Abstract
Neuroscientific studies on agency focus rather exclusively on the notion of who initiates and regulates actions, not on the notion of why the person does. The present study focused on the latter to investigate two different reasons underlying personal agency. Using event-related functional magnetic resonance imaging, we scanned 16 healthy human subjects while they imagined the enactment of volitional, agentic behavior on the same task but either for a self-determined and intrinsically motivated reason or for a non-self-determined and extrinsically motivated reason. Results showed that the anterior insular cortex (AIC), known to be related to the sense of agency, was more activated during self-determined behavior while the angular gyrus, known to be related to the sense of loss of agency, was more activated during non-self-determined behavior. Furthermore, AIC activities during self-determined behavior correlated highly with participants’ self-reported intrinsic satisfactions. We conclude that self-determined behavior is more agentic than is non-self-determined behavior and that personal agency arises only during self-determined, intrinsically motivated action.
Keywords: agency, anterior insular cortex, fMRI, intrinsic motivation, self-determined motivation
INTRODUCTION
People engage in volitional action for two different reasons—to pursue intrinsic satisfactions, such as personal interest, and to pursue extrinsic contingencies, such as a promised reward (Deci et al., 1999; Sansone and Harackiewicz, 2000; Lepper et al., 2005). In both cases, the person has the subjective experience of personal causation and of being the agentic cause of the ensuing motoric action. What distinguishes between the two types of motivation is the reason why the person engages in the volitional, agentic action. If the reason is incentive-based (e.g. ‘I read the book to get extra-credit points’), then the volitional action is actually extrinsically motivated (EM) in that it is both environmentally generated and environmentally regulated. That is, as environmental incentives arise, change, and disappear, the person’s volitional and agentic action changes in kind. However, if the reason is self-based (e.g. ‘I read the book because it interests me’), then the volitional action is intrinsically motivated (IM) in that it is truly self-generated and self-regulated. That is, as self-satisfactions (e.g. interest, enjoyment and autonomy) arise, change and disappear, the person’s volitional and agentic action changes in kind.
Neuroscience-based agency research focuses rather exclusively on the notion of who initiates and regulates the person’s action, not on the notion of why the person does. That is, numerous studies have tried to determine the neural substrates of agency by examining the neural differences between self-generated vs other generated behavior. In these studies, the contrast is between personal action (e.g. move a joystick) that is caused by self vs caused by someone else, such as the experimenter (e.g. ‘I held the joystick and I moved it’ vs ‘I held the joystick but, actually, the experimenter moved it’). Findings from these studies suggest that the neural activities of the prefrontal cortex, insular cortex, cerebellum and motor-related regions (e.g. supplementary motor area, pre-supplementary motor area, precentral gyrus and postcentral gyrus) are related to the execution, observation, or imagination of self-generated behavior (Gallagher, 2000; Haggard, 2008; Nachev et al., 2008). Specifically, some studies demonstrated that the activities of the anterior insular cortex (AIC) were more associated with self-generated (i.e. more agentic in this literature) behavior, while the activities of the angular gyrus were more associated with other generated (i.e. less agentic in this literature) behavior (Farrer and Frith, 2002; Farrer et al., 2003).
Volitional, agentic, self-generated action is clearly different from non-volitional, non-agentic, other generated action, but this study argues further that two important types of motivation exist within the first category of action. Specifically, this study was designed to identify the neural differences between volitional, agentic behavior that is self-determined and IM (e.g. ‘I did it for personal interest, not for environmental reward’) vs volitional, agentic behavior that is non-self-determined and EM (e.g. ‘I did it for environmental reward, not for personal interest’). Our research strategy was to have people imagine the personal enactment of volitional, agentic behavior on the same task but for two different reasons. For the self-determined (i.e. intrinsic) reasons, we borrowed those reasons for action emphasized by self-determination theorists, including acting out of interest, enjoyment, autonomy, and competence (Deci and Ryan, 1985; Ryan and Deci, 2000). What these reasons have in common is an inner endorsement of one’s action, which is the sense that action is self-authored, emanates from the self, and is one’s own (Ryan and Deci, 2000). For the non-self-determined (i.e. extrinsic) reasons, we included acting in order to attain an attractive environmental reward or incentive, including money, a high grade, a prize and extra-credit points. What these reasons have in common is an environmental reason for action, which is the sense that action is environmentally authored and emanates from the presence vs the absence of attractive environmental contingencies. Imagination of behaviors was used as the experimental paradigm because numerous studies have shown that imagining behavior has worked as well as the actual execution of behavior for generating a sense of volition and agency (Decety et al., 1994; Gallese and Goldman, 1998; Ruby and Decety, 2001). Importantly, this strategy had the advantage of allowing us to sample neural activities in response to a broad and representative range of self-determined vs non-self-determined reasons for acting.
