Abstract
Although it has been suggested that social deficits of autism spectrum disorders (ASDs) are related to reward circuitry dysfunction, very little is known about the neural reward mechanisms in ASD. In the current functional magnetic resonance imaging study, we investigated brain activations in response to both social and monetary reward in a group of children with ASD, relative to matched controls. Participants with ASD showed the expected hypoactivation in the mesocorticolimbic circuitry in response to both reward types. In particular, diminished activation in the nucleus accumbens was observed when money, but not when social reward, was at stake, whereas the amygdala and anterior cingulate cortex were hypoactivated within the ASD group in response to both rewards. These data indicate that the reward circuitry is compromised in ASD in social as well as in non-social, i.e. monetary conditions, which likely contributes to atypical motivated behaviour. Taken together, with incentives used in this study sample, there is evidence for a general reward dysfunction in ASD. However, more ecologically valid social reward paradigms are needed to fully understand, whether there is any domain specificity to the reward deficit that appears evident in ASD, which would be most consistent with the ASD social phenotype.
Keywords: autism spectrum disorders, reward, functional magnetic resonance imaging, limbic system, nucleus accumbens
INTRODUCTION
A salient behavioural feature of individuals with autism spectrum disorders (ASDs) is decreased social motivation, which presents as a lack of interest in attending to social stimuli, or in seeking and enjoying reciprocal social interactions (Levy et al., 2009; Chevallier et al., 2012). It has been hypothesized that the lack of social motivation might be attributed to dysfunction of the mesocorticolimbic reward circuitry comprising, amongst others, the amygdala, ventral striatum (including nucleus accumbens/NAcc), anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC) (Dawson et al., 2005; Schultz, 2005).
Despite the influence of the ‘social motivation deficit hypothesis’ in current theories of ASD, very few studies have been conducted to test it. The first functional magnetic resonance imaging (fMRI) study on reward functioning in autism examined brain activation during a monetarily rewarded, sustained attention task and found aberrant brain responses in the ACC among adults with ASD (Schmitz et al., 2008). Dichter et al. (2012a) applied an anticipation paradigm and also reported aberrant brain activation in the reward circuitry in adults with ASD, particularly decreased NAcc activation during the anticipation of money, but not during the anticipation of typical autism-specific objects of interest (e.g. trains). However, both studies exclusively tested adults and did not include social incentives. Thus, it remained unclear to what extent neural reward dysfunction may also be observed for social rewards in individuals with ASD, particularly affected children. Scott-Van Zeeland et al. (2010a) were the first to compare neural activation in response to both monetary and social reward in an implicit learning task and found diminished NAcc activation in response to both reward types in autistic children, with the most pronounced reduction in responses observed for social rewards. Taken together, these results indicate that developmental abnormalities in reