Abstract
The neuropeptide oxytocin functions as a hormone and neurotransmitter and facilitates complex social cognition and approach behavior. Given that empathy is an essential ingredient for third-party decision-making in institutions of justice, we investigated whether exogenous oxytocin modulates empathy of an unaffected third-party toward offenders and victims of criminal offenses. Healthy male participants received intranasal oxytocin or placebo in a randomized, double-blind, placebo-controlled, between-subjects design. Participants were given a set of legal vignettes that described an event during which an offender engaged in criminal offenses against victims. As an unaffected third-party, participants were asked to rate those criminal offenses on the degree to which the offender deserved punishment and how much harm was inflicted on the victim. Exogenous oxytocin selectively increased third-party decision-makers’ perceptions of harm for victims but not the desire to punish offenders of criminal offenses. We argue that oxytocin promoted empathic concern for the victim, which in turn increased the tendency for prosocial approach behavior regarding the interpersonal relationship between an unaffected third-party and a fictional victim in the criminal scenarios. Future research should explore the context- and person-dependent nature of exogenous oxytocin in individuals with antisocial personality disorder and psychopathy, in whom deficits in empathy feature prominently.
Keywords: oxytocin, empathy, third-party decision-making, neurolaw, antisocial personality disorder, psychopathy
INTRODUCTION
Converging evidence reveals that oxytocin, a neuropeptide that is produced in the hypothalamus and released into both the brain and the bloodstream, broadly influences social behavior including pair bonding/attachment, peer recognition and social memory (Lee et al., 2009; Ebstein et al., 2010). For example, oxytocin receptor knockout mice demonstrate several aberrations in social behaviors, including aggression and mother–offspring interaction (Nishimori et al., 2008) that can be fully restored by injection of oxytocin (Ferguson et al., 2001). Since neuropeptides cross the blood–brain barrier after intranasal administration (Born et al., 2002), oxytocin can also be used in humans to investigate its effects on the central nervous system (Singer et al., 2008). Recent studies have demonstrated that oxytocin functioning both as a hormone and neurotransmitter is a crucial mediator in the regulation of complex social cognition and behavior (for reviews, see Bartz et al., 2011; Kemp and Guastella, 2011; Meyer-Lindenberg et al., 2011; Striepens et al., 2011). Importantly, emerging human evidence points to the conclusion that the social effects of oxytocin are often moderated by either contextual factors determined by features of the situation in which oxytocin is administered or by stable characteristics of the individuals to whom oxytocin is administered (Bartz et al., 2011).
The neurobehavioral effects of oxytocin on empathy, defined as the capacity to share and understand the feelings of others (de Vignemont and Singer, 2006), are currently the focus of much research. Neuroimaging research suggests that we understand other people’s affective states via activation of neural networks usually involved in processing our own affective states, regardless of whether the stimuli to which empathy is directed is negative or positive (Lamm et al., 2007; Singer et al., 2008). Moreover, the magnitude of empathy-related brain activation is positively associated with individual differences (Jabbi et al., 2007; Lamm et al., 2007; Singer et al., 2008) as measured by self-report questionnaires such as the Interpersonal Reactivity Index (Davis, 1983). Previous studies investigating the effect of exogenously administered intranasal oxytocin on measures of empathy, have either shown no effect on empathy for pain (Singer et al., 2008), an indirect effect on empathy accuracy moderated by individual differences (Bartz et al., 2010) or a direct effect on emotional but not cognitive empathy for positive or negative stimuli (Hurlemann et al., 2010).
