Abstract
Although neuroimaging studies strongly implicate the medial prefrontal cortex (ventral and dorsal), cingulate gyrus (anterior and posterior), precuneus and temporoparietal cortex in mediating self-referential processing (SRP), little is known about the neural bases mediating individual differences in valenced SRP, that is, processes intrinsic to self-esteem. This study investigated the neural correlates of experimentally engendered valenced SRP via the Visual–Verbal Self-Other Referential Processing Task in 20 women with fMRI. Participants viewed pictures of themselves or unknown other women during separate trials while covertly rehearsing ‘I am’ or ‘She is’, followed by reading valenced trait adjectives, thus variably associating the self/other with positivity/negativity. Response within dorsal and ventral medial prefrontal cortex, cingulate cortex and left temporoparietal cortex varied with individual differences in both pre-task rated self-descriptiveness of the words, as well as task-induced affective responses. Results are discussed as they relate to a social cognitive and affective neuroscience view of self-esteem.
Keywords: self-referential processing, implicit social cognition, self-esteem
INTRODUCTION
Trait self-esteem, or the tendency to evaluate oneself positively rather than negatively, is a robust predictor of mental health and well-being (Baumeister et al., 2003). Neuroimaging studies strongly implicate the medial prefrontal cortex (ventral and dorsal), cingulate gyrus (anterior and posterior), precuneus and temporoparietal cortex (reviews by Qin and Northoff, 2011; Qin et al., 2012a; van der Meer et al., 2010) in mediating our ability to consciously reflect about ourselves, that is, self-referential processing (SRP; Northoff et al., 2006). However, little is known about the neural bases mediating individual differences in valenced SRP, that is, why most people tend to think about themselves positively, whereas others regard themselves negatively, cognitive and affective processes that are intrinsic to self-esteem.
Previous studies investigating valenced SRP measured neural response while healthy participants explicitly judged the self-descriptiveness of trait adjectives (e.g. ‘liked’ vs ‘disliked’, ‘success’ vs ‘failure’) (Fossati et al., 2003; Moran et al., 2006; Yoshimura et al., 2009) or self-relevance of valenced pictures (Phan et al., 2004; Lemogne et al., 2011), tasks not unlike completing a self-esteem questionnaire within the scanner. However, healthy individuals typically endorse positive stimuli (e.g. the words ‘liked’, ‘success’) as more self-descriptive than negative stimuli (e.g. ‘disliked’, ‘failure’) (Mezulis et al., 2004), confounding valence with self-descriptiveness, and rendering these designs less sensitive to detecting neural processes mediating negatively valenced SRP. Limitations inherent to the use of direct survey-based measures of SRP also include susceptibility to self-presentational biases and the likelihood that not all valenced self-representations are fully accessible to conscious reflection (e.g. Gawronski, 2009). To circumvent these concerns, priming methodologies are increasingly used in experimental social psychology as indirect measures of associations between valence and self-representation (reviews by Buhrmester et al., 2011; Zeigler-Hill and Jordan, 2010). However, no previous studies have utilized these methods in order to assess the neural processes mediating valenced SRP (for a study examining implicit SRP of stimuli that were not overtly valenced, however, see Moran et al., 2009).
The present study addressed the above limits of past literature by directly comparing the neural correlates of valenced SRP with valenced ‘other-referential processing’ (ORP) using a priming methodology. Specifically, to obviate the effect of the self-positivity bias, we previously designed a ‘Visual–Verbal Self-Other Referential Processing Task’ (VV-SORP-T; see Figure 1) that directly engenders valenced SRP and ORP (Frewen and Lundberg, 2012). The VV-SORP-T requires participants to covertly rehearse the words ‘I am’ or ‘He/She is’ when presented with either their own or another person’s picture and then read positive or negative words, thereby experimentally engendering an association between the self/other and positivity/negativity on different trials (e.g. ‘I am’ … ‘disliked’). The encoded representation (e.g. ‘I am’ … ‘disliked’) may or may not match individuals’ internal self-representations (e.g. ‘I am’ … ‘liked’) as determined by individual differences in trait self-esteem. Participants monitor and report their affective response to the task, the outcome of which we further interpret as partly reflecting the match between the task-induced encoded representation and internal representations. Specifically, matching negative self-representations (e.g. ‘I am’ … ‘disliked’) are more likely to engender negative affect and matching positive self-representations (e.g. ‘I am’ … ‘liked’) are more likely to engender positive affect. The impact of the task on cognitive processing is also measured indirectly via response time (RT) providing a conjoint passive button-pressing requirement. We previously demonstrated in young adults that the VV-SORP-T is sensitive to individual differences in valenced SRP such that individuals with lower explicit self-esteem (as indexed by the Rosenberg Self-Esteem Scale; Rosenberg, 1965) are more likely to experience negative affect during negative SRP, less likely to experience positive affect during positive SRP, and evidence slower RT particularly during negative SRP (Frewen and Lundberg, 2012).
Fig. 1.
Illustration of one block of the VV-SORP-T. The individual shown in the photograph is the second author. Participants posed for their own photographs in neutral expression as for a passport application. Photographs of strangers (‘other’-condition) were taken from the NimStim set (Tottenham et al., 2009) and matched to the participant as closely as possible for the following attributes: ethnicity, hair colour and hair length. Participants viewed the photographs and silently rehearsed ‘I am’ (for the self) or ‘She is’ (for the other), and then silently read the words, thus associating the self/other with positivity/negativity on different trials.
In the present fMRI study we investigated the neural correlates of cognitive and affective processes relating to individual differences in self-esteem by examining variability in the BOLD response to the VV-SORP-T in 20 women. Consistent with previous research, we expected relatively few differences between valenced SRP and valenced ORP at the group level (Yoshimura et al., 2009). However, we hypothesized that individual differences in valenced adjective endorsement, and affective responses to the VV-SORP-T, would predict between-person variation in the BOLD response within regions of interest including within the medial prefrontal cortex, cingulate gyrus, temporoparietal cortex and amygdala.
METHODS
Participants
Twenty women varying from young to middle adulthood (18–52 years, M age = 27.80, s.d. = 8.33) recruited by advertisement from the general community took part in this study. Participants’ ethnic status was distributed as follows: European–Caucasian (n = 12, 60%), East Indian (n = 3, 15%), Asian (n = 2, 10%), African (n = 2, 10%) and Middle Eastern (n = 1, 5%). As a group, participants reported normative levels of trait self-esteem [Rosenberg Self-Esteem Scale (Rosenberg, 1965): M = 22.61, s.d. = 6.01, Range 13–30] and self-critical thinking [Cognitive Distortion Scale–Self-Criticism subscale (Briere, 2000); M = 14.22, s.d. = 6.25, Range 8–33]. Current or past psychiatric history, head injury with loss of consciousness and left-handedness were study exclusion criteria as assessed by structured clinical interviews.
