator region (InR), which functions in positioning the RNA polymerase II initiation complex and may help direct the transcriptional effects of cell-specific factors at the promoter (Heckert et al., 1992; Smale and Kadonaga, 2003). Transient transfection experiments using sequential deletions of the 5000bp promoter defined a region from −100bp to +123bp, relative to the first transcriptional start site (i.e.: −100bp corresponds to 198bp upstream of the start of translation) that represents the minimal promoter sequence required to maintain transcriptional activity (Fig. 2; Heckert et al., 1998; Heckert et al., 1992; Linder et al., 1994; Goetz et al., 1996). These studies also revealed two regions that repressed promoter activity (−100bp to −220bp and −2700bp to −3600bp), suggesting that transcriptional inhibitors bind in these regions, although the mechanisms remain uncharacterized (Fig. 2).
Figure 2. Transcriptional activity of the Fshr promoter.
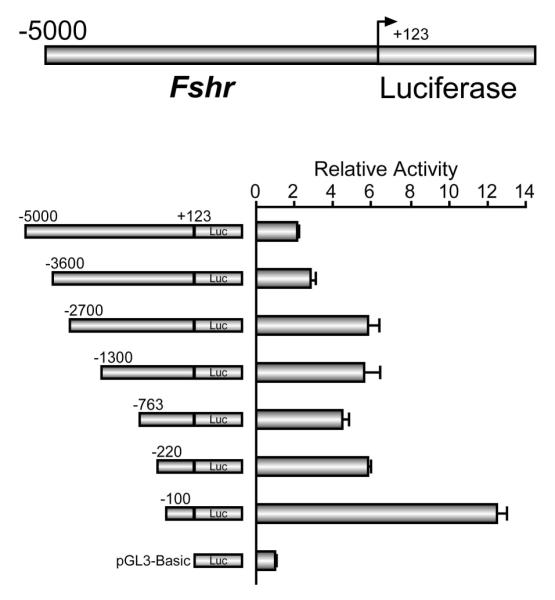
Promoter regions containing sequentially shorter amounts of Fshr 5′ flanking sequence, ranging from −5000bp through −100bp relative to the first Fshr transcriptional start site and extending to +123bp were placed immediately 5′ to the firefly luciferase reporter gene in pGL3-Basic. At the top, the longest promoter construct −5000bp/+123bp is shown as an example. Each of the constructs was transfected into the mouse Sertoli cell line MSC-1 along with a control plasmid (RSV-βgalactosidase), and the luciferase/βgalactosidase activity of each plasmid is shown relative to the luciferase/βgalactosidase activity of promoter-less pGL3-Basic. As little as 223bp of promoter sequence overlapping the transcriptional start sites was required to maintain transcriptional activity in transient transfections (−100bp/+123bp). Deletion mutagenesis also revealed two repressive regions are present in the promoter (−100 to −220 and −2.7kb to −3.6kb). Reprinted with permission from Heckert et al., 1998 (Copyright 1998, The Endocrine Society).
B. Elements within the Fshr promoter
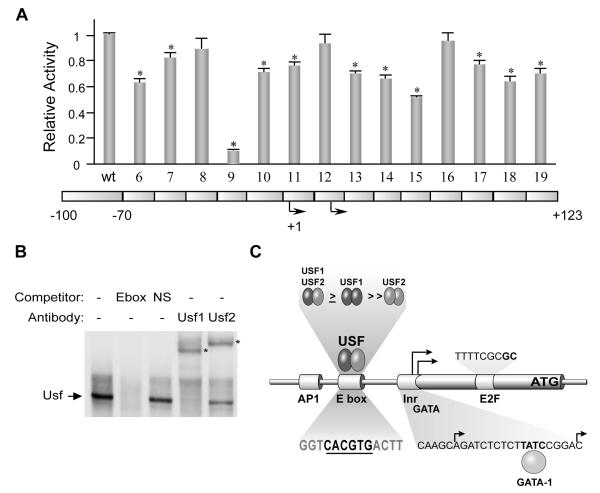
To identify important regulatory elements within the active promoter, sequences within the −100/+123 region were systematically mutated and functionally analyzed by transient transfection analysis (Fig. 3A). These block-replacement mutagenesis studies identified a small 14bp segment immediately upstream of the transcriptional start sites as the primary source of promoter activity (Fig. 3A, mutant 9; Heckert et al., 1998). This mutation included deletion of an E-box (5′-CACGTG-3′), a well-described element known to bind transcription factors in the basic helix-loop-helix family. Point mutations in core and flanking bases of the E-box abolished its activity, demonstrating its importance for promoter activity (Heckert et al., 1998). In addition to the E-box, mutation in a region of the first exon containing a putative E2F site (5′-GTTTTCGCGCT-3′) reduced promoter activity (Fig. 3A, mutant 15; Heckert et al., 1998; Kim and Griswold, 2001). Mutation of an inverted GATA site (5′-CTTATCCG-3′) in the InR reduced promoter activity in primary Sertoli cells, but demonstrated little contribution to promoter activity in the mouse Sertoli cell line MSC-1 (Fig. 3A, mutant 12; Heckert et al., 1998; Kim and Griswold, 2001). In conjunction with the minimal promoter region, studies have also demonstrated that the orphan nuclear receptor SF-1 activates transcription through sequence upstream of the promoter (between −743bp and −2700bp) (Heckert, 2001; reviewed in Heckert, 2005). Overall, the prominent role of the E-box in Fshr promoter activation, its functional conservation across species, and implications that it coordinates the activities of other transcription factors emphasizes the fundamental nature of the E-box in basal promoter function.