The capacity to empathize is a central ingredient of third-party decision-making in institutions of justice. To maintain social norms, civil societies are required to punish the intentional acts of an offender who harms a victim, typically via an unaffected third-party, the legal system (Fehr and Fischbacher, 2004; Spitzer et al., 2007). Previous studies demonstrated that third-party punishment has distinct determinants, which probably relate to distinct feelings such as empathy linked to the interest and welfare of others rather than one’s own (e.g. Carlsmith et al., 2002; Zeelenberg et al., 2008). In this study, we explored whether exogenous oxytocin modulates empathy of an unaffected third-party toward offenders and victims of criminal offenses. Applying a randomized, double-blind, placebo-controlled, between-subject design, healthy male participants received intranasal oxytocin or placebo and were given a set of legal vignettes that described an event during which an offender named ‘John’ engages in criminal offenses. An example was: ‘John is offended by a woman’s mocking remark and decides to hurt her badly. At work the next day, when no one else is around, he picks up a letter opener from his desk and stabs her. She later dies from the wound’. Participants were asked to rate those criminal offenses based on the degree to which the offender deserved punishment and how much harm was inflicted on the victim. Importantly, the offenses depicted in the vignettes represented ∼94.9% of the actual criminal offenses committed in the USA (i.e. sexual assault: 0.8% of all offenses; robbery: 2.5%; assault: 19.0%; household burglary: 14.0% and theft: 58.6%) and varied systematically on levels of severity ranging from low severity (e.g. theft by taking, theft by fraud, property destruction), middle severity (e.g. assault, burglary, robbery), to high severity (e.g. kidnapping, rape, negligent homicide, manslaughter, murder and torture) (Robinson and Kurzban, 2007).
The goal of this study was to investigate whether oxytocin modulates empathy toward offenders and victims of criminal offenses by modifying the third-party’s punishment and harm ratings. Modifications of both punishment and/or harm ratings are conceivable based on the assumption that empathy is an essential affective mental state for prosocial approach behavior and that oxytocin facilitates prosocial approach behaviors (Bartz et al., 2011; Kemp and Guastella, 2011; Meyer-Lindenberg et al., 2011; Striepens et al., 2011). Our findings provide the first evidence that exogenous administration of oxytocin selectively increased perceptions of harm for victims but not the desire to punish offenders of criminal offenses in healthy men.
METHODS
Subjects
Fifty-four healthy male college students (mean age ± s.d.: 24.2 ± 1.7) were randomly assigned to an oxytocin (n = 27) group or a placebo (n = 27) group. Inclusionary criteria were that participants had to be males between the ages of 18 and 30 years, in good health, and able to understand the informed consent as assessed by the study physician. Participants were excluded if they had evidence of medical or psychiatric disorder that would compromise study participation, current or past history of drug or alcohol abuse or dependence, a history of hypersensitivity to OXT or vehicle, presence of or history of clinically significant allergic rhinitis, smoked more than 10 cigarettes per day and had experiences of any trauma involving either injury or threat of injury to themselves or a close family/friend member or of being a victim of or having witnessed a violent crime (Assessment tool: clinical interview and Symptom Checklist 90-Revised) (Buckelew et al., 1988). Participants intranasally self-administered a single dose of 40 IU oxytocin (Syntocinon spray, Pharmaworld, Zurich, Switzerland) or placebo (containing the carrier without the neuropetide) 45 min before the experiment. Although most studies reporting effects of intranasal oxytocin administration use doses of 24 IU (MacDonald et al., 2011), we chose the dose of 40 IU as there is objective evidence that this dose given intranasally yields evidence of increases in cerebrospinal fluid levels as previously shown for vasopressin, a similar peptide to oxytocin (Born et al., 2002). Other studies using cognitive paradigms have also used a 40-IU dose (Zak et al., 2007; Ditzen et al., 2009). Participants gave written consent to participate for financial compensation in the experiment that was approved by the George Mason University Human Subjects Research Board.
Stimuli and material
Psychological testing included experimental and control measures: For the experimental measure, 20 legal vignettes (S1–S20), each composed of a header and a scenario consisting of two to three sentences, were randomly presented to participants on a computer screen during the study (Robinson and Kurzban, 2007) (see Supplementary Table S1). A previous study has shown that individuals demonstrate an astonishingly high agreement in their group rank-ordering when asked to rank-order those offenses on blameworthiness for the offender (Robinson and Kurzban, 2007). Kendall’s coefficient of concordance was 0.95 (a value of 1.0 would indicate a perfect agreement and a value of 0.0 no agreement whatsoever), suggesting that individuals are consistent in how they blame the offender for different degrees of legal norm violations based on harm caused to the victims of criminal offenses. Based on this evidence, the legal vignettes were divided into three groups: low-level severity (S1–S7), middle-level severity (S8–S14) and high-level severity (S15–S20) allowing us to investigate whether the effect of oxytocin on punishment and harm ratings is moderated by the severity of the offenses. During the experiment, participants were asked to rate each vignette on Likert scales on how much the offender deserved punishment (1 = least punishment, 100 = most punishment) and caused harm to the victim (1 = no harm, 100 = extreme harm).
