Abstract
M protein mutant vesicular stomatitis virus is an attractive candidate oncolytic virus for the treatment of metastatic colorectal cancer due to its ability to kill cancer cells that are defective in their antiviral responses. The oncolytic activity of recombinant wild-type and M protein mutant vesicular stomatitis viruses was determined in RKO, Hct116 and LoVo colorectal cancer cells, as well as in human fibroblast and hepatocyte primary cultures. RKO and Hct116 cells were sensitive to both viruses, whereas LoVo cells were resistant. [35S]methionine labeling experiments and viral plaque assays showed that sensitive and resistant colorectal cancer cells supported viral protein and progeny production after infection with either virus. Colorectal cancer cells were pretreated with β-interferon and infected with vesicular stomatitis virus to evaluate the extent to which interferon signaling is downregulated in colorectal cancer cells. Although colorectal cancer cells retained some degree of interferon signaling, this signaling did not negatively impact the oncolytic effects of either virus in sensitive cells. Murine xenografts of RKO cells were effectively treated by intratumoral injections with M protein mutant virus, whereas LoVo xenografts were resistant to treatment with this virus. These results suggest that M protein mutant vesicular stomatitis virus is a good candidate oncolytic virus for the treatment of selected metastatic colorectal cancers.
Keywords: vesicular stomatitis virus, oncolytic virus, colorectal cancer, type I interferon, xenograft
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in the United States. It is estimated that 147 000 individuals are diagnosed with invasive CRC every year.1 Although progress has been made in many aspects of combating this disease—including improved techniques in early diagnosis, surgery and systemic therapy—nearly a third of all patients die of metastatic disease, and thus highlighting the need for novel approaches to the treatment of advanced CRC. Treatment of advanced CRC with oncolytic viruses has the potential to improve the outcomes for this cohort of patients.
Cancer cells are generally more susceptible to viral infections than normal cells because they often suppress their antiviral responses in favor of the development of enhanced proliferative signaling.2,3 In fact, it has been known for many years that most tumor cells are resistant to the antiproliferative effects of interferons (IFNs) due to various defects in the IFN signal-transduction pathway.4 Defects in the antiviral IFN signaling transduction pathway make these cells correspondingly more susceptible to infection with a variety of viruses including vesicular stomatitis virus (VSV).5–9
VSV, the prototypical member of the family Rhabdoviridae, is one of several viruses currently being developed as oncolytic agents for antitumor therapies.5 An attractive candidate as an oncolytic agent, VSV is a potent inducer of apoptosis with many types of tumor cells susceptible to infection and killing by VSV.5,10–13 Moreover, VSV is highly sensitive to the antiviral effects of IFN and therefore replicates selectively in cancer cells that have defects in the IFN pathway. The selectivity of VSV for cancer cells can be enhanced by introducing mutations in the matrix (M) protein. The M protein of VSV is a multifunctional protein that plays a major role in virus assembly as well as in the inhibition of host gene expression. The inhibition of host gene expression suppresses the antiviral responses of host cells. This inhibition occurs at multiple levels, including transcription, nuclear-cytoplasmic RNA transport and translation. 14–16 The inhibitory effects of M protein on host gene expression are genetically separable from its virus assembly functions, so that a number of different mutations render the M protein defective in its ability to inhibit host gene expression without compromising its ability to function in virus assembly.17,18 Because the inhibition of host gene expression suppresses the production of IFNs and other antiviral proteins in infected cells, mutations in the M protein allow infected cells with intact IFN signaling to mount antiviral responses. As a result, normal cells, which have intact antiviral responses, are able to produce IFN and other antiviral cytokines in response to infection with M protein mutant VSV, thereby enhancing the virus’ specificity for cancer cells. Our group has generated genetically engineered recombinant viruses derived from cDNA clones containing M protein mutations, such as the M51R virus, which contains a single M51R amino-acid substitution in the M protein, that is selective for cancer cells.5 Such M protein mutants appear to be strong candidates for oncolytic viral therapy as they are attenuated for replication in normal tissues, but replicate as well in recombinant virus with wild-type (wt) M protein (rwt virus) in cancers that have defective antiviral responses. Previous work has described the oncolytic effects of wtVSV against a variety of malignancies including colon cancer.3,19,20 Stojdl et al.20 found that six of seven CRC cell lines from the NCI 60 cell line panel were sensitive to VSV oncolysis. However, the basis for sensitivity versus resistance to VSV in CRC cell lines has not been addressed. We therefore sought to investigate the ability of this virus to replicate and induce cell death in CRC cells with a variety of molecular phenotypes. The cell lines chosen for this work have BRAF (RKO) and KRas (Hct116) mutations, as well as overexpression of the c-myc proto-oncogene (LoVo).
In the work presented herein, we found that human CRC cell lines differ in their susceptibility to both rwt and M protein mutant (rM51R-M) VSV. Interestingly, all CRC cell lines supported the production of viral progeny and protein production after infection with rwt and M51R VSV. IFN-blocking experiments demonstrated that the sensitivity of CRC cells to VSV-induced cell death occurred independent of defects in the IFN signaling pathway. Taken together, these results suggest that the mechanism that determines the sensitivity of CRC cells to M51R VSV is based not on the cell line’s sensitivity to IFN, but on the effectiveness of the cell death pathways induced by viral infection. Established xenografts derived from CRC cells were treated with M51R VSV to determine if M51R VSV can effectively treat CRC tumors. Intratumoral injections of murine xenografts established with sensitive CRC cells with M51R VSV resulted in the inhibition of tumor growth relative to untreated controls. On the other hand, treatment of xenografts of resistant cells did not result in significant inhibition of growth. Hence, we propose that oncolytic VSV can effectively treat selected CRCs.
Materials and methods
Cells and viruses
The recombinant viruses rwt and rM51R-M, and rGFP-M51R- M were isolated from infectious VSV cDNA clones, and virus stocks were prepared and their titers determined by plaque in BHK cells as described.21–23 RKO, Hct116 and LoVo tumor cells were from the American Type Culture Collection and were grown in McCoy’s 5A medium (containing 10% fetal bovine serum, penicillin/streptomycin and L-glutamine) and Ham’s F12K (containing 10% fetal bovine serum, penicillin/streptomycin, L-glutamine and 1.5 g l−1 NaCHO3), respectively. Human dermal fibroblasts (CC2511; Clonetics, San Diego, CA) and primary hepatocytes (HU0796 and HU0803; CellzDirect, Pittsboro, NC) were maintained in the medium prescribed by the manufactures. Only cell lines that tested negative for mycoplasma at 6-month intervals were used for the experiments described herein. Cells were grown in monolayers to about 70–90% confluence and infected in small volumes at the multiplicities of infection (MOIs) specified for each experiment.
Expression of green fluorescent protein
RKO, Hct116 and LoVo cells were seeded in six-well plates and infected with rGFP-M51R VSV at MOIs of 10, 20 and 50 for 8 h. All cells were harvested by scraping cells into the medium with sterile cell lifters, and cells were collected into microcentrifuge tubes. Cells were gently pelleted and washed in ice-cold fluorescence-activated cell sorter buffer (100 ml of phosphate-buffered saline (PBS) and 5ml of fetal bovine serum). A Beckman Dickinson FACSCalibur flow cytometer was used to quantify green fluorescent protein (GFP) cell surface fluorescence.
Cell viability assays
RKO, Hct116 and LoVo CRC cells were plated in a 96-well plate at 4500 cells per well. CC2511 human dermal fibroblasts as well as HU0796 and HU0803 primary hepatocytes were plated at a density of 3000 cells per well. Cells were infected with wt and mutant viruses at an MOI of 0, 0.1, 1, 5 or 10 plaque forming units (PFU) per cell. At 24 and 48 h postinfection, live cells were measured by an MTS assay (CellTiter 96 Aqueous One Solution Cell Proliferation Assay; Promega, Madison, WI) according to the manufacturer’s instructions.