VV-SORP-T
The VV-SORP-T involved three components: (i) completion of a paper-and-pencil survey asking about the descriptiveness of negative and positive traits for the self vs others (completed outside scanning), (ii) completion of an experimental task while undergoing fMRI and (iii) a post-task rating questionnaire asking about affective responses (completed outside scanning). The instructions given for the VV-SORP-T are reported verbatim in supplementary data to our prior report (Frewen and Lundberg, 2012).
Approximately 2 weeks prior to scanning, within a battery of related questionnaires, participants rated for each of 10 positive and 10 negative words ‘how much each word describes (i) how you think about yourself, and describes (ii) how you think about other people, in general’ on 11-point (0–10) scales anchored by ‘Not at all’ (0), ‘Moderately’ (5) and ‘Completely’ (10). The adjective list was the same as that used in Frewen and Lundberg (2012), originally based on that used in Frewen et al. (2011), and covered social (e.g. loved, rejected) and achievement-related (e.g. successful, incompetent) themes. We conceptualize such scores as indicative of trait self-esteem and consistent with that assumption adjective endorsement scores correlated r = 0.73 with Rosenberg Self-Esteem Scale scores in our prior study (Frewen and Lundberg, 2012). We did not purposely match the negative and positive word sets we used for frequency of general use as this would have violated natural usage within the English language (Loumann et al., 2012), nor did we seek to equate the word sets for salience as this would also be contrary to norms (e.g. Baumeister et al., 2001). Nevertheless, post hoc comparisons revealed the word sets to be statistically comparable in terms of length in letters, frequency of use within the English language relative Hyperspace Analogue of Language (HAL) norms (Lund and Burgess, 1996), as well as normed mean reaction time in lexical decision and naming, all as investigated and compiled within the English Lexicon Project (Balota et al., 2007; number of letters, P = 0.24; normed frequency of use, P = 0.42; log-frequency of use, P = 0.69; RT in lexical decision, P = 0.23; RT in naming, P = 0.27). Furthermore, no differences were observed in arousal ratings relative to the Affective Norms for English Words (Bradley and Lang, 1999) for the subset of the words we used that are contained therein (n = 12 of 20; P = 0.27).
Figure 1 illustrates how the experimental component of the VV-SORP-T was conducted. Participants’ photographs were taken in neutral expression (instructions were to pose ‘as if for a passport photograph’) using a standard-use electronic camera (4.1 megapixels) against an off-white office wall. Photographs were then standardized in order to match in essential respects those used in the development of the NimStim set of facial expressions (Tottenham et al., 2009). The latter were used as pictures for a comparison ‘other’ (i.e. a female was selected from the NimStim set for each study participant, matched as closely as possible for ethnicity, hair colour and hair length). Before beginning the VV-SORP-T participants were habituated to the photographs for 6–10 s (as desired) in order to reduce their novelty, with the ‘other’ instructed to be regarded as ‘a typical person they might meet in their day-to-day life but presently do not know personally’. This manipulation was intended to limit error associated with responding to specific persons as has been used in previous fMRI studies (e.g. individual differences in how one regards former American President George Bush; Kelley et al., 2002).
Instructions underscored that completing the VV-SORP-T would require participants ‘to do three things: 1) internally rehearse statements and read words, 2) press response buttons on a keypad, and 3) all the while pay close attention to how you are feeling throughout the different parts of the task’. Participants were instructed to view a fixation cross (presented for 12 s in between task-blocks) until they were presented with the word ‘SELF’ or ‘OTHER’ (for 3 s) signalling which of the respective pictures they were about to see. Upon seeing their own or the other person’s photograph (also presented for 3 s), they silently rehearsed to themselves ‘I am’ or ‘She is’, respectively, and then pressed a keypad button with either their index or middle finger (counter-balanced). Participants were then presented with a single positive or negative word for 3 s, asked to silently read the word and then pressed another keypad button with their other finger. Four additional pictures and words were then presented following the same ‘picture-then-word’ rotation, with the identical picture displayed in all cases, and the words being of common valence. Therefore the stimulus presentations were blocked in terms of the conditions Reference (Self vs Other, i.e. photographs) and Valence (words), creating four trial types: self-negative (S-N), self-positive (S-P), other-negative (O-N) and other-positive (O-P). Participants were not instructed that they ‘should try to press the buttons as fast as possible’ as is often done in social cognition experiments. In contrast, participants were instructed only to press the buttons ‘so that we can assess afterwards whether you are paying attention to and completing the task’. This passive orientation was intended to focus attention towards introspection and interoception with participants reminded repeatedly of the importance of ‘paying close attention to how you are feeling throughout the different parts of the task’.
While undergoing FMRI, participants were presented with eight-blocks in each of three 6-min runs in which the self and other photographs were presented in combination with two negative and two positive word lists. The order of the eight blocks within runs was fully randomized within and across participants. A full 6-min practice run was also completed outside of the scanner in an office setting ∼30 min before scanning in order to normalize participants to the task.
Immediately after completing the experimental task component and exiting the scanner, participants were asked open-ended and percentage rating-scale questions about their response to the four experimental conditions (S-N, S-P, O-N, O-P). The percentage rating-scale asked participants to rate from 0 (‘Not at all’) to 100% (‘Strongly’), with 50% indicating ‘Moderately’, ‘ … how much you felt certain specific feelings in response to each picture and word type combination’. Ratings were provided for the following five negative affective states: ‘Anger’, ‘Sad’, ‘Anxiety-Fear’, ‘Disgust’, ‘Bad About Self’, and for two positive affective states: ‘Happy’ and ‘Good about Self’. As previously noted we conceptualize affective responses to the task as providing an additional measure of relevance to individual differences in self-esteem-related processes. Specifically, our prior study observed that individuals with lower trait self-esteem reported experiencing greater negative affect during S-N trials, and lesser positive affect during S-P trials (Frewen and Lundberg, 2012). Results for quantitative ratings collected from the present sample are presented herein and open-ended comments are included as Supplementary Table S1.
Procedure
All procedures were approved by the health sciences research ethics board of Western University in London, Ontario, Canada. As noted previously, participants were assessed for study inclusion criteria and completed a short questionnaire battery including the adjective rating component of the VV-SORP-T ∼2 weeks prior to scanning. Participants completed a single-block practice version of the experimental component of the VV-SORP-T in an office setting ∼30 min prior to fMRI scanning, and three blocks of the experimental component of the VV-SORP-T while undergoing fMRI. Participants then rated their affective response to the VV-SORP-T immediately post-scan. The entire experiment took ∼75 min to complete.