Figure 3. Fshr promoter activity requires the E-box and Usf1/2.
(A) Systematic block-replacement mutations were introduced into the Fshr (−220/+123) luciferase promoter construct and each mutant promoter construct was transfected into MSC-1 cells along with the control plasmid (RSV-βgalactosidase). The luciferase/βgalactosidase activity of each construct is shown normalized to the luciferase/βgalactosidase activity of the wildtype promoter. A diagram of the promoter below notes the position of each mutation directly below the bar showing transcriptional activity of that mutant. Bent arrows signify the two transcriptional start sites. Asterisks above the bars indicate a statistically significant change in promoter activity. Activity of the Fshr promoter was largely contained within a small region 20bp upstream of the transcriptional start site (mutant 9) which contained a conserved E-box element. Adapted with permission from Heckert et al., 1998 (Copyright 1998, The Endocrine Society) (B) Electrophoretic mobility shift assay was performed with nuclear proteins isolated from immature mouse testes and a radiolabeled probe containing the Fshr E-box (5′-TCTTGGTGGGTCACGTGACTTTGCCCGT-3′) as described (Heckert et al., 2000). When included in the reactions, antibodies against Usf1 and Usf2 cross-reacted with the major specific binding complex to form supershifted complexes (*), implicating Usf1 and Usf2 as components of the E-box binding complex (arrow). Homologous (E-box) or nonspecific competitors (NS) competitors were added at 100-fold molar excess and 2μg of antibody was included in noted reactions (see Heckert et al., 2000). (C) A model of Fshr promoter function emphasizes Usf1/2 binding to the E-box and includes contributions from E2F, GATA and AP-1 elements. Sequences of the E-box (underlined), InR (GATA site bolded), and E2F site are shown. Bolded bases in the E2F site are most functionally relevant. The relative abundance of Usf hetero- and homodimeric binding complexes in the testis is noted (unpublished data). Adapted with permission from Heckert, 2005 (Copyright 2005, Elsevier Academic Press).
C. The E-box and Usf are central to Fshr transcription
The critical role of the E-box to Fshr promoter activity underscored the need to identify the proteins that bind and mediate its activity, in order to elucidate the transcriptional mechanism of Fshr. Protein-DNA binding studies using electrophoretic mobility shift assays demonstrated that the E-box bound hetero- and homodimers of the basic helix-loop-helix transcription factors upstream stimulatory factor 1 (Usf1) and Usf2 (Fig. 3B & C; Goetz et al., 1996; Heckert et al., 1998; Heckert et al., 2000; Xing and Sairam, 2001). Usf binding to the E-box was abolished by mutations in the element that also eliminated promoter activity in transient transfection experiments (Heckert et al., 1998). Importantly, studies also demonstrated that overexpression of Usf proteins in transfected cells activated the Fshr promoter, while mutant proteins lacking a transactivation domain inhibited promoter activity (Heckert et al., 2000). The E-box and its binding proteins are also required for activation of the promoter by SF-1, indicating that the Usf proteins coordinate Fshr regulation through the E-box (Heckert, 2001). These studies clearly implicated the E box, Usf1, and Usf2 as the primary factors coordinating transcriptional activity of the Fshr promoter, but did not provide any evidence for their role in vivo.
The first implication that the E-box and Usf proteins functioned in vivo came from epigenetic studies showing that global demethylation of cells in culture resulted in activation of the normally silent Fshr gene, which correlated with binding status of the E-box (McGuinness et al., 1994; Griswold and Kim, 2001). Additional evidence from in vivo genomic footprinting demonstrated the E-box was bound by proteins in Sertoli cells, but not in non-expressing cells, indicating that, within the endogenous gene’s promoter, the E-box is occupied and thus functional only in expressing cells (Heckert et al., 2000). This finding, however, did not address the identity of the binding proteins. Evidence that the Usf proteins occupy the Fshr E-box, in vivo, (i.e. on the endogenous gene) was recently provided by studies employing chromatin immunoprecipitation (Fig. 4 and data not shown, manuscript in preparation). In these studies, antibodies against Usf1 or Usf2 specifically immunoprecipitated the Fshr promoter from cross-linked chromatin prepared from mouse Sertoli cells (Fig. 4; manuscript in preparation). Thus, PCR-amplification of the promoter was much greater in chromatin samples immunoprecipitated with Usf antibodies than in those immunoprecipitated with a non-specific IgG antibody (Fig. 4). Importantly, there was no significant difference in the Usf or IgG samples when the amplification was performed on a region 5000bp upstream (Fig. 4). While these data provide strong evidence for Usf/E-box interactions, in vivo, the resolution of this technique is restricted to the size of chromatin fragments immunoprecipitated, thus, precluding precise identification of the Usf binding site. Despite this limitation, in vivo binding of the Usf proteins to the E-box seems certain, given the accumulated evidence supporting their joint function.
Figure 4. Usf binds the Fshr promoter, in vivo.