For the ‘control measures’, collected 1–2 weeks before the oxytocin experiment, the following self-report scales/questionnaires were administered: empathy (Interpersonal Reactivity Index: perspective taking, empathic concern, personal distress, fantasy) (Davis, 1983), altruism (Rushton Altruism Scale) (Rushton et al., 1981), attachment styles (Relationship Scale Questionnaire: secure, dismissive, preoccupied, fearful) (Griffin and Bartholomew, 1994), personality styles (NEO Five-Factor Inventory: extraversion, agreeableness, conscientiousness, neuroticism, openness to experience) (Costa and McCrae, 1992), anxiety (State-Trait Anxiety Inventory, STAI: state and trait) (Spielberger, 1983) and affect (Positive Affect and Negative Affect Schedule, PANAS: positive and negative affects) (Watson et al., 1988). STAI and PANAS were administered three times during the experiment: before the drug administration, 45 min after the drug administration and after the experiment. After the oxytocin experiment, participants were asked about their belief regarding the received treatment condition (administration of OXT: 0 = no, 1 = yes, 2 = unsure) (See Supplementary Data on control measures).
Data analysis
Data analysis was carried out using SPSS 14.0 (SPSS Inc., Chicago, USA) with alpha set to P < 0.05 (two tailed). Data were normally distributed (Kolmogorov–Smirnov test) and assumptions for analyses of variance (Bartlett’s test) were not violated. First, drug effects on mean scores of ratings were determined using a mixed 2 × 3 × 2 analysis of covariance (ANCOVA) with judgment (punishment, harm) and severity (low, middle, high) as within-subjects factors, group (oxytocin, placebo) as a between-subjects factor and belief about treatment condition (no, yes, unsure), facets of trait empathy (i.e. perspective taking, empathic concern, personal distress, fantasy), attachment styles (secure, dismissive, preoccupied, fearful), personality styles (extraversion, agreeableness, conscientiousness, neuroticism, openness) and altruism as covariates. Second, in planned follow-up analyses ratings between groups were compared using independent-samples t-tests. Effect sizes (Cohen’s d) were calculated representing the observed difference in ratings between groups (d = 0.2 indicates a small effect size, d = 0.5 a medium effect size and d = 0.8 a large effect size) (Cohen, 1988). Finally, treatment group effects on ‘control measures’ were determined using independent-samples t-tests to rule out alternative explanations due to psychological traits, mood changes during the experiment and participants’ beliefs about the received treatment condition.
RESULTS
The ANCOVA revealed only a significant covariate effect on ratings for empathic concern [F(1,37) = 5.46, P < 0.05] but not for other facets of trait empathy [perspective talking: F(1,37) = 0.39, P = 0.536; fantasy: F(1,37) = 0.28, P = 0.598]; personal distress: F(1,37) = 0.09, P = 0.768), attachment styles [secure: F(1,37) = 0.03, P = 0.857, dismissive: F(1,37) = 0.42, P = 0.521, preoccupied: F(1,37) = 0.01, P = 0.958, fearful: F(1,37) = 1.86, P = 0.181], personality styles [extraversion: F(1,37) = 0.01, P = 0.921, agreeableness: F(1,37) = 0.82, P = 0.372, conscientiousness: F(1,37) = 0.55, P = 0.464, neuroticism: F(1,37) = 0.02, P = 0.897, openness: F(1,37) = 0.16, P = 0.689], altruism [F(1,37) = 0.33, P = 0.571] and belief about treatment condition [F(1,37) = 0.01, P = 0.957]. The covariate effect revealed a positive association between empathic concern and ratings (parameter estimates [mean ± s.e.m]: punishment ratings, beta = 1.01 ± 0.42, t = 2.37, P < 0.05; harm ratings, beta = 0.69 ± 0.39, t = 2.24, P < 0.05), indicating that the higher participants’ trait empathic concern were, the higher were their mean punishment and harm ratings (see Supplementary Table S2 for bivariate Pearson correlations between ratings and trait variables).