To formulate the experimental hypotheses, we argue that EM behavior and non-self-generated behavior are functional equivalents in everyday practice, as both are generated and regulated by the offering of attractive incentives and consequences (e.g. ‘my boss offered a bonus if I worked, my teacher promised extra-credit points if I studied’), even if the EM behavior is volitional and agentic in the sense that the person initiates the action. Recognizing this, we hypothesized that non-self-determined EM reason for acting would be related to angular gyrus activities (Farrer and Frith, 2002; Farrer et al., 2003). Likewise, we argue that IM behavior and self-generated behavior are functional equivalents, as both are generated and regulated by the experiencing of self-satisfactions (e.g. ‘I worked because it was fun, I studied because I felt curious about it’). Recognizing this, we hypothesized that self-determined IM reasons for acting would be associated with AIC activities (Farrer and Frith, 2002; Farrer et al., 2003). To confirm that these hypothesized AIC activations were linked to the expectation of intrinsic satisfactions, we identified whether participants’ subjective experiences of perceived autonomy and perceived competence correlated with the extent of their AIC activity (as well as with other neural activities) during self-determined behavior. We assessed these two particular types of intrinsic satisfaction because they constitute the definitional basis of intrinsic motivation: ‘Intrinsic motivation is based on the innate, organismic needs for competence, and self-determination. It energizes a wide variety of behaviors and psychological processes for which the primary rewards are the experiences of effectance and autonomy’ (Deci and Ryan, 1985, p. 32).
METHODS
Participants
Sixteen Korean undergraduates (8 females and 8 males; aged 19–24 years), who were recruited from a large university in Korea, participated in the fMRI study. They were right-handed and had no history of neurological illness. All participants provided informed consent in accordance with the regulations of the Institutional Review Board of Korea University and received compensation for their participation.
Stimuli
Korean phrases were used to describe situations from the following three conditions: self-determined IM reasons for action, non-self-determined EM reasons for action, and neutral reasons for action (Neutral). The phrases were created for the purposes of this study and written to reflect the theoretical postulates and operational definitions from self-determination theory (SDT; Deci and Ryan, 1985; Ryan and Deci, 2000). Each phrase consisted of two parts, one part described a familiar situation to imagine acting on (e.g. writing a paper, participating in a project) and the other part provided one of the three different reasons for acting. The situations used and the reasons for acting were based on examples and experimental manipulations utilized in the SDT literature. In the IM condition, the phrases described situations with reasons for acting that featured internal causalities, such as interest, enjoyment, or perceived autonomy (e.g. writing an enjoyable paper). In the EM condition, the phrases described situations with reasons for acting that featured attractive extrinsic incentives (e.g. writing an extra-credit paper). In the Neutral condition, the phrases described situations with only a neutral reason for acting (e.g. writing a class paper). The within-situation phrases were matched in terms of sentence structure and the number and length of the words used (Table 1).
Table 1.
Examples of phrases for each experimental condition used in the experimental task
| IM phrases | EM phrases | Neutral phrases |
|---|---|---|
| Writing an enjoyable paper | Writing an extra-credit paper | Writing a class paper |
| Working with freedom | Working for incentives | Working with time to spare |
| Participating in a fun project | Participating in a money-making project | Participating in a routine project |
| Having options and choices | Having prizes and awards | Having things to do |
| Studying for fun | Studying for a grade | Studying because it is time |
| Feeling curious | Feeling rewarded | Feeling neutral |
| Feeling interested | Anticipating a prize | Feeling normal |
To verify the suitability of the phrases, a pilot test was conducted in advance. A total of 23 Korean undergraduates (10 females, 13 males; aged 20–28 years), recruited from the same large university in Korea, participated. In this computerized pilot test, participants rated 180 phrases with 60 phrases from each of the three experimental conditions. During the pilot test, one phrase, randomly selected from the array of 180 phrases, was presented sequentially, and participants were asked to rate each described situation on a series of four 1–7 uni-polar scales in terms of how behaviorally energizing, intrinsically motivating, extrinsically motivating and attractive it was perceived to be. Prior to viewing the phrases, participants received instructions on the conceptual definitions of what constituted behavioral energization, intrinsic motivation, extrinsic motivation and attractiveness. Data of one male participant were excluded from the analyses because his responses were not recorded (equipment failure). Results from the pilot test appear in the ‘Results’ section. From this larger pool of 180 phrases included in the pilot test, we selected 150 (50 situations, each depicting 3 different reasons for acting) for the actual study that were rated as expected on these four dependent measures.
Task and procedure
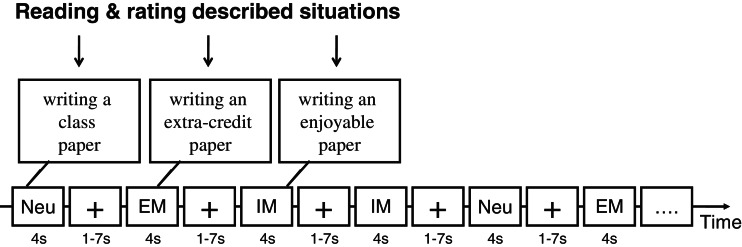
An event-related experimental design was used in which participants viewed the phrases one-at-a-time in a random order. Overall, the procedure utilized two separate runs, and each run lasted for 12 min 30 s and consisted of 75 trials (25 phrases from each of the three experimental conditions). In each trial (Figure 1), one phrase, randomly selected from the whole array of phrases, was presented for 4 s, and a fixation cross was presented at the inter-trial interval (ITI) for an average of 3 s (1000–7000 ms). Participants rated how much they wanted to engage in each of the described situations on a 1–4 scale (‘Do you want to do this?’: 1 = not at all; 2 = a little; 3 = some; 4 = a great deal). By asking the participants to make this engagement rating, the experimental procedure encouraged the participants to mentalize the described situation. To indicate their judgments, participants were asked to press one of the four buttons using their right-hand fingers.