Apoptosis assay
The percentage of infected cells undergoing DNA fragmentation characteristic of apoptosis was determined by TdT-mediated dUTP nick-end labeling (assay) (TUNEL) analysis (In Situ Cell Death Detection Kit, Roche Diagnostics, Indianapolis, IN). RKO, LoVo and Hct116 CRCs were infected with rwt and rM51R-M viruses at the indicated MOIs. At 24 and 48 h postinfection, cells were harvested, fixed in paraformaldehyde and permeabilized. Cells were incubated with the TUNEL reaction mixture containing TdT and fluorescein-dUTP to free 3′-OH ends in the DNA as described.24 The incorporated fluorescein was analyzed and quantitated by flow cytometry on a Becton Dickinson FACSCalibur flow cytometer.
Quantification of host and viral protein synthesis
RKO, Hct116 and LoVo cells were plated at 450 000 cells per dish in 60mm dishes. The cells were infected with rwt or M51R VSV at an MOI of 10 PFU per cell. At 4, 8 and 12 h, cells were labeled with a 15-min pulse of [35S]methionine (200 μCi ml−1) in a small volume of methionine-free medium. Cells were washed with PBS, and extracts were prepared by harvesting cells in radioimmunoprecipitation assay buffer.25 Cell extracts were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the fixed and dried gels were analyzed by phosphorescence imaging. Radioactivity of N protein bands and background host proteins (three sections from each lane excluding viral protein bands) was quantified with the ImageQuant software (Molecular Dynamics Inc., Sunnyvale, CA).21
Growth curves
RKO, Hct116 and LoVo cells were plated in 100mm dishes. Cells were infected with rwt or M51R VSV in duplicate at MOIs of 0.1 or 10 PFU per cell for 1 h. The inoculum was removed and the plates were washed with PBS to remove any of the original infecting viruses. Fresh media (13 ml) was placed on the plates. At appropriate time points, the plates were gently rocked to mix the media and a 1 ml aliquot was removed and stored at −80° C. The viruses were titered as described previously.5
IFN responsiveness
RKO, LoVo and Hct116 cells were plated onto 96-well dishes and pretreated with different concentrations of Universal Type I Interferon (6.4–800 IU ml−1) (PBL Interferon Source, Piscataway, NJ). Cell viability was measured by MTS assays (CellTiter 96 Aqueous One Solution Cell Proliferation Assay; Promega). Controls included mock-treated cells infected with VSV and IFN-treated cells that were not challenged with VSV.
Tumor treatment
RKO and LoVo cells were harvested from semiconfluent cultures and checked for animal pathogens (service from RADIL, Columbia, MO). Cells were suspended at 2×106 cells/0.2 ml PBS and injected subcutaneously in a flank of Balb/c nude mice. Animals were monitored for tumor development three times a week by visual inspection and palpation of the injection site. Animals with palpable tumors had their tumor volume measured by calipers and the volume calculated using the formula: volume=(width)2×length/2. Tumors were evaluated, and when the tumors had reached the threshold size (3.5–4mm on an axis with the other axis being non-negligible), the animal was subjected to treatment with either intratumoral injection of culture medium (n=18) or intratumoral injection of 6×108 PFU of M51R VSV in culture medium (n=18). Tumor volume was measured 3 days a week with calipers, as described above, and animal mass was also measured. Two mice were selected from each group to be killed at 2 and 4 days after treatment and the tumor removed for later analysis via viral plaque assays and immunohistochemistry techniques.
Viral titers from xenografts
Tumor xenografts were homogenized in PBS and the supernatants were stored in −80 ° C. The supernatants were thawed, serially diluted and the virus titer was determined using BHK cells.
Immunohistochemistry
Harvested tissues were fixed in 4% paraformaldehyde overnight, embedded in paraffin and sectioned at 5 μm. Sections were stained with hematoxylin and eosin for histological examination or used in immunohistochemical staining. For immunohistochemical staining, cells were fixed in descending series of ethanol washes, quenched with 0.3% peroxide in PBS and blocked in 5% goat serum. Serial sections were incubated overnight with antibodies against the viral envelope glycoprotein (rabbit anti-G; Research Diagnostics Inc., Flanders, NJ). Secondary antibody (biotinylated anti-rabbit from BioGenex Super-sensitive Detection Kit, San Ramon, CA) was incubated on sections at room temperature for 30 min. Primary antibody detection was accomplished using a streptavidin alkaline phosphatase detection kit (Supersensitive Detection Kit; BioGenex). Vector Red Substrate Kit No. 1 for alkaline phosphatase (Vector Laboratories Inc., Burlingame, CA) was used to visualize the antibody–antigen complex. Nuclei were counterstained using Mayer’s hematoxylin. Negative controls consisted of histological sections processed without the addition of primary antibody, but incubated instead with 1% goat serum or mouse immunoglobulin G (Reagent Grade, 0.33 mg ml−1; Sigma, Saint Louis, MO).
Statistical analysis
We utilized a paired Student’s t-test to analyze significances of differences between groups at individual time points for experiments containing two experimental groups. Repeated measures analysis of variance was used to determine significant differences between groups at different time points. Repeated measures analysis of variance was used to assess the significance of differences in tumor size relative to that seen before treatment. Specifically, we tested whether there were significant differences in the mean percent change in tumor volume between the treatment and control groups overtime. Values were considered statistically significant if P-values are <0.05.
Results
Differences exist in the theoretical versus actual multiplicities of infection in CRC cells
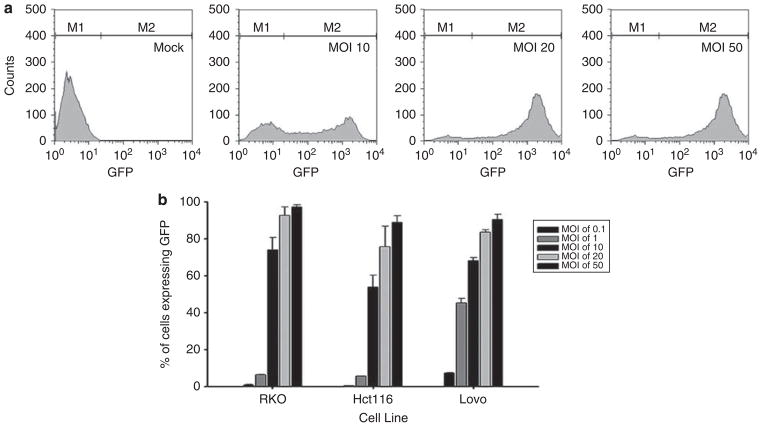
Virus doses in cell culture are defined by the MOI in PFUs per cell. However, viral titers are typically determined in sensitive cell lines, such as BHK in the case of VSV. Thus, the actual MOI may differ from the theoretical MOI. It is widely recognized in virology that viral titers vary if assayed on different cell types.25,26 To determine if the viral concentrations obtained from titration of VSV on BHK cells corresponded with those observed in our panel of CRC cells, we determined the MOI required for synchronous infection of RKO, Hct116 and LoVo cells. The cells were infected with rGFP-M51R VSV at theoretical MOIs of 10, 20 and 50 PFU per cell. After 8 h of incubation, cells were harvested and levels of GFP expression were determined by flow cytometry.
