Abstract
Background
Profibrotic cells derived from circulating CD14+ monocytes include fibrocytes and alternatively activated macrophages. These cells are associated with interstitial lung disease (ILD) and are implicated in the pathogenesis of Systemic Sclerosis (SSc); however, the simultaneous presence of profibrotic cells and their associated mediators in the circulation of these patients has not been defined. We hypothesized that monocytes from patients with SSc-related ILD (SSc-ILD) would show profibrotic characteristics when compared with normal controls.
Methods
We recruited patients with SSc-ILD (n=12) and age-matched normal controls (n=27) and quantified circulating collagen producing cells by flow cytometry for CD45 and pro-Collagen I. The in vitro alternative activation potential of CD14+ monocytes was assessed using flow cytometry for the scavenger receptor CD163, and by ELISA for CCL18 and IL-10 secretion. Profibrotic mediators in plasma were quantified using Luminex-based assays.
Results
The concentration of circulating collagen producing cells was increased in the SSc-ILD patients when compared to controls. These cells were composed of both CD34+ fibrocytes and a population of CD34+CD14+ cells. Cultured CD14+ monocytes from SSc-ILD patients revealed a profibrotic phenotype characterized by expression of CD163 and by enhanced secretion of CCL18 and IL-10 in response to pro-inflammatory activation. Plasma levels of IL-10, MCP-1, IL-1RA, and TNF levels were significantly elevated in the plasma of the SSc-ILD cohort. Subgroup analysis of the normal controls revealed that unlike the subjects ≤ 35 years, subjects ≥ 60 years old showed higher levels of circulating CD34+CD14+ cells, collagen producing CD14+ monocytes, CD163+ monocytes, IL-4, IL-10, IL-13, MCP-1, and CCL-18.
Conclusions
These data indicate that the blood of patients with SSc-ILD and of healthy aged controls is enriched for fibrocytes, profibrotic monocytes, and fibrosis-associated mediators. Investigations defining the factors responsible for this peripheral blood profile may provide new insight into SSc-ILD as well as the pathophysiology of aging.
Keywords: alternative activation, fibrocytes, macrophages, pulmonary fibrosis, scleroderma
Introduction
Systemic sclerosis (SSc), or scleroderma, is a multisystem autoimmune disease characterized by progressive cutaneous and visceral fibrosis. With advances in the treatment of SSc-related renal disease, pulmonary involvement has emerged as the greatest cause of mortality in SSc (1). The lungs of patients with SSc frequently show the pathologic hallmarks of interstitial lung disease (SSc-ILD), with replacement of the normal lung architecture with inflamed and fibrotic tissue that is ineffective for gas exchange. Approximately 42% of patients with SSc-ILD will die of disease progression within ten years of diagnosis (1). While treatment with cyclophosphamide has shown benefit in delaying disease progression, the overall clinical benefit is minor, and many patients relapse after treatment cessation (2). In addition, the prevalence of gastroesophageal reflux disease (GERD) in these patients increases their risk for poor outcomes following lung transplantation (3, 4). Thus, closer investigation of the pathogenesis of SSc-ILD has the potential to impact the proximate cause of death of most patients.
The CD14+ monocyte fraction of peripheral blood contains precursors to several mature cell lineages, including fibrocytes (5) and macrophages (6). Abnormalities of both of these cell types have been documented in scleroderma (7-9), although their specific association with SSc-ILD is less well-defined. While all monocytes express CD14, these cells nevertheless exhibit considerable heterogeneity in terms of their differentiation and activation potential (6). CD14+ monocyte precursors give rise to fibrocytes in animal models of renal fibrosis (10) and in studies of cultured human peripheral blood cells (5). Fibrocytes are identified by their co-expression of the stem cell marker CD34, the leukocyte common antigen CD45, and extracellular matrix proteins such as collagen (11). An increasing amount of data highlight the association between elevated levels of peripheral blood fibrocytes and diverse forms of localized or diffuse organ remodeling such as renal fibrosis (12), cirrhosis (13), atherosclerosis (14) and nephrogenic systemic fibrosis (15). Multiple studies also demonstrate that elevated fibrocyte levels are found in the blood and in the lungs of patients with different types of pulmonary fibrosis (16-19). While fibrocytes may be found in the skin lesions of patients with SSc (20) and may play a role in the disease, a relationship between fibrocytes and SSc-ILD has not been investigated.
Most CD14+ monocytes differentiate into tissue macrophages, which can display distinct phenotypes of activation (21). In the lung, an important distinction is made along the lines of Th1 and Th2 inflammation. Th1, or “classically” activated, macrophages (M1) arise in response to priming with interferon-γ or IL-1 and are identified by the expression of IL-12, TNF, and the chemokine CXCL10; these cells display a phenotype that is associated with tumor resistance, the destruction of intracellular parasites, and tissue damage. Th2, or “alternatively” activated (M2), macrophages are derived following exposure to IL-4 or IL-13 and are identified in part by expression of the scavenger receptor CD163, the IL-1RII decoy receptor, mannose receptors, and by increased IL-10 and CCL18 production (22, 23). Most of these markers are associated with such diverse diseases as atopy (24), renal disease, and cirrhosis (25). CCL18 is a monocyte-derived chemokine that induces collagen production by fibroblasts; it also is strongly associated with lung fibrosis (26) and has been shown recently to predict clinical outcomes in patients with IPF (27). Stimulation of CD14+ cells with the bacterial cell wall component lipopolysaccharide (LPS) can induce an M1 or M2 phenotype depending upon the activation potential of the precursor cell (24).
Alveolar macrophages obtained from patients with SSc demonstrate a distinct M2 phenotype (28) accompanied by high levels of CCL18 production (27, 29). However, there are scant data regarding the alternative activation potential of monocytes derived from the peripheral blood of patients with this disease. Clarification of this concept could lead to the development of novel biomarkers to define disease activity and could provide novel targets for therapeutic intervention. Based on these considerations, we hypothesized that the peripheral blood of patients with SSc-ILD would contain monocytic precursors that are skewed towards the profibrotic phenotype of fibrocytes and alternatively activated macrophages. In addition, we undertook to define the soluble mediators associated with this cellular profile in the hope of gaining further insight into the factors promoting the development of ILD in patients with SSc.
Materials and Methods
Patients
All studies were performed with Human Investigations Committee approval at Yale University School of Medicine. Patients followed at the Interstitial Lung Diseases Clinic at Yale University School of Medicine were eligible to enroll. All patients were greater than 18 years of age who had been diagnosed with SSc based on the American College of Rheumatology (ACR) criteria and demonstrated ILD on high resolution CT scan. Healthy, age-matched controls were recruited from the greater New Haven area. Exclusion criteria included current or recent use of immunosuppression, chronic infection such as HIV or hepatitis, known pulmonary hypertension, cardiovascular, renal or neoplastic disease, and inability to provide informed consent. Following enrollment and informed consent, demographic data concerning age, race, sex, and co-morbid conditions were collected on all patients. In addition, data regarding restrictive ventilatory defect (decreased forced vital capacity, or FVC) and diffusion impairment (decreased DLCO) were obtained from chart abstraction on the patients with SSc-ILD.
