Abstract
Background
Covault et al. (2007) reported that the common functional polymorphism, 5-HTTLPR, in the serotonin transporter gene moderated the association between past-year stressful events and daily reports of drinking in a sample of European-American (EA) college students. We examined this effect in college students of African descent.
Methods
Students recruited at a Historically Black University (n=564) completed web-based measures of past-year stressful life experiences and daily reports of drinking and heavy drinking over a 30-day period. Participants were genotyped for the tri-allelic 5-HTTLPR polymorphism and dichotomized as low-activity S’ allele carriers or high-activity L’ homozygotes. Generalized linear models were used to examine the effects of life stress, genotype, and their interaction on the two drinking measures.
Results
In students who completed 15 or more daily surveys (n=393), there was a significant interaction of past-year stressful events, 5-HTTLPR genotype, and gender on the number of drinking days (p=0.002). Similar findings were obtained in relation to heavy drinking days (p=0.007). Men showed a main effect of past-year stressful events on both drinking outcomes (p’s<0.001), but no main or moderator effects of genotype. In women, the S’ allele moderated the impact of past-year life stressors on the frequency of drinking and heavy drinking days (p’s<0.001).
Conclusions
In college students of African descent, past-year stressful events were associated with more frequent drinking and heavy drinking, an effect that was moderated by the 5-HTTLPR polymorphism. However, in contrast to the findings in EA students, in the current sample, 5-HTTLPR moderated the association only among women.
Keywords: gene-environment interaction, college student drinking, alcohol, 5-HTTLPR, daily reports
Introduction
Although drinking is common among young adults irrespective of whether they attend college, college students drink more frequently and intensively than their non-college peers (Jackson et al. 2006, O’Malley and Johnston 2002). Whereas most college students mature out of heavy episodic drinking (Jessor et al. 1991), maladaptive drinking during college places many individuals at risk for abuse and dependence and related problems, both concurrently (Carey and Correia 1997, Read et al. 2003, Simons et al. 2005) and later in life (Jessor et al. 1991, O’Neil et al. 2001). Thus, understanding the determinants of heavy drinking among college students has important public health implications.
One of the determinants of heavy drinking in college students appears to be variation in the gene encoding the serotonin transporter. Serotonin (5-HT) is a monoamine neurotransmitter, the activity of which affects alcohol consumption (Heinz et al. 2003; Higley and Linnoila 1997; LeMarquand et al. 1994). The 5-HT transporter (5-HTT), by mediating the re-uptake of 5-HT following synaptic release, is a key regulator of serotonergic tone. A common, functional variable number of tandem (22 bp) repeat polymorphism identified in the 5-HTT linked promoter region (5-HTTLPR) of SLC6A4, the gene encoding 5-HTT, yields long (L; 16 repeats) and short (S; 14 repeats) alleles. The L allele of 5-HTTLPR was associated with greater transcriptional activity than the S allele (Heils et al., 1996; Lesch et al., 1996). A single nucleotide polymorphism (A/G) in the L allele modifies its function, rendering the transcriptional rate of the LG allele similar to that of the S allele (Hu et al. 2006). The polymorphism is thus tri-allelic, with two low-activity alleles (S and LG, referred to here as S’) and a high-activity allele (LA, referred to here as L’). Examination of the tri-allelic polymorphism is of particular importance in individuals of African descent as the S allele is less common in this group, but these individuals have a greater frequency of LG alleles than individuals of European descent, resulting in an approximately equal frequency of S’ alleles in the two populations (Hu et al., 2006).
The functional variation in 5-HTTLPR has been shown to exert both main and moderator effects on drinking behaviors in humans, though the results have not been entirely consistent. Turker et al. (1998) found that young adults with high alcohol tolerance had a higher frequency of the 5-HTTLPR S allele. College students homozygous for the S allele reported more episodes of binge drinking and drinking to “get drunk” than L-allele homozygotes (Herman et al. 2003). In adolescents and young adults, the 5-HTTLPR S allele was significantly associated with higher levels of drinking in young adulthood, but not in adolescence (Guo et al. 2007). van der Zwaluw (2010) found that the S allele was associated with a steeper increase in drinking among adolescents in a five-year, multi-wave longitudinal study. In social drinkers, the S allele was significantly associated with greater alcohol consumption (Munafo et al. 2005). This sample also showed an interaction of genotype by gender, such that men with the S allele reported a higher level of alcohol consumption, whereas heterozygous women had a higher level of consumption than either homozygote group. Consistent with an earlier meta-analysis (Feinn et al. 2005), a recent meta-analysis (McHugh et al. 2010) showed a modest, but significant association of the S allele with risk of alcohol dependence. However, Nilsson et al. (2005) found that adolescents heterozygous for the 5-HTTLPR polymorphism were more likely to report a high frequency of intoxication and consumed more alcohol than either homozygote group. In that study, there was also an interaction effect of 5-HTTLPR with environment, such that heterozygotes with family relations self-rated as “neutral” or “bad” had the greatest risk for frequent intoxication and those with “bad” family relations reported the greatest annual alcohol consumption. Hopfer et al. (2005) found no effect of 5-HTTLPR genotype on drinking behavior in an adolescent twin and sibling pair sample followed for 6 years. Further, in a study of individuals followed from ages 14 to 24, Olsson et al. (2005) found a protective effect of the 5-HTTLPR S allele on binge drinking in young adulthood.