Histograms from a series of experiments were gated on GFP expression to analyze the percentage of CRC cells that express GFP (Figure 1). Figure 1a shows sample histograms of LoVo cells that were mock infected or infected at MOIs of 10, 20 or 50. Interestingly, upon infection at MOIs of 10 and 20, there were two distinct populations of cells expressing either a low or high level of GFP. On the other hand, in cells infected at an MOI of 50, there was a single population of cells expressing a high level of GFP. Results with all three cell lines indicated that MOIs between 20 and 50 were required to synchronously infect CRC cells such that over 90% of cells expressed GFP (Figure 1b). Although these findings suggest that the effective MOI of rVSV in our panel of CRC is approximately 20–50% that expected from BHK titration, we do not expect that they will make a significant difference in the interpretation of the results described herein.
Figure 1.
Establishment of synchronous infection of rGFP-M51R vesicular stomatitis virus (VSV) in colorectal cancer (CRC) cells. CRC cells were infected at various multiplicities of infection (MOIs) (0.1–50) for 8 h. Cells were then analyzed for green fluorescent protein (GFP) expression by flow cytometry. (a) Representative histograms for LoVo cells show different populations of cells expressing low and high levels of GFP in cells infected at MOIs of 10, 20 and 50. (b) The percentage of CRC cells expressing GFP on their surface for each condition; the data are representative of three independent experiments.
CRC cells display differential sensitivity to the oncolytic effects of VSV
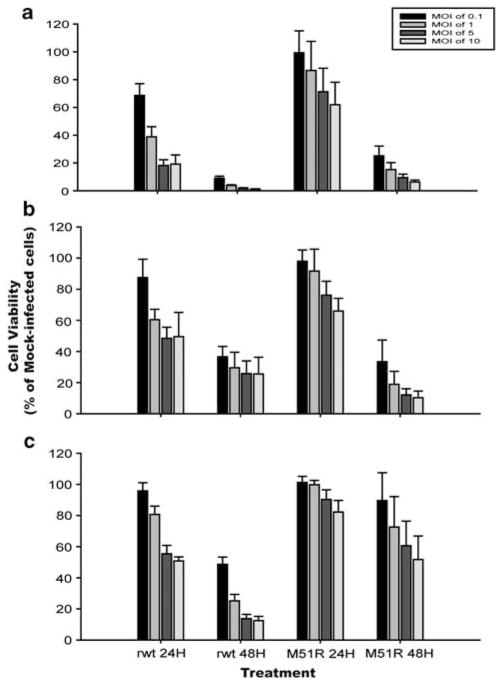
Three primary human cell cultures (CC2511 human dermal fibroblasts as well as HU0796 and HU0803 primary hepatocytes) and three CRC cell lines (RKO, Hct116 and LoVo) were tested for their sensitivity to the oncolytic activity of VSV. Two different viruses were tested, rwt virus, which contains a wt M protein, and M51R virus, which is an isogenic M protein mutant virus that is defective in its ability to inhibit host gene expression. Primary cell cultures and CRC cells were infected with rwt or M51R viruses at varying MOIs, and cell viability was assayed at 24 and 48 h postinfection using MTS assay (Figures 2 and 3). All primary cell cultures were resistant to the cytolytic effects of VSV (Figure 2). In cultures infected at an MOI of 0.1 PFU per cell, killing of a substantial percentage of cells requires multiple-cycle replication and spread of the virus throughout the culture. Of the three CRC cell lines, RKO cells were the most sensitive to the cytolytic activity of VSV, with cell viability reduced to approximately 10–20% of mock-infected controls by 48 h postinfection with either virus in both single- and multiple-cycle infections (Figure 3a). The time course of cell killing by rwt virus was slightly more rapid than that of M51R virus, which is most apparent at the 24-h time point. LoVo cells were the most resistant to VSV cytolysis of the three CRC cell lines, with viability of 50–60% of control at 48 h postinfection with M51R virus, even at high MOI (Figure 3c). Hct116 cells (Figure 3b) were intermediate in their sensitivity between RKO and LoVo cells, and the three cell lines were killed more rapidly by rwt virus than M51R virus.
Figure 2.
Viability of primary cell cultures infected with recombinant wild type (rwt) and M51R vesicular stomatitis virus (VSV). CC2511 human dermal fibroblasts (a), HU0796 primary hepatocyte culture (b) and HU0803 primary hepatocyte cultures (c) cells were infected with rwt and M51R VSV at the indicated MOIs. At 48 h postinfection, live cells were measured by MTS assay. Data are expressed as a percentage of the cell viability of mock-infected cells and represent the means±s.d. of at least four independent experiments.
Figure 3.
Viability of colorectal cancer (CRC) cells infected with recombinant wild type (rwt) and M51R vesicular stomatitis virus (VSV). RKO (a), Hct116 (b) and LoVo (c) cells were infected with rwt and M51R VSV at the indicated MOIs. At the indicated times postinfection, live cells were measured by MTS assay. Data are expressed as a percentage of the cell viability of mock-infected cells and represent the means±s.d. of at least four independent experiments.
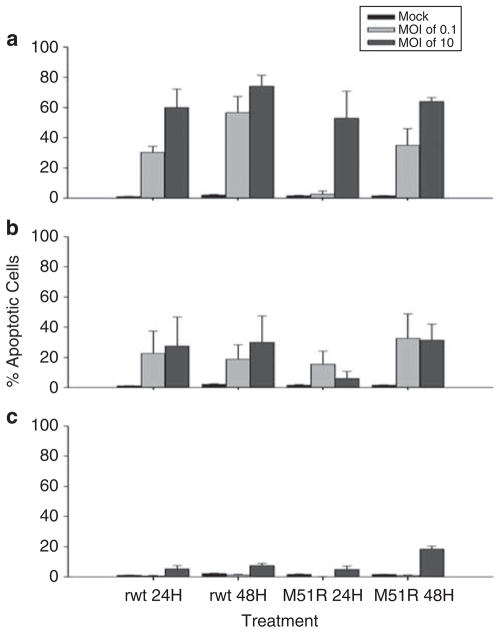
The oncolytic activity of VSV in CRC cells was further tested by quantifying virus-induced apoptosis by the number of cells undergoing DNA fragmentation determined by TUNEL assay (Figure 4). Under most conditions, RKO cells had the highest percentage of TUNEL-positive cells, and LoVo cells had the lowest percentages, with Hct116 cells intermediate between the two, consistent with the data in Figure 3. In the case of LoVo cells, there was a low percentage of TUNEL-positive cells even under conditions that resulted in a substantial loss of viability determined by MTS assay, such as 48 h postinfection with rwt at an MOI of 10 PFU per cell. This suggests that loss of metabolic activity measured by MTS assay is not as strongly coupled to apoptotic pathways that result in DNA fragmentation in LoVo cells compared with other cell types, such as RKO cells.
Figure 4.
Infection with vesicular stomatitis virus (VSV) results in apoptosis in colorectal cancer (CRC) cells. RKO (a), Hct116 (b) and LoVo (c) were treated with recombinant wild type (rwt) and M51R VSV under multiple-cycle (multiplicity of infection (MOI) of 0.1 PFU per cell) or single-cycle (MOI of 10 PFU per cell) infection conditions. At 24 or 48 h postinfection, the cells were harvested, fixed and permeabilized. The cells were then incubated with the TUNEL reaction mixture according to the manufacturer’s directions, and the incorporated fluorescein was analyzed and quantitated by flow cytometry. Data are expressed as the mean±s.d. of three independent experiments.