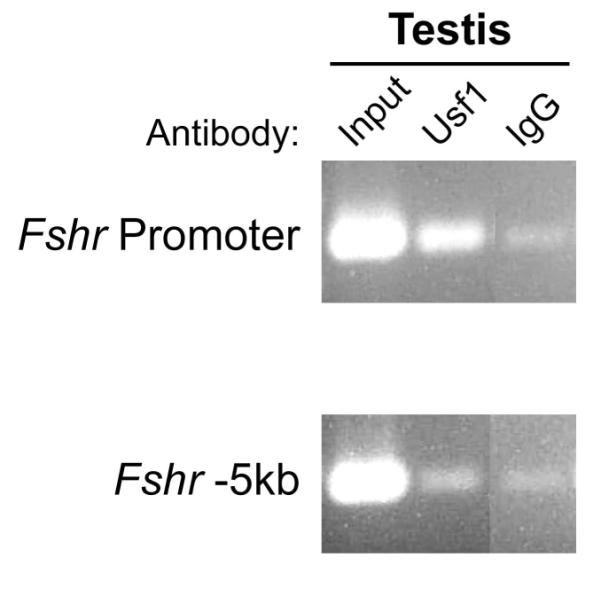
Chromatin immunoprecipitation was employed to analyze Usf1 and Usf2 binding to the Fshr E-box. Sertoli cells were isolated from mice and used to generate the chromatin employed in the assay (Heckert et al., 2000; Hermann and Heckert, 2005). Immunoprecipitation was performed either with an antibody to Usf1 or normal IgG, which was included as a negative control. Precipitated chromatin was PCR-amplified with primers to detect either the Fshr promoter or a region located 5kb upstream of Fshr exon 1. Samples containing a portion of the chromatin input served as positive controls. The rabbit anti-Usf1(C-20X) IgG was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
The question of whether the Usf proteins are required for Fshr expression, in vivo, was further addressed by evaluating Fshr expression in mice lacking Usf1 or Usf2. Results indicated that levels of ovarian Fshr mRNA were reduced by nearly 1.7-fold in immature Usf1 and Usf2 knockout mice, when compared to wildtype littermates, with no apparent change in testis Fshr expression (manuscript in preparation). Importantly, Fshr expression in knockout gonads was not accompanied by changes in SF-1 expression, another putative Usf target gene, revealing that the impact on Fshr in the ovary was not due to diminished SF-1 activity (manuscript in preparation). This study revealed that, in the context of an intact animal, normal Fshr expression in the ovary depends, in part, on the presence of both Usf1 and Usf2, but surprisingly, its expression in the testis did not require the presence of both, despite abundant evidence implicating these proteins. The apparent inconsistency suggests a mechanism whereby homodimers of either protein supports Fshr expression in Sertoli cells. Unfortunately, the embryonic lethality of the Usf1 Usf2 double knockout mice has prevented testing this hypothesis, and thus, confirming a role for the Usf proteins in Fshr expression in the testis. Despite the inability to demonstrate an impact of Usf deficiency on testicular Fshr expression, the preponderance of data clearly implicates Usf in the control of Fshr transcription through the E-box.
IV. Distal regulatory elements are required for Fshr expression
A. Transgenic analysis of Fshr transcriptional regulation
Given the critical role of the E-box in Fshr transcription, it is tempting to speculate that the element and its major binding proteins, Usf1 and Usf1, direct cell specificity. However, as discussed below, cell-specific expression of Fshr requires sequences located very distant from the promoter’s E-box. Furthermore, Usf1 and Usf2 are ubiquitous proteins, and thus, their participation in cell-specific regulation of Fshr could not be direct, but would require an additional layer of regulation, such as cell-specific dimerization, co-activators, or modifications to the proteins themselves (i.e.: phosphorylation) (Murre et al., 1989; Gregor et al., 1990; Kirschbaum et al., 1992; Sirito et al., 1994; Luo and Sawadogo, 1996; Viollet et al., 1996; Qyang et al., 1999). However, transient transfection experiments demonstrating significant Fshr promoter activity in a variety of cell types, including those that do not express Fshr, suggest the Usf proteins can ubiquitously activate the Fshr promoter, if the E box is accessible, and do not require additional regulatory mechanisms to induce transcription (Heckert et al., 1998).
The most compelling data that the E-Box and its binding proteins do not direct cell-specific expression of Fshr comes from studies employing transgenic mice. Four studies have tested the ability of constructs containing the Fshr promoter region to direct cell-specific expression of reporter genes, in vivo (Linder et al., 1994; Heckert et al., 2000; Nordhoff et al., 2003; Hermann et al., 2006). Three out of the four implicated a promoter deficient in the necessary information for cell-specificity (Heckert et al., 2000; Nordhoff et al., 2003; Hermann et al., 2006). In one study, 5000bp and 198bp Fshr promoters were used to drive expression of Cre recombinase and transgene expression was examined in 11 tissues from 16 transgenic lines (8 for each transgene; Heckert et al., 2000). While transgene expression was detected in the testes and ovaries, ectopic transgene expression was also observed in a number of different tissues, particularly the brain, eyes and heart, indicating neither promoter sufficiently prevented inappropriate transgene expression (Heckert et al., 2000). Moreover, both promoters were inactive in immature testes and further examination of mice carrying the 198bp promoter revealed testicular transgene expression reflected its production in germ cells, not Sertoli cells. Thus, the results indicated that the Fshr promoter could not activate Fshr expression in Sertoli cells (Heckert et al., 2000). The absence Cre expression from either transgene in immature testes agreed with this finding, since Sertoli cells express Fshr at that stage (Heckert et al., 2000). Importantly, another transgenic study using the human FSHR promoter demonstrated that 1500bp of sequence failed to appropriately regulate expression in mice (Nordhoff et al., 2003). These transgenic studies indicated that cis-regulatory elements located outside the promoter are required for proper cell-specific expression of Fshr.