Using daily reports of drinking obtained via an Internet-based survey and one-time self-reports of past-year negative life events, we reported that S-allele homozygotes showed a positive association between negative life events and drinking frequency (Covault et al. 2007). Kaufman et al. (2007) found that children with the S allele who had been maltreated during childhood began drinking at a significantly earlier age than L-allele homozygotes. However, Laucht et al. (2009) found that both childhood and recent psychosocial adversity increased the risk of recent hazardous drinking, an effect that was moderated by the L’L’ genotype and was seen only in males. Tartter and Ray (2011) recently reported that, in a group of heavy drinkers drawn from different populations, L-allele homozygotes reported heavier alcohol consumption during the month prior to assessment. This effect was moderated by depressive symptoms, such that L-allele homozygotes with greater depressive symptoms reported heavier drinking than those with lower depressive symptom scores.
Because the vast majority of participants in studies of the moderating role of the 5-HTTLPR polymorphism were of European descent, it is of interest to determine whether this effect is seen in other populations. Thus, we examined the interactive effects of negative life events and 5-HTTLPR genotype on drinking behavior in college students of African descent. We employed an observational study design in which we measured, via the Internet, past-year life stress in a baseline assessment and alcohol use during 30 days of daily diary reporting.
Methods
Participants
Students were recruited from introductory courses in psychology, business, engineering, etc., through flyer distribution, advertisements in campus newspaper, email distribution, and proactive face-to-face recruitment. Interested students were invited to attend one of several informational sessions (with up to 4 students/session), during which research assistants explained study procedures in greater detail and obtained written informed consent. Participants provided saliva samples for DNA extraction and were given the secure website address, their password and username, and instructions on how to complete the initial and daily surveys. Students were given up to four weeks to complete the initial survey, after which the daily survey began. All participants began the daily phase on the same pre-designated day.
The daily survey required 5-7 minutes to complete. It was programmed to allow access only between 2:30 and 7:00 PM to coincide with the end of the school day for most undergraduate students, but before they begin their activities for the evening, to avoid having participants report when they might be under the influence of alcohol. An email reminder was forwarded each day prior to 2:30 PM. Participants who were unable to complete the survey before 7:00 PM could request a missed survey password, which allowed them until 9:00 PM to complete the survey or a late link, which permitted them until 11:00 AM the following day to complete the survey. If a daily survey was not completed, the participant was reminded by email to complete the next day’s survey. Further, if participants failed to complete a daily survey, during their next login, the server queried them on their drinking during missed intervals lasting up to 3 days.
Participants were paid for their participation. All participants gave written informed consent to participate in the protocol, which was approved by the institutional review boards at Howard University and the University of Connecticut Health Center.
Psychological and behavioral measures
Baseline assessment measures included the 36-item Life Events Scale for Students (LESS) (Clements and Turpin 1996; Linden 1984), an empirically derived inventory of stressful life events adapted from the Social Readjustment Rating Scale (Holmes & Rahe, 1967) for use with college students. Using this checklist, students identified stressful life events that had happened to them over the past year (e.g., broke up with a boyfriend or girlfriend; failed a course; had family health problems; had financial problems). We used only the 25 unambiguously negative events (see Covault et al., 2007), which were summed to yield a score of 0-25.
Each daily diary asked students to report the number of alcoholic drinks they consumed during the previous night (having been informed that 1 drink equaled one 12-oz can or bottle of beer, one 5-oz glass of wine, one 12-oz. wine cooler or 1.5-oz measure of liquor straight or in a mixed drink). From these data, we computed two aggregate daily variables for each person: drinking frequency to assess the regularity with which students drank 1 or more drinks, and heavy drinking frequency to assess the regularity of heavy drinking (days on which men consumed 5 or more drinks and women consumed 4 or more drinks).