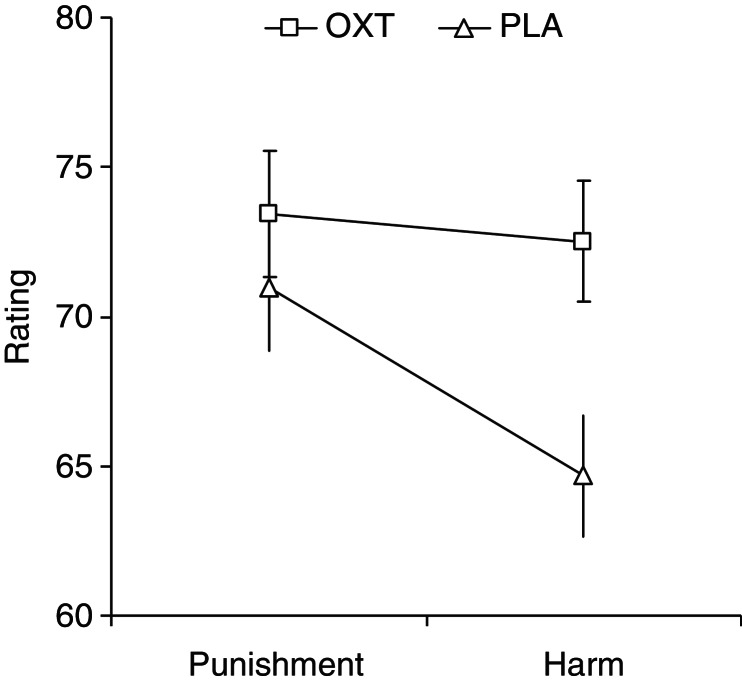
After determining the influence that covariates had on the ratings, the means for ratings were adjusted in the ANCOVA model to identify what effect the independent variables (e.g. group: oxytocin, placebo; severity: low, middle, high) had on the ratings after the effects of the covariates were removed. The ANCOVA revealed a significant interaction effect for rating × group [F(1,37) = 4.88, P < 0.05], indicating that the administration of exogenous oxytocin had an effect on ratings. Planned follow-up analyses demonstrated that the oxytocin group gave higher harm ratings than the placebo group [t(52) = 2.63, P < 0.011, Cohen’s d = 0.74; 95% confidence interval of the difference, CI: 1.6–11.9], whereas no significant differences were observed for punishment ratings [t(52) = 0.81, P = 0.420, Cohen’s d = 0.22; 95% CI: −3. to 7.9] (Figure 1).
Fig. 1.
Rating (adjusted means ± s.e.m.) for criminal offenses: The oxytocin (OXT) group compared to the placebo (PLA) group produced significantly higher harm ratings for the victim but not for deserved punishment of the offender.
The ANCOVA revealed a significant main effect for severity [F(2,74) = 6.77, P < 0.005] (see Supplementary Figure S1), indicating that punishment and harm ratings increased with the degree of severity. However, no significant interaction effects were observed for severity × group [F(2,74) = 0.21, P = 0.808] and rating × severity × group [F(2,74) = 1.38, P = 0.258], indicating that the effect of oxytocin on ratings was not moderated by the severity of the offenses (Supplementary Figure S2). All other main and interaction effects were not significant.
For the ‘control measures’, no significant group differences were found for psychological trait measures (empathy: perspective taking, empathic concern, personal distress, fantasy; altruism; attachment styles: secure, dismissive, preoccupied and fearful; personality styles: extraversion, agreeableness, conscientiousness, neuroticism, openness to experience and trait anxiety) and mood measures (state anxiety, positive and negative affect) during the experiment (Table 1).
Table 1.