Fig. 1.
The experimental task and the experimental design are presented. In each trial, one phrase, randomly selected from the whole array of phrases, was presented sequentially, and participants were asked to rate how much they ‘want to engage in’ each of the described situations. Neu: neutral.
Before entering the fMRI setting, participants completed the Basic Psychological Needs Scale (described below) and received the task instruction. Once the participant was situated in the fMRI setting, he or she completed four practice trials. During the fMRI scan, functional images were acquired while participants performed the experimental task, and then anatomic images were acquired. At the end of the experiment, participants were debriefed about the experiment and received their compensation for participation.
Measure
Basic Psychological Needs Scale
The Basic Psychological Needs Scale (BPNS; Gagné, 2003; see Appendix A) featured 13 items to assess the extent to which the psychological experiences of autonomy and competence occur in the person’s life. Sample items are ‘I feel like I can decide for myself how to live my life’ (autonomy) and ‘Most days I feel a sense of accomplishment from what I do’ (competence). The scale has been widely used and has been shown to produce acceptable psychometric properties (Gagné, 2003; Wei et al., 2005; Niemiec et al., 2009). In this study, we used the Korean translated version of the BPNS (Jang et al., 2009). To complete the scale, participants rated on a 1–7 Likert scale (Strongly disagree–Strongly agree) how true each statement was for them in general. Participants’ responses on the BPNS scale showed acceptable reliability in this study (13 items, α = 0.83).
fMRI data acquisition
Imaging was performed with a 3T Trio MRI scanner (Siemens, Erlangen, Germany). First, 32-slice functional images were acquired using a T2*-weighted gradient-echo echo planar imaging (EPI) sequence sensitive to blood oxygenation level-dependent (BOLD) contrast. The following imaging parameters were used: TR = 2000 ms, TE = 30 ms, flip angle = 90°, FOV = 224 × 224, in-plane resolution = 3.5 × 3.5 mm, and slice thickness = 4 mm (no gap). High-resolution T1-weighted structural images were acquired by a MP-RAGE sequence. These images were used for anatomical localization in order to facilitate the precise determination of the structures corresponding to the functional activation foci. The following imaging parameters were used for the high-resolution T1-weighted structural images: TR = 1900 ms, TE = 2.52 ms, flip angle = 9°, FOV = 256 × 256 and slice thickness = 1 mm (no gap).
fMRI data analysis
The brain images were analyzed using AFNI (Cox, 1996; http://afni.nimh.nih.gov). The first three images of each run were discarded to allow hemodynamics and MRI signals to reach a steady state. In pre-processing, the functional images were checked to determine whether there were signal artifacts which could be made by participants’ head movement, scanner irregularities and so on. Then, the functional images were interpolated to the same time point at the beginning of the TR for slice timing correction. After the functional images of each participant were aligned to the structural images of each participant, the aligned functional images were registered to the base volume for head motion correction. These motion-corrected brain images were spatially smoothed with a 5 mm full-width at half-maximum (FWHM) Gaussian kernel. After the values of background voxels (i.e. voxels outside the brain) were excluded, the functional data were normalized as a percent of the mean for running future statistical analyses. The functional images of each run were separately pre-processed, and then the two runs of each participant were concatenated before individual analyses.
In individual analyses, the pre-processed time-series data were analyzed by a general linear model (GLM). In the GLM, hemodynamic response functions (HRF) of nine regressors were computed. Three regressors were for the experimental conditions of IM, EM and Neutral. To control for the effects of head motion artifacts, six regressors for head motion parameters were included as covariates in the model.
For group analyses, each individual’s statistical data were transformed to MNI space. First, the high-resolution structural images of each participant were transformed to the standardized structural images. Then, the functional images of each participant were transformed to the standardized high-resolution structural images of each participant. At that time, the functional images were resampled to 2 × 2 × 2 mm3 voxels.
In the group analyses, subtraction analyses were conducted to compare the neural differences between the IM and EM conditions. A regression analysis was also conducted to examine the correlations between participants’ BPNS scores and the neural activities in the IM condition. For correcting multiple comparison inferences in these whole-brain analyses, the cluster-wise threshold (corrected P < 0.05) was employed based on Monte-Carlo simulations (Forman et al., 1995), which was determined by both voxel-wise threshold (P < 0.005) and cluster size (n = 53, a minimum volume of 424 mm3). The significant activations for the subtraction analyses and the regression analysis were reported as MNI coordinates. To see the neural activation magnitudes of regions of interests (ROIs), the BOLD signal changes in the ROIs were extracted. To avoid the issue of non-independence bias (Kriegeskorte et al., 2009), a leave-one-subject-out (LOSO) method (Esterman et al., 2010) was used. In the LOSO method, we repetitively left out one participant’s data as we ran 16 independent GLMs using the 15 remaining participants’ data to define independent clusters of the ROIs, so to extract the BOLD signal changes in the clusters from the left-out participant’s data.