Imaging took place at the Robarts Research Institute in London, Ontario, Canada. All imaging data were collected using a 3.0 Tesla whole-body MRI scanner (Magnetom Tim Trio, Siemens Medical Solutions, Erlangen, Germany) with the manufacturer’s 32-channel phased array head coil. Orthogonal scout images were collected and used to prescribe a tri-dimensional T1-weighted anatomical image of the whole head with 1 mm isotropic resolution (MP-RAGE, TR/TE/TI = 2300/2.98/900 ms, flip angle = 9°, FOV (x, y, z) = 256 × 240 × 192 mm, acc. factor = 4, total acq. time = 3 min 12 s). The anatomical volume was used to determine the angle of the transverse plane passing through both the anterior and posterior commeasures mid-sagittaly and as the source image for inter-individual spatial normalization. A set of 64 contiguous, 2 mm thick imaging planes for BOLD fMRI were prescribed parallel to the AC–PC plane and positioned to ensure coverage of the top of the brain. BOLD fMRI images were acquired with the manufacturer’s standard gradient echo EPI pulse sequence (single-shot blipped EPI) using an interleaved slice acquisition order and tri-dimensional prospective acquisition correction (3D-PACE). EPI volumes were acquired with 2 mm isotropic resolution and the following parameters: FOV = 192 × 192 mm, 94 × 94 matrix, TR/TE = 3000/20 ms, flip angle = 90°, 64 slices, 178 measurements. Before completing the VV-SORP-T while undergoing fMRI a ‘resting-state’ functional scan of each participant’s brain was also acquired, to be described elsewhere.
Data preparation and statistical analysis
Across blocks and runs for each of the four experimental conditions (S-N, S-P, O-N, O-P), VV-SORP-T survey scores were summed, and button-press RT and affect ratings were averaged. The effect of experimental condition on each of these variables was examined by ANOVA with results reported in Table 1.
Table 1.
Descriptive statistics and paired comparisons between conditions of the VV-SORP-T
| S-N | S-P | O-N | O-P | S-N vs S-P |
S-N vs O-N |
S-P vs O-P |
O-N vs O-P |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M (s.d.) | M (s.d.) | M (s.d.) | M (s.d.) | t | d′ | t | d′ | t | d′ | t | d′ | |
| Survey | 3.95 (6.50) | 86.79 (10.05) | 8.05 (10.37) | 72.79 (14.88) | 28.99 | 6.48 | 2.45 | 0.55 | 4.64 | 1.04 | 13.96 | 3.12 |
| NA | 15.53 (19.30) | 2.84 (9.32) | 15.05 (15.53) | 2.32 (5.97) | 3.77 | 0.86 | 0.11 | 0.02 | 0.40 | 0.09 | 4.05 | 0.93 |
| PA | 14.34 (17.75) | 75.26 (24.12) | 6.97 (13.71) | 33.55 (31.31) | 10.64 | 2.44 | 2.34 | 0.54 | 5.26 | 1.21 | 4.37 | 1.00 |
| RT | 758 (291) | 774 (302) | 732 (283) | 759 (303) | 0.62 | 0.14 | 1.55 | 0.35 | 0.67 | 0.15 | 1.56 | 0.35 |
n = 19 (one subject was missing affect rating data). Degrees of freedom are thus for multivariate ANOVA F(4,15), univariate ANOVA F(1,18) and for post hoc paired t-tests t(18). For paired comparisons between conditions, the effect size d′ is noted. Multivariate tests were statistically significant for Reference, F(4,15) = 11.56, P < 0.001, η2 < 0.76, Valence, F(4,15) = 139.05, P < 0.001, η2 = 0.97 and Reference-x-Valence, F(4,15) = 8.14, P = 0.001, η2 = 0.69. For survey endorsement, the results of univariate ANOVA were: Reference, F(1,18) = 11.79, P = 0.003, η2 = 0.40, Valence, F(1,18) = 458.64, P < 0.001, η2 = 0.96 and Reference-x-Valence, F(1,18) = 20.47, P < 0.001 η2 = 0.53. For negative affect (NA) ratings, the results of univariate ANOVA were: Reference, F(1,18) = 0.04, P = 0.85, η2 < 0.01, Valence, F(1,18) = 25.11, P < 0.001, η2 = 0.58 and Reference-x-Valence, F(1,18) = 0.00, P = 0.99, η2 < 0.01. For positive affect (PA) ratings, the results of univariate ANOVA were: Reference, F(1,18) = 27.31, P < 0.001, η2 = 0.60, Valence, F(1,18) = 92.89, P < 0.001, η2 = 0.84 and Reference-x-Valence, F(1,18) = 20.61, P < 0.001, η2 = 0.53. Finally, for RT (ms), the results of univariate ANOVA were: Reference, F(1,18) = 3.04, P = 0.10, η2 = 0.14, Valence, F(1,18) = 1.73, P = 0.21, η2 = 0.09 and Reference-x-Valence, F(1,18) = 0.18, P = 0.68, η2 = 0.01.
Analyses of the BOLD signal were conducted via SPM8 (Welcome Department of Imaging Neuroscience, University College, London, UK). Standard preprocessing was conducted within SPM8, with volumes realigned to the first functional image acquired (unidirectional movements were <4 mm from origin in all cases), normalized to a common EPI template [rendering 2 mm3 voxels in accordance with the coordinate system of the Montreal Neurological Institute (MNI)], and data smoothed across 8 mm (FWHM). A canonical haemodynamic response function was modelled as a response to each stimulus in individual participants (first-level), with group-averaged results evaluated as random effects (second-level). The BOLD response observed during each of the four task trials relative to between-block fixation was examined via the general linear model. Planned contrasts also compared response occurring during S-N relative to S-P trials, S-N relative to O-N trials, and S-P relative to O-P trials, thus examining the effects of Valence within Reference, and Reference within Valence. Group-averaged results for these contrasts are reported in Table 2 and Figure 2. We also report the results of main effect contrasts for Reference and Valence in Supplementary Table S2 and Supplementary Figures F1 and F2.
Table 2.