Participants
A total of 564 participants completed a baseline survey and at least one daily survey. We limited our analysis to participants who provided 15 or more daily survey responses (n=415), self-identified as being of African descent (n=398, including individuals of African, Afro-Caribbean or African-American ethnicity), and for whom we had complete tri-allelic 5-HTTLPR genotype results (N=393). Males were less likely than females to complete at least 15 surveys (67% vs. 79%; χ2(1)=10.5, p=0.001). Participants completing fewer than 15 daily surveys did not differ significantly from those completing 15-30 surveys on age (p=0.90), mean number of past year life stressors (p=0.10), mean number of drinking days (p=0.38) or heavy drinking days (p=0.063) in the month prior to study entry, or the number of S’ alleles (p=0.37).
The mean age of included participants was 20.1 years (SD=1.6) and 58% were women. Participants were approximately evenly distributed among the four undergraduate years (freshman: 23%, sophomore: 25%, junior: 19%, senior: 24%), with a smaller percentage of students (9%) in a fifth year of undergraduate study. About equal proportions of participants from each of the three study years [2008/2009: n=119 (30%), 2009/2010: n=128 (33%), 2010/2011: n=146 (37%)] were included in the analysis. The mean number of diary reports was 23.8 (SD=4.4), which was similar across the three study years (2008/2009: M = 23.0, SD = 4.0; 2009/2010: M = 24.2, SD = 4.4; 2010/2011: M = 24.1, SD = 4.5).
Genotyping Procedure
Genomic DNA was extracted from saliva samples using a commercial DNA isolation protocol (Oragene, DNA Genotek, Kanata, Ontario, Canada). Average yield per participant was 89 μg (range 1 to 468 μg; SD=71μ g). The 5-HTTLPR polymorphism was genotyped using a TaqMan™ 5’nuclease assay modified from that originally described by Hu et al. (2005). Twenty five μ l PCR reactions prepared in 96-well assay plates contained 200 nM each of forward and reverse primers (GCAACCTCCCAGCAACTCCCTGTA and GAGGTGCAGGGGGATGCTGGAA), 120nM of an L- allele-specific Fam-labeled probe and 60 nM of a Vic-labeled internal control probe whose target is present in the PCR amplicon for both L and S alleles (6FAM-TGCAGCCCCCCCAGCATCTCCC-MGB and VIC-TCCCCCCCTTCACCCCTCGCGGCATCC-MGB; ABI-Applied Biosystems Inc., Foster City, CA), 1M Betaine, 1X ABI TaqMan Universal master mix, and 25 ng of genomic DNA. Samples were heated to 95°C for 10 minutes to activate Taq DNA polymerase, followed by 40 thermal cycles of 98°C for 15 sec, followed by 62.5°C for 90 sec. The number of L alleles (0, 1, or 2) for each participant was identified by examination of scatter plots of endpoint Fam vs. Vic fluorescence levels captured using an ABI 7900 Sequence Detection System.
A second TaqMan 5’nuclease allelic discrimination assay served to distinguish LA versus LG alleles by using the same primers and amplification conditions as for the L versus S allele assay but using LA versus LG allele-specific probes, (6FAM-CCCCCC- TGCACCCCCAGCATCCC-MGB and VIC-CCCCTGCACCCCCGGCATCCCC-MGB, respectively). In the TaqMan assay, XL alleles appear as L allele on the scatter plot. Thus, in this study, because gels were not used to discriminate S and L alleles, we do not know the number of XL carriers. The XL allele is, thus, coded as an L allele.
At least 10% of samples for each assay plate were repeated. Among 149 repeat assays for the S vs. L allele no discrepancies were observed. One TaqMan based genotype call for LG vs. LA was discrepant among 159 repeated samples. We previously validated the closed-tube fluorescent assay of 5-HTTLPR S vs. L alleles by comparing results obtained for 300 DNA samples using the 5’nuclease TaqMan assay with those from a traditional 5-HTTLPR agarose gel-based PCR fragment length assay, which yielded 100% agreement between methods (Herman et al. 2010). Because the LG/LG cluster is less reliably defined in this assay, when all LG/LG samples underwent independent DNA sequence analysis to confirm genotype, 12% were found to be LG/LA heterozygotes and recoded accordingly. Twenty-three LG/LA heterozygotes were also sequenced with 100% confirmation of genotype.