RESULTS
Pilot test results
Analyses from the pilot test data confirmed that the phrases in the IM and EM conditions worked as intended. Specifically, participants rated the phrases in the IM condition as more intrinsically motivating than they rated the phrases in the EM condition, t(20) = 5.77, P < 0.05 (Ms, 5.8 vs 4.5; Figure 2A), rated the phrases in the EM condition as more extrinsically motivating than they rated the phrases in the IM condition, t(20) = 6.58, P < 0.05 (Ms, 6.0 vs 4.7; Figure 2A), and rated the phrases in both the IM and EM conditions as more behaviorally energizing, F(2,20) = 49.64, P < 0.05 (IM, EM and Neutral Ms, 5.9 = 5.7 > 4.9; Figure 2B) and as more positively valenced, F(2,20) = 55.33, P < 0.05 (IM, EM and Neutral Ms, 5.7 = 5.5 > 4.7; Figure 2C), than they rated the phrases in the Neutral condition.
Fig. 2.
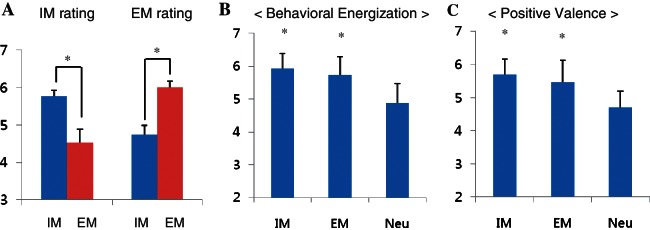
Results of the pilot test. Results confirmed that the phrases in the IM, EM, and Neutral conditions produced their intended effects. That is, the phrases in the IM condition were rated as higher in IM and as lower in EM than were the phrases in the EM condition (A). Further, the phrases in the IM and EM conditions were rated similarly high in terms of behavioral energization (B) and positive valence (C) and both were rated as higher than were the phrases in the Neutral condition. *P < 0.05. Neu: neutral.
Behavioral results
Participants’ ratings of how much they wanted to engage in the described situations varied significantly across the three conditions, as the situations described in the IM and EM phrases were both perceived as more motivating than were those described in the Neutral phrases, F(2,14) = 71.48, P < 0.05 (IM, EM, Neutral Ms, 3.3 = 3.2 > 2.4; Figure 3A).
Fig. 3.
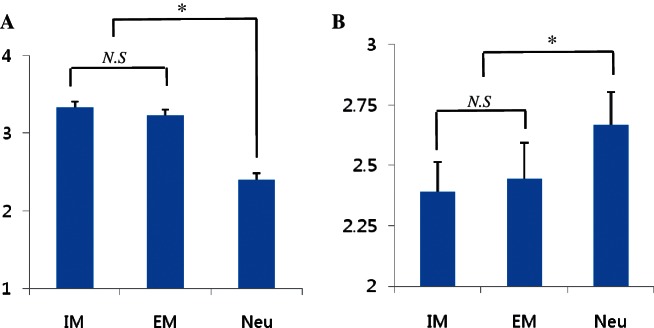
Participants’ mean behavioral energization rating (A) and mean RT (B) for each of the three experimental conditions. Results confirmed that behavioral energization ratings in the IM and EM conditions were not significantly different while both were significantly higher than the ratings in the Neutral condition (A). Results also confirmed that the RTs in the IM and EM conditions were not significantly different while both were significantly shorter than the RTs in the Neutral condition (B). * P < 0.05. N.S: non-significant. Neu: neutral.
Participants’ reaction times (RT) indicating extent of judgmental simplicity in deciding how much they wanted to engage in the described situations varied significantly across the three conditions, as the situations described in the IM and EM phrases were both responded to more quickly than were those described in the Neutral phrases, F(2,14) = 10.82, P < 0.05 (IM, EM, Neutral Ms, 2392.6 = 2443.3 < 2668.3; Figure 3B).
fMRI results
Results of the subtraction analyses (Table 2) showed that the left AIC (Figure 4A) as well as the left superior temporal gyrus (STG), the bilateral cerebellum, the left posterior insular cortex, the bilateral supplementary motor area (SMA), the right dorsolateral prefrontal cortex (DLPFC), the right occipital lobe, the right precentral gyrus, the right postcentral gyrus and the right middle frontal gyrus were more activated in the IM condition than in the EM condition (corrected P < 0.05). BOLD signal changes of the AIC across conditions appear in Figure 4B.
Table 2.