Group-level differences between VV-SORP-T trial types
| Conditions | Regions | kP<0.005 | kSVC | Z | x | y | z |
|---|---|---|---|---|---|---|---|
| S-N > Fixation | Posterior mid-cingulate | 204 | 66a | 4.81 | 4 | −28 | 36 |
| Right superior parietal cortex | 210 | – | 4.21 | 50 | −52 | 40 | |
| DMPFC | 121 | 32b | 3.77 | 2 | 22 | 42 | |
| S-P > Fixation | MPFC | 97 | – | 3.56 | −2 | 64 | −6 |
| Left middle frontal gyrus | 151 | – | 3.54 | −22 | 58 | 16 | |
| O-P > Fixation | Right DLPFC | 85 | – | 3.84 | 56 | 16 | 10 |
| Right temporal pole | 188 | – | 3.47 | 52 | 18 | −28 | |
| O-N > Fixation | Right posterior insula | 168 | – | 3.87 | 38 | −22 | 8 |
| Left posterior insula | 83 | – | 3.14 | −44 | −16 | −2 | |
| Right middle frontal gyrus | 87 | 28c | 3.67 | 56 | 34 | 10 | |
| Left middle frontal gyrus | 283 | – | 3.67 | −44 | 52 | 16 | |
| Left middle frontal gyrus | 267 | 132d | 3.38 | −42 | 14 | 22 | |
| Left precentral gyrus | 503 | – | 3.64 | −50 | −18 | 36 | |
| Left precentral gyrus | 110 | – | 3.36 | −28 | −20 | 50 | |
| Left posterior mid-cingulate | 70 | – | 3.35 | −14 | −28 | 42 | |
| Left cuneus | 204 | – | 3.20 | −24 | −74 | 30 | |
| Left cuneus | 124 | – | 3.00 | −10 | −72 | 10 | |
| S-N > S-P | Posterior mid-cingulate | 67 | – | 3.76 | 4 | −28 | 36 |
| Right superior parietal cortex | 88 | – | 3.51 | 46 | −52 | 44 | |
| S-P > S-N | No significant results | – | – | – | – | – | – |
| S-N > O-N | No significant results | – | – | – | – | – | – |
| S-P > O-P | No significant results | – | – | – | – | – | – |
All ROIs were prescribed from Moran et al. (2006). aAt ROI −3, 19, 38, PSVC < 0.01.
bAt ROI −3, 19, 38, PSVC < 0.01.
cAt ROI 50, 24, 10, PSVC = 0.02.
dAt ROI −56, 15, 10, PSVC < 0.02.
Fig. 2.
BOLD response during the four conditions of the VV-SORP-T vs baseline fixation. Response during S-N trials is shown in red, during S-P trials in green, during O-N trials in magenta, during O-P trials in yellow. Voxel-wise P < 0.005 with a cluster threshold k ≥ 67 voxels.
Of primary interest to this study, however, was a multiple regression analysis associating individual differences in survey and affective response scores with between-participant variability in the BOLD contrast between S-N trials and both fixation and O-N trials, and S-P trials and both fixation and O-P trials. Note that we preferred to evaluate BOLD correlations with adjective endorsement rather than Rosenberg Self-Esteem scores in this study, which allowed direct examination of associations between response to a common stimulus set (words) evaluated in differing contexts (i.e. paper-and-pencil survey rating of self and other descriptiveness vs performance of the experimental component of the VV-SORP-T). Results concerning individual differences are reported in Tables 3 and 4, and Figures 3 and 4.
Table 3.
Individual differences in response to S-N trials of VV-SORP-T
| Conditions | Predictors | Direction of correlation | Regions | kP<0.005 | kSVC | Z | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| S-N > Fixation | S-N Survey | + | L-MPFC | 168 | 7a | 4.10 | −12 | 48 | −6 |
| + | Cerebellum | 79 | – | 3.51 | 22 | −54 | 2 | ||
| + | VACC/VMPFC | 78 | 26b | 3.40 | −2 | 28 | −12 | ||
| + | L-IFG | 67 | – | 3.14 | −42 | 26 | −10 | ||
| S-N NA | + | No significant results | – | – | – | – | – | – | |
| S-N > O-N | S-N Survey | + | Supplementary motor area | 108 | 61c | 4.37 | −2 | 10 | 54 |
| + | R caudate | 82 | – | 3.91 | 18 | −2 | 0 | ||
| + | L superior temporal gyrus | 68 | – | 3.58 | −52 | −20 | 6 | ||
| + | PCC (retrosplenial cortex) | 81 | – | 3.43 | 10 | −36 | 2 | ||
| + | L superior temporal gyrus | 113 | – | 3.39 | 58 | −42 | 0 | ||
| + | R superior frontal gyrus | 67 | – | 3.37 | 44 | 40 | 28 | ||
|
+ | L-pACC (wm) | 93 | – | 4.08 | −16 | 32 | −6 | |
| + | R amygdala–parahippocampal gyrus | 81 | – | 3.93 | 20 | 4 | −22 | ||
| + | L superior temporal gyrus | 309 | – | 3.85 | −62 | 0 | 8 | ||
| + | L occipital cortex | 70 | – | 3.78 | −24 | −48 | 2 | ||
| + | L post-central gyrus (wm) | 67 | – | 3.68 | −22 | −24 | 34 | ||
| + | R fusiform gyrus | 87 | – | 3.60 | 18 | −72 | −10 |
(wm), white matter.
aAt ROI −6, 42, −12, Lemogne et al., 2011.
bAt ROI 0, 22, −9, Moran et al., 2006.
cAt ROI −3, 14, 49, Moran et al., 2006.
Table 4.
Individual differences in response to S-P trials of the VV-SORP-T
| Conditions | Predictors | Direction of correlation | Regions | kP<0.005 | kSVC | Z | x | y | z |
|---|---|---|---|---|---|---|---|---|---|
| S-P > Fixation | S-P Survey | + | R precuneus | 85 | – | 3.92 | 18 | −42 | 60 |
| + | L middle frontal gyrus (wm) | 91 | – | 3.60 | −22 | 40 | 18 | ||
| + | L post-central gyrus | 143 | – | 3.44 | −34 | −32 | 58 | ||
| + | L superior temporal gyrus | 107 | – | 3.26 | −48 | −14 | 34 | ||
| − | R amygdala–parahippocampal gyrus | 148 | – | 3.67 | 36 | 0 | −20 | ||
| − | R temporal pole | 119 | – | 3.53 | 58 | −6 | −18 | ||
|
+ | R temporal pole | 102 | – | 4.51 | 62 | −6 | −16 | |
| + | DMPFC | 237 | 87a | 3.84 | 6 | 50 | 20 | ||
| + | L-TPJ (wm) | 104 | – | 3.75 | −36 | −60 | 14 | ||
| + | L-TPJ | 102 | 48b | 3.69 | −50 | −54 | 18 | ||
| + | L posterior insula (wm) | 104 | – | 3.65 | −24 | −22 | 12 | ||
| + | L post-central gyrus | 117 | – | 3.65 | 14 | −24 | 66 | ||
| + | L-superior frontal gyrus | 163 | – | 3.51 | 14 | 40 | 52 | ||
| − | No significant results | – | – | – | – | – | – | ||
| S-P > O-P | S-P Survey | + | No significant results | – | – | – | – | – | – |
| − | R-DMPFC | 88 | 18c | 4.38 | 14 | 30 | 46 | ||
| − | L-TPJ | 74 | 22d | 4.28 | −54 | −56 | 32 | ||
| − | VMPFC | 456 | 48e | 4.26 | −4 | 32 | −26 | ||
| − | R-IFG | 118 | – | 3.97 | 38 | 58 | −2 | ||
| − | L-IFG | 145 | – | 3.81 | −36 | 50 | −4 | ||
| − | R-temporal pole | 124 | – | 3.37 | 40 | 0 | −20 | ||
| − | L-IFG | 91 | – | 3.36 | −20 | 40 | −14 | ||
|
+ | VMPFC | 73 | 20f | 4.28 | −2 | 30 | −24 | |
| + | DMPFC | 229 | 35g | 4.10 | 12 | 32 | 46 | ||
| + | L-TPJ | 108 | 31h | 4.08 | −52 | −60 | 16 | ||
| + | L-IFG | 169 | – | 4.03 | −20 | 24 | −16 | ||
| + | R-caudate | 159 | – | 3.75 | 10 | 14 | 4 | ||
| + | Precuneus | 186 | – | 3.70 | −2 | −60 | 38 | ||
| + | L-cerebellum | 75 | – | 3.34 | −16 | −62 | −34 | ||
| + | VMPFC | 95 | – | 2.93 | −8 | 52 | −20 |
(wm), white matter.