Statistical Analysis
Sample descriptive measures include frequencies (and percentages) for categorical variables and means (with standard deviations) for continuous variables. Generalized linear models with binomial probability distributions and logit link functions were used to predict the number of drinking days and the number of heavy drinking days during the 30-day study period. The following predictors: gender, life stress, and 5-HTTLPR genotype, coded as S’ (S or LG allele) carriers or L’ (LA) homozygotes were modeled together in a single, multivariable equation for each of the two outcome measures. The models included all main and interaction effects of LESS score, 5-HTTLPR genotype, and gender. The number of daily reports completed served as the number of trials for the two binomial outcome variables, thereby controlling for differences among the students in the rate of survey completion. In addition, year in school (freshman to fifth-year student, treated as a continuous variable) was included as a covariate because of its linear relationship with both drinking outcomes. Significant interaction effects were followed up by simple effects analyses to identify the nature and direction of the higher-level interactions. As described below, 2.5% of individuals abstained during the monitoring period. Because in the analysis of drinking days, we were interested in identifying predictors of the level of drinking (including abstinence), abstinent individuals were retained. However, because the analysis of predictors of heavy drinking focuses on the transition from drinking to heavy drinking, abstinent individuals were excluded.
Results
Descriptive Statistics
The number of reported stressful life events ranged from 0-18, with a mean of 5.7 (SD=3.6). The bi-allelic genotype distribution was 224 (57%) LL, 143 (36%) LS, and 26 (7%) SS. The tri-allelic genotype distribution was 95 (24%) L’L’, 193 (49%) L’S’, and 105 (27%) S’S’. Whether considered as a bi-allelic or tri-allelic genotype, the distributions were consistent with Hardy-Weinberg Equilibrium expectations [χ2(1)=0.24 and 0.12 and p=0.62 and 0.73, respectively). The observed frequencies of S, LA and LG alleles (25%, 49%, and 26%, respectively) were similar to those reported by Hu et al. (2006) for a sample of 624 African-American participants (25%, 51%, and 24%, respectively). Although the frequency of the S and L alleles differs substantially by population, the S’ and L’ allele frequencies are similar across populations (Hu et al. 2006). That fact, combined with the functional nature of the polymorphism, argues against the potential for stratification bias in our findings due to individuals of African descent being admixed with those of European descent.
Drinking Behavior
During the study, students reported drinking on an average of 6.8 days (SD=4.7), or 24% of reported days. This is similar to the frequency of drinking in Covault et al.’s (2007) sample of EA students, in which 20-25% of reported days were drinking days across the two sampling periods. The current sample reported drinking heavily on 2.6 days (SD=2.9), which comprised 9% of reported days or 38.2% of drinking days. This contrasts with the EA sample in Covault et al. (2007), in which heavy drinking comprised 13-16% of reported days or more than 60% of drinking days.
A total of 10 students (2.5%) reported no drinking during the monitoring period. Students consumed a mean of 3.9 drinks per drinking day (SD=2.6). Men drank on significantly more days [M=7.6 (SD=5.2) vs. 6.2 (SD=4.3), p=0.006], consumed more drinks per drinking day [M=4.9 (SD=3.3) vs. 3.2 (SD=1.7), p<0.001], and had more heavy drinking days [M=3.1 (SD=3.0) vs. 2.3 (SD=2.6), p=0.007] than women.
Drinking Days
This analysis, which was conducted with a sample of 393 students, showed a significant three-way interaction of LESS score, 5-HTTLPR genotype, and gender [χ2(1)=9.61, p=0.002] on drinking days. Follow-up analyses revealed a significant two-way interaction of LESS score and 5-HTTLPR for women [χ2(1)=12.62, p<0.001], but not men [χ2(1)=1.25, p=0.26]. As shown in Figure 1, in women with one or two S’ alleles, there was a positive association between the number of life stressors during the year preceding study participation and the propensity to drink during study participation [B=0.05, p<0.001, OR=1.05 (95% confidence interval=1.03-1.07)]. In contrast, in women homozygous for the L’ allele, the relationship between reported stressors and the propensity to drink approached significance in the opposite direction [B= -0.04, p=0.054, OR=0.96 (0.92-1.00)]. In men, the propensity to drink was positively associated with the number of life stressors, irrespective of genotype [B=0.08, p<0.001, OR=1.09 (1.07-1.11)].
Figure 1. Predicted Probability of Any Drinking.