Results of the subtraction analysis between the IM condition and the EM condition
| Region | BA | Volume | Side | MNI Coordinates |
Maximum t-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| IM–EM | |||||||
| Superior temporal gyrus | 22 | 8544 | L | −64 | −30 | 16 | 8.04 |
| Cerebellum | 2864 | R | 14 | −46 | −50 | 6.34 | |
| 960 | L | −16 | −60 | −42 | 5.33 | ||
| 904 | R | 34 | −40 | −42 | 5.47 | ||
| 864 | L | −10 | −44 | −44 | 6.54 | ||
| Insular cortex | 13 | 2064 | L | −34 | 4 | 8 | 6.80 |
| 13 | 808 | L | −32 | −6 | 22 | 5.41 | |
| Supplementary motor area | 6 | 1216 | R | 22 | −4 | 64 | 5.44 |
| 6 | 504 | L | −14 | −6 | 70 | 5.54 | |
| Dorsolateral prefrontal cortex | 44 | 752 | R | 58 | 10 | 26 | 6.69 |
| Occipital lobe | 18 | 616 | R | 20 | −68 | 0 | 4.53 |
| Precentral gyrus | 4 | 560 | R | 32 | −18 | 48 | 5.16 |
| Postcentral gyrus | 2 | 520 | R | 32 | −32 | 60 | 6.18 |
| Middle frontal gyrus | 8 | 456 | R | 30 | 16 | 48 | 5.90 |
| EM–IM | |||||||
| Angular gyrus | 39 | 480 | L | −44 | −54 | 52 | 4.62 |
Note: The cluster-wise threshold (correct P < 0.05) is determined by voxel-wise threshold (P < 0.005), the connectivity radius (2.0 mm), the minimum volume (53 contiguous voxels, 424 mm3), and the FWHM (5 mm).
Fig. 4.
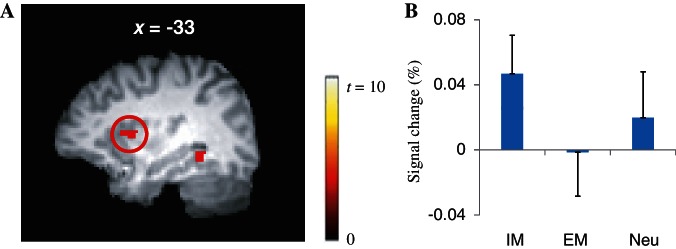
There were significantly greater brain activations of the AIC in the IM condition than in the EM condition (A). The BOLD signal changes of the AIC across conditions are presented (B). Neu: neutral.
The subtraction analysis further showed that the left angular gyrus (Figure 5A) was more activated in the EM condition than in the IM condition (corrected P < 0.05). BOLD signal changes of the angular gyrus across conditions appear in Figure 5B.
Fig. 5.
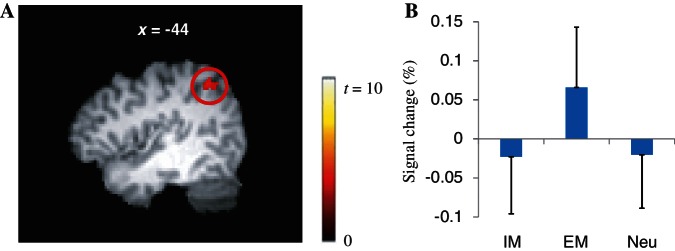
There were significantly greater brain activations of the angular gyrus in the EM condition than in the IM condition (A). The BOLD signal changes of the angular gyrus across conditions are presented (B). Neu: neutral.
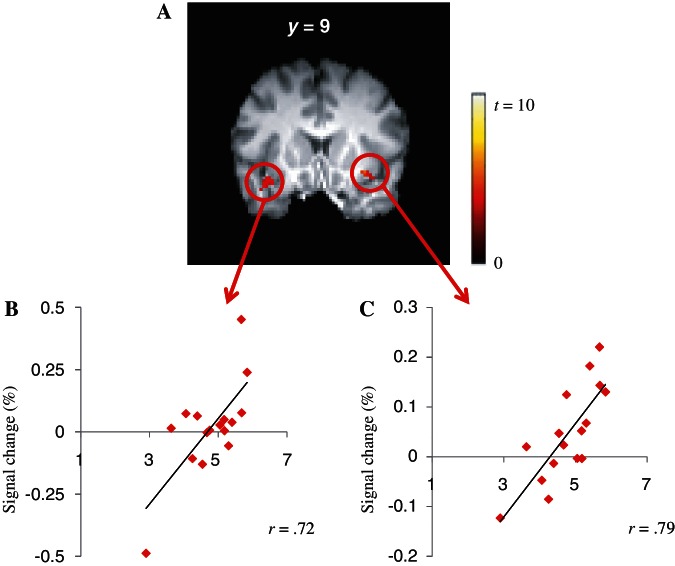
Only the extent to which participants showed AIC activations in the IM condition correlated significantly with their self-reported intrinsic satisfactions (i.e. scores of autonomy and competence on the BPNS; Table 3; Figure 6A). The scatter plots showing the relations of participants’ self-reported intrinsic satisfactions with the left AIC activities (Figure 6B) and with the right AIC activities (Figure 6C) are presented.
Table 3.
Results of the regression analysis between participants’ intrinsic satisfactions and neural activities in the IM condition
| Region | BA | Volume | Side | MNI Coordinates |
Maximum t-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Insular cortex | 13 | 760 | L | −36 | 6 | −8 | 5.52 |
| 13 | 464 | R | 32 | 18 | −10 | 6.91 | |
Note: The cluster-wise threshold (correct P < 0.05) is determined by voxel-wise threshold (P < 0.005), the connectivity radius (2.0 mm), the minimum volume (53 contiguous voxels, 424 mm3), and the FWHM (5 mm).