Descriptive (mean ± s.e.m.) and inferential statistics for the control measures of the oxytocin experiment
| Measurement/group | Placebo | Oxytocin | t-statistics (df = 52); CIa |
|---|---|---|---|
| Age (years) | 23.8 ± 1.8 | 24.2 ± 1.5 | t = 1.38, P = 0.174; CI: −0.3 to 1.6 |
| Dispositional empathy (IRI) | |||
| Perspective taking | 25.3 ± 0.9 | 24.4 ± 1.0 | t = −0.65, P = 0.520; CI: −3.8 to 1.9 |
| Fantasy | 26.2 ± 1.0 | 25.3 ± 0.9 | t = −0.66, P = 0.514; CI: −3.8 to 1.9 |
| Emphatic concern | 26.2 ± 0.9 | 27.1 ± 0.9 | t = 0.45, P = 0.448; CI: −1.5 to 3.4 |
| Personal distress | 17.5 ± 1.0 | 17.6 ± 0.9 | t = 0.06, P = 0.956; CI: −2.6 to 2.7 |
| Altruism (RAS) | 62.0 ± 2.5 | 60.6 ± 2.4 | t = −0.40, P = 0.694; CI: −8.3 to 5.6 |
| Attachment styles (RSQ) | |||
| Secure | 3.3 ± 0.1 | 3.5 ± 0.1 | t = 0.46, P = 0.462; CI: −0.2 to 0.5 |
| Fearful | 2.5 ± 0.2 | 2.8 ± 0.2 | t = 1.88, P = 0.066; CI: −0.1 to 0.9 |
| Preoccupied | 2.9 ± 0.1 | 2.8 ± 0.2 | t = −0.54, P = 0.592; CI: −0.5 to 0.3 |
| Dismissing | 3.3 ± 0.1 | 3.4 ± 0.1 | t = 1.01, P = 0.316; CI: −0.2 to 0.5 |
| Personality styles (NEO-FFI) | |||
| Neuroticism | 1.6 ± 0.1 | 1.6 ± 0.1 | t = −0.04, P = 0.972; CI: −0.4 to 0.3 |
| Extraversion | 2.5 ± 0.1 | 2.6 ± 0.1 | t = 0.60, P = 0.547; CI: −0.2 to 0.4 |
| Openness | 2.4 ± 0.1 | 2.3 ± 0.1 | t = −0.94, P = 0.349; CI: −0.4 to 0.2 |
| Agreeableness | 2.4 ± 0.1 | 2.3 ± 0.1 | t = −0.52, P = 0.609; CI: −0.3 to 0.2 |
| Conscientiousness | 2.5 ± 0.1 | 2.6 ± 0.1 | t = 0.95, P = 0.347; CI: −0.2 to 0.6 |
| Trait anxiety (STAI) | 2.3 ± 0.1 | 2.2 ± 0.1 | t = 0.64, P = 0.529; CI: −0.1 to 0.2 |
| State anxiety (STAI) | |||
| Time 1 | 2.4 ± 0.1 | 2.4 ± 0.1 | t = 0.92, P = 0.365; CI: −0.1 to 0.2 |
| Time 2 | 2.3 ± 0.1 | 2.3 ± 0.1 | t = 0.14, P = 0.886; CI: −0.1 to 0.1 |
| Time 3 | 2.3 ± 0.1 | 2.3 ± 0.1 | t = 0.31, P = 0.756; CI: −0.1 to 0.2 |
| Positive and negative affect (PANAS) | |||
| Time 1 | 2.7 ± 0.1 | 2.7 ± 0.1 | t = 0.95, P = 0.347; CI: −0.1 to 0.3 |
| Time 2 | 2.5 ± 0.1 | 2.5 ± 0.1 | t = 0.90, P = 0.372; CI: −0.1 to 0.3 |
| Time 3 | 2.5 ± 0.1 | 2.5 ± 0.1 | t = 0.18, P = 0.861; CI: −0.2 to 0.3 |
a95% Confidence interval of the difference.
IRI = Interpersonal Reactivity Index; RAS = Rushton Altruism Scale; RSQ = Relationship Scale Questionnaire; NEO-FFI = NEO Five-Factor Inventory; STAI = State-Trait Anxiety Inventory; PANAS = Positive and Negative Affect Scale; Time 1 = before drug administration; Time 2 = 45 min after drug administration; Time 3 = after experiment.
DISCUSSION
The goal of the study was to investigate whether exogenous administration of oxytocin modulates empathy towards offenders and victims of criminal offenses. The capacity to empathize is a central ingredient for third-party decision-making for settings in institutions of justice, where an individual as a member of the society might be called to serve as a juror in a criminal trial. As a juror this individual is asked to make decisions as an unaffected third-party about a criminal offense by determining the degree to which the offender deserved punishment based on the harm that was caused to the victim. Using a double-blind procedure, we demonstrated that, compared to placebo, oxytocin selectively increased the perceptions of harm for victims but not the desire to punish offenders independently of the severity of the criminal offenses. We argue that oxytocin facilitated empathic concern for the victim, because of an association between the trait empathic concern subscale and experimental ratings, after controlling for alternative explanations due to other psychological traits, mood changes during the experiment, and participants’ beliefs about the received treatment condition.
Emerging evidence points to the conclusion that the social effects of oxytocin are often moderated by contextual factors such as features of the situation in which oxytocin is administered (Bartz et al., 2011). Overall, our finding is consistent with previous studies demonstrating that oxytocin facilitates prosocial approach behavior, including trust, generosity and cooperation (Kosfeld et al., 2005; Bartz and Hollander, 2006; Hurlemann et al., 2010) as well as perception of others in ways that facilitate affiliation/bonding (Buchheim et al., 2009; Theodoridou et al., 2009). However, our findings cannot be explained by cooperation toward familiar others vs strangers (Declerck et al., 2010), since offender and victim were strangers (i.e. fictional characters) for the participants. Nor can the findings be explained by liking of individuals belonging to one’s own group vs belonging to another group (De Dreu et al., 2011), since participants acted as an unaffected third-party and were not members of the offender or victim group. Instead, in the context of institutions of justice, we argue that oxytocin increased the state empathic concern of an unaffected third-party decision-maker toward the victim but not the offender of criminal offenses, leading to a selectively increase in perceptions of harm for victims but not in desires to punish offenders. Individuals experience feelings of sympathy, compassion and concern for unfortunate others, if they consider how those have been hurt or harmed (Batson et al., 2007; Nelissen and Zeelenberg, 2009). Importantly, individuals tend to help others more frequently under conditions of empathic concern in what appears to be an altruistically motivated effort to improve the other’s well-being (Batson et al., 1997; Penner et al., 2005). A previous study using a multifaceted empathy test revealed that intranasal oxytocin increases emotional but not cognitive empathy to both positive and negative valence stimuli (Hurlemann et al., 2010). The drug effect reported in our study is consistent with this separation between emotional and cognitive aspects of empathy, since ratings in the experiment were only associated with trait measures on empathic concern (extent of individuals’ feelings of warmth, compassion and concern for others) but not with perspective taking (extent to which individuals spontaneously try to adopt others’ points of view), fantasy (extent to which individuals identify with fictional characters) or personal distress (extent of individuals’ anxiety and discomfort as a result of another’s negative experience).
Emerging evidence points further to the conclusion that the social effects of oxytocin are often moderated by stable characteristics of the individuals to whom oxytocin is administered (Bartz et al., 2011). A previous study applied an empathic accuracy task and demonstrated that oxytocin improved empathy accuracy only for less socially proficient individuals as determined by a self-report instrument (i.e. ‘Autism spectrum Quotient’) predicting social–cognitive performance (Bartz et al., 2010). This finding suggests that oxytocin should benefit only those individuals who are less attuned to the social environment. Importantly, participants in our study were only healthy men and previous investigations have shown that men frequently score lower on standard tests of empathy than women (Baron-Cohen and Wheelwright, 2004; Toussaint and Webb, 2005), a difference reflected in the recruitment of different brain networks (Schulte-Ruther et al., 2008). Therefore, oxytocin administration may increase empathy responses in men to levels found in untreated women, which has been recently confirmed (Hurlemann et al., 2010). In addition to those exogenous oxytocin effects, a recent gene association study demonstrated that a specific polymorphism (rs53576) of the oxytocin receptor gene modulates dispositional empathy (Rodrigues et al., 2009). Compared with individuals homozygous for the G allele of rs53576 (GG), individuals with one or two copies of the A allele (AG/AA) exhibited lower dispositional empathy as measured by the Interpersonal Reactivity Index (Davis, 1983). Moreover, a recent neuroimaging study has shown a volume reduction in the hypothalamus as the primarily region for the synthesis of oxytocin in carriers of the oxytocin receptor allele rs53576A that is associated with an increased risk for autistic spectrum disorders, and a sex-dependent mechanism impacting the structure and function of hypothalamic–limbic circuits that are of potential clinical and translational significance (Tost et al., 2010).
There are some limitations in our study that deserve discussion. First, since we did not directly assess state empathic concern in our study, future investigations have to validate our findings by implementing empathy state measures, for example, by using questions from the empathy trait scale and making them specific to the victim and offender in the legal vignettes. Moreover, although we found no association between oxytocin and the desire to punish offenders of criminal offenses, another interpretation might be that the ratings for punishment may not reflect participants’ empathic concern but instead their degree of empathic anger (Vitaglione and Barnett, 2003) or their beliefs about how justice should be served in society. Second, since our study only included men who read vignettes about a male offender, future studies should examine whether the observed findings can be generalized to both genders. Finally, since participants made third-party decisions regarding fictional characters of criminal scenarios, where their decisions involved no costs for themselves, future studies have to clarify if the reported findings for institutions of justice are similar to third-party decision-making in the context of economic exchange utilizing economic game paradigms with monetary compensation.
In conclusion, our findings provide the first demonstration that oxytocin selectively increased perceptions of harm for victims but not the desire to punish offenders of criminal offenses in healthy men. Given the selective effect found in our study, future research should explore the context- and person-dependent nature of exogenous oxytocin administration in individuals with psychiatric disorders, such as antisocial personality disorder and psychopathy which are far more common among males. One strategic therapeutic approach might be the combination of oxytocin pharmacotherapy with a psychosocial intervention design to target specific cognitive outcomes that improve victim empathy.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
FUNDING
This research was supported in part by the Intramural Research Program of the NIH, NIDA and funded by Air Force Office of Scientific Research (AFOSR) Grant FA9550-10-1-0385 to Raja Parasuraman and the Center of Excellence in Neuroergonomics, Technology, and Cognition (CENTEC).
Supplementary Material
Acknowledgments
We thank Samuel Moyer, Christina Cary and Steven Scheid for research assistance.
REFERENCES
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders. 2004;34(2):163–175. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Hollander E. The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Hormones and Behavior. 2006;50(4):518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Science. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, et al. Oxytocin selectively improves empathic accuracy. Psychological Science. 2010;21(10):1426–1428. doi: 10.1177/0956797610383439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson C, Kennedy C, Nord L, et al. Anger at unfairness: Is it moral outrage? European Journal of Social Psychology. 2007;37:1272–1285. [Google Scholar]
- Batson C, Sager K, Garts E, Kang M, Rubchinsky K, Dawson K. Is empathy-induced helping due to self-other merging? Journal of Personality and Social Psychology. 1997;73:495–509. [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neuroscience. 2002;5(6):514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Buchheim A, Heinrichs M, George C, et al. Oxytocin enhances the experience of attachment security. Psychoneuroendocrinology. 2009;34(9):1417–1422. doi: 10.1016/j.psyneuen.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckelew S, Burk J, Brownelee-Duffeck M, Frank R, DeGood D. Cognitive and somatic aspects of depression among a rehabilitation sample: Reliability and validity of SCL-90-R research subscales. Rehabilitation Psychology. 1988;33:67–75. [Google Scholar]
- Carlsmith K, Darley J, Robinson P. Why do we punish? Deterrence and just deserts as motives for punishment. Journal of Personality and Social Psychology. 2002;83:284–299. doi: 10.1037/0022-3514.83.2.284. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Costa P, McCrae R. Revised NEO Personality Inventory (NEO PI-R) Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc; 1992. [Google Scholar]
- Davis M. Measuring individual differences in empathy: evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44:113–126. [Google Scholar]
- De Dreu CK, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJ. Oxytocin promotes human ethnocentrism. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(4):1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vignemont F, Singer T. The empathic brain: how, when and why? Trends in Cognitive Science. 2006;10(10):435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Declerck CH, Boone C, Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Hormones and Behavior. 2010;57(3):368–374. doi: 10.1016/j.yhbeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biological Psychiatry. 2009;65(9):728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Israel S, Chew SH, Zhong S, Knafo A. Genetics of human social behavior. Neuron. 2010;65(6):831–844. doi: 10.1016/j.neuron.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Fehr E, Fischbacher U. Third-party punishment and social norms. Evolution and Human Behavior. 2004;25:63–87. [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. Journal of Neuroscience. 2001;21(20):8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D, Bartholomew K. Models of the self and other: fundamental dimensions underlying measures of adult attachment. Journal of Personality and Social Psychology. 1994;67(3):430–445. [Google Scholar]
- Hurlemann R, Patin A, Onur OA, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. Journal of Neuroscience. 2010;30(14):4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34(4):1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Kemp A, Guastella A. The role of oxytocin in human affect: a novel hypothesis. Psychological Science. 2011;20(4):222–231. [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007;19(1):42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: the great facilitator of life. Progress in Neurobiology. 2009;88(2):127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36(8):1114–1126. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews. Neuroscience. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Nelissen R, Zeelenberg M. Moral emotions as determinants of third-party punishment: anger, guilt, and the functions of altruistic sanctions. Judgment and Decision Making. 2009;4(7):543–553. [Google Scholar]
- Nishimori K, Takayanagi Y, Yoshida M, Kasahara Y, Young LJ, Kawamata M. New aspects of oxytocin receptor function revealed by knockout mice: sociosexual behaviour and control of energy balance. Progress in Brain Research. 2008;170:79–90. doi: 10.1016/S0079-6123(08)00408-1. [DOI] [PubMed] [Google Scholar]
- Penner L, Dovidio J, Piliavin J, Schroeder D. Prosocial behaviour: multilevel perspectives. Annual Review of Psychology. 2005;56:365–392. doi: 10.1146/annurev.psych.56.091103.070141. [DOI] [PubMed] [Google Scholar]
- Robinson P, Kurzban R. Concordance and conflict in intuitions of justice. Minnesota Law Review. 2007;91:1829–1893. [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(50):21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton J, Chrisjohn R, Fekken G. The altruistic personality and the self-report altruism scale. Personality & Individual Differences. 1981;50:1192–1198. [Google Scholar]
- Schulte-Ruther M, Markowitsch HJ, Shah NJ, Fink GR, Piefke M. Gender differences in brain networks supporting empathy. Neuroimage. 2008;42(1):393–403. doi: 10.1016/j.neuroimage.2008.04.180. [DOI] [PubMed] [Google Scholar]
- Singer T, Snozzi R, Bird G, et al. Effects of oxytocin and prosocial behavior on brain responses to direct and vicariously experienced pain. Emotion. 2008;8(6):781–791. doi: 10.1037/a0014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C. Manual for the State-Trait Anxiety Inventory (STAI), Form Y. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Spitzer M, Fischbacher U, Herrnberger B, Gron G, Fehr E. The neural signature of social norm compliance. Neuron. 2007;56(1):185–196. doi: 10.1016/j.neuron.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Striepens N, Kendrick KM, Maier W, Hurlemann R. Prosocial effects of oxytocin and clinical evidence for its therapeutic potential. Frontiers in Neuroendocrinology. 2011;32:426–450. doi: 10.1016/j.yfrne.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. Oxytocin and social perception: oxytocin increases perceived facial trustworthiness and attractiveness. Hormones and Behavior. 2009;56(1):128–132. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(31):13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint L, Webb J. Gender differences in the realtionship between empathy and forgiveness. The Journal of Social Psychology. 2005;145:673–685. doi: 10.3200/SOCP.145.6.673-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaglione G, Barnett M. Assessing a new dimension of empathy: empathic anger as a predictor of helping and punishing desires. Motivation and Emotion. 2003;27:301–325. [Google Scholar]
- Watson D, Clark L, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007;2(11):e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeelenberg M, Nelissen R, Breugelmans S, Pieters R. On emotion specificity in decision making: Why feeling is for doing. Judgment and Decision Making. 2008;3:18–27. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.