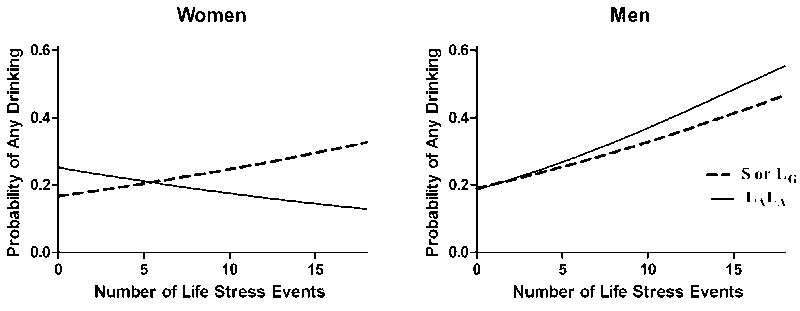
This model includes the effects of genotype, past-year negative life events (LESS score), and outcomes from the daily diary survey, with the average value of year in school as a covariate. Line symbols: dashed=S’ carriers; solid=L’ homozygotes. Among women, but not men, the interaction of genotype × LESS score is significant (p< .001, S’-carriers vs. L’L’).
Heavy Drinking Days
Excluding the 10 abstinent individuals, this analysis was conducted in a sample of 383 individuals. It showed a significant three-way interaction of LESS score, 5-HTTLPR genotype, and gender [χ2(1)=7.27, p=0.007] on the propensity to drink heavily. Follow-up analyses also revealed a significant two-way interaction of LESS score and 5-HTTLPR genotype on this outcome for women [χ2(1)=14.95, p<0.001], but not men [χ2(1)=0.44, p=0.51]. As shown in Figure 2, women with one or two S’ alleles showed a positive association between the number of life stressors during the year preceding study participation and the propensity to drink heavily during study participation [B=0.07, p<0.001, OR=1.08 (1.05-1.11)]. In contrast, in women homozygous for the L’ allele, there was a significant inverse relationship between reported stressors and the propensity to drink heavily [B=-0.08, p=0.023, OR=0.92 (0.86-0.99)]. In men, heavy drinking was significantly positively associated with life stressors, irrespective of genotype [B=0.09, p<0.001, OR=1.10 (1.07-1.12)].
Figure 2. Predicted Probability of Heavy Drinking.
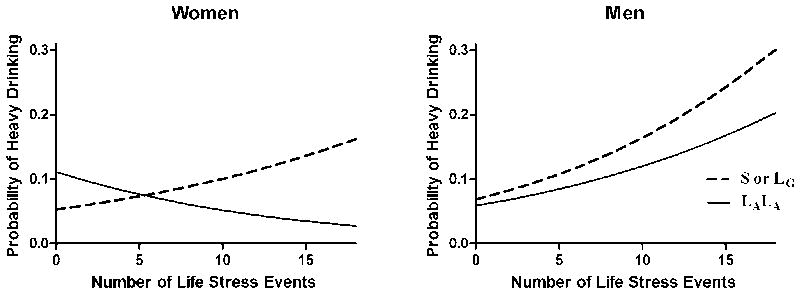
This model includes the effects of genotype, past-year negative life events (LESS score), and outcomes from the daily diary survey, with the average value of year in school as a covariate. Line symbols: dashed=S’ carriers; solid=L’ homozygotes. Among women, but not men, the interaction of genotype × LESS score is significant (p< .001, S’-carriers vs. L’L’).
Discussion
Our results demonstrating the interaction of 5-HTTLPR genotype and negative life events on the risk of any drinking and heavy drinking in a college student sample of African descent is the first evidence of this gene × environment interaction on drinking behavior in this population. The findings are similar to those that we reported previously, in that, based on the application of similar methods in a sample of EA college students we saw significant positive associations between stressful life events in the preceding year and subsequent drinking levels (Covault et al. 2007). As in the initial study, we used daily Internet reporting to obtain drinking data close to its real-time occurrence, relatively free of recall error and bias, as well as the same measure of life stress.
In contrast to our earlier findings, in the present study the observed effects differed significantly by gender, with women showing evidence of moderation by 5-HTTLPR genotype, whereas men showed no interaction with genotype. Although most studies showing an interaction effect of 5-HTTLPR with environment factors on behavioral outcomes have not shown an interaction with gender (Caspi et al., 2010), several studies reported that only women showed an interaction of the S allele with life stressors. For example, in four studies of adolescent depression there were higher rates among girls than boys with the SS genotype in the context of exposure to either domestic abuse/maltreatment (Aslund et al., 2009) or family/socioeconomic stress (Eley et al., 2004; Sjoberg et al., 2006; Hammen et al., 2010). Studies in adult populations have shown gender differences in the moderation by the S allele of the effects of caregiver stress on depressive symptoms in two separate samples (Brummett et al., 2008) or of the effects of unemployment stress on mental and physical distress (Grabe et al., 2005). Our results add to this growing literature showing a greater moderating effect of the low-activity 5-HTTLPR allele on the impact of life stressors on drinking behavior in women of African ancestry.
These findings must be viewed in the context of several limitations. First, our study population was restricted to college students. Further, in the present sample, heavy drinking was less common than is often reported for college students. Participants in this study were students at a Historically Black University with its own drinking norms, and thus the findings may also not generalize to students of African descent at universities in which such students are in the minority. Similar studies of students at other universities and of young adults not attending college are needed to examine the generality of our findings. Second, the substantial and variable time elapsed between past-year stressful life events and current alcohol use behaviors could have attenuated the effects of interest and the cross-sectional correlational design limits the interpretation of causation. Third, the presence of greater life stress in the past year in some individuals could reflect a lifelong history of greater stressors in these individuals, including events occurring during early childhood development. Such early life stressors have been reported to interact with the 5-HTTLPR S allele in children to produce higher rates of depression (Kaufman et al. 2004) and earlier alcohol use (Kaufman et al. 2007), and greater levels of alcohol intake by non-human primates (Barr et al. 2004). Finally, because the aim of the current study was to replicate among students of African descent the methods we employed in our prior study of the effects of 5-HTTLPR variation on drinking behavior in EA college students (Covault et al. 2007), we did not examine the potential moderation by depressive symptoms of the effects of either 5-HTTLPR or life stress. Future studies will benefit from designs that collect information on a range of early life adversity and that examine depressive symptoms as a potential moderator of these relations.
As described earlier, the literature on the main and moderating effects of the 5-HTTLPR polymorphism on risk of drinking or heavy drinking is mixed. Analysis of the main effects of the 5-HTTLPR polymorphism on the risk of depressive symptoms and disorders have also been controversial (Lohoff 2010). Further, meta-analyses of the growing literature on the moderating effect of 5-HTTLPR on risk of depression following stressful events have yielded very different conclusions regarding the validity of the association (Risch et al. 2009, Karg et al. 2011). Discrepant findings in the literature could, to a limited degree, be due to the fact that some studies report findings in relation to the bi-allelic (S/L) genotype and others the tri-allelic genotype (S’/L’). The extent to which this difference could explain the mixed literature will require a systematic comparison of the two approaches. More likely sources of discrepant findings across studies are differences in how alcohol use has been measured across the studies and to noteworthy differences in the types of stressful life events, which have varied dramatically across studies in their recency, chronicity and severity, as well as in how these life stressors were measured.
The modest nature of the effects exerted by the 5-HTTLPR polymorphism on drinking behavior could also contribute to the lack of consistent findings in this area. Drinking behavior is a complex phenotype, so that a full understanding of the underlying mechanisms must take into account substantial environmental (including cultural) differences and effects of other genetic moderators that likely contribute to variation in drinking behavior. Studies of epigenetic modifications contributing to the regulation of SLC6A4 expression may help to elucidate the etiology of this and other complex phenotypes (Philibert et al. 2008).
In conclusion, our findings suggest that, in the presence of multiple or repeated life stress, female university students of African descent with at least one copy of the 5-HTTLPR S’ allele have a higher risk of drinking and heavy drinking and are therefore exposed to the potential adverse effects associated with that behavior. This finding clearly warrants further study and, if replicated, these findings would have important implications for personalized prevention and intervention programs to reduce college student drinking.
Acknowledgments
This study was supported by NIH grants R21 AA017584, K24 AA13736, P20 AA014643, M01 RR06192, M01RR10284, and UL1RR031975. We greatly appreciate the assistance of Nicholas Maltby for web programming; Pamela Fall, Kaitlin Miller and Christine Abreu for technical assistance in DNA isolation and genotyping; and Aquil Meeks, Nnenna Kalu, Gloria Cain, Vanessa Marshall and Breana Sewell for the daily implementation of the study. Dr. Henry R. Kranzler has been a paid consultant for Alkermes, GlaxoSmithKline, Roche, Lundbeck, and Gilead. He has received research support from Merck. He also reports associations with Eli Lilly, Janssen, Schering Plough, Lundbeck, Alkermes, GlaxoSmithKline, Abbott, and Johnson & Johnson, as these companies provide support to the ACNP Alcohol Clinical Trials Initiative (ACTIVE) and Dr. Kranzler receives support from ACTIVE.
Footnotes
All other authors have no disclosures to make.
References
- Aslund C, Leppert J, Comasco E, Nordquist N, Oreland L, Nilsson KW. Impact of the interaction between the 5HTTLPR polymorphism and maltreatment on adolescent depression. A population-based study. Behavior Genetics. 2009;39(5):524–31. doi: 10.1007/s10519-009-9285-9. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Archives of General Psychiatry. 2004;61(11):1146–52. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Boyle SH, Siegler IC, Kuhn CM, Ashley-Koch A, Jonassaint CR, Zuchner S, Collins A, Williams RB. Effects of environmental stress and gender on associations among symptoms of depression and the serotonin transporter gene linked polymorphic region (5-HTTLPR) Behavior Genetics. 2008;38(1):34–43. doi: 10.1007/s10519-007-9172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey KB, Correia CJ. Drinking motives predict alcohol-related problems in college students. Journal of Studies on Alcohol. 1997;58(1):100–5. doi: 10.15288/jsa.1997.58.100. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. The American Journal of Psychiatry. 2010;167(5):509–27. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements K, Turpin G. The Life Events Scale for Students: Validation for use with British Samples. Person Individ Diff. 1996;20:747–751. [Google Scholar]
- Covault J, Tennen H, Armeli S, Conner TS, Herman AI, Cillessen AH, Kranzler HR. Interactive effects of the serotonin transporter 5-HTTLPR polymorphism and stressful life events on college student drinking and drug use. Biological Psychiatry. 2007;61(5):609–16. doi: 10.1016/j.biopsych.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Molecular Psychiatry. 2004;9(10):908–15. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2005;133B(1):79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Lange M, Wolff B, Volzke H, Lucht M, Freyberger HJ, John U, Cascorbi I. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Molecular Psychiatry. 2005;10(2):220–4. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- Guo G, Wilhelmsen K, Hamilton N. Gene-lifecourse interaction for alcohol consumption in adolescence and young adulthood: five monoamine genes. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2007;144B(4):417–23. doi: 10.1002/ajmg.b.30340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C, Brennan PA, Keenan-Miller D, Hazel NA, Najman JM. Chronic and acute stress, gender, and serotonin transporter gene-environment interactions predicting depression symptoms in youth. J Child Psychol Psychiatry. 2010;51(2):180–187. doi: 10.1111/j.1469-7610.2009.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. Journal of Neurochemistry. 1996;66(6):2621–4. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Gorey JG, Bennet A, Suomi SJ, Weinberger DR, Higley JD. Serotonin transporter availability correlates with alcohol intake in non-human primates. Molecular Psychiatry. 2003;8(2):231–4. doi: 10.1038/sj.mp.4001214. [DOI] [PubMed] [Google Scholar]
- Herman AI, Conner TS, Anton RF, Gelernter J, Kranzler HR, Covault J. Variation in the gene encoding the serotonin transporter is associated with a measure of sociopathy in alcoholics. Addiction Biology. 2011;16(1):124–32. doi: 10.1111/j.1369-1600.2009.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman AI, Philbeck JW, Vasilopoulos NL, Depetrillo PB. Serotonin transporter promoter polymorphism and differences in alcohol consumption behaviour in a college student population. Alcohol and Alcoholism. 2003;38(5):446–9. doi: 10.1093/alcalc/agg110. [DOI] [PubMed] [Google Scholar]
- Higley JD, Linnoila M. A nonhuman primate model of excessive alcohol intake. Personality and neurobiological parallels of type I- and type II-like alcoholism. Recent Developments in Alcoholism. 1997;13:191–219. [PubMed] [Google Scholar]
- Holmes TH, Rahe RH. The Social Readjustment Rating Scale. Journal of Psychosomatic Research. 1967;11(2):213–8. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Hopfer CJ, Timberlake D, Haberstick B, Lessem JM, Ehringer MA, Smolen A, Hewitt JK. Genetic influences on quantity of alcohol consumed by adolescents and young adults. Drug and Alcohol Dependence. 2005;78(2):187–93. doi: 10.1016/j.drugalcdep.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism, Clinical and Experimental Research. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. American Journal of Human Genetics. 2006;78(5):815–26. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JE, Doescher MP, Hart LG. Problem drinking: rural and urban trends in America, 1995/1997 to 2003. Preventive Medicine. 2006;43(2):122–4. doi: 10.1016/j.ypmed.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Jessor R, Donovan JE, Costa FM. Beyond Adolescence: Problem Behavior and Young Adult Development. New York: Cambridge University Press; 1991. [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68(5):444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Crouse-Artus M, Lipschitz D, Krystal JH, Gelernter J. Genetic and environmental predictors of early alcohol use. Biological Psychiatry. 2007;61(11):1228–34. doi: 10.1016/j.biopsych.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(49):17316–21. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laucht M, Treutlein J, Schmid B, Blomeyer D, Becker K, Buchmann AF, Schmidt MH, Esser G, Jennen-Steinmetz C, Rietschel M, et al. Impact of psychosocial adversity on alcohol intake in young adults: moderation by the LL genotype of the serotonin transporter polymorphism. Biological Psychiatry. 2009;66(2):102–9. doi: 10.1016/j.biopsych.2009.02.010. [DOI] [PubMed] [Google Scholar]
- LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biological Psychiatry. 1994;36(5):326–37. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Linden W. Development and initial validation of a life event scale for students. Canadian Counsellor. 1984;18:106–110. [Google Scholar]
- Lohoff FW. Overview of the genetics of major depressive disorder. Current Psychiatry Reports. 2010;12(6):539–46. doi: 10.1007/s11920-010-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Hofmann SG, Asnaani A, Sawyer AT, Otto MW. The serotonin transporter gene and risk for alcohol dependence: a meta-analytic review. Drug and Alcohol Dependence. 2010;108(1-2):1–6. doi: 10.1016/j.drugalcdep.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Lingford-Hughes AR, Johnstone EC, Walton RT. Association between the serotonin transporter gene and alcohol consumption in social drinkers. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2005;135B(1):10–4. doi: 10.1002/ajmg.b.30162. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Sjoberg RL, Damberg M, Alm PO, Ohrvik J, Leppert J, Lindstrom L, Oreland L. Role of the serotonin transporter gene and family function in adolescent alcohol consumption. Alcoholism, Clinical and Experimental Research. 2005;29(4):564–70. doi: 10.1097/01.alc.0000159112.98941.b0. [DOI] [PubMed] [Google Scholar]
- O’Malley PM, Johnston LD. Epidemiology of alcohol and other drug use among American college students. Journal of Studies on Alcohol Supplement. 2002;(14):23–39. doi: 10.15288/jsas.2002.s14.23. [DOI] [PubMed] [Google Scholar]
- O’Neill SE, Parra GR, Sher KJ. Clinical relevance of heavy drinking during the college years: cross-sectional and prospective perspectives. Psychology of Addictive Behaviors. 2001;15(4):350–9. doi: 10.1037//0893-164x.15.4.350. [DOI] [PubMed] [Google Scholar]
- Olsson CA, Byrnes GB, Lotfi-Miri M, Collins V, Williamson R, Patton C, Anney RJ. Association between 5-HTTLPR genotypes and persisting patterns of anxiety and alcohol use: results from a 10-year longitudinal study of adolescent mental health. Molecular Psychiatry. 2005;10(9):868–76. doi: 10.1038/sj.mp.4001677. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Sandhu H, Hollenbeck N, Gunter T, Adams W, Madan A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2008;147B(5):543–9. doi: 10.1002/ajmg.b.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read JP, Wood MD, Kahler CW, Maddock JE, Palfai TP. Examining the role of drinking motives in college student alcohol use and problems. Psychology of Addictive Behaviors. 2003;17(1):13–23. doi: 10.1037/0893-164x.17.1.13. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301(23):2462–71. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Gaher RM, Oliver MN, Bush JA, Palmer MA. An experience sampling study of associations between affect and alcohol use and problems among college students. Journal of Studies on Alcohol. 2005;66(4):459–69. doi: 10.15288/jsa.2005.66.459. [DOI] [PubMed] [Google Scholar]
- Sjoberg RL, Nilsson KW, Nordquist N, Ohrvik J, Leppert J, Lindstrom L, Oreland L. Development of depression: sex and the interaction between environment and a promoter polymorphism of the serotonin transporter gene. The International Journal of Neuropsychopharmacology. 2006;9(4):443–9. doi: 10.1017/S1461145705005936. [DOI] [PubMed] [Google Scholar]
- Tartter MA, Ray LA. The serotonin transporter polymorphism (5-HTTLPR) and alcohol problems in heavy drinkers: moderation by depressive symptoms. Frontiers in Psychiatry. 2011;2:49. doi: 10.3389/fpsyt.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turker T, Sodmann R, Goebel U, Jatzke S, Knapp M, Lesch KP, Schuster R, Schutz H, Weiler G, Stober G. High ethanol tolerance in young adults is associated with the low-activity variant of the promoter of the human serotonin transporter gene. Neuroscience Letters. 1998;248(3):147–50. doi: 10.1016/s0304-3940(98)00347-4. [DOI] [PubMed] [Google Scholar]
- van der Zwaluw CS, Engels RC, Vermulst AA, Rose RJ, Verkes RJ, Buitelaar J, Franke B, Scholte RH. A serotonin transporter polymorphism (5-HTTLPR) predicts the development of adolescent alcohol use. Drug and Alcohol Dependence. 2010;112(1-2):134–9. doi: 10.1016/j.drugalcdep.2010.06.001. [DOI] [PubMed] [Google Scholar]