Fig. 6.
AIC activities in the IM condition were the only neural activities which correlated positively with participants’ perceived intrinsic satisfactions (A) The scatter plot between the BOLD signal changes of the left AIC (B) and the right AIC (C) in the IM condition and participants’ BPNS scores are presented.
DISCUSSION
When participants imagined self-determined action, they showed greater AIC activity than when they imagined non-self-determined action, even while imagining the same action. Given that such neural activity is associated with a sense of volition and agency (Ruby and Decety, 2001; Farrer and Frith, 2002; Farrer et al., 2003), this finding suggests that self-determined IM behavior is more volitional and agentic than is non-self-determined EM behavior. This finding supports the conclusion that self-generated agentic action arises for two different reasons—intrinsically from what SDT labels as truly self-determined motivation as people engage in action from internal causalities and for internal satisfactions vs extrinsically from what SDT labels as non-self-determined motivation as people engage in action from environmental causalities and for extrinsic incentives and rewards. The novelty of this finding is that it means that a crucial distinction needs to be made within the category of ‘volitional, agentic, self-generated behavior’ so to distinguish self-determined (IM) volitional, agentic, self-generated behavior from non-self-determined (EM) volitional, agentic, self-generated behavior. In fact, the neural activities observed in the EM condition (greater angular gyrus activations, lesser AIC activations) look like that observed in previous studies of non-agentic other generated behavior.
Agency means having a sense of ‘my own voluntary behavior’ (Deci and Ryan, 1991; Gallagher, 2000; Bandura, 2001). However, ‘my own’ has two different meanings. In one sense, ‘my own’ is determined by who generates behavior, and this is the distinction between the agentic feelings that ‘I’ generated the behavior vs the non-agentic feeling that ‘other’ generated the behavior (Gallagher, 2000). In another sense, ‘my own’ is determined by why I generate behavior (Deci and Ryan, 1991). This approach to the study of agency argues, first, that two types of motivation exist within self-generated agentic action, secondly, that self-generated behavior enacted for the pursuit of environmental contingencies is best conceptualized as non-self-determined behavior, and thirdly, that this distinction between the two types of motivation is a practically important one in that people (e.g. students, workers and athletes) function more positively in a wide variety of important ways when self-determined and IM vs when non-self-determined and EM (with positive functioning being indicated by the extent of learning, engagement, performance, achievement, and well-being outcomes; Deci et al., 1981; Black and Deci, 2000; Vansteenkiste et al., 2004; Jang et al., 2009).
The insular cortex is involved in many different functions (Craig, 2009; Singer et al., 2009). Of particular importance to this study is the role that insula activations play in the representation of bodily states (Damasio, 1999). Bodily state information is stored in and retrieved from the somatosensory map, and the insular cortex works for the function of this somatosensory map. Therefore, the activities of the insular cortex, including the AIC, have been repeatedly observed in studies on psychological processes influenced by bodily information, such as feeling processes (Damasio et al., 2000; Craig, 2009), feeling-based decision-making (Bechara and Damasio, 2005), bodily needs and urges (Brody et al., 2002; Critchley et al., 2004; Pelchat et al., 2004), cravings from bodily addictions (Naqvi et al., 2007; Goldstein et al., 2009; Naqvi and Bechara, 2009), and so on. Insular cortex activities, particularly AIC activities, highlighted in the empirical study of agency can be understood in this same vein (Gallagher, 2000; Ruby and Decety, 2001; Farrer and Frith, 2002). That is, bodily information during self-initiated motor activity is monitored by the insular cortex, and its activities then provide the neural basis of the sense of agency (Farrer et al., 2003). AIC activity correlated significantly and positively with participants’ subjective experiences of perceived intrinsic satisfaction (i.e. perceived autonomy, perceived competence). These correlations reported in Figure 6 are important because they show the close relation between AIC activations and a striving for intrinsic satisfaction—for the psychological need satisfaction that is so central to the experience of intrinsic motivation.
The neural results of this study also revealed that the left angular gyrus was the only brain region showing greater neural activations during the sense of non-self-determined behavior than during the sense of self-determined behavior. Neural evidence suggests that these neural activities are associated with the sense of a loss of agency. Even though the right hemisphere of these posterior parietal regions were emphasized as dominant regions activated by the sense of loss of agency, the left hemisphere has been also observed to be activated together with the right hemisphere in many studies on agency (Farrer and Frith, 2002; Farrer et al., 2003). These results again suggest that non-self-determined behavior is less agentic than is self-determined behavior. Why this is so is because participants appeared to sense low degrees of self-authored agency when their actions were linked to extrinsic reasons for acting.
The posterior parietal regions tend to be more activated during the observation or imagination of other generated behavior, compared to self-generated behavior (Ruby and Decety, 2001; Farrer and Frith, 2002). Therefore, these brain regions have been generally assumed to be activated as other generated behavior reduces the sense of agency. In this study, the posterior parietal regions were more activated when participants imagined self-generated behavior that was initiated and regulated by environmental forces (i.e. extrinsic reasons for acting) than when participants imagined self-generated behavior that was initiated and regulated by the self (i.e. intrinsic reasons for acting). Considering these posterior parietal regions are known to be related to the understanding of social knowledge (Fogassi et al., 2005; Culham and Valyear, 2006; Chiao et al., 2009), perceiving environmental influences on self-related processes is assumed to reduce the sense of agency.