CRC cells support VSV protein synthesis
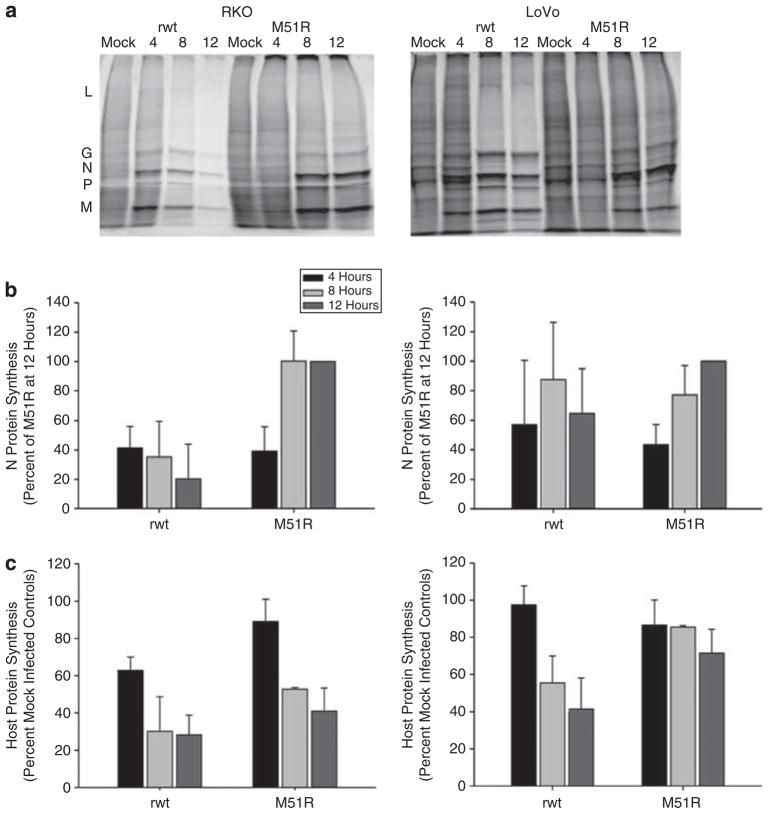
The permissiveness of CRC cells to VSV infection was evaluated by determining the time course of viral protein expression in the sensitive RKO cells as well as the resistant LoVo CRC cells. Figure 5 shows the rates of VSV and host protein synthesis in RKO and LoVo CRC cell lines. Cells were mock infected or infected with rwt or M51R virus at an MOI of 10 PFU per cell. At 4, 8 and 12 h postinfection, cells were pulsed with [35S]methionine and harvested for analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and phosphorescence imaging. Figures 5a and d show representative images for RKO and LoVo cells, respectively, at different times postinfection. Both cell lines support high levels of viral protein synthesis as shown by the intense labeling of viral L, G, N, P and M proteins above the background labeling of host proteins. Also apparent in Figures 5a and d is the rapid inhibition of host protein synthesis in cells infected with rwt virus. The data from multiple experiments were quantified by determining the density of the VSV N band (the most abundant viral protein) and are expressed in Figures 5b and e as a percentage of the level of N protein level 12 h after infection with M51R virus. In VSV-sensitive RKO cells viral protein synthesis peaked early after rwt VSV infection and declined at late times postinfection, which is a typical pattern for cells that are permissive for VSV.5,18 The peak of viral protein expression is seen at 4 h after infection of RKO cells with rwt virus, while it is seen at 8 h after infection of RKO cells with M51R virus. Identical experiments in the less sensitive Hct116 cell line demonstrated similarly high levels of VSV viral protein production (data not shown). In the less sensitive LoVo CRC cells, peak viral gene expression was delayed to 8 h after infection with rwt virus, and 12 h after infection with M51R virus. From these data, one can conclude that all cell lines support a high level of viral protein synthesis after infection with VSV viruses. However, there is a delay in viral gene expression in cells infected with M51R virus and there is a further delay in LoVo cells relative to RKO cells.
Figure 5.
Analysis of protein synthesis in RKO and LoVo cells infected with recombinant wild type (rwt) and M51R vesicular stomatitis virus (VSV). RKO (a–c) and LoVo (d–f) cells were infected at a multiplicity of infection (MOI) of 10 PFU per cell. At various times postinfection, cells were labeled with [35S]methionine. Lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and phosphorescence imaging. Representative images for various times postinfection are shown in panel a and panel d. Viral proteins are indicated to the left of panel a. The radioactivity of the VSV N protein band was quantified and is shown for RKO cells in panel b and for LoVo cells in panel e. The radioactivity of background host protein synthesis (taken from three sections of each lane that exclude viral protein bands) was quantified and is shown for the RKO cells in panel c and LoVo cells in panel f. Data are expressed as the mean of each experimental result ±s.d. of four independent experiments.
The inhibition of host cell proteins after infection with rwt and M51R VSV was evaluated by determining the time course of host protein expression in RKO and LoVo cells. Host protein synthesis was determined by analyzing radioactivity in three areas in each lane devoid of viral protein bands. Host protein synthesis in RKO and LoVo cells is shown in Figures 5e and f as a percentage of host protein synthesis in mock-infected cells. In RKO cells, host protein synthesis was reduced to 60% of control by 4 h postinfection with rwt VSV and to about 30% at 8 h. As expected, infection with M51R-M mutant resulted in a much less significant reduction in protein synthesis as these cells experienced only a 15% reduction in host protein synthesis at 4 h postinfection and 50% reduction at 8 h. On the other hand, less sensitive LoVo CRC cells undergo a delayed inhibition of host protein synthesis after infection with rwt VSV relative to RKO cells. Host protein synthesis in rwt-infected LoVo cells was reduced to 40% of mock-infected cells at 12 h. These data indicate that wt M protein promotes the inhibition of host protein synthesis in infected cells. In addition, they also show that the sensitivity of cells to killing by wtVSV parallels with host protein synthesis inhibition by VSV.
CRC cells support VSV progeny production
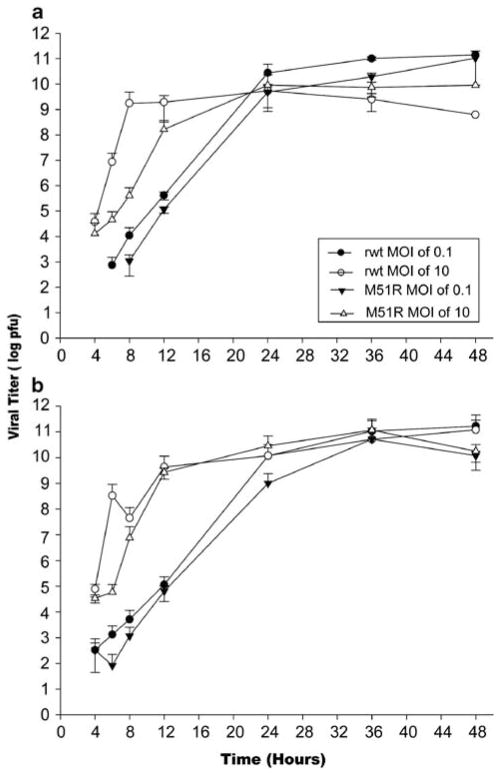
The therapeutic effect of oncolytic VSV will depend on its ability to replicate throughout the tumor. The ability of rwt and M51R viruses to produce viral progeny was determined in CRC cells under single- and multiple-cycle infection conditions. In the single-cycle infection experiments, cells were infected at an MOI of 10 PFU per cell and the yield of progeny virus was determined by plaque assay over a 48-h time course (Figure 6). As expected from their high levels of viral protein synthesis, all cell lines produced progeny virus between 108 and 109 PFU per ml by 12 h postinfection with both rwt and M51R VSV.
Figure 6.
Colorectal cancer (CRC) cells support the production of viral progeny. RKO (a) and LoVo (b) cells were treated with recombinant wild type (rwt) and M51R vesicular stomatitis virus (VSV) under multiple-cycle (multiplicity of infection (MOI) of 0.1 PFU per cell) or single-cycle (MOI of 10 PFU per cell) infection conditions. At various times postinfection, small aliquots of the supernatant were removed to determine the amount of progeny virus by plaque assay. Data are expressed as the mean of each experimental result ±s.d. of four independent experiments.
As an extension of these findings, we sought to determine whether antiviral responses affect the spread of virus to surrounding uninfected cells by testing the ability of rwt and M51R VSV to replicate in CRC cells under multiple-cycle infections. In these experiments, cells were infected with an MOI of 0.1 PFU per cell. Both viruses reached titers as high as those observed in the single-cycle growth experiment, although there was a delay in growth due to the kinetics of virus spread. These findings demonstrate that CRC cells are highly permissive for VSV infection and are unable to prevent the spread of virus throughout culture.
IFN signaling does not negatively impact the oncolytic effects of VSV in CRC cells
The production of IFN by adjacent immune, stromal and CRC cells can impact the virus’ ability to infect and replicate in surrounding cells. Delineating the extent to which IFN signaling is downregulated in these cells is important as it can enhance our understanding of a major factor that can modulate the clinical potential of VSV. As expected, CRC cells did not produce IFN after treatment with VSV (data not shown). Most commonly, IFN determines the sensitivity of cancer cells to oncolytic viruses. However, because other cell types in the tumor microenvironment may produce IFN, we sought to evaluate whether the sensitivity of CRC cells to VSV was affected by IFN treatment.
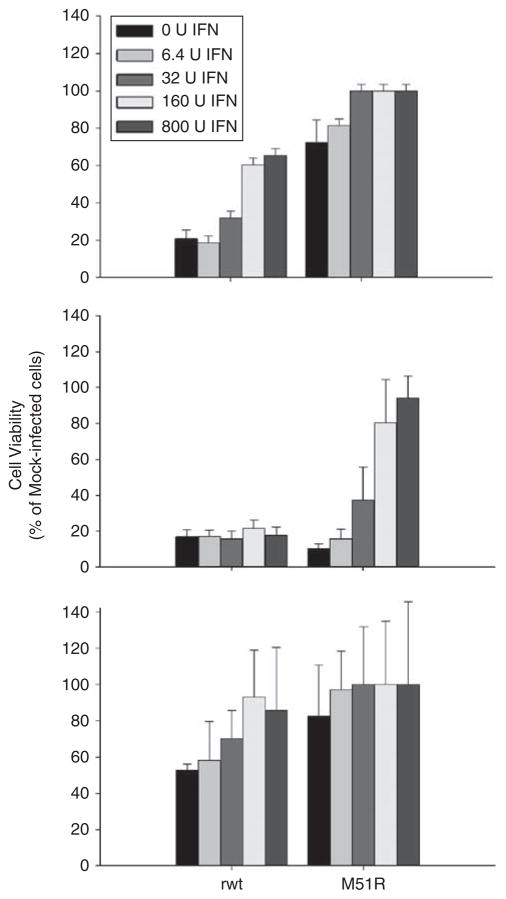
Cells that are responsive to IFN exhibit an IFN dose-dependent resistance to viral infections, whereas cells that are not responsive to IFN show no difference in cytopathic effect regardless of IFN concentration. RKO, Hct116 and LoVo CRC cells were seeded into 96-well dishes and incubated with increasing doses of Universal Type I Interferon. After 8 h of IFN pretreatment, the cells were challenged with rwt and M51R VSV at an MOI of 5 PFU per cell. At 24 and 48 h postinfection, cell viability was measured by MTS assay. Data in Figures 7a and b show that pretreatment with high doses of IFN protected RKO and Hct116 cells from M51R-induced cell death at 48 h postinfection, thus indicating that they are responsive to the antiviral effects of IFN. As demonstrated in Figure 7c, pretreatment of LoVo cells had minimal effects, given the modest cytopathic effect of M51R virus on these cells. In addition, preliminary data show that IFN-responsive genes are upregulated in all CRC cell lines after infection with M51R VSV. These results indicate that unlike many cancers, CRC cells retain some level of sensitivity to IFN signaling.5,20
Figure 7.
Responsiveness of colorectal cancer (CRC) cells to interferon (IFN). RKO (a), Hct116 (b) and LoVo (c) cells were incubated with varying concentrations of IFN (6.4–800 IU ml−1) for 8 h and challenged with recombinant wild type (rwt) or M51R vesicular stomatitis virus (VSV) at a multiplicity of infection (MOI) of 5 PFU per cell. At 24 h postinfection, cell viability was measured by MTS assay. Controls included IFN-treated cells that were not challenged with VSV. Data are expressed as the percentage of mock-infected cells±s.d. of three independent experiments.
rM51R-M virus effectively kills RKO tumor cells in vivo
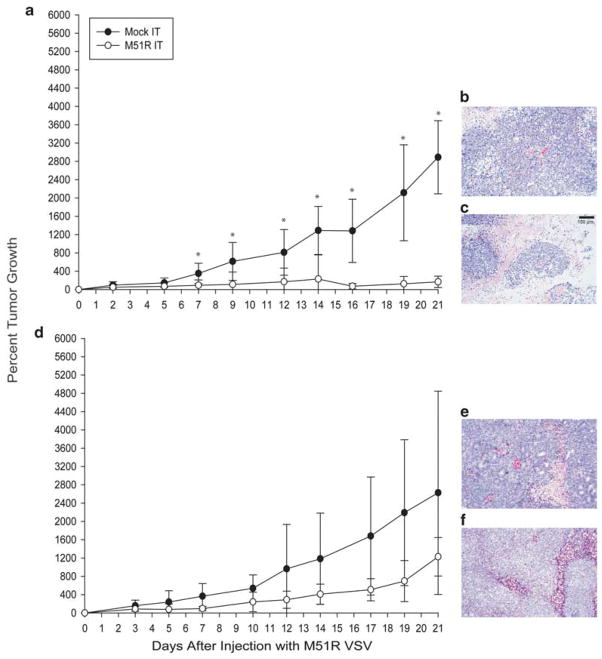
The ability of M51R VSV to treat CRC xenografts in athymic nude mice was determined. In these experiments, VSV-sensitive RKO and VSV-resistant LoVo cells were injected subcutaneously into the flanks of athymic nude mice and the resultant tumors were treated with intratumoral injections of either saline or rM51R-M virus. Tumor volume was measured three times a week as an indicator of treatment effect. Xenografts established from RKO tumor cells did not grow after treatment with M51R virus, while mock-treated tumors continued to increase in size (Figure 8a). After day 7, the percent change in volume of tumor treated with M51R virus was significantly lower than mock-infected tumors (P=0.031). Statistical analysis revealed that this difference remained significant throughout the course of the experiment (P=0.001 on day 21 post-treatment). Histological examination of RKO tumors by hematoxylin and eosin staining at day 21 postinfection showed that mock-infected cells had well-defined boarders and hyperchromatic nuclei (Figure 8b). The cytoplasm of the mock-infected cells was vesicular and eosinophilic, with evidence of mitosis. In contrast, tumors treated with rM51R-M virus were extensively necrotic, characterized by loss of nuclear staining, increased cytoplasmic eosinophilia and loss of cellular detail and cell boarders (Figure 8c). The additional tissue eosinophilia in tumors infected with rM51R-M virus was due to infiltrating erythrocytes as a result of hemorrhaging. These data support the idea that the remaining tumor mass at day 21 consisted largely of dead or dying tumor cells. Although similar treatment with LoVo tumors resulted in a slight decrease in tumor volume relative to mock-treated controls, this difference was not statistically significant (Figure 8d). Similar histological results were seen to a lesser degree in LoVo tumors (Figures 8e and f). Taken together, these data support our hypothesis that M51R VSV can be used to treat established CRC tumors. In addition, in vitro sensitivity of CRC cells to treatment with M51R VSV correspond with sensitivity of established xenografts of these tumors to oncolytic VSV.
Figure 8.
Treatment of xenografts derived from RKO and LoVo cells with rM51R-M virus. RKO (a) and LoVo (d) cells were injected subcutaneously in the flank of athymic nude mice. Animals with palpable tumors were randomly separated into two experimental groups and were injected in the tumor with 6×108 PFU of M51R virus (n=10) in culture medium or with culture medium alone (n=7) as a negative control. Tumor volume was measured with calipers. Data are shown as the percentage of original tumor size on day 0 (mean±s.d.). Histological examination of RKO and LoVo xenografts by routine hematoxylin and eosin staining at 21 days after intratumoral injection with saline (b and e) or M51R (c and f) VSV.
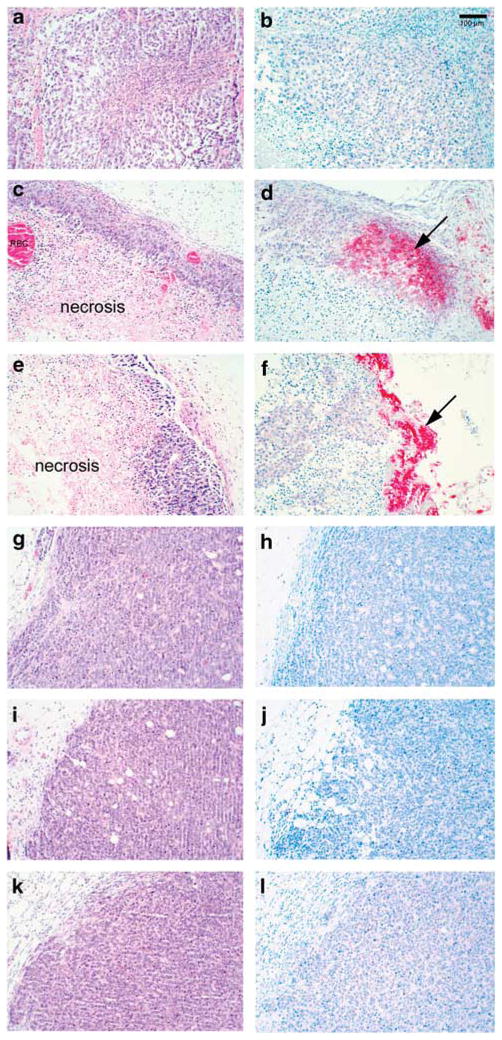
Virus replication and spread in the tumors were determined by viral plaque assays and immunohistochemistry analysis for viral G protein at days 2 and 4 post-treatment. Treatment of RKO xenografts resulted in the production of viral progeny at both 2 and 4 days after treatment with M51R VSV, whereas treatment of resistant LoVo cells did not result in appreciable titers of M51R VSV (Table 1). Immunohistochemical analysis of tumors from mice at days 2 and 4 post-treatment was carried out with antibodies against the viral G protein to evaluate viral protein production in CRC tumors after infection with rM51R-M virus. As expected, treatment of RKO xenografts resulted in significant necrosis at 2 and 4 days post-treatment (Figures 9a, c and e). In addition, viral G protein was readily apparent in RKO cells treated with M51R VSV (Figures 9b, d and f). In contrast, little cytopathic effect is seen in LoVo xenografts at 2 and 4 days post-treatment (Figures 9g, i and k) and no viral G protein is seen in these tumors over an identical time course (Figures 9h, j and l). These data indicate that tumors that are sensitive to the oncolytic effects of VSV support viral progeny production and spread throughout the tumor, whereas resistant tumors are able to inhibit viral progeny production.
Table 1.
Viral titers from CRC xenografts treated with M51R VSV
| Control
|
2 Days
|
4 Days
|
||||
|---|---|---|---|---|---|---|
| Animal 1 | Animal 2 | Animal 1 | Animal 2 | Animal 1 | Animal 2 | |
| RKO | 0 | 0 | 3.40×107 | 6.00×107 | 1.03×107 | 1.15×107 |
| LoVo | 0 | 0 | 5.00×102 | 3.00×103 | 0 | 0 |
Abbreviations: CRC, colorectal cancer; PBS, phosphate-buffered saline; VSV, vesicular stomatitis virus.
RKO and LoVo xenografts were treated with M51R VSV for the times indicated. The tumors were then homogenized in PBS and viral titers of the supernatants determined as described previously. Sensitive RKO cells support viral replication long after a single treatment with M51R, whereas very low viral titers were seen after a single treatment of LoVo CRC xenografts with M51R virus.
Figure 9.
Viral G production after treatment of RKO and LoVo xenografts with rM51R-M virus. Xenografts derived from RKO and LoVo cells were injected subcutaneously in the flank of athymic nude mice. Animals with palpable tumors were randomly separated into two experimental groups (8 animals/group) and were injected in the tumor with 6×108 PFU of M51R virus (n=4) in culture medium or with culture medium alone (n=4) as a negative control. RKO (a–f) and LoVo (g–l) tumors were harvested at 2 (c–d, i–j) and 4 (e–f, k–l) days for histological examination by routine hematoxylin and eosin staining (a, c, e, g, i, k) as well as immunohistochemical staining for viral G protein (b, d, f, h, j, l).
Discussion
Previous reports demonstrate that VSV can treat a variety of malignancies including prostate, breast and hepatocellular cancers.5,27–29 Given the clinical outcomes of patients with metastatic CRC, it is clear that innovative approaches such as VSV are needed for this disease. The work described herein establishes a conceptual framework for understanding the potential clinical utility of VSV and other oncolytic viruses for the treatment of CRC by advancing three important points. First, M protein mutant VSV can treat susceptible CRC cells, whereas normal cells are resistant to its cytopathic effects. Second, although CRC cells retain some sensitivity to the antiviral effects of IFN, they are defective in their antiviral responses and therefore support viral protein and progeny production. Finally, VSV can effectively treat established CRC tumors derived from susceptible cells, but VSV is not as effective in treating tumors that are more resistant to the oncolytic effects of VSV.
It is well-established that M protein is intimately involved in the activation of apoptosis, which is one of the principal cytopathic effects of VSV infection.21,27,30,31 Previous data suggest that the role of M protein in the regulation of apoptosis during a virus infection is dependent on cell type. Studies with rwt and rM51R-M viruses have shown that in some cell types, such as HeLa cells, the wt M protein accelerates VSV-induced apoptosis by inhibiting host gene expression and activation of the intrinsic apoptotic pathway. However, in other cell types, such as BHK cells, the rM51R-M virus induces apoptosis more rapidly than rwt virus, indicating that the extrinsic signaling pathway is the major inducer of apoptosis in these cells.16,21 Interestingly, later work from our group found that mutation of M protein did not have a deleterious effect on the induction of apoptosis in LNCaP and PC-3 prostate tumor cells in single-cycle infection experiments.5 Because one of the main concerns regarding the use of viral vectors for tumor therapies is their safety in patients, our aim is to develop viruses based on VSV that were less pathogenic than their wt counterparts. Previous experiments that evaluate the efficacy of VSV as a vector for antitumor therapies used naturally occurring wt and mutant strains of VSV in mice.3,20,32 In this study, we tested the effectiveness of the less pathogenic recombinant strains of VSV as agents for CRC tumor therapy with the idea that IFN-inducing viruses, such as rM51R-M virus, would be safer in vivo due to their inability to suppress an effective antiviral response in normal tissue. Our results demonstrated no difference in the oncolytic effects of VSV in sensitive CRC cell lines after infection with either rwt or M51R VSV. In addition, infection with M51R virus did not result in cell death in a panel of normal cells. Taken together, these findings support the use of M51R mutant VSV in the treatment of CRC. Furthermore, they suggest that M protein mutants of VSV offer great promise as backbones from which additional viral vectors will be constructed.
One of the underlying assumptions in the use of oncolytic viruses is that tumor cells that are defective in their antiviral responses would be sensitive to the cytopathic effects of VSV, whereas resistant cells would have intact antiviral signaling, thus preventing viral protein production and replication. We found that although IFN signaling is somewhat intact in both sensitive and resistant CRC cell lines, all of the CRC cell lines support viral protein production and viral progeny production. Based on these data, it is unlikely that antiviral factors contribute to the resistance of LoVo cells to VSV killing in cell culture. Instead, these results suggest that the mechanism that determines the sensitivity of CRC cells to M51R VSV is based not on the cell line’s sensitivity to IFN, but on the effectiveness of the cell death pathways induced by viral infection. Despite the fact that all of these cell lines are derived from metastatic CRC tumors, numerous molecular differences between these cell types have been described, several of which may contribute to their differential susceptibility to VSV.
Several studies have demonstrated the effectiveness of VSV as an oncolytic agent for antitumor therapies in experimental animals with a variety of tumor types These tumors include glioblastomas13,33 myc and ras transformed cell lines32 and human melanoma xenografts in nude mice.3 In addition, VSV represses the growth of several different metastatic tumor models in immuno-competent mice,10,12,34–36 and hepatocellular carcinomas in rats.29 Mutations of the Mprotein result in viruses with dramatically reduced pathogenicity in both nude mice and immunocompetent mice.5,20 Consistent with these earlier results, we did not observe symptoms of virus infection in M51R virus-treated nude mice. Thus, M protein mutant viruses would not only be effective oncolytic viruses for selected CRC tumors due to their ability to kill tumor cells, but would also be safer viruses due to their ability to induce antiviral responses and spare normal cells. Similar strategies for enhancing the safety of VSV vectors include pretreating mice with IFN,3,28 engineering an IFN gene into the VSV genome,37 and using naturally occurring IFN-inducing mutants of VSV.3,38,39 Our results with the treatment of CRC tumor xenografts are consistent with these previous studies in other histologies (Figure 7). The effectiveness of VSV in reducing tumor volume varies considerably among these different tumor models, and our results showing that a single treatment of a well-established tumor derived from RKO cells with M51R virus led to tumor response in some cases without causing disease in nude mice stands among the most successful examples. The fact that mice bearing tumors derived from LoVo cells were resistant to treatment with VSV indicates that VSV is not effective at treating all CRC tumors. Thus, any future strategy for using VSV for CRC is likely to involve testing individual tumors for their susceptibility to virus infection. We are in the process of analyzing patient tumors for their susceptibility to VSV infection and killing. Future studies will allow us to determine the degree of differences present in CRC tumors and develop viruses that will more successfully treat such tumors.
Analysis of tumor xenografts at 2 and 4 days after treatment demonstrates that the xenografts derived from the sensitive RKO cells are able to support viral progeny and protein production. On the other hand, tumor xenografts derived from the resistant LoVo cells did not support significant viral progeny production and they did not support viral G-protein expression (Table 1 and Figure 9). It is possible that in certain cases, the host may prematurely clear rM51R-M virus before it can effectively spread throughout the tumor. Given that CRC cells retain some level of IFN responsiveness, it is reasonable to conclude that xenografts from VSV-resistant LoVo cells sensitive to the IFN and other antiviral cytokines produced by surrounding normal cells and cells of the innate immune system, thereby resulting in the clearance of virus from the tumor. Future efforts will include strategies that inhibit the very proximal components of type I IFN signaling pathway in host cells, thus allowing us to determine the balance necessary between viral and host factors for the development of recombinant viruses for more effective antitumor therapies.
The data presented herein suggest that M51-R VSV can serve as an effective treatment for CRC. However, barriers remain to the development of M51R VSV as therapy for metastatic CRC. The first barrier to moving M51R VSV to the clinic setting is that there are no validated data on the molecular determinants of sensitivity of CRC cells to M51R VSV. Although CRC cells differed in their sensitivity to the oncolytic effects of both rwt and M51-R VSV, all CRC cells supported viral replication and protein production when treated with VSV in vitro. These findings suggest that the differential induction of cell death is predicated on modulation of apoptosis in CRC cells. Although oligonucleotide microarray data from prostate cancer cells suggests that sensitivity to M51R VSV is tied to the expression of antiviral genes,25 data from our group indicate that the same principles do not apply to CRC cells. We have identified gene signatures related to cell death pathways that correspond with sensitivity to M51R VSV in CRC. Our group will seek to further advance the potential clinical utility of M51R VSV by validating our oligonucleotide microarray findings on the molecular determinants of sensitivity to M51R VSV, and subsequently devising tests that can predict benefit from treatment of metastatic CRC tumors with M51R VSV. Secondly, identification of molecular targets that can predict sensitivity to M51R VSV will also allow us to design M51R VSV-based vectors that can effectively treat a broad range of phenotypes of metastatic CRC tumors.
Another barrier to the development of M51R VSV in the clinical setting is that it is unclear what proportion of metastatic colorectal tumors in the clinical setting are susceptible to the oncolytic effects of M51R VSV, as was highlighted in recent meetings of the NIH Recombinant DNA Advisory Committee.40,41 Given the inherent weaknesses of evaluating novel therapies with established cell lines, future work should focus on treating a series of patient-derived xenografts from CRC tumors with M51R to delineate the proportion of tumors seen in the clinical setting that are sensitive to the oncolytic effects of M51-R virus.
Acknowledgments
We thank Shelby Puckett and Margie McKenzie (Wake Forest University School of Medicine, Department of Biochemistry) for their assistance with viral plaque assays and 35S-labeling experiments, Hermina Borgerink (Wake Forest University School of Medicine, Department of Comparative Medicine) for staining of tissue sections, Gregory Russell (Wake Forest University School of Medicine, Division of Public Health Sciences) for statistical analysis and Mary Cromer (Wake Forest University School of Medicine, Comprehensive Cancer Center) for editorial assistance. This work was supported by Grant no. K08-CA131482 from the National Cancer Institute (JS), 63527 from the Robert Wood Johnson Foundation Harold Amos Faculty Development Award (JS) and R01-AI32983 from the National Institute of Allergy and Infectious Diseases (DL).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran S, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life. 2000;50:135–138. doi: 10.1080/713803696. [DOI] [PubMed] [Google Scholar]
- 3.Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 4.Wong LH, Krauer KG, Hatzinisiriou I, Estcourt MJ, Hersey P, Tam ND, et al. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3gamma. J Biol Chem. 1997;272:28779–28785. doi: 10.1074/jbc.272.45.28779. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed M, Cramer SD, Lyles DS. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology. 2004;330:34–49. doi: 10.1016/j.virol.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 6.Kirn DH, Wang Y, Le BF, Bell J, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haralambieva I, Iankov I, Hasegawa K, Harvey M, Russell SJ, Peng KW. Engineering oncolytic measles virus to circumvent the intracellular innate immune response. Mol Ther. 2007;15:588–597. doi: 10.1038/sj.mt.6300076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elankumaran S, Chavan V, Qiao D, Shobana R, Moorkanat G, Biswas M, et al. Type I interferon-sensitive recombinant Newcastle disease virus for oncolytic virotherapy. J Virol. 2010;84:3835–3844. doi: 10.1128/JVI.01553-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naik S, Russell SJ. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin Biol Ther. 2009;9:1163–1176. doi: 10.1517/14712590903170653. [DOI] [PubMed] [Google Scholar]
- 10.Chang G, Xu S, Watanabe M, Jayakar HR, Whitt MA, Gingrich JR. Enhanced oncolytic activity of vesicular stomatitis virus encoding SV5-F protein against prostate cancer. J Urol. 2010;183:1611–1618. doi: 10.1016/j.juro.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Moussavi M, Fazli L, Tearle H, Guo Y, Cox M, Bell J, et al. Oncolysis of prostate cancers induced by vesicular stomatitis virus in PTEN knockout mice. Cancer Res. 2010;70:1367–1376. doi: 10.1158/0008-5472.CAN-09-2377. [DOI] [PubMed] [Google Scholar]
- 12.Galivo F, Diaz RM, Thanarajasingam U, Jevremovic D, Wongthida P, Thompson J, et al. Interference of CD40L-mediated tumor immunotherapy by oncolytic vesicular stomatitis virus. Hum Gene Ther. 2010;21:439–450. doi: 10.1089/hum.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wollmann G, Rogulin V, Simon I, Rose JK, van den Pol AN. Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells. J Virol. 2010;84:1563–1573. doi: 10.1128/JVI.02040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan H, Yoza BK, Lyles DS. Inhibition of host RNA polymerase II-dependent transcription by vesicular stomatitis virus results from inactivation of TFIID. Virology. 1998;251:383–392. doi: 10.1006/viro.1998.9413. [DOI] [PubMed] [Google Scholar]
- 15.Yuan H, Puckett S, Lyles DS. Inhibition of host transcription by vesicular stomatitis virus involves a novel mechanism that is independent of phosphorylation of TATA-binding protein (TBP) or association of TBP with TBP-associated factor subunits. J Virol. 2001;75:4453–4458. doi: 10.1128/JVI.75.9.4453-4458.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopecky SA, Lyles DS. Contrasting effects of matrix protein on apoptosis in HeLa and BHK cells infected with vesicular stomatitis virus are due to inhibition of host gene expression. J Virol. 2003;77:4658–4669. doi: 10.1128/JVI.77.8.4658-4669.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed M, Lyles DS. Identification of a consensus mutation in M protein of vesicular stomatitis virus from persistently infected cells that affects inhibition of host-directed gene expression. Virology. 1997;237:378–388. doi: 10.1006/viro.1997.8808. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed M, McKenzie MO, Puckett S, Hojnacki M, Poliquin L, Lyles DS. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J Virol. 2003;77:4646–4657. doi: 10.1128/JVI.77.8.4646-4657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang TG, Ebert O, Shinozaki K, Garcia-Sastre A, Woo SL. Oncolysis of hepatic metastasis of colorectal cancer by recombinant vesicular stomatitis virus in immune-competent mice. Mol Ther. 2003;8:434–440. doi: 10.1016/s1525-0016(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 20.Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 21.Kopecky SA, Willingham MC, Lyles DS. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J Virol. 2001;75:12169–12181. doi: 10.1128/JVI.75.24.12169-12181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitlow ZW, Connor JH, Lyles DS. Preferential translation of vesicular stomatitis virus mRNAs is conferred by transcription from the viral genome. J Virol. 2006;80:11733–11742. doi: 10.1128/JVI.00971-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitlow ZW, Connor JH, Lyles DS. New mRNAs are preferentially translated during vesicular stomatitis virus infection. J Virol. 2008;82:2286–2294. doi: 10.1128/JVI.01761-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart JH, Tran TL, Levi N, Tsai WS, Schrump DS, Nguyen DM. The essential role of the mitochondria and reactive oxygen species in cisplatin-mediated enhancement of fas ligand-induced apoptosis in malignant pleural mesothelioma. J Surg Res. 2007;141:120–131. doi: 10.1016/j.jss.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 25.Carey BL, Ahmed M, Puckett S, Lyles DS. Early steps of the virus replication cycle are inhibited in prostate cancer cells resistant to oncolytic vesicular stomatitis virus. J Virol. 2008;82:12104–12115. doi: 10.1128/JVI.01508-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyles DS, Rupprecht CE. Rhabdoviridae. In: Howley DKaP., editor. Fields Virology. 5. Vol. 2. Lippencott, Williams and Wilkins; USA: 2007. pp. 1364–1402. [Google Scholar]
- 27.Shi W, Tang Q, Chen X, Cheng P, Jiang P, Jing X, et al. Antitumor and antimetastatic activities of vesicular stomatitis virus matrix protein in a murine model of breast cancer. J Mol Med. 2009;87:493–506. doi: 10.1007/s00109-009-0444-5. [DOI] [PubMed] [Google Scholar]
- 28.Shinozaki K, Ebert O, Suriawinata A, Thung SN, Woo SL. Prophylactic alpha interferon treatment increases the therapeutic index of oncolytic vesicular stomatitis virus virotherapy for advanced hepatocellular carcinoma in immune-competent rats. J Virol. 2005;79:13705–13713. doi: 10.1128/JVI.79.21.13705-13713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altomonte J, Braren R, Schulz S, Marozin S, Rummeny EJ, Schmid RM, et al. Synergistic antitumor effects of transarterial viroembolization for multifocal hepatocellular carcinoma in rats. Hepatology. 2008;48:1864–1873. doi: 10.1002/hep.22546. [DOI] [PubMed] [Google Scholar]
- 30.Du XB, Lang JY, Xu JR, Lu Y, Wen YJ, Zhao JM, et al. Vesicular stomatitis virus matrix protein gene enhances the antitumor effects of radiation via induction of apoptosis. Apoptosis. 2008;13:1205–1214. doi: 10.1007/s10495-008-0253-2. [DOI] [PubMed] [Google Scholar]
- 31.Pearce AF, Lyles DS. Vesicular stomatitis virus induces apoptosis primarily through Bak rather than Bax by inactivating Mcl-1 and Bcl-XL. J Virol. 2009;83:9102–9112. doi: 10.1128/JVI.00436-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balachandran S, Porosnicu M, Barber GN. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. J Virol. 2001;75:3474–3479. doi: 10.1128/JVI.75.7.3474-3479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozduman K, Wollmann G, Piepmeier JM, van den Pol AN. Systemic vesicular stomatitis virus selectively destroys multifocal glioma and metastatic carcinoma in brain. J Neurosci. 2008;28:1882–1893. doi: 10.1523/JNEUROSCI.4905-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obuchi M, Fernandez M, Barber GN. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J Virol. 2003;77:8843–8856. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J, Thompson J, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 36.Luo S, Chen P, Luo ZC, Zhang P, Sun P, Shi W, et al. Combination of vesicular stomatitis virus matrix protein gene therapy with low-dose cisplatin improves therapeutic efficacy against murine melonoma. Cancer Sci. 2010;101:1219–1225. doi: 10.1111/j.1349-7006.2010.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saloura V, Wang LC, Fridlender ZG, Sun J, Cheng G, Kapoor V, et al. Evaluation of an attenuated vesicular stomatitis virus vector expressing interferon-beta for use in malignant pleural mesothelioma: heterogeneity in interferon responsiveness defines potential efficacy. Hum Gene Ther. 2010;21:51–64. doi: 10.1089/hum.2009.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu L, Huang TG, Meseck M, Altomonte J, Ebert O, Shinozaki K, et al. rVSV(M Delta 51)-M3 is an effective and safe oncolytic virus for cancer therapy. Hum Gene Ther. 2008;19:635–647. doi: 10.1089/hum.2007.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alain T, Lun X, Martineau Y, Sean P, Pulendran B, Petroulakis E, et al. Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production. Proc Natl Acad Sci USA. 2010;107:1576–1581. doi: 10.1073/pnas.0912344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Services USDoHaH. Recombinant DNA Advisory Committee. US Department of Health; Rockville, MD: 2008. Minutes of the Recombinant DNA Advisory Committee 12/3–4/2008. [Google Scholar]
- 41.Services USDoHaH. Recombinant DNA Advisory Committee. United States Department of Health and Human Services; Bethesda, MD: 2008. Minutes of the Recombinant DNA Advisory Committee 9/9–10/08. [Google Scholar]