Processing of blood for flow cytometry
Following informed consent and enrollment, 30 ml of heparinized peripheral blood was drawn from study subjects, anonymized, and transported to the laboratory. The peripheral blood mononuclear cells (PBMCs) were isolated from the whole blood using Ficoll-paque based separation (Stem Cell Technologies). Total viable cells were quantified using Tryphan blue exclusion. Total blood fibrocyte levels and fibrocyte percentages were assessed using a minor modification of previously published protocols (17). Briefly, 106 cells were suspended in 100 μl of buffer and nonspecific antibody binding was minimized with 5 μl of FcR blocking agent (Miltenyi). Cells then were stained according to manufacturer's instructions with 2 μl of anti-CD45-PE (eBIoscience) or anti-CD45-PerCP (BD) antibodies, washed, and fixed with Cytofix/Cytoperm kit (BD Pharmingen) in order to permeabilize the cells. Cells were incubated with 1 μl of rat-anti-human pro-Col-Iα antibody (Clone M58, Chemicon) or isotype control (Clone 43414, R&D Systems), washed, and finally counter-stained with goat-anti-rat FITC-labeled secondary antibody (Invitrogen) at 1:500 dilution. Nonspecific secondary antibody binding was further blocked with normal goat serum (10% in the secondary antibody dilution).
CD14+ Monocyte Culture
An aliquot of PBMCs separated from the freshly collected patient blood was enriched for CD14+ cells using MACS negative selection. Assessment of purity by flow cytometry for CD14 revealed that after one selection, cells were >80% CD14 positive. A second purification procedure yielded a purity of > 90% (data not shown). Thus, the negative selection was performed twice. The remaining cells were plated into DMEM containing 10% fetal bovine serum ± 1 μg/ml LPS (Peprotech) as previously described (29). After 48 hours of culture, cell morphology was assessed visually and the cells were harvested and stained for the alternatively activated macrophage phenotype and collagen production with anti-CD163-PE (Dako) and anti-CD68-FITC (BD).
Quantification of Leukocyte Subsets
Total lymphocytes and monocytes were quantified on PBMCs using a flow cytometry assessment of FSC vs SSC characteristics. These percentages then were multiplied by total post-Ficoll cell counts to determine the total concentration of lymphocytes and monocytes per ml of blood.
Flow Cytometry
Flow cytometry was performed using a BD FACSCalibur. Data were analyzed using Flow Jo v 7.5 software (TreeStar, Inc).
ELISA
ELISA for CCL18, IL10, and M-CSF were performed on plasma or culture supernatant according to the manufacturer's instructions (R&D Systems). Multianalyte assessment on plasma was performed using Luminex technology as previously described (30).
Statistical analysis
Statistical analyses were performed using Prism 5 software (GraphPad, Inc). Normally distributed data were assessed using Student's t-test. Data not normally distributed were assessed using Mann-Whitney U test. Pearson's correlation coefficient was used to determine correlations. Categorical variables were compared using Wilcoxon rank-sum test. Significance was defined as p<0.05.
Results
Subjects
Thirty-nine subjects were recruited for the initial studies. Of these, 27 were normal controls with no history of SSc-ILD. Twelve patients with SSc-ILD were recruited. Baseline characteristics of the studied groups are shown in Table 1. There was no difference between groups in terms of age, race, sex, history of hypertension, or hyperlipidemia. While only three control subjects had a history of GERD, all patients with SSc-ILD had a history of this disease (p<0.03). Patients with SSc-ILD demonstrated moderate restrictive ventilatory defect (FVC = 64.58 ± 3.44%) and a severely impaired diffusion capacity (DLCO = 49.67 ± 16.96%). Because control subjects self-identified as normal, no pulmonary testing is available for these subjects.
Table 1.
Characteristics of Subjects
| Characteristic | Control (n=27) | SSc-ILD (n=12) | p-value |
|---|---|---|---|
| Age, years | 51.71±-3.91 | 47.42±-3.13 | 0.49 |
| Race – white | 21/27 | 10/12 | 0.39 |
| Sex – female | 16/27 | 9/12 | 0.79 |
| Hypertension | 8/27 | 4/12 | 0.42 |
| GERD | 3/27 | 12/12 | 0.03 |
| Hyperlipidemia | 4/27 | 3/12 | 0.78 |
| FVC, percent predicted | NA | 64.58+/-3.44 | NA |
| DLCO, percent predicted | NA | 49.67+/-16.96 | NA |
Data are expressed as mean ± SEM.
Peripheral blood CD45+/pro-Col-Iα+ cells concentrations are increased in SSc-ILD
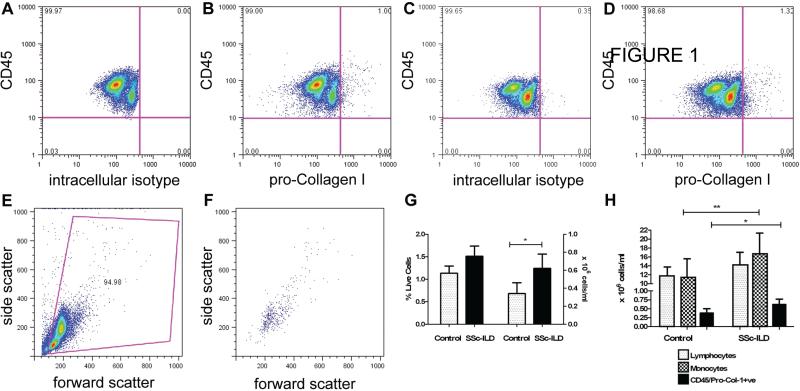
We first determined whether the blood of SSc-ILD patients was enriched for collagen-expressing cells using flow cytometry. Here, flow cytometry of PBMCs stained with CD45 and intracellular isotype control revealed a significant shift to the right. This control was used to set the negative gate for pro-collagen staining (Figure 1A) and isotype staining was subtracted from true positive staining to determine the percentage of double-positive cells. This number then was multiplied by the total post-Ficoll PBMC count and divided by the input volume to determine concentrations of CD45+/pro-Col-Iα+ cells/ml.
Figure 1. CD45+/pro-Col-1α+ cells in SSc-ILD.
A: CD45-PE vs. intracellular isotype control + anti-rat-FITC analyses on blood from a representative normal subject. This control was used to set negative gates. X-axis: rat IgGk intracellular isotype. Y-axis: anti-CD45-PE. B: CD45+/pro-Col-1α+ cells in normal blood. X-axis: pro-Col-1α stained with anti-rat secondary antibody. Y-axis: anti-CD45-PE. C: Anti CD45-PE vs. intracellular isotype control and anti-rat FITC performed on blood from patient with SSc-ILD. This control was used to set negative gates for the SSc-ILD sample. X-axis: rat IgGk intracellular isotype. Y-axis: anti-CD45-PE. D: CD45+/pro-Col-1α+ cells in SSc-ILD blood, same axes as 1B. E: FSC vs. SSC analysis of PBMCs from the sample in (D). F: FSC vs. SSC analysis of PBMCs from the double positive population in (D). G. Comparison of fibrocyte percentages (left axis) and quantities (right axis) in normal (n = 27, gray bar) and SSc-ILD subjects (n=12, black bar). Fibrocyte quantities but not percentages are increased in SSc-ILD. H: Comparison of lymphocytes (dotted bar), monocytes (checked bar) and fibrocytes (black bar) fibrocyte concentrations in normal control (left) and SSC-ILD blood (right). Monocyte quantities are increased in patients with SSc-ILD. Data are expressed as mean ± SEM. *p<0.05, *p<0.01.
Using this approach we found a double positive population of CD45+/pro-Col-Iα+ monocytes in all subjects (Figure 1B-F). We did not detect a difference in the percentage of CD45+/pro-Col-Iα+ cells (expressed relative to total PBMCs) between normal and SSc-ILD patients (p<0.07, Figure 1G, Table 2). However, there was a significant increase in the total number of CD45+/pro-Col-Iα+ cells in the blood of SSc-ILD patients when compared to controls (p<0.05, Figure 1G). These cells displayed forward-scatter (FSC) versus side-scatter (SSC) characteristics that gated to the monocyte fraction of PBMCs (Figure 1F, Table 2). These data indicate that PBMCs from patients with SSc-ILD are enriched for Col-Iα production.