Other neural activities, which were more activated in the IM condition than in the EM condition, suggest a possibility that participants were more cognitively engaged during the imagination of self-determined behavior than during the imagination of non-self-determined behavior. This is because the motor-related regions (e.g. SMA, precentral gyrus, and post-central gyrus), the DLPFC and the cerebellum were expected to be activated by the experimental paradigm. The motor-related regions are generally understood to be linked to motor enactment or motor imagery, the DLPFC is linked to cognitive control over motor-related processes, and the cerebellum is linked to the prediction of consequences of these motor-related processes (Lotze et al., 1999; Blakemore and Decety, 2001; Blakemore et al., 2001; Bunge et al., 2001; Miller and Cohen, 2001). Therefore, more neural activities of these brain regions in the IM condition can be interpreted as more cognitive engagement for motor-related processes during the sense of self-determined behavior. Previous studies on agency have also repeatedly observed that self-generated behavior is linked to greater activations in these same regions that we observed in our IM condition (Frith et al., 1991; Gallagher, 2000; Ruby and Decety, 2001; Farrer and Frith, 2002).
Three aspects of the methodology limit the conclusions that can be drawn from this study. The first limitation is that participants imagined enacting self-generated behavior, rather than actually acting it out. While previous research has shown that imagining such behavior works as well as actually executing it in terms of generating a sense of agency (Ruby and Decety, 2001; Brooks et al., 2011), it still seems necessary to test our hypothesis using actual action. The second limitation is that participants imagined their reasons for acting, rather than experienced the self-satisfaction and reward receipt directly. This is a limitation because additional neural activity may emerge with the actual receipt of intrinsic satisfaction and extrinsic reward that were not observed in this study (e.g. reward-related striatal activations). A third limitation concerns a possible alternative interpretation as to why participants in the IM condition showed increased neural activity in the motor-related regions, DLPFC, and cerebellum than did participants in the EM condition. Our interpretation was that the imagination of self-determined behavior was more cognitively engaging than was the imagination of non-self-determined behavior. An alternative interpretation, however, might be that some other property of the stimulus phrases might have produced these neural differences. For example, the reasons depicted in the IM phrases might have simply been easier for participants to mentalize than where the reasons depicted in the EM phrases. The lack of RT differences between the two conditions argues against this alternative interpretation, but we acknowledge that we cannot rule it out because we did not a priori match the stimulus phrases across conditions in terms of how easy to mentalize they were.
CONCLUSION
Within the neuroscientific conceptualization of agentic behavior, an important motivational distinction needs to be made between that which is self-determined and IM vs that which is non-self-determined and EM. This distinction is justified by the increased AIC and decreased angular gyrus activations observed during self-determined, IM, self-generated agentic action vs the decreased AIC and increased angular gyrus activations observed during non-self-determined, EM, self-generated agentic action.
Acknowledgments
This research was supported by the WCU (World Class University) Program funded by the Korean Ministry of Education, Science and Technology, consigned to the Korea Science and Engineering Foundation (Grant no. R32-2008-000-20023-0).
Appendix A
Basic Psychological Needs Scale (BPNS)
Items to assess perceived autonomy:
I feel like I am free to decide for myself how to live my life.
I feel pressured in my life. (R)
I generally feel free to express my ideas and opinions.
In my daily life, I frequently have to do what I am told. (R)
People I interact with on a daily basis tend to take my feelings into consideration.
I feel like I can pretty much be myself in my daily situations.
There is not much opportunity for me to decide for myself how to do things in my daily life. (R)
Items to assess perceived competence:
Often, I do not feel very competent. (R)
People I know tell me I am good at what I do.
I have been able to learn interesting new skills recently.
Most days I feel a sense of accomplishment from what I do.
In my life I do not get much of a chance to show how capable I am. (R)
I often do not feel very capable. (R)
Where (R) represents reverse scored.