Prompted by these initial findings, a more recent transgenic study utilized a substantial amount of genomic sequence as a transgene in order to secure a proper expression profile. Thus, a 413kb region of the rat genome, including Fshr and a significant amount of flanking sequence, was used as a transgene to evaluate Fshr expression, in vivo (Hermann et al., 2006). Despite the large amount of sequence, the transgene never expressed in the ovary or immature testis of transgenic mice. Reminiscent of the previous plasmid transgenic studies, germ cells from the adult testis abundantly expressed the transgene, while no expression was observed in Sertoli cells. Thus, not only did this study substantiate previous findings indicating a deficiency in the promoter, but it extended the placement of critical regulatory elements to a region outside the transgene sequence (i.e.: 97kb upstream of exon 1 to 57kb downstream of exon 10). In contrast to these three reports, one transgenic study suggested cell-specificity was obtained with the Fshr promoter (Linder et al., 1994). However, the limited scope of the study obscured accurate evaluation of cellular specificity, and thus, the majority of evidence supports a role for distal cis-regulatory elements in Sertoli and granulosa cell Fshr transcription and preventing ectopic gene expression.
B. Identification of distal Fshr regulatory elements
Interest in the identity of regulatory sequences that restrict Fshr expression to Sertoli and granulosa cells prompted a thorough evaluation of the genomic sequence in the chromosomal region that houses Fshr. However, pinpointing the relevant regulatory sequences in the vicinity of Fshr presented a significant challenge given its size, the potential for regulatory elements to act at very large distance (i.e. as far 1 Mb), the lack of a defined, transcriptionally-competent locus for Fshr, and the knowledge that at least some important elements reside outside the region defined by the 413kb transgene (Bishop et al., 2000; Lettice et al., 2002; Qin et al., 2004; Sagai et al., 2005; Hermann et al., 2006). To facilitate the search, comparative genomics and DNase I hypersensitivity (DHS) mapping were employed to identify evolutionarily conserved and DNase I hypersensitive sequences, both hallmarks of transcriptional regulatory regions.
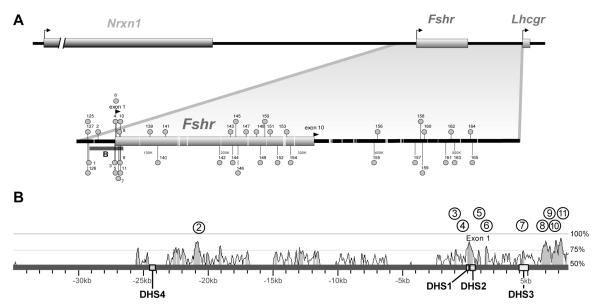
Sequence comparison across the Fshr locus was first reported in a study that used a pairwise alignment strategy to directly compare the rat and human sequences. This study identified 156 co-linear, conserved sites in the vicinity of Fshr and was combined with a functional screen for chromatin modifications using DHS mapping (Fig. 5A and B; Hermann and Heckert, 2005). A conserved, hypersensitive site (DHS3/site7) was identified in the first intron, and transient transfection analysis showed it acted as a silencing element (Fig. 5B; Hermann and Heckert, 2005). Further characterization showed that inhibitory activity of the element was primarily restricted to non-expressing cells, suggesting that it bound proteins that silence Fshr activity in order to restrict inappropriate expression (Hermann and Heckert, 2005). Evaluation of the binding proteins revealed that octamer transcription factor 1 and a GATA factor modulate activity of the site (Hermann and Heckert, 2005). While this combined approach of comparative genomics and DHS mapping proved an effective means to identify regulatory elements, the evaluation of more than 100 conserved sites as potential regulatory sequences, even in combination with DHS mapping, is an unrealistic task. However, with completion of additional genome sequences and development of more sophisticated software programs, comparative studies can now be used more effectively, by including sequences of more divergent species, to identify the most highly conserved sequences, and thus, ones most likely to be functional. (Groves, 2001; Nobrega and Pennacchio, 2004).
Figure 5. Fshr regulatory elements revealed by comparative genomics and DHS mapping.
(A) At the top, the chromosomal environment of Fshr and its neighboring genes (Nrxn1 and Lhcgr) is shown. Below, a portion of this region from the rat and human FSHR loci was compared by pairwise sequence analysis to identify conserved sequenced (≥75% sequence identity over 100bp or more; Hermann and Heckert, 2005). Identified conserved sequences are noted by numbered balloons. The Fshr coding region is noted (shaded rectangle), white lines in the base sequence represent repeats, and the black bar below indicates the region shown in part B. (B) DNase I hypersensitivity (DHS) mapping performed across a 45kb segment of the 5′flanking sequence of the gene using Sertoli cells and non-expressing peritubular myoid cells identified four hypersensitive sites (DHS1-4; Hermann and Heckert, 2005). DHS1, 2 and 4 were specific to Sertoli cells, while DHS3 was observed in both cell types. The position of the DHS sites (numbered boxes) are shown on the sequence (black line), and a percent identity curve above the line shows the percent identity between human and rat Fshr (vertical axis) at any given position on the sequence (horizontal axis), illustrating the position of the evolutionary conserved sequences over this segment of Fshr. The curve was generated by the VISTA genome browser by comparing human and rat Fshr (http://pipeline.lbl.gov; Couronne et al., 2003). Shading beneath the curve indicates ≥75% sequence identity and the position of conserved sites identified by pairwise analysis are noted by numbered circles (Hermann and Heckert, 2005). The position of exon 1 is noted.
Subsequent sequence analysis of Fshr, employing multiple genomes and more stringent parameters, revealed only the most highly conserved sequences within the Fshr locus, reducing the number of candidate sites to seven (Hermann et al., 2006). Each of the seven evolutionary conserved regions (ECRs1-7) were evaluated by transient transfection analysis in Sertoli or myoid cells and shown to have associated transcriptional activity, and two exhibited differential effects in expressing and non-expressing cells. These results suggested the ECRs house distal regulatory elements, two of which are involved in cell-specific transcriptional control. Importantly, the absence of six ECRs from the large genomic transgene and the transcriptional activity associated with each ECR is persuasive evidence that these highly conserved regions function as distal elements required for appropriate Fshr regulation.
V. Distal regulatory elements and Fshr transcription
A wealth of evidence thus indicates that distal cis-regulatory elements participate in Fshr transcriptional control and candidates for these elements (i.e.: ECRs1-7) have been identified based on their evolutionary conservation, as described above. Several models have been proposed to account for the action of distally located cis-acting sequences in Fshr transcription (Hermann et al., 2006). In one model, cell-specific transcription factors bind distal Fshr enhancers in Sertoli and granulosa cells, which, in turn, interact with proteins bound to the promoter (i.e.: Usf1 and Usf2 at the E-box) to activate Fshr expression, either through direct protein-protein interaction or indirectly via participation of bridging factors (Fig. 6). These interactions likely help recruit histone modifying enzymes to establish a local chromatin conformation that favors Fshr transcriptional activity (Fig. 6; Jenuwein and Allis, 2001). Alternatively, an active region of the Fshr locus could be protected from transcriptionally repressive heterochromatin in expressing Sertoli and granulosa cells by proteins that recruit histone acetyltransferases to distal insulators at its boundaries (Fig. 6). Moreover, in non-expressing cells, inappropriate Fshr activation by an exogenous enhancer might be prevented by enhancer-blocking proteins that bind distal insulators. Finally, an “active chromatin hub” model proposes that proteins bound to distal elements might direct displacement of Fshr to transcription foci in nuclei of Sertoli and granulosa cells to facilitate its expression. Although data for these models are compelling for other genes, the precise roles of distal regulatory elements in Fshr transcription will only emerge as specific cis-acting elements and their cognate binding proteins are defined within each site and the ability of these sequences to restrict Fshr expression to Sertoli and granulosa cells is tested in animal models.
Figure 6. Proposed model of Fshr transcription.
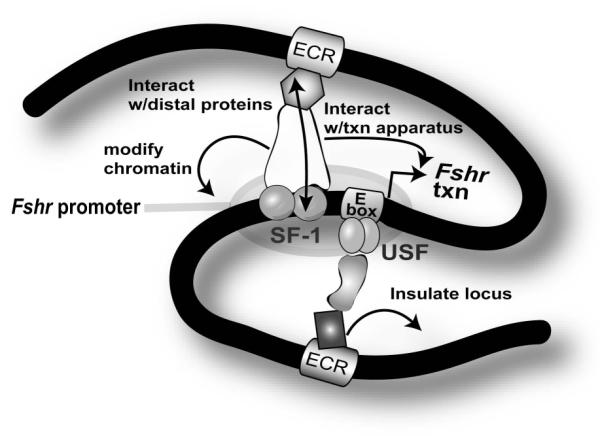
Transcription factors (shaded objects) bind cis-regulatory elements (rectangles) on Fshr locus DNA (black line). Within the promoter region, Usf1 and Usf2 bind to the E-box and SF-1 binds to a region upstream of the transcriptional start site (bent arrow). Other distally-located elements are proposed to reside within evolutionarily conserved regions (ECRs). While some transcription factors bind to DNA, others form bridges between bound transcription factors. In Sertoli and granulosa cells, proteins bound to distal enhancers might interact with transcription factors bound to the Fshr promoter via chromatin loops, inducing secondary modifications to the local chromatin environment and interactions with the transcriptional apparatus to activate Fshr transcription. Proteins bound to distal insulators might protect Fshr from the surrounding heterochromatin environment or block action of neighboring genes’ enhancers.
VI. Summary and Conclusions
Progress towards understanding Fshr transcriptional regulation has recently gained momentum by studies that have revisited the long-standing mechanisms of promoter function in the context of the whole animal and others which exploited genome sequence information to help identify new regulatory components. While the role of Usf in Fshr transcription was recently confirmed, in vivo, the studies also revealed an entirely new concept of Fshr regulation by demonstrating that gene expression is differentially affected by loss of Usf in Sertoli and granulosa cells. More extensive comparative analysis of the transcriptional characteristics of these two cell types will help explain how Fshr is differentially regulated in the testis and ovary. Despite our advances, though, the precise mechanisms controlling cell-specific and temporal expression are still unknown. Several lines of evidence indicate distal cis-regulatory elements (located outside of the promoter region) control cell-specific expression of Fshr. Candidate sequences were identified (i.e.: ECRs1-7) using comparative genomics but their roles in Fshr transcription await further characterization to define specific functional sequences and their cognate binding proteins. Irrespective of the true nature of these seven sequences, identifying regulatory elements and their binding proteins that are key to cell-specificity will add great insight to our understanding of Fshr regulation and gene expression specificity in Sertoli and granulosa cells and it seems certain that comparative genomics will pave the way.
Acknowledgements
The authors wish to thank Dr. R. R. M. Maran for critical reading of this manuscript, Kaori Hornbaker for assistance with mouse colony maintenance and cell culture, Dr. Lane Christenson for assistance with ChIP, and the Kansas IDeA Network of Biomedical Research Excellence (K-INBRE) for access to Discovery Studio Gene (Accelrys Inc, San Diego, CA).
The research was supported by the National Institutes of Child Health & Human Development (HD35217 to LLH), a University of Kansas Medical Center Biomedical Training fellowship to BPH and the University of Kansas Medical Center’s Center for Reproductive Sciences (NICHD SCCPRR; U54-HD33994).
Footnotes
Reprint requests and any correspondence to LLH.
References
- Bishop CE, Whitworth DJ, Qin Y, Agoulnik AI, Agoulnik IU, Harrison WR, Behringer RR, Overbeek PA. A transgenic insertion upstream of Sox9 is associated with dominant XX sex reversal in the mouse. Nat.Genet. 2000;26:490–494. doi: 10.1038/82652. [DOI] [PubMed] [Google Scholar]
- Bluhm AP, Toledo RA, Mesquita FM, Pimenta MT, Fernandes FM, Ribela MT, Lazari MF. Molecular cloning, sequence analysis and expression of the snake Follicle-stimulating hormone receptor. Gen.Comp Endocrinol. 2004;137:300–311. doi: 10.1016/j.ygcen.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Bockers TM, Nieschlag E, Kreutz MR, Bergmann M. Localization of Follicle-stimulating hormone (FSH) immunoreactivity and hormone receptor mRNA in testicular tissue of infertile men. Cell Tissue Res. 1994;278:595–600. doi: 10.1007/BF00331379. [DOI] [PubMed] [Google Scholar]
- Camp TA, Rahal JO, Mayo KE. Cellular localization and hormonal regulation of Follicle-stimulating hormone and Luteinizaing hormone receptor messenger RNAs in the rat ovary. Mol.Endocrinol. 1991;5:1405–1417. doi: 10.1210/mend-5-10-1405. [DOI] [PubMed] [Google Scholar]
- Couronne O, Poliakov A, Bray N, Ishkhanov T, Ryaboy D, Rubin E, Pachter L, Dubchak I. Strategies and tools for whole-genome alignments. Genome Res. 2003;13:73–80. doi: 10.1101/gr.762503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankbar B, Brinkworth MH, Schlatt S, Weinbauer GF, Nieschlag E, Gromoll J. Ubiquitous expression of the androgen receptor and testis-specific expression of the FSH receptor in the cynomolgus monkey (Macaca fascicularis) revealed by a ribonuclease protection assay. J.Steroid Biochem.Mol.Biol. 1995;55:35–41. doi: 10.1016/0960-0760(95)00148-s. [DOI] [PubMed] [Google Scholar]
- Goetz TL, Lloyd TL, Griswold MD. Role of E box and initiatory region in the expression of the rat Follicle-stimulating hormone receptor. J.Biol.Chem. 1996;271:33317–33324. doi: 10.1074/jbc.271.52.33317. [DOI] [PubMed] [Google Scholar]
- Gregor PD, Sawadogo M, Roeder RG. The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimer. Genes and Development. 1990;4:1730–1740. doi: 10.1101/gad.4.10.1730. [DOI] [PubMed] [Google Scholar]
- Griswold MD, Kim JS. Site-specific methylation of the promoter alters deoxyribonucleic acid-protein interactions and prevents Follicle-stimulating hormone receptor gene transcription. Biol.Reprod. 2001;64:602–610. doi: 10.1095/biolreprod64.2.602. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Dankbar B, Gudermann T. Characterization of the 5′ flanking region of the human Follicle-stimulating hormone receptor gene. Mol Cell Endocrinol. 1994;102:93–102. doi: 10.1016/0303-7207(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Gromoll J, Pekel E, Nieschlag E. The structure and organization of the human Follicle-stimulating hormone receptor gene. Genomics. 1996;35:308–311. doi: 10.1006/geno.1996.0361. [DOI] [PubMed] [Google Scholar]
- Groves CP, editor. Primate Taxonomy. Smithsonian Institute Press; Washington, D.C.: 2001. [Google Scholar]
- Heckert LL. Activation of the rat Follicle-stimulating hormone receptor promoter by Steroidogenic factor 1 is blocked by Protein kinase A and requires Upstream stimulatory factor binding to a proximal E box element. Mol Endocrinol. 2001;15:704–715. doi: 10.1210/mend.15.5.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckert LL. Structure and Regulation of the FSH Receptor Gene. In: Skinner MK, Griswold MD, editors. Sertoli Cell Biology. Elsevier Academic Press; San Diego, CA: 2005. pp. 281–302. [Google Scholar]
- Heckert LL, Daggett MA, Chen J. Multiple promoter elements contribute to activity of the Follicle- stimulating hormone receptor (FSHR) gene in testicular Sertoli cells. Mol Endocrinol. 1998;12:1499–1512. doi: 10.1210/mend.12.10.0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckert LL, Daley IJ, Griswold MD. Structural organization of the Follicle-stimulating hormone receptor gene. Mol.Endocrinol. 1992;6:70–80. doi: 10.1210/mend.6.1.1738373. [DOI] [PubMed] [Google Scholar]
- Heckert LL, Griswold MD. Expression of Follicle-stimulating hormone receptor mRNA in rat testes and Sertoli cells. Mol.Endocrinol. 1991;5:670–677. doi: 10.1210/mend-5-5-670. [DOI] [PubMed] [Google Scholar]
- Heckert LL, Sawadogo M, Daggett MA, Chen J. The USF proteins regulate transcription of the Follicle-stimulating hormone receptor but are insufficient for cell-specific expression. Mol.Endocrinol. 2000;14:1836–1848. doi: 10.1210/mend.14.11.0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Heckert LL. Silencing of Fshr occurs through a conserved, hypersensitive site in the first intron. Mol.Endocrinol. 2005;19:2112–2131. doi: 10.1210/me.2004-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Hornbaker KI, Maran RRM, Heckert LL. Distal regulatory elements are required for Fshr expression, in vivo. Mol Cell Endocrinol. 2006 doi: 10.1016/j.mce.2006.01.017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde A, Lambert A, Saumande J, Silversides DW, Lussier JG. Structure of the bovine Follicle-stimulating hormone receptor complementary DNA and expression in bovine tissues. Mol.Reprod.Dev. 1994;39:127–135. doi: 10.1002/mrd.1080390202. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I, Eskola V, Pakarinen P, Matikainen T, Sprengel R. The murine Luteinizing hormone and Follicle-stimulating hormone receptor genes: transcripional initiation sites, putative promoter sequences, and promoter activity. Mol.Cell.Endocrinol. 1992;88:55–66. doi: 10.1016/0303-7207(92)90009-u. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Kim JS, Griswold MD. E2F and GATA-1 are required for the Sertoli cell-specific promoter activity of the Follicle-stimulating hormone receptor gene. J Androl. 2001;22:629–639. [PubMed] [Google Scholar]
- Kirschbaum BJ, Pognonec P, Roeder RG. Definition of the transcriptional activation domain of recombinant 43-kilodalton USF. Mol.Cell Biol. 1992;12:5094–5101. doi: 10.1128/mcb.12.11.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliesch S, Penttila TL, Gromoll J, Saunders PT, Nieschlag E, Parvinen M. FSH receptor mRNA is expressed stage-dependently during rat spermatogenesis. Mol Cell Endocrinol. 1992;84:R45–R49. doi: 10.1016/0303-7207(92)90039-9. [DOI] [PubMed] [Google Scholar]
- Kwok HF, So WK, Wang Y, Ge W. Zebrafish gonadotropins and their receptors: I. Cloning and characterization of zebrafish Follicle-stimulating hormone and Luteinizing hormone receptors--evidence for their distinct functions in follicle development. Biol.Reprod. 2005;72:1370–1381. doi: 10.1095/biolreprod.104.038190. [DOI] [PubMed] [Google Scholar]
- Lettice LA. Disruption of a long-range cis-acting regulator for Shh causes preaxial polydactyly. Proc.Natl.Acad.Sci.U.S.A. 2002;99:7548–7553. doi: 10.1073/pnas.112212199. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder CC, Heckert LL, Goetz TL, Griswold MD. Follicle-stimulating hormone receptor gene promoter activity. Endocrine. 1994;2:957–966. [Google Scholar]
- Luo X, Sawadogo M. Functional domains of the transcription factor USF2: atypical nuclear localization signals and context-dependent transcriptional activation domains. Mol Cell Biol. 1996;16:1367–1375. doi: 10.1128/mcb.16.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiske D, Pask AJ, Shaw JM, Shaw G. Structure and expression of the Follicle-stimulating hormone receptor gene in a marsupial, Macropus eugenii. Mol.Reprod.Dev. 2002;63:24–31. doi: 10.1002/mrd.10161. [DOI] [PubMed] [Google Scholar]
- McGuinness MP, Linder CC, Morales CR, Heckert LL, Pikus P, Griswold MD. Relationship of a mouse Sertoli cell line (MSC-1) to normal Sertoli cells. Biol.Reprod. 1994;51:116–124. doi: 10.1095/biolreprod51.1.116. [DOI] [PubMed] [Google Scholar]
- Murre C. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. others. [DOI] [PubMed] [Google Scholar]
- Nobrega MA, Ovcharenko I, Afzal V, Rubin EM. Scanning human gene deserts for long-range enhancers. Science. 2003;302:413. doi: 10.1126/science.1088328. [DOI] [PubMed] [Google Scholar]
- Nobrega MA, Pennacchio LA. Comparative genomic analysis as a tool for biological discovery. J.Physiol. 2004;554:31–39. doi: 10.1113/jphysiol.2003.050948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordhoff V, Gromoll J, Foppiani L, Luetjens CM, Schlatt S, Kostova E, Huhtaniemi I, Nieschlag E, Simoni M. Targeted expression of human FSH receptor Asp567Gly mutant mRNA in testis of transgenic mice: role of human FSH receptor promoter. Asian.J.Androl. 2003;5:267–275. [PubMed] [Google Scholar]
- Oktay K, Briggs D, Gosden RG. Ontogeny of Follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J.Clin.Endocrinol.Metab. 1997;82:3748–3751. doi: 10.1210/jcem.82.11.4346. [DOI] [PubMed] [Google Scholar]
- Ovcharenko I, Loots GG, Nobrega MA, Hardison RC, Miller W, Stubbs L. Evolution and functional classification of vertebrate gene deserts. Genome Res. 2005;15:137–145. doi: 10.1101/gr.3015505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Kong LK, Poirier C, Truong C, Overbeek PA, Bishop CE. Long-range activation of Sox9 in Odd Sex (Ods) mice. Hum.Mol Genet. 2004;13:1213–1218. doi: 10.1093/hmg/ddh141. [DOI] [PubMed] [Google Scholar]
- Qyang Y, Luo X, Lu T, Ismail PM, Krylov D, Vinson C, Sawadogo M. Cell-type-dependent activity of the ubiquitous transcription factor USF in cellular proliferation and transcriptional activation. Mol.Cell Biol. 1999;19:1508–1517. doi: 10.1128/mcb.19.2.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannikki AS, Zhang F-P, Huhtaniemi IT. Ontogeny of Follicle-stimulating hormone receptor gene expression in the rat testis and ovary. Mol.Cell.Endocrinol. 1995;107:199–210. doi: 10.1016/0303-7207(94)03444-x. [DOI] [PubMed] [Google Scholar]
- Sagai T, Hosoya M, Mizushina Y, Tamura M, Shiroishi T. Elimination of a long-range cis-regulatory module causes complete loss of limb-specific Shh expression and truncation of the mouse limb. Development. 2005;132:797–803. doi: 10.1242/dev.01613. [DOI] [PubMed] [Google Scholar]
- Sairam MR, Subbarayan VSR. Characterization of the 5′ flanking region and potential control elements of the ovine Follitropin receptor gene. Molecular Reproduction and Development. 1997;48:480–487. doi: 10.1002/(SICI)1098-2795(199712)48:4<480::AID-MRD8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Sirito M, Lin Q, Maity T, Sawadogo M. Ubiquitous expression of the 43- and 44-kDa forms of transcription factor USF in mammalian cells. Nucleic Acid Res. 1994;22:427–433. doi: 10.1093/nar/22.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sites CK, Patterson K, Jamison CS, Degen SJ, LaBarbera AR. Follicle-stimulating hormone (FSH) increases FSH receptor messenger ribonucleic acid while decreasing FSH binding in cultured porcine granulosa cells. Endocrinology. 1994;134:411–417. doi: 10.1210/endo.134.1.8275955. [DOI] [PubMed] [Google Scholar]
- Smale ST, Kadonaga JT. The RNA polymerase II core promoter. Annu.Rev.Biochem. 2003;72:449–479. doi: 10.1146/annurev.biochem.72.121801.161520. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M, Manna PR, Huhtaniemi I. Molecular cloning of the mouse Follicle-stimulating hormone receptor complementary deoxyribonucleic acid: functional expression of alternatively spliced variants and receptor inactivation by a C566T transition in exon 7 of the coding sequence. Biol.Reprod. 1999;60:1515–1527. doi: 10.1095/biolreprod60.6.1515. [DOI] [PubMed] [Google Scholar]
- Tisdall DJ, Watanabe K, Hudson NL, Smith P, McNatty KP. FSH receptor gene expression during ovarian follicle development in sheep. J.Mol.Endocrinol. 1995;15:273–281. doi: 10.1677/jme.0.0150273. [DOI] [PubMed] [Google Scholar]
- Viollet B, Lefrancois-Martinez AM, Henrion A, Kahn A, Raymondjean M, Martinez A. Immunochemical characterization and transacting properties of upstream stimulatory factor isoforms. J.Biol.Chem. 1996;271:1405–1415. doi: 10.1074/jbc.271.3.1405. [DOI] [PubMed] [Google Scholar]
- Xing W, Sairam MR. Characterization of regulatory elements of ovine Follicle-stimulating hormone (FSH) receptor gene: the role of E-box in the regulation of ovine FSH receptor expression. Biol.Reprod. 2001;64:579–589. doi: 10.1095/biolreprod64.2.579. [DOI] [PubMed] [Google Scholar]
- Xu Z, Garverick HA, Smith GW, Smith MF, Hamilton SA, Youngquist RS. Expression of Follicle-stimulating hormone and Luteinizing hormone receptor messenger ribonucleic acids in bovine follicles during the first follicular wave. Biol.Reprod. 1995;53:951–957. doi: 10.1095/biolreprod53.4.951. [DOI] [PubMed] [Google Scholar]
- Yamamura N, Takeishi M, Goto H, Tagami M, Mizutani T, Miyamoto K, Doi O, Kamiyoshi M. Expression of messenger RNA for gonadotropin receptor in the granulosa layer during the ovulatory cycle of hens. Comp Biochem.Physiol A Mol.Integr.Physiol. 2001;129:327–337. doi: 10.1016/s1095-6433(00)00350-0. [DOI] [PubMed] [Google Scholar]
- You S, Bridgham JT, Foster DN, Johnson AL. Characterization of the chicken Follicle-stimulating hormone receptor (cFSH-R) complementary deoxyribonucleic acid, and expression of cFSH-R messenger ribonucleic acid in the ovary. Biol.Reprod. 1996;55:1055–1062. doi: 10.1095/biolreprod55.5.1055. [DOI] [PubMed] [Google Scholar]
- Yuan W, Lucy MC, Smith MF. Messenger ribonucleic acid for insulin-like growth factors-I and -II, insulin-like growth factor-binding protein-2, gonadotropin receptors, and steroidogenic enzymes in porcine follicles. Biol.Reprod. 1996;55:1045–1054. doi: 10.1095/biolreprod55.5.1045. [DOI] [PubMed] [Google Scholar]