aAt ROI 2, 55, 17, Yoshimura et al., 2009.
bAt ROI −52, −56, 22, van Overwalle, 2009.
cAt ROI 6, 27, 42, Lemogne et al., 2011.
dTwo clusters within ROI −52, −56, 22, van Overwalle, 2009.
eAt ROI −3, 36, −18, Phan et al., 2004.
fAt ROI −3, 36, −18, Phan et al., 2004.
gAt ROI 6, 27, 42, Lemogne et al., 2011.
hAt ROI −52, −56, 22, van Overwalle, 2009.
Fig. 3.
BOLD response during S-N trials vs baseline fixation (BL) and O-N trials. Within the legend, positive correlations are denoted with a plus symbol; there were no significant negative correlations. Positive correlation between survey endorsement of negative traits and response during S-N trials (>BL) is shown in red. Positive correlation between survey endorsement of negative traits and response during S-N trials (>O-N trials) is shown in magenta. Positive correlation between experienced negative affect and response during S-N trials (>O-N trials) is shown in blue. Voxel-wise P < 0.005 with a cluster threshold k ≥ 67 voxels.
Fig. 4.
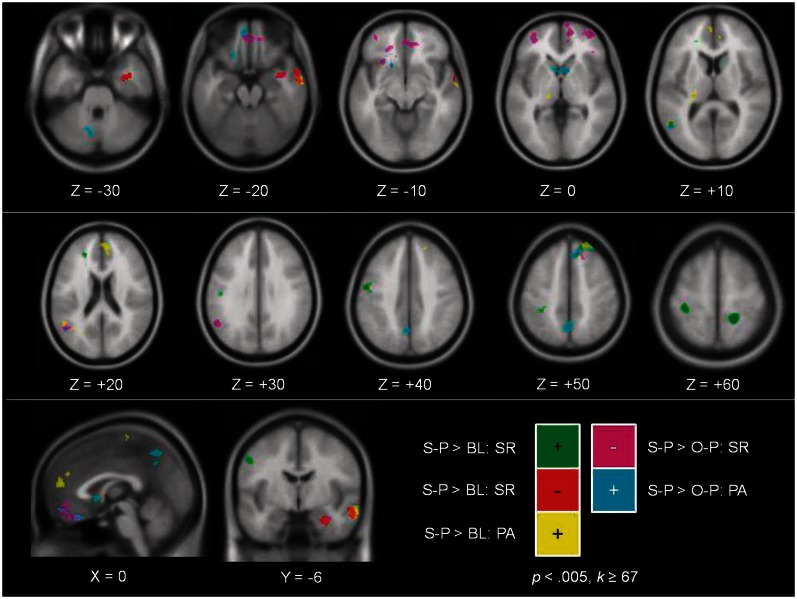
BOLD response during S-P trials vs baseline fixation (BL) and O-P trials. Within the legend, positive correlations are denoted with a plus symbol, and negative correlations are denoted with a minus symbol. Regarding survey endorsement of positive traits and response during S-P trials (>BL), positive correlations are shown in green and negative correlations are shown in red. Positive correlation between experienced positive affect and response during S-P trials (>BL) is shown in yellow. Negative correlation between survey endorsement of positive traits and response during S-P trials (>O-P trials) is shown in magenta. Positive correlation between experienced positive affect and response during S-P trials (>O-P trials) is shown in cyan. Voxel-wise P < 0.005 with a cluster threshold k ≥ 67 voxels.
We report within Tables clusters of size k ≥ 67 voxels (approximating the volume of the smoothing kernel) with uncorrected voxel-wise P < 0.005 as a criterion selected so as to balance risk against type-I and type-II errors (Lieberman and Cunningham, 2009). To examine the location of BOLD responses we observed in relation to previous studies, we also report the number of voxels that fell within an 8-mm radius (equally the smoothing kernel) of coordinates reported in recent meta-analyses of SRP (van der Meer et al., 2010; Qin and Northoff, 2011; Qin et al., 2012a) and key study results (Phan et al., 2004; Moran et al., 2006; Lemogne et al., 2009, 2011; Yoshimura et al., 2009); an ROI for the left and right temporoparietal junction (TPJ) was also prescribed from van Overwalle’s (2009) meta-analysis of neuroimaging studies of social cognition (as calculated in Frewen et al., 2010). Voxels in ROI analyses include those exhibiting P < 0.05 after correction for multiple comparisons (family-wise error rate) within the indicated centred spherical search volume, denoted kSVC for Small-Volume Corrected. Cluster loci are labelled by the voxel exhibiting maximal effect size within MNI space.
RESULTS
Self-report, experiential and behavioural response
Table 1 reports the descriptive and inferential statistics describing self-report and behavioural response to the VV-SORP-T. Replicating previous results (Frewen and Lundberg, 2012), survey endorsements were higher for S-P as compared with O-P (d′ = 1.04), consistent with the self-positivity bias. Positive affect was also higher during S-P than O-P trials (d′ = 1.21), although negative affect was not significantly higher during S-N than O-N trials (d′ = 0.02). Finally, RT was marginally slower during S-N than O-N trials (d′ = 0.35), and during S-P than O-P trials (d′ = 0.15).