Table 2.
Fibrocyte Percentages in Peripheral Blood and CCL18 and IL-10 Levels in Culture Supernatant (Control vs. SSc-ILD)
| Control (n=27) | SSc-ILD (n=12) | p-value | |
|---|---|---|---|
| Fibrocyte percentage | 1.13 ± 0.16 | 1.34 ± 0.25 | 0.22 |
| Fibrocyte Number (× 106) | 0.34 ± 0.12 | 0.61 ± 0.160 | 0.031 |
| - LPS, % CD163+ | 5.817 ± 1.88 | 7.812 ± 2.120 | 0.21 |
| + LPS, % CD163+ | 7.91 ± 1.805 | 11.16 ± 1.500 | 0.19 |
| - LPS, CCL18, pg/ml | 67.08 ± 26.43 | 172.1 ± 143.9 | 0.6 |
| + LPS, CCL18, pg/ml | 156.4 ± 44.09 | 703.6 ± 159.3 | 0.0009 |
| - LPS, IL-10, pg/ml | 209.6 ± 56.95 | 58.42 ± 20.92 | 0.03 |
| + LPS, IL-10, pg/ml | 370.2 ± 71.29 | 1388 ± 256.6 | 0.04 |
Correlation of PBMC subsets with CD45+/pro-Col-Iα+ cells
Subsets of PBMCs including lymphocytes and monocytes are both believed to be important for the appearance of collagen producing cells in the peripheral blood (5, 31, 32). In order to explore this concept further, we quantified the numbers of lymphocytes and monocytes in the blood of SSc-ILD patients and normal controls and determined whether either or both of these cell types correlate with the CD45+/pro-Col-Iα+ cells. We found an increase in circulating monocytes in the blood of patients with SSc-ILD (Figure 1H). This increase in monocytes approached a moderate correlation with CD45+/pro-Col-Iα+ cells (r value 0.58, p=0.06). Although we did not observe an increase in the absolute number of circulating lymphocytes in patients with SSc-ILD versus normal controls, there was a strong and significant correlation between lymphocytes and CD45+/pro-Col-Iα+ cells in the blood of patients with SSc-ILD (r value 0.64, p<0.03). In contrast, monocytes displayed a very strong association with CD45+/pro-Col-Iα+ cells in the control population (r value 0.86, p<0.0001) and there was no association between lymphocytes and CD45+/pro-Col-Iα+ cells in the controls (r value 0.21, p = 0.27). These data indicate that lymphocytes but not monocytes correlate with the increase in CD45+/pro-Col-Iα+ cells in patients with SSc-ILD.
SSc-ILD monocytes increase CD163 expression in response to LPS
The expression of CD163 signifies the alternatively activated macrophage, and this marker has been used previously to enumerate this macrophage sub-population in a number of pathologic conditions including pulmonary fibrosis and SSc-ILD (33, 34). CD163+ expression in response to LPS stimulation also has been reported to identify CD14+ cells that are skewed towards alternative activation in response to LPS stimulation in the blood of patients with atopy (24). CD163 expression is also enhanced in the lungs of patients with many types of pulmonary fibrosis (27) so we expected that the blood of patients with SSc-ILD would show increased CD163 expression in response to LPS stimulation when compared to normal controls.
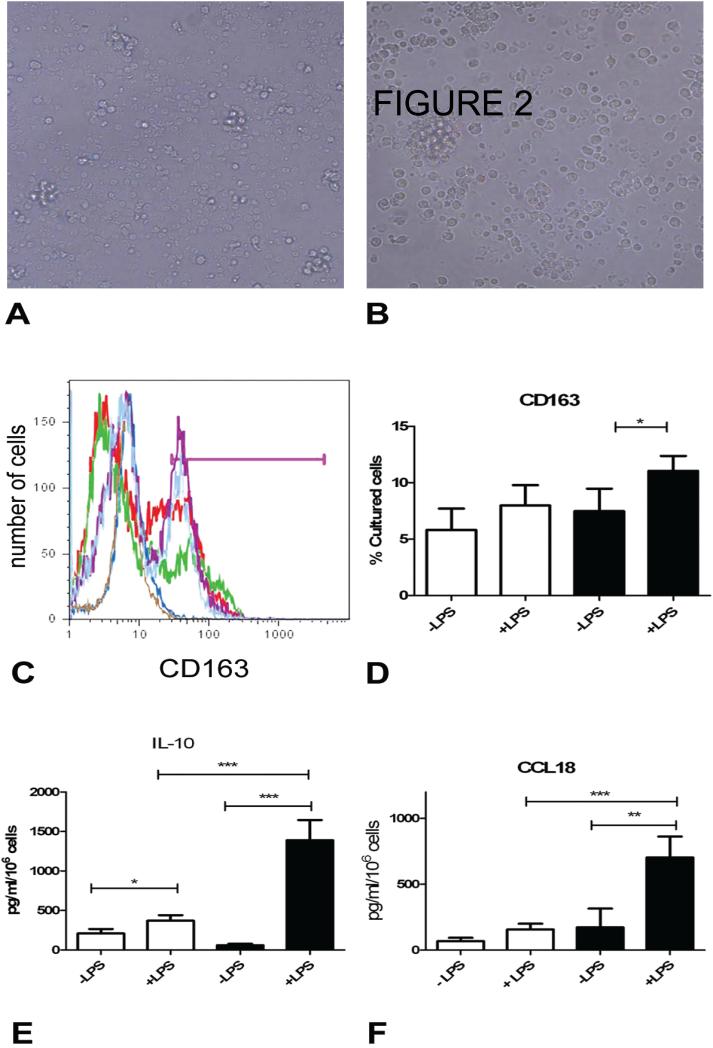
Because CD163 has recently been reported to be expressed on cultured fibrocytes (35), we first assessed cultured cells for the characteristic spindle-shaped morphology of fibrocytes. We found that CD14+ monocytes maintained a rounded morphology after 48 hours in culture and that no cells showed the spindle shaped morphology of fibrocytes (Figure 2 A, B). These data indicate that cells expressing CD163 in this system are unlikely to be mature fibrocytes. We next performed flow cytometry on these cultured cells. Our results showed that unstimulated CD14+ monocytes from control subjects displayed different levels of CD163 expression. LPS stimulation increased CD163 expression by 47.5% in the patients with SSc-ILD but had no effect on its expression in the control population (Table 2, Figure 2C, D). Interestingly, monocytes from some controls displayed very little CD163 expression while monocytes from other controls showed very high expression of this receptor (Figure 2C). These data indicate that CD14+ monocyte CD163 expression is differentially influenced by LPS activation in the SSc-ILD patients.
Figure 2. Alternative macrophage activation in SSc-ILD.
A, B: Light microscopy of CD14+ peripheral blood monocytes that were cultured in the absence (A) and presence (B) of LPS. C: Overlay of histograms of CD163 staining on cultured CD14+ monocytes. Brown: young control, no LPS. Dark blue: young control +LPS. Green: aged control, no LPS. Red: aged control, +LPS. Light blue: SSc-ILD, no LPS. Purple: SSc-ILD, + LPS. The pink bar indicates the positive gate. D: Percentage of CD14+ cells that express CD163 following 48 hours in culture. White bar: control, black bar: SSc-ILD. E: CCL-18 secretion following 48 hours in culture. White bar: control, black bar: SSc-ILD. *p<0.05, **p<0.01, ***p<0.001. F: IL-10 secretion following 48 hours in culture. White bar: control (n = 27). Black bar: SSc-ILD (n = 12).*p<0.05, **p<0.01, ***p<0.001. All data are expressed as mean ± SEM.
LPS-stimulated CCL18 and IL-10 secretion by monocytes is enhanced in SSc-ILD
We next assessed functional differences in monocytes obtained from these patients. CCL18 is a monocyte-derived chemokine that induces T-cell migration and collagen production by fibroblasts. CCL18 is elevated in the lungs of patients with SSc-ILD and is a biomarker of disease progression in patients with IPF. We found a substantial increase in both baseline and LPS-stimulated CCL18 production in the SSc-ILD monocytes compared to normal controls (p<0.01 and <0.001 respectively, Figure 2E, Table 2). Because IL-10 has been shown to promote CCL18 production in human cells (27, 36) and perpetuate fibrosis in murine models (37), we measured this cytokine as well. Unstimulated monocytes obtained from both normal controls and SSc-ILD patients produced equivalent levels of IL-10; however, LPS stimulation of monocytes from patients with SSc-ILD produced nearly triple the amount of IL-10 as those from normal controls (Figure 2F, Table 2). These data suggest that nonspecific stimulation with LPS induces a profibrotic phenotype in monocytes from patients with SSC-ILD.
Multianalyte ELISA analysis of SSc-ILD patient plasma reveals an increase in profibrotic mediators
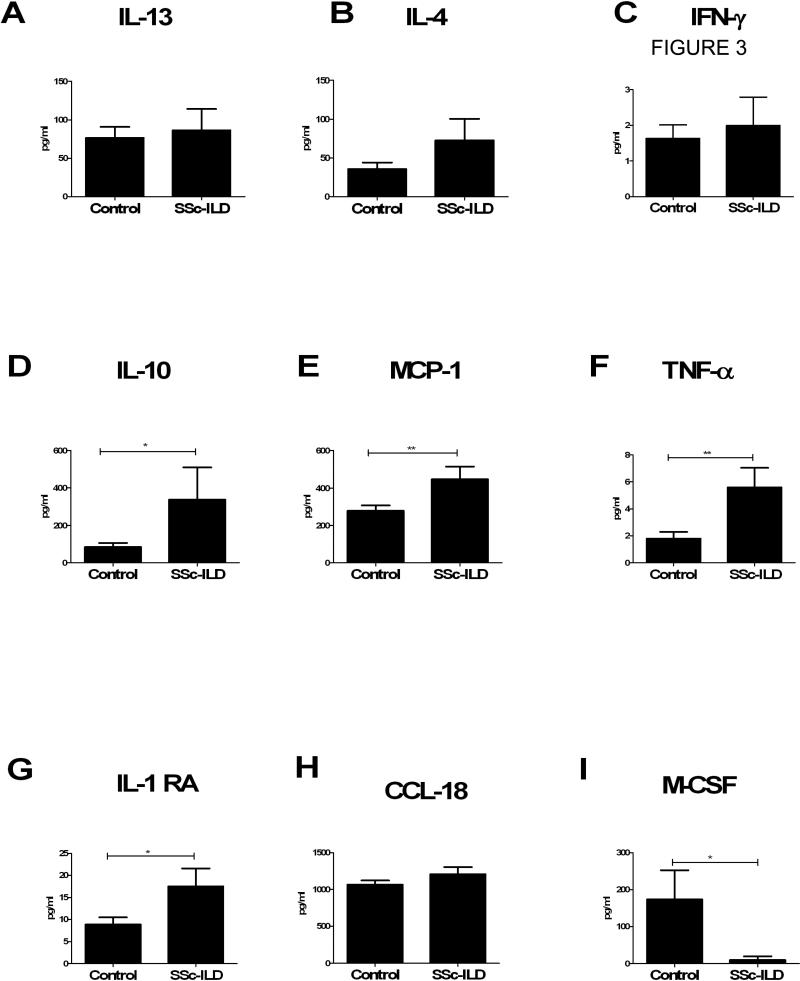
In order to gain a better understanding of the soluble factors associated with a fibrogenic monocyte phenotype, we performed multianalyte ELISA on plasma samples obtained from the SSc-ILD patient and control groups. Because of the increased number of fibrocytes and the alternative activation of LPS-stimulated monocytes found in SSc-ILD patients, we expected to find an increase in Th2 cytokines (which promote fibrocyte differentiation and M2 activation) and decreased Th1 cytokines (which inhibit these processes). Interestingly, neither the circulating levels of the Th2 cytokines, IL-13 and IL-4, or the Th1 cytokine, IFN-γ, differed between the two groups (Figure 3A-C). We found significantly higher levels of IL-10 (p< 0.04, Figure 3D), the chemokine MCP-1 (p<0.01, Figure 3E), which affects the trafficking of both fibrocytes and monocytes, TNF-α, which can both promote and suppress fibrosis (p< 0.01, Figure 3F), and IL-1RA (p<0.02, Figure 3G) which functions to suppress inflammation. CCL18 levels did not differ between these groups (Figure 3H). In addition, M-CSF, which induces the development of macrophages from CD34+ hematopoietic stem cells, was significantly reduced in the patients with SSc-ILD compared to normal controls (p<0.05, Figure 3I). These data are also shown in Table 3.
Figure 3.
Multianalyte ELISA comparisons of plasma cytokines in control (n = 27, left) vs. SSc-ILD subjects (n = 11, right). A: IL-13 B: IL-4. C: IFN-γ. D: IL-10 E: MCP-1. F: TNFα. G: IL-1 RA. H: CCL18. I: M-CSF. All data are expressed as pg/ml (mean ± SEM). *p<0.05. **p<0.01. ***p<0.001.
Table 3.
Plasma Cytokine Levels (Control vs. SSc-ILD)
| Control (n=27) | SSc-ILD (n=11) | p-value | |
|---|---|---|---|
| MCP-1, pg/ml | 300.2 ± 43.25 | 557.7 ± 96.08 | 0.01 |
| IL-13, pg/ml | 120.9 ± 12.95 | 119.5 ± 30.77 | 0.925 |
| IL-4, pg/ml | 59.90 ± 13.25 | 72.48 + 27.94 | 0.62 |
| IFN-γ, pg/ml | 1.710 ± 0.830 | 1.991 ± 0.811 | 0.87 |
| TNF-α, pg/ml | 3.400 ± 0.750 | 5.581 ± 1.467 | 0.01 |
| IL-1 RA, pg/ml | 111.2 ± 18.72 | 382.8 ± 165.4 | 0.02 |
| IL-10, pg/ml | 8.850 ± 1.164 | 18.94 ± 3.495 | 0.04 |
| CCL18, pg/ml | 1.067 ± 56.01 | 1209 ± 96.25 | 0.21 |
| M-CSF, pg/ml | 174.0 ± 78.80 | 9.971 ± 8.234 | 0.05 |
Subgroup analysis of control group blood
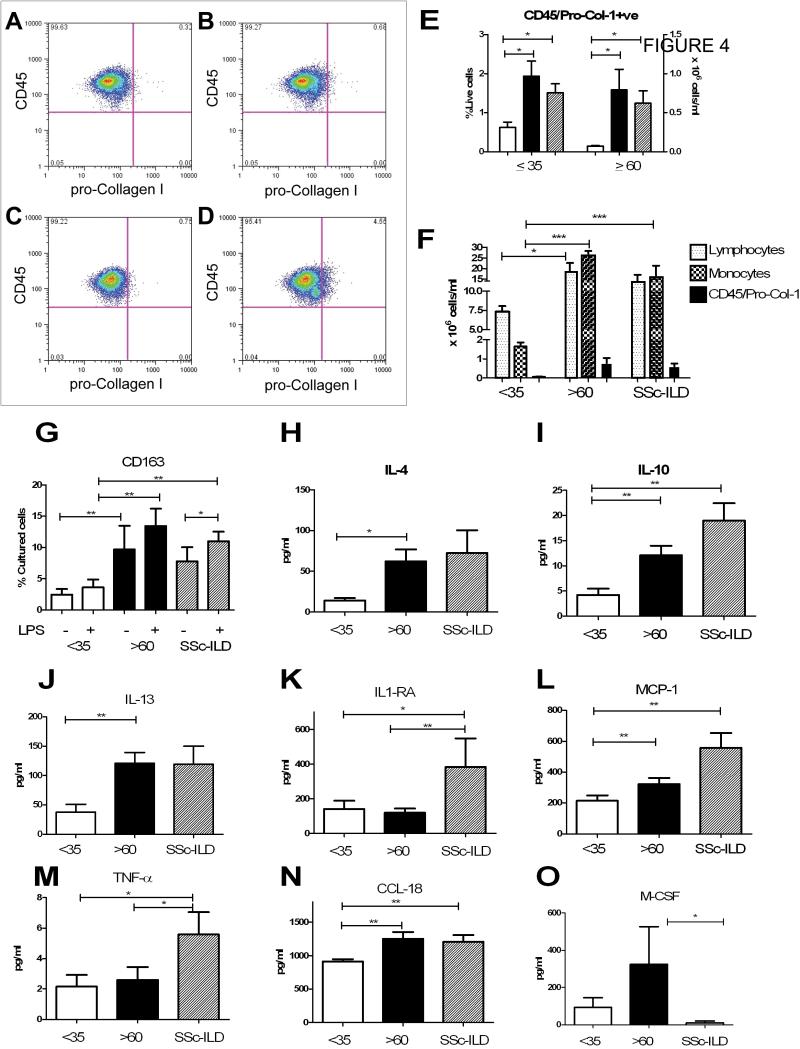
Further analysis of the data from control samples revealed an unexpected age-dependent pattern; when compared to the younger controls, blood from older controls showed a higher percentage and quantity of circulating CD45+/pro-Col-Iα+ cells (Figure 4 A-E, Table 4) and PBMCs, which contained increased lymphocytes and monocytes than the younger patients (Figure 4F Table 4). CD45+/pro-Col-Iα+ cells and total monocytes were very strongly correlated in the older subjects (r value 0.81, p<0.00016), while no correlation existed between these cell types in the younger subjects (r value 0.35, p=0.24). There was no association with lymphocytes in either of these populations (data not shown). In addition, there was an increase in both baseline and LPS stimulated CD163 expression on monocytes obtained from the older individuals (Figure 4G). While there was no difference in baseline or LPS stimulated CCL18 or IL-10 secretion (data not shown), we did observe increased circulating IL-4 (p<0.03), IL-10 (p<0.004), IL-13 (p<0.004), MCP-1 (p<0.05), and CCL-18 (p<0.01) in the older controls. TNF-α and IFN-γ did not differ between these groups (Table 4 and Figure 4H-O). This analysis also demonstrated that concentrations of CCL-18, IL-10 and MCP-1 were elevated in the SSc-ILD when compared to the young controls (p <0.01 all comparisons, Figure 4I, N, L). IL-4 and IL-13 in the SSc-ILD patients did not differ from either the young or old subsets (Figure 4H, J). IL-1RA and TNFα were elevated in the SSc-ILD patients when compared to both young and older subjects (p<0.05 all comparisons, Figure 4J, K,). Interestingly, M-CSF was lower in the SSc-ILD than in the aged controls (p<0.05, Figure 5 0). These data are the first to suggest that the blood of healthy but aged controls displays a distinct profibrotic signature that is unique from that of SSc-ILD patients.
Figure 4.
Subgroup analysis of peripheral blood monocyte subpopulations and fibrogenic mediators in young vs. aged controls. A: Staining with anti-CD45-PE vs. intracellular isotype control antibody used to set negative gates. B:CD45+/pro-Col-1α+ staining (CD45-PE vs. pro-Col-Iα) in young healthy control. C: Anti-CD45-PE vs intracellular isotype control antibody used to set negative gates. D: CD45+/pro-Col-1α+ staining (CD45-PE vs. pro-Col-Iα) in aged healthy control. E: Fibrocyte percentages (left axis) and quantities (right axis) in young subjects (white bar), aged subjects (black bar) and SSc-ILD patients (slanted lines). F: Comparison of lymphocytes (dotted line), monocytes (checked bar) and CD45+/pro-Col-Iα+ cells (black bar) in (left to right) young, aged, and SSc-ILD subjects. G: CD14+ monocyte expression of CD163 after 48 hours in culture. H-O: Concentrations of the plasma cytokines IL-4 (H), IL-10 (I), IL-13 (J), IL-1 RA (K), MCP-1 (L), TNFα (M), CCL-18 (N) and M-CSF (O) in young controls (n = 10, white bar), aged controls ( n = 12, black bar) and patients with SSc-ILD (n = 11, slanted bar). All data are expressed as mean ± SEM. *p<0.05. **p<0.01. ***p<0.001.
Table 4.
Subgroup Analysis: Fibrocyte Percentages, CD163 Expression, and Cytokine Levels (Older vs. Younger Controls)
| <35 years (n=10) | > 60 years (n=12) | p-value | |
|---|---|---|---|
| Fibrocyte percentage | 0.620 ± 0.014 | 1.932 ± 0.3900 | 0.001 |
| Fibrocyte concentration | 0.070 ± 0.010 | 0.790 ± 0.265 | 0.007 |
| Lymphocyte concentration | 7.380 ± 0.680 | 18.31 ± 4.310 | 0.03 |
| Monocyte concentration | 1.661 ± 0.221 | 27.06 ± 1.312 | 0.0001 |
| - LPS, percent CD163+ | 2.465 ± 0.900 | 9.724 ± 3.744 | 0.02 |
| + LPS, percent CD163+ | 3.632 ± 1.261 | 13.42 ± 2.766 | 0.01 |
| IL-4 (pg/ml) | 14.22 ± 2.99 | 62.47 ± 14.51 | 0.003 |
| IL-10 (pg/ml) | 4.169 ± 1.285 | 12.10 ± 1.88 | 0.004 |
| IL-13 (pg/ml) | 37.71 ± 13.27 | 121.2 ± 18.41 | 0.004 |
| IL-1RA (pg/ml) | 140.7 ± 48.76 | 118.6 ± 25.16 | 0.04 |
| MCP-1 (pg/ml) | 214.8 ± 34.59 | 322.5 ± 39.84 | 0.05 |
| TNFα (pg/ml) | 2.158 ± 0.76 | 2.591 ± 0.852 | 0.70 |
| CCL-18 (pg/ml) | 913.3 ± 33.92 | 1251 ± 102.1 | 0.01 |
| M-CSF (pg/ml) | 93.02 ± 51.60 | 324.5 ± 201.6 | 0.42 |
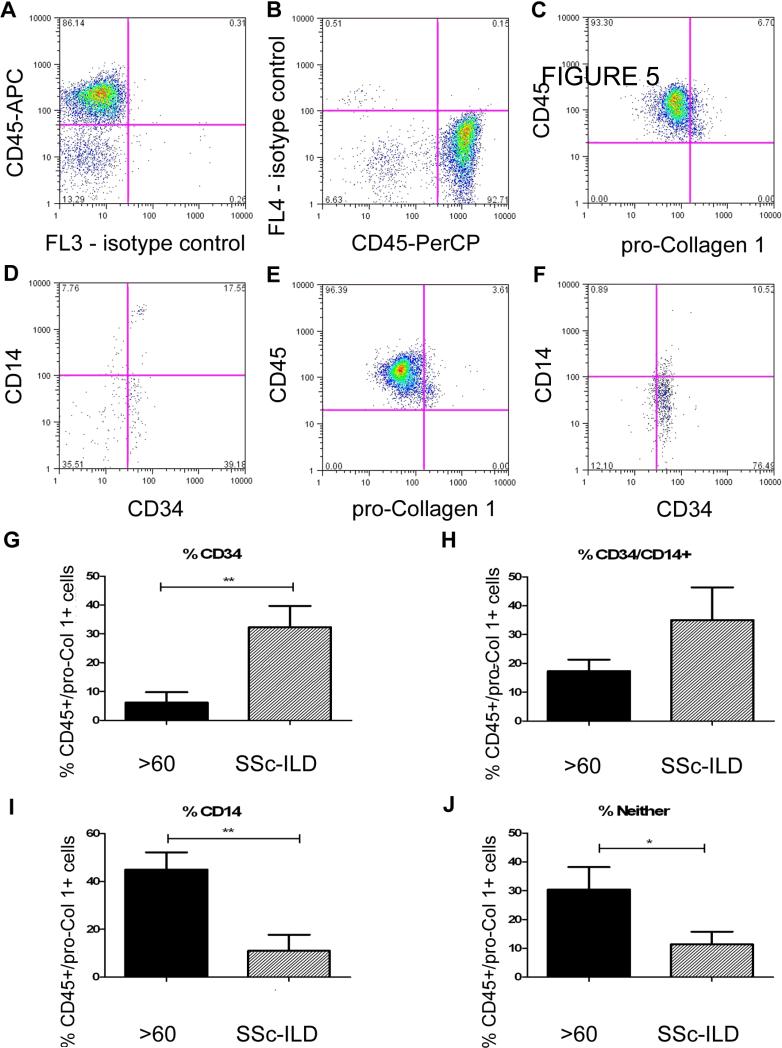
Figure 5.
Phenotype of CD45+/pro-Col-1α+ cells in SSc-ILD and aged, healthy controls A. PerCP isotype control (X axis) vs. Anti-CD45-APC (Y axis).This control was used to set the negative gate for PerCP. B. Anti-CD45-PerCP isotype control (X axis) vs. APC isotype control (Y axis). This control was used to set the negative gate for APC. C. Anti-pro-Col-Iα (X axis) vs. anti-CD45 (Y axis) on PBMCs from a subject > 60 years old. D. Anti-CD34 (X axis) vs anti-CD14 (Y axis) on the double positive population seen in C. E. Anti-pro-Col-1α (X axis) vs. anti-CD45 (Y axis) on PBMCs from a patient with SSc-ILD. F. Anti-CD34 (X axis) vs anti-CD14 (Y axis) on the double positive population seen in E. G-J: Comparison of CD34+ CD14- (G), CD14+ CD34+ (H), CD34-CD14+ (I) and CD14-CD34- (J) Populations in the pro-Col-1α+ cells obtained from (left to right) subjects greater than 60 years old (n = 10, black bar) and those with SSc-ILD (n = 7, slanted bar). Data are expressed as mean ± SEM. *p<0.05, **p<0.01.
Variable phenotype of CD45+/pro-Col-Iα+ cells in SSc-ILD vs. aged controls
Because we found differences in the circulating factors accompanying increased CD45+/pro-Col-Iα+ cells in the blood of healthy aged controls compared with that of patients with SSc-ILD we sought to more accurately phenotype the CD45+/pro-Col-Iα+ cells found in the blood of these individuals. Accordingly, we recruited an additional seven patients with SSc-ILD and ten aged controls and repeated the CD45+/pro-Col-Iα+ analysis with the addition of two additional markers, CD14 and CD34. These cell surface markers were selected to determine whether monocytes or fibrocytes accounted for the increase in circulating collagen-producing cells. Our initial results confirmed our prior studies, showing that both of these groups contained elevated numbers of CD45+/pro-Col-Iα+ cells expressing cells in the circulation (data not shown). When we gated on this dual positive population and quantified CD14 and CD34 expression, we found that the Collagen I expressing cells in all subjects expressed CD14 and CD34 to varying degrees (Figure 5A-F). In the patients with SSc-ILD, the collagen expressing cells were primarily CD34+ (32.31 ± 7.432% vs. 6.134 ± 3.641 %, p<0.004, Figure 5G) or CD34+ CD14+ (35.01 ± 11.34% vs. 17.26 ± 4.072%, p = 0.18, Figure 5H). In contrast, CD45+/pro-Col-Iα+ cells from the aged patients were enriched for CD14 expression (44.96 ± 7.18% vs. 10.98 ± 6.712, p<0.01, Figure 5I) as well as for a population of cells that expressed neither marker (30.44 ± 7.814% vs. 11.35 ± 4.412%, p<0.05, Figure 5J). These results demonstrate that different cell populations account for the increase in collagen-producing cells found in the circulation of subjects with SSc-ILD and in that of healthy aged controls.
Discussion
Our data show that monocytes obtained from the blood of patients with SSc-ILD display a fibrogenic phenotype. Specifically, the blood of patients with SSc-ILD shows increased numbers of fibrocytes and collagen-producing monocytes when compared to healthy age-matched controls. In addition, LPS stimulation of CD14+ monocytes from these patients led to substantially increased expression of CD163 and the profibrotic mediators CCL18 and IL-10. Also, the blood of patients with SSc-ILD contained significantly higher concentrations of IL-10, MCP-1, TNF-α, and IL-1 RA. Lastly, the blood of aged healthy controls contained increased quantities of collagen-producing monocytes, an increase in CD163 expressing monocytes, and higher circulating levels of CCL-18, IL-4, IL-10, IL-13 and MCP-1 when compared to younger individuals. These data provide new information regarding the relationship between fibrogenic monocyte precursors, SSc-ILD, and normal aging.
Circulating cells that express Collagen I are associated with an expanding repertoire of human diseases including renal fibrosis (12), cirrhosis (13), scleroderma (7), nephrogenic systemic fibrosis (38), pulmonary fibrosis (16-18), and asthma (39, 40). Most of these studies have used the combination of CD45 and pro-Col-Iα to identify these cells and have assumed them to be fibrocytes. However, a recent study demonstrates some overlap, in the markers used to identify fibrocytes and particular macrophage subsets (35, 41). Our data indicate that the blood of patients with scleroderma and healthy aged controls is enriched for collagen-producing cells. In the scleroderma patients, this increase appears to relate to an increase in fibrocytes (identified by the expression of CD45, CD34, and pro-collagen I) as well as an intermediate cell population characterized by the expression of CD45, CD14, CD34, and pro-collagen I. Because CD34 is a marker of progenitor cells, these results may indicate either decreased terminal differentiation of fibrocytes in the SSc-ILD patients or an increase in the efflux of progenitor cells from the bone marrow. In contrast, in aged subjects the increase in circulating CD45+/pro-Col-Iα+ cells appears to result from increased collagen production by CD14+ monocytes as well as a population of CD45+/pro-Col-Iα+ cells expressing neither CD34 nor CD14. While the precise identity and significance of these cells in aging remains to be determined, the increased fibrocyte levels detected in our SSc-ILD patient cohort may promote lung remodeling by a number of mechanisms including direct matrix production, antigen presentation and immune stimulation, and cytokine and growth factor production (42). Alternately, the increase in fibrocytes observed in our SSc-ILD cohort may represent a host response to fibrosis and an attempt to normalize repair.
Alternatively activated macrophages displaying high level production of IL-10 and CCL18 (43) have been found in the lungs of patients with SSc (43). Our data show that LPS stimulated monocytes from patients with SSc-ILD demonstrate enhanced CD163 expression, and increased CCL18 and IL-10 secretion when compared to monocytes from age-matched normal controls, thus confirming the fibrogenic potential of this cell population. These findings also agree with a recent report that LPS-activated dendritic cells from patients with SSc produce greater levels of CCL18 and IL10 in vitro (36). While one recent report suggests that cultured fibrocytes can express CD163 (35), the round rather than spindle shaped morphology of CD163+ cells in our studies supports the contention that these cells are macrophages (35). Because of evidence that circulating monocytes give rise to alveolar macrophages (44), it is intriguing to find evidence for a fibrogenic phenotype within monocytes that is detectable prior to the entry of these cells into diseased lungs. This finding supports the idea that monocyte phenotypes may be pre-programmed to adopt their ultimate activation state, perhaps through genetic or epigenetic alterations in progenitor cells. It also is noteworthy that in both aged patients and in those with SSc-ILD, cultured monocytes adopt this phenotype in response to LPS, which stimulates the TLR4 receptor (24). These data suggest that the monocytes of these patients are inappropriately skewed towards an alternatively activated phenotype, which could account for both the propensity to develop fibrosis and the abnormalities in host defense that may be seen in both aged patients (45) and in those with SSc-ILD (46).
The “type II hypothesis of fibrosis” posits that an imbalance between Th1 and Th2 cytokines alters tissue microenvironments towards a profibrotic phenotype (47). Indeed, Th2 cytokines regulate differentiation and activation of both fibrocytes (48) and M2s (23). Indeed, we detected an increase in IL-10, MCP-1, IL-1 RA, and TNFα, as well as decreased M-CSF in the blood of patients with SSc-ILD when compared to both older and younger controls. This combination of cytokines may contribute to the increase in CD34+ fibrocyte numbers observed in the SSc-ILD group as well as to the increased CCL18 and IL10 levels seen in response to LPS stimulation of SSc-ILD monocytes. In contrast, the blood of aged healthy controls contained increased IL-4, IL-10, IL-13, MCP-1, and CCL18, but decreased TNFα when compared to young healthy controls. However, these mediators did not differ between aged subjects and those with SSc-ILD. Given the differences in CD45+/pro-Col-Iα+ cell phenotypes seen in our cohort, it is possible that this combination of cytokines may induce collagen expression in monocytes, but that IL-1 RA and TNFα are required for the maintenance of CD34 expression. It also is possible that the increase in M-CSF detected in the aged controls compared with SSc-ILD subjects drives CD14+ cells away from the fibrocyte phenotype.
One particularly intriguing and unexpected finding concerns the age-dependent changes in the quantities of circulating CD45+/pro-Col-Iα+ cells that we observed. While age-related changes in monocyte quantity and activation (49), and blood levels of MCP-1 (50) and IL13 (51) have been reported, to our knowledge these are the first data linking the accumulation or trafficking of collagen-expressing cells in the blood with normal aging. Our data show an increase in lymphocytes and monocytes in the aged population and a very strong association between this monocytosis and collagen production by CD14+ cells. It is possible that had we examined complete blood counts from these patients prior to Ficoll purification the results would have differed, as the Ficoll-based cell separation can cause a variable loss of cells (52), or that our inclusion of healthy elderly subjects accounts for this increase in cell counts, as prior work has shown that leukocyte counts of aged and frail individuals are significantly lower than those of aged but healthy subjects (53). It also is possible that these findings result from the higher observed plasma levels of IL-4, IL-10, IL-13, CCL-18, and MCP-1 in the blood of aged individuals. Given the limitations of clinical data, we also cannot rule out the possibility that these findings may be related to undiagnosed medical conditions, bone marrow abnormalities, or immunosenescence. We suggest based on these findings that circulating fibrocytes and collagen-producing monocytes may have an additional physiologic role in the maintenance of tissue homeostasis during normal aging.
In conclusion, these data strengthen the association between fibrocytes, alternatively activated macrophages, and SSc-ILD. The distinct profibrotic phenotype of circulating monocytes in SSc-ILD implies that abnormalities in differentiation, recruitment, trafficking, activation, and clearance of these cells may be important factors in the pathogenesis of lung fibrosis in this patient population.
Acknowledgments
Funding Sources: This work was supported in part by awards from the NIH (P30AR053495-01A1, K08 HL079066, UL1 RR024139, N01 AI50031), an Edward Mallinckrodt, Jr. Scholar Award, funds from the Yale Department of Medicine, and Promedior, Inc. (All to E.L.H.)
Disclosures
LM is an employee of and RB is a member of the Scientific Advisory Board of Promedior, Inc. RB is a co-inventor on a patent describing the therapeutic utility of fibrocytes.
Abbreviations
- ILD
Interstitial Lung Disease
- SSc
systemic sclerosis
- IPF
Idiopathic Pulmonary Fibrosis
- CT
computed tomography
- LPS
lipopolysaccharide
- M1
classically activated macrophage
- M2
alternatively activated macrophage
- FVC
forced vital capacity
- PBMCs
peripheral blood mononuclear cells
- DLCO
diffusing capacity of the lung for carbon monoxide
- FSC
forward scatter
- SSC
side scatter
- GERD
gastroesophageal reflux disease
- pro-Col-Iα
pro-Collagen Iα
- Col-Iα
Collagen Iα
- TLR4
toll-like receptor 4
- ELISA
enzyme-linked immunosorbent assay
References
- 1.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66(7):940–4. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 3.D'Ovidio F, Singer LG, Hadjiliadis D, Pierre A, Waddell TK, de Perrot M, et al. Prevalence of gastroesophageal reflux in end-stage lung disease candidates for lung transplant. Ann Thorac Surg. 2005;80(4):1254–60. doi: 10.1016/j.athoracsur.2005.03.106. [DOI] [PubMed] [Google Scholar]
- 4.D'Ovidio F, Mura M, Tsang M, Waddell TK, Hutcheon MA, Singer LG, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129(5):1144–52. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 5.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166(12):7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 6.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 7.Katebi M, Fernandez P, Chan ES, Cronstein BN. Adenosine A2A receptor blockade or deletion diminishes fibrocyte accumulation in the skin in a murine model of scleroderma, bleomycin-induced fibrosis. Inflammation. 2008;31(5):299–303. doi: 10.1007/s10753-008-9078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36(4):598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Ihn H. [Systemic sclerosis]. Nippon Rinsho. 2009;67(3):519–22. [PubMed] [Google Scholar]
- 10.Niedermeier MRB, Gomez M, Denzel A, Schmidbauer K, Gobel N, Talke Y, Scweda F, Mack M. CD4+ T cells control the differentiatin of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0906070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1(1):71–81. [PMC free article] [PubMed] [Google Scholar]
- 12.Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, et al. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci U S A. 2006;103(38):14098–103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol. 2006;45(3):429–38. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87(9):858–70. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 15.Vakil V, Sung JJ, Piecychna M, Crawford JR, Kuo P, Abu-Alfa AK, et al. Gadolinium-containing magnetic resonance image contrast agent promotes fibrocyte differentiation. J Magn Reson Imaging. 2009;30(6):1284–8. doi: 10.1002/jmri.21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersson-Sjoland A, de Alba CG, Nihlberg K, Becerril C, Ramirez R, Pardo A, et al. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008 doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353(1):104–8. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 18.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, et al. Circulating Fibrocytes Are an Indicator for Poor Prognosis in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2009 doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 19.Murray Lynne A., Rosada Rogerio, Moreira Ana Paula, Joshi Amrita, Kramer Michael S., Hesson David P., Argentieri Rochelle L., Peng Xueyang, Mathai Susan, Homer Robert J., Elias Jack A., van Rooijen Nico, Lee Chun Geun, Gulati Mridu, Hogaboam Cory M., Herzog Erica L. Modulation of M2 Macrophage Function Ameliorates TGF - Driven Lung Fibrosis. 2009. in revision.
- 20.Aiba S, Tabata N, Ohtani H, Tagami H. CD34+ spindle-shaped cells selectively disappear from the skin lesion of scleroderma. Arch Dermatol. 1994;130(5):593–7. [PubMed] [Google Scholar]
- 21.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115(1):56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 23.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177(10):7303–11. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 24.Prasse A, Germann M, Pechkovsky DV, Markert A, Verres T, Stahl M, et al. IL-10-producing monocytes differentiate to alternatively activated macrophages and are increased in atopic patients. J Allergy Clin Immunol. 2007;119(2):464–71. doi: 10.1016/j.jaci.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 25.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104(1):27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 26.Hieshima K, Imai T, Baba M, Shoudai K, Ishizuka K, Nakagawa T, et al. A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1 alpha/LD78 alpha and chemotactic for T lymphocytes, but not for monocytes. J Immunol. 1997;159(3):1140–9. [PubMed] [Google Scholar]
- 27.Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, Goldmann T, et al. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173(7):781–92. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- 28.Meneghin A, Hogaboam CM. Infectious disease, the innate immune response, and fibrosis. J Clin Invest. 2007;117(3):530–8. doi: 10.1172/JCI30595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasse A, Pechkovsky DV, Toews GB, Schafer M, Eggeling S, Ludwig C, et al. CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis. Arthritis Rheum. 2007;56(5):1685–93. doi: 10.1002/art.22559. [DOI] [PubMed] [Google Scholar]
- 30.Ding Y, Boguslawski EA, Berghuis BD, Young JJ, Zhang Z, Hardy K, et al. Mitogen-activated protein kinase kinase signaling promotes growth and vascularization of fibrosarcoma. Mol Cancer Ther. 2008;7(3):648–58. doi: 10.1158/1535-7163.MCT-07-2229. [DOI] [PubMed] [Google Scholar]
- 31.Niedermeier M, Reich B, Gomez MR, Denzel A, Schmidbauer K, Gobel N, et al. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci U S A. 2009;106(42):17892–7. doi: 10.1073/pnas.0906070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160(1):419–25. [PubMed] [Google Scholar]
- 33.Higashi-Kuwata N, Makino T, Inoue Y, Takeya M, Ihn H. Alternatively activated macrophages (M2 macrophages) in the skin of patient with localized scleroderma. Exp Dermatol. 2009;18(8):727–9. doi: 10.1111/j.1600-0625.2008.00828.x. [DOI] [PubMed] [Google Scholar]
- 34.Moriyama H, Kobayashi M, Takada T, Shimizu T, Terada M, Narita J, et al. Two-dimensional analysis of elements and mononuclear cells in hard metal lung disease. Am J Respir Crit Care Med. 2007;176(1):70–7. doi: 10.1164/rccm.200601-134OC. [DOI] [PubMed] [Google Scholar]
- 35.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4(10):e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Lieshout AW, Vonk MC, Bredie SJ, Joosten LB, Netea MG, van Riel PL, et al. Enhanced interleukin-10 production by dendritic cells upon stimulation with Toll-like receptor 4 agonists in systemic sclerosis that is possibly implicated in CCL18 secretion. Scand J Rheumatol. 2009:1–9. doi: 10.1080/03009740802572467. [DOI] [PubMed] [Google Scholar]
- 37.Lee CG, Homer RJ, Cohn L, Link H, Jung S, Craft JE, et al. Transgenic overexpression of interleukin (IL)-10 in the lung causes mucus metaplasia, tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J Biol Chem. 2002;277(38):35466–74. doi: 10.1074/jbc.M206395200. [DOI] [PubMed] [Google Scholar]
- 38.Bucala R. Circulating fibrocytes: cellular basis for NSF. J Am Coll Radiol. 2008;5(1):36–9. doi: 10.1016/j.jacr.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171(1):380–9. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 40.Wang CH, Huang CD, Lin HC, Lee KY, Lin SM, Liu CY, et al. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med. 2008;178(6):583–91. doi: 10.1164/rccm.200710-1557OC. [DOI] [PubMed] [Google Scholar]
- 41.Schnoor M, Cullen P, Lorkowski J, Stolle K, Robenek H, Troyer D, et al. Production of type VI collagen by human macrophages: a new dimension in macrophage functional heterogeneity. J Immunol. 2008;180(8):5707–19. doi: 10.4049/jimmunol.180.8.5707. [DOI] [PubMed] [Google Scholar]
- 42.Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol. 2007;82(3):449–56. doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- 43.Luzina IG, Atamas SP, Wise R, Wigley FM, Xiao HQ, White B. Gene expression in bronchoalveolar lavage cells from scleroderma patients. Am J Respir Cell Mol Biol. 2002;26(5):549–57. doi: 10.1165/ajrcmb.26.5.4683. [DOI] [PubMed] [Google Scholar]
- 44.Everhart MB, Han W, Parman KS, Polosukhin VV, Zeng H, Sadikot RT, et al. Intratracheal administration of liposomal clodronate accelerates alveolar macrophage reconstitution following fetal liver transplantation. J Leukoc Biol. 2005;77(2):173–80. doi: 10.1189/jlb.1203647. [DOI] [PubMed] [Google Scholar]
- 45.Caruso C, Buffa S, Candore G, Colonna-Romano G, Dunn-Walters D, Kipling D, et al. Mechanisms of immunosenescence. Immun Ageing. 2009;6:10. doi: 10.1186/1742-4933-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Juarez M, Misischia R, Alarcon GS. Infections in systemic connective tissue diseases: systemic lupus erythematosus, scleroderma, and polymyositis/dermatomyositis. Rheum Dis Clin North Am. 2003;29(1):163–84. doi: 10.1016/s0889-857x(02)00100-x. [DOI] [PubMed] [Google Scholar]
- 47.Sime PJ, O'Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol. 2001;99(3):308–19. doi: 10.1006/clim.2001.5008. [DOI] [PubMed] [Google Scholar]
- 48.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83(6):1323–33. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadeghi HM, Schnelle JF, Thoma JK, Nishanian P, Fahey JL. Phenotypic and functional characteristics of circulating monocytes of elderly persons. Exp Gerontol. 1999;34(8):959–70. doi: 10.1016/s0531-5565(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 50.Gerli R, Monti D, Bistoni O, Mazzone AM, Peri G, Cossarizza A, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. 2000;121(1-3):37–46. doi: 10.1016/s0047-6374(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 51.Shearer GM. Th1/Th2 changes in aging. Mech Ageing Dev. 1997;94(1-3):1–5. doi: 10.1016/s0047-6374(96)01849-0. [DOI] [PubMed] [Google Scholar]
- 52.Hokland P, Heron I. The Isopaque-Ficoll method re-evaluated: selective loss of autologous rosette-forming lymphocytes during isolation of mononuclear cells from human peripheral blood. Scand J Immunol. 1980;11(3):353–6. doi: 10.1111/j.1365-3083.1980.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 53.Kapasi ZF, Ouslander JG, Schnelle JF, Kutner M, Fahey JL. Effects of an exercise intervention on immunologic parameters in frail elderly nursing home residents. J Gerontol A Biol Sci Med Sci. 2003;58(7):636–43. doi: 10.1093/gerona/58.7.m636. [DOI] [PubMed] [Google Scholar]