REFERENCES
- Bandura A. Social cognitive theory: an agentic perspective. Annual Review of Psychology. 2001;52:1–26. doi: 10.1146/annurev.psych.52.1.1. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR. The somatic marker hypothesis: a neural theory of economic decision. Games and Economic Behavior. 2005;52:336–72. [Google Scholar]
- Black AE, Deci EL. The effects of instructors’ autonomy support and students’ autonomous motivation on learning organic chemistry: a self-determination theory perspective. Science Education. 2000;84:740–56. [Google Scholar]
- Blakemore SJ, Decety J. From the perception of action to the understanding of intention. Nature Reviews Neuroscience. 2001;2:561–7. doi: 10.1038/35086023. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Frith CD, Wolpert DM. The cerebellum is involved in predicting the sensory consequences of action. NeuroReport. 2001;12:1879–84. doi: 10.1097/00001756-200107030-00023. [DOI] [PubMed] [Google Scholar]
- Brody A, Mandelkern MA, London ED, et al. Brain metabolic changes during cigarette craving. Archives of General Psychiatry. 2002;59:1162–72. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brooks SJ, O'Daly OG, Uher R, et al. Differential neural responses to food images in women with bulimia versus anorexia nervosa. PLoS One. 2011;6(7):e22259. doi: 10.1371/journal.pone.0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JDE. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–86. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Oby ER, Zhang L, Parrish T, Bridge DJ. Neural representations of social status hierarchy in human inferior parietal cortex. Neuropsychologia. 2009;47:354–63. doi: 10.1016/j.neuropsychologia.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews of Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Current Opinion in Neurobiology. 2006;16:205–12. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt; 1999. [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Decety J, Perani D, Jeannerod M, et al. Mapping motor representations with positron emission tomography. Nature. 1994;371:600–2. doi: 10.1038/371600a0. [DOI] [PubMed] [Google Scholar]
- Deci EL, Ryan RM. Intrinsic Motivation and Self-Determination in Human Behavior. New York: Plenum; 1985. [Google Scholar]
- Deci EL, Ryan RM. A motivational approach to self: Integration in personality. In: Dienstbier R, editor. Nebraska Symposium on Motivation: Vol. 38. Perspectives on Motivation. Lincoln: University of Nebraska Press; 1991. pp. 237–88. [PubMed] [Google Scholar]
- Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychological Bulletin. 1999;125:627–68. doi: 10.1037/0033-2909.125.6.627. [DOI] [PubMed] [Google Scholar]
- Deci EL, Schwartz AJ, Sheinman L, Ryan RM. An instrument to assess adults’ orientations toward control versus autonomy with children: reflections on intrinsic motivation and perceived competence. Journal of Educational Psychology. 1981;73:642–50. [Google Scholar]
- Esterman M, Tamber-Rosenau BJ, Chiu YC, Yantis S. Avoiding nonindependence in fMRI data analysis: leave one subject out. NeuroImage. 2010;50:572–6. doi: 10.1016/j.neuroimage.2009.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer C, Frith CD. Experiencing oneself vs. another person as being the cause of an action: the neural correlates of the experience of agency. NeuroImage. 2002;15:596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- Farrer C, Franck N, Georgieff N, Frith CD, Decety J, Geannerod M. Modulating the experience of agency: a positron emission tomography study. NeuroImage. 2003;18:324–33. doi: 10.1016/s1053-8119(02)00041-1. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston K, Liddle PF, Frackowiak RSJ. Willed action and the prefrontal cortex in man: a study with PET. Proceedings of the Royal Society of London. 1991;244:241–6. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–7. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gagné M. The role of autonomy support and autonomy orientation in prosocial behavior engagement. Motivation and Emotion. 2003;27:199–223. [Google Scholar]
- Gallagher S. Philosophical conceptions of the self: Implications for cognitive science. Trends in Cognitive Sciences. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends in Cognitive Sciences. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, et al. The neurocircuitry of impaired insight in drug addiction. Trends in Cognitive Sciences. 2009;13:372–80. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggard P. Human volition: towards a neuroscience of will. Nature Reviews Neuroscience. 2008;9:934–46. doi: 10.1038/nrn2497. [DOI] [PubMed] [Google Scholar]
- Jang H, Reeve J, Ryan RM, Kim A. Can self-determination theory explain what underlies the productive, satisfying learning experiences of collectivistically-oriented South Korean adolescents? Journal of Educational Psychology. 2009;101:644–61. [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Reviews Neuroscience. 2009;12:535–40. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper MR, Corpus JH, Iyengar SS. Intrinsic and extrinsic motivational orientations in the classroom: age differences and academic correlates. Journal of Educational Psychology. 2005;97:184–96. [Google Scholar]
- Lotze M, Montoya P, Erb M, et al. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. Journal of Cognitive Neuroscience. 1999;11:491–501. doi: 10.1162/089892999563553. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience. 2008;9:856–69. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends in Neurosciences. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–4. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemiec CP, Ryan RM, Deci EL. The path taken: consequences of attaining intrinsic and extrinsic aspirations in post-college life. Journal of Research in Personality. 2009;43:291–306. doi: 10.1016/j.jrp.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. NeuroImage. 2004;23:1486–93. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effect of subjective perspective taking during simulation of action: a PET investigation of agency. Nature Neuroscience. 2001;4:546–50. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American Psychologist. 2000;55:68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- Sansone C, Harackiewicz JM. Intrinsic and Extrinsic Motivation: The Search for Optimal Motivation and Performance. San Diego, CA: Academic Press; 2000. [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy, and uncertainty. Trends in Cognitive Sciences. 2009;13:334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Vansteenkiste M, Simons J, Lens W, Sheldon KM, Deci EL. Motivating learning, performance, and persistence: the synergistic role of intrinsic goals and autonomy-support. Journal of Personality and Social Psychology. 2004;87:246–60. doi: 10.1037/0022-3514.87.2.246. [DOI] [PubMed] [Google Scholar]
- Wei M, Shaffer PA, Young SK, Zakalik RA. Adult attachment, shame, depression, and loneliness: the mediation role of basic psychological needs satisfaction. Journal of Counseling Psychology. 2005;52:591–601. [Google Scholar]