Further replicating previous results (Frewen and Lundberg, 2012), participants who described themselves more positively (S-P survey endorsement) experienced less negative affect during S-N trials (r = −0.77, P < 0.001), less negative affect during S-P trials (r = −0.57, P = 0.006) and greater positive affect during S-P trials (r = 0.52, P = 0.011). In comparison, associations between S-N survey endorsement and affective responses were non-significant.
fMRI-BOLD response
Group-level differences between trial types
Figure 2 and Table 2 report significant responses observed as specific to each of the four distinct trial types at the group level in comparison with between-block fixation (in Figure 2, S-N = red, S-P = green, O-N = magenta, O-P = yellow). S-N trials activated three clusters: the posterior mid-cingulate (at ROI 0, −13, 31, Moran et al., 2006; kSVC = 66), right superior parietal cortex and dorsal ACC-MPFC (at ROI −3, 19, 38, Moran et al., 2006; kSVC = 32). S-P trials activated two clusters: ventral MPFC and left middle frontal cortex. O-P trials activated two clusters: right DLPFC and right temporal pole. Finally, response during O-N trials was more distributed, with the maximal effect size observed within the right posterior insula, and additional activations observed within the left posterior insula, right middle frontal gyrus (at ROI 50, 24, 10, Moran et al., 2006; kSVC = 28), left middle frontal gyrus (at ROI −56, 15, 10, Moran et al., 2006; kSVC = 132), left precentral gyrus, left posterior mid-cingulate and left cuneus.
Planned contrasts examining the effects of Valence within SRP and Reference within Valence are also reported in Table 2. S-N trials were associated with greater response than S-P trials within two regions: the posterior mid-cingulate and right superior parietal cortex. In contrast, S-P trials were not associated with greater response in comparison with S-N trials in any brain region, and contrasts of Reference within Valence were non-significant.
Individual differences in response to S-N trials
Table 3 and Figure 3 report correlations between self-report and affective responses, on the one hand, and response during S-N trials, relative to both between-block fixation and O-N trials, on the other. Concerning the contrast S-N > fixation, a positive correlation was observed between how negatively participants regarded themselves and response within the ventral MPFC-ACC (including within ROI 0, 22, −9, Moran et al., 2006; kSVC = 26). In addition, women who rated themselves more negatively demonstrated increased response within left VMPFC (including within ROI −6, 42, −12, Lemogne et al., 2011; kSVC = 7). In comparison, there were no significant correlations with variability in negative affective response.
Concerning the contrast S-N > O-N, participants who regarded themselves more negatively demonstrated less response within the supplementary motor area (including within ROI −3, 14, 49, Moran et al., 2006; kSVC = 61) and retrosplenial cortex. In comparison, participants who experienced greater negative affect exhibited greater response within the parahippocampal gyrus and right amygdala.
Individual differences in response to S-P trials
Table 4 and Figure 4 report correlations across the whole-brain between self-report and affective responses, on the one hand, and BOLD response during S-P trials, relative to both between-block fixation and O-P trials, on the other. Concerning the contrast S-P > fixation, a negative correlation was observed between how positively participants regarded themselves and response within the right parahippocampal gyrus/amygdala and right temporal pole. A positive correlation was observed between positive affect experienced during S-P trials and response within the DMPFC (within ROI 2, 55, 17, Yoshimura et al., 2009; kSVC = 87), left TPJ (within ROI −52, −56, 22, van Overwalle, 2009; kSVC = 48) and right temporal pole.
Concerning the contrast S-P > O-P, a negative correlation was observed between how positively participants regarded themselves and response within VMPFC (at ROI −3, 36, −18, Phan et al., 2004; kSVC = 48), right DMPFC (at ROI 6, 27, 42, Lemogne et al., 2011; kSVC = 18), left TPJ (two clusters within ROI −52, −56, 22, van Overwalle, 2009; kSVC = 22 and 36), right temporal pole and bilateral inferior frontal gyri. In comparison, the more positive affect participants experienced during S-P trials, the greater was their response within many of the same regions, specifically VMPFC (at ROI −3, 36, −18, Phan et al., 2004; kSVC = 20), DMPFC (at ROI 6, 27, 42, Lemogne et al., 2011; kSVC = 35), left TPJ (within ROI −52, −56, 22, van Overwalle, 2009; kSVC = 31), left inferior frontal gyrus, as well as within the precuneus.
DISCUSSION
How people represent themselves in comparison with others, and the role played by affective processing in such representations, are matters of significant interest to a social cognitive and affective neuroscience of core personality constructs including trait self-esteem. We investigated the neural correlates of self-esteem-related processes in response to the VV-SORP-T using an individual differences design.
Although recent meta-analyses confirm greater response within MPFC, perigenual ACC and PCC during SRP than during ORP (van der Meer et al., 2010; Qin and Northoff, 2011; Qin et al., 2012b), individual studies using relatively neutral adjectives rarely observe this effect (e.g. Ochsner et al., 2005; cf Heatherton et al., 2006; Yaoi et al., 2009). Yoshimura et al. (2009), using valenced adjectives, similarly observed few differences between SRP and ORP. Within the present study, the spatial maps obtained relative to fixation differed between valenced SRP and ORP (Figure 2), while null effects were observed when conditions were directly compared, as conducted by Yoshimura et al. (2009). We speculate that the neural correlates of SRP and ORP will differ principally in so far as SRP is regarded as more affectively salient (e.g. Tacikowski et al., 2011). In other words, we expect that trait endorsement must not only differentiate the self from others, but this result must sufficiently matter to participants to evoke corresponding differences in the BOLD signal. The use of valenced adjectives as in Yoshimura et al.’s (2009) study and the present one cannot assure this because, as was clearly the case in the present study, most participants will endorse robustly positive views of both themselves and others, leading trials requiring negative SRP and ORP to be regarded as relatively neutral and irrelevant (Frewen and Lundberg, 2012).
In contrast to the modal self-positivity bias, however, a certain number of participants with lower self-esteem will endorse relatively negative views of themselves and a corresponding range of affective responses when such representations are primed such as via the VV-SORP-T. Consistent with expectations, in the present study these individual differences dovetailed considerably with between-subject variability in the BOLD response within ROIs including MPFC, PCC, left temporoparietal cortex and right amygdala. These potentials for heterogeneity across subjects in experiential response to a common stimulus pattern make them well suited to the study of the neural correlates of personality and individual differences (Varela, 1996; Lutz and Thompson, 2003). Further knowledge about the neural underpinnings of negative SRP may also enlighten our understanding of psychiatric disorders associated with maladaptive SRP including depression (Grimm et al., 2009; Johnson et al., 2009; Lemogne et al., 2009) and post-traumatic stress disorder (Frewen et al., 2011).
The present findings are consistent with and further inform current theorizing about the neural correlates of the ‘emotional self’ (Fossati et al., 2003). In particular, it has been posited that ventral MPFC (inclusive of ventral ACC; Moran et al., 2006; Yoshimura et al., 2009) may be particularly associated with SRP that is affectively (and perhaps uniquely negatively) salient, whereas dorsal MPFC may be particularly involved in conscious, reflective processes that are either neutral or positive in nature (van der Meer et al., 2010; Heatherton, 2011). Consistent with previous observations, relative to fixation, we observed ventral MPFC/ACC response during negative SRP particularly in women who regarded themselves more negatively (see Figure 2, x = 0, z = −10, red blobs), but dorsal MPFC response particularly in women who experienced greater positive affect during positive SRP (see Figure 3, x = 0, z = +10 and +20, yellow blobs). However, when contrasting response occurring during positive SRP with positive ORP, ventral MPFC regions were particularly involved such that, interestingly, response was increased as a function of increasing positive affect but decreasing self-regard (see Figure 2, x = 0, z = −20, cyan and magenta blobs, respectively). This dissociation suggests the merit of Phan et al.’s (2004) distinction between the significance of referential vs affective ratings and may inform interpretations regarding the ‘validity’ of direct (e.g. self-report survey) vs indirect (e.g. task-induced affective response) assessments of self-esteem-related processes (Buhrmester et al., 2011; Zeigler-Hill and Jordan, 2010). Our findings that decreasing self-regard predicted positive SRP response (Figure 2 magenta) agree with the hypothesis of VMPFC involvement in negative SRP. However, the dissociation with positive affective experience within a brain region strongly associated with reward requires interpretation. One interpretation is that VMPFC response is particularly increased in mediating positive affect in individuals for which positive affect is otherwise not easily activated, such as in individuals disposed towards alexithymia (Berthoz et al., 2002) and anhedonia (Keedwell et al., 2005; Harvey et al., 2007). However, that the same dissociation was observed concerning response within the left TPJ, which is widely implicated in social cognition and mentalizing (e.g. van Overwalle, 2009), suggests individual differences during ORP likely complicate interpretation. Consistent with this, self-reports obtained from the present participants as well as those collected from participants in a previous study suggest that response during ORP within the VV-SORP-T represents anything but simply a neutral comparator condition (Frewen and Lundberg, 2012). Investigation of individual differences in affective response during ORP as a predictor of the BOLD response could clarify this concern, but were considered beyond the scope of the present project given its focus on valenced SRP as it relates to self-esteem. It should also be noted that Lemogne and colleagues observed increasing response during SRP within dorsal MPFC in both depressed individuals (Lemogne et al., 2009) and individuals high in trait negative affect (Lemogne et al., 2011), which challenges a model emphasizing only the ventral MPFC in the affective aspects of SRP.
Besides response within higher cortical areas, Yoshimura and colleagues recently revealed a dissociation between left vs right amygdala response and negative vs positive SRP, respectively (Yoshimura et al., 2009). The right amygdala has also been associated with social emotional processing (Britton et al., 2006; Frewen et al., 2010; Heatherton, 2011) as is inherent to the VV-SORP-T (see Frewen and Lundberg, 2012, for descriptions of socio-emotional responses during ORP including guilt, shame and envy). In our study, however, not only those women who experienced greater negative affect during negative SRP, but also those women who regarded themselves less positively before positive SRP, exhibited an increased right amygdala response. If right amygdala response is to be interpreted as signifying a negative self-appraisal within the context of SRP (Yoshimura et al., 2009), our individual difference effects extend the significance of right amygdala response to positively valenced SRP. However, our results may qualify the finding in suggesting that the amygdala response may signify the outcome of the appraisal, that is, the experienced negativity of a stimulus or task, rather than the negativity inherent to the stimulus or task, per se. In other words, even objectively positive stimuli may be responded to as if they are negative (e.g. Lemogne et al., 2009, 2011; Frewen et al., 2012a, b), with the right amygdala response during SRP perhaps revealing the valence or salience of the result of that appraisal.
The loci of activations observed within the present study overlapped most closely with those observed by Moran et al. (2006), Phan et al. (2004) and Lemogne et al. (2011), the only other studies, to our knowledge, directly addressing between-subject variability in SRP using a correlational design. MPFC response within the present study was more inferior to loci summarized by recent meta-analyses (wherein z-values are typically > 5; van der Meer et al., 2010; Qin and Northoff, 2011; Qin et al., 2012a), but consistent with that observed with the methodology of Phan et al. (2004; also used by Lemogne et al., 2011). Provided current models emphasize VMPFC in the affective aspects of SRP (van der Meer et al., 2010; Heatherton, 2011), this confluence of findings for the VV-SORP-T and Phan et al. methodology are interesting provided that both methods likely encourage affective processing more greatly than do most other standard judgments tasks (the Phan et al. method through the use of arousing pictures and the VV-SORP-T by engendering an association between self and valence and encouraging attention to that association). Overlapping responses observed herein with those observed by Moran et al. occurred in regions that differentiated reaction time in Moran et al.’s study, implicating these regions in online SRP. Future neuroimaging studies might compare different SRP tasks to provide a more nuanced assessment of the specific subprocesses involved in SRP.
Limitations of the present study should be addressed in future work. We recruited only female participants for the present study due to widely known gender differences in trait self-esteem and associated risk for depression (Hyde et al., 2008); future studies may wish to directly investigate the neural basis for these gender differences. Clarity of interpretation could have been enhanced had we also administered neutral words and assessed affective response to the task not only subjectively but also via peripheral physiological measures of arousal. It should further be noted that contrasts of both SRP and ORP with passive fixation may be underpowered due to similarity between the neural processes involved in SRP, ORP and the passive resting state (Qin and Northoff, 2011); inclusion of active control tasks other than passive fixation would be useful in future studies, which might utilize a rest-stimulus interaction paradigm to examine valenced SRP and ORP (Northoff et al., 2010). Finally, the external ‘real-world’ validity of the VV-SORP-T for predicting socio-emotional behaviour remains to be established.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This research was supported by a New Investigator Fellowship to P.A.F. from the Ontario Mental Health Foundation.
REFERENCES
- Balota DA, Yap MJ, Cortese MJ, et al. The English Lexicon Project. Behavior Research Methods. 2007;39:445–59. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5:323–70. [Google Scholar]
- Baumeister RF, Campbell JD, Krueger JI, Vohs KD. Does high self-esteem cause better performance, interpersonal success, happiness, or healthier lifestyles? Psychological Science in the Public Interest. 2003;4(1):1–44. doi: 10.1111/1529-1006.01431. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Artiges E, Van de Moortele P-F, et al. Effect of impaired recognition and expression of emotions on frontocingulate cortices: an fMRI study of men with alexithymia. American Journal of Psychiatry. 2002;159:961–7. doi: 10.1176/appi.ajp.159.6.961. [DOI] [PubMed] [Google Scholar]
- Buhrmester MD, Blanton H, Swann WB. Implicit self-esteem: nature, measurement, and a new way forward. Journal of Personality and Social Psychology. 2011;100(2):365–85. doi: 10.1037/a0021341. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective norms for English words (ANEW): Instruction Manual and affective ratings. 1999. Technical Report C-1, The Center for Research in Psychophysiology, University of Florida. [Google Scholar]
- Briere J. Cognitive Distortion Scales: Professional Manual. Odessa, FL, US: Psychological Assessment Resources; 2000. [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Welsh RC, Berridge KC, Liberzon I. Neural correlates of social and nonsocial emotions: an fMRI study. NeuroImage. 2006;31:397–409. doi: 10.1016/j.neuroimage.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, et al. In search of the emotional self: an fMRI study using positive and negative emotional words. The American Journal of Psychiatry. 2003;160:1938–45. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dean J, Lanius RA. Assessment of anhedonia in psychological trauma: development of the Hedonic Deficit and Interference Scale. European Journal of Psychotraumatology. 2012a;3:8585. doi: 10.3402/ejpt.v3i0.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Lanius RA. Assessment of anhedonia in psychological trauma: psychometric and neuroimaging perspectives. European Journal of Psychotraumatology. 2012b;3:8587. doi: 10.3402/ejpt.v3i0.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Neufeld RWJ, Densmore M, Stevens TK, Lanius R. Neuroimaging social emotional processing in women: fMRI study of script-driven imagery. Social Cognitive Affective Neuroscience. 2010;6:375–92. doi: 10.1093/scan/nsq047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Neufeld RWJ, Densmore M, Stevens T, Lanius RA. Self-referential processing in women with PTSD related to childhood abuse: affective and neural response. Psychological Trauma: Theory, Research, Practice, and Policy. 2011;3:317–28. [Google Scholar]
- Frewen PA, Lundberg E. Visual–Verbal Self/Other-Referential Processing Task: direct vs. indirect assessment, valence, and experiential correlates. Personality and Individual Difference. 2012;52(4):509–14. [Google Scholar]
- Gawronski B. Ten frequently asked questions about implicit measures and their frequently supposed, but not entirely correct answers. Canadian Psychology. 2009;50(3):141–50. [Google Scholar]
- Grimm S, Jutta E, Boesiger P, et al. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Human Brain Mapping. 2009;30:2617–27. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Molecular Psychiatry. 2007;12:767–75. doi: 10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- Heatherton TF. Neuroscience of self and self-regulation. Annual Review of Psychology. 2011;62:363–90. doi: 10.1146/annurev.psych.121208.131616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience. 2006;1(1):18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: integrating affective, biological and cognitive models to explain the emergence of the gender difference in depression. Psychological Review. 2008;115:291–313. doi: 10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Nolen-Hoeksema S, Mitchell KJ, Levin Y. Medial cortex activity, self-reflection and depression. Social Cognitive and Affective Neuroscience. 2009;4:313–27. doi: 10.1093/scan/nsp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry. 2005;58:843–53. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(5):785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Lemogne C, Gorwood P, Bergouingnan L, Pélissolo A, Lehéricy S, Fossato P. Negative affectivity, self-referential processing and the cortical midline structures. Social Cognitive and Affective Neurosceince. 2011;6:426–33. doi: 10.1093/scan/nsq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, le Bastard G, Mayberg H, et al. In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Social Cognitive and Affective Neuroscience. 2009;4(3):305–12. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4(4):423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loumann IM, Danforth CM, Harris KD, Bliss CA, Dodds PS. Positivity of the English language. PLoS One. 2012;7:e29484. doi: 10.1371/journal.pone.0029484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund K, Burgess C. Producing high-dimensional semantic spaces from lexical co-occurrence. Behavior Research Methods, Instruments and Computers. 1996;28:203–8. [Google Scholar]
- Lutz A, Thompson E. Neurophenomenology: integrating subjective experience and brain dynamics in the neuroscience of consciousness. Journal of Consciousness Studies. 2003;9–10:31–52. [Google Scholar]
- Mezulis AH, Abramson LY, Hyde JS, Hankin BL. Is there a universal positivity bias in attributions? A meta-analytic review of individual, developmental, and cultural differences in the self-serving attributional bias. Psychological Bulletin. 2004;130(5):711–47. doi: 10.1037/0033-2909.130.5.711. [DOI] [PubMed] [Google Scholar]
- Moran JM, Heatherton TF, Kelley WM. Modulation of cortical midline structure by implicit and explicit self-relevance evaluation. Social Neuroscience. 2009;4(3):197–211. doi: 10.1080/17470910802250519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18(9):1586–94. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain-a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Northoff G, Qin P, Nakao T. Rest-stimulus interaction in the brain: a review. Trends in Neuroscience. 2010;33:277–84. doi: 10.1016/j.tins.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. NeuroImage. 2005;28(4):797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. NeuroImage. 2004;21(2):768–80. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Qin P, Liu Y, Shi J, et al. Anterior cingulate activity and the self in disorders of consciousness. Human Brain Mapping. 2012a;31:1993–2002. doi: 10.1002/hbm.20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Liu Y, Shi J, et al. Dissociation between anterior and posterior cortical regions during self-specificity and familiarity: a combined fMRI-meta-analytic study. Human Brain Mapping. 2012b;33(1):154–64. doi: 10.1002/hbm.21201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? NeuroImage. 2011;57(3):1221–33. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- Tacikowski P, Brechmann A, Marchewka A, Jednoróg K, Dobrowolny M, Nowicka A. Is it about the self or the significance? An fMRI study of self-name recognition. Social Neuroscience. 2011;6(1):98–107. doi: 10.1080/17470919.2010.490665. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neuroscience and Biobehavioral Reviews. 2010;34(6):935–46. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2009;30(3):829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela FJ. Neurophenomenology: a methodological remedy for the hard problem. Journal of Consciousness Studies. 1996;3(4):330–50. [Google Scholar]
- Yaoi K, Osaka N, Osaka M. Is the self special in the dorsomedial prefrontal cortex? An fMRI study. Social Neuroscience. 2009;4(5):455–63. doi: 10.1080/17470910903027808. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Ueda K, Suzuki S, Onoda K, Okamoto Y, Yamawaki S. Self-referential processing of negative stimuli within the ventral cingulate gyrus and right amygdala. Brain and Cognition. 2009;69:218–25. doi: 10.1016/j.bandc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Zeigler-Hill V, Jordan CH. Two faces of self-esteem: implicit and explicit forms of self-esteem. In: Gawronski B, Payne K, editors. Handbook of Implicit Social Cognition. NewYork, NY: Guilford Press; 2010. pp. 392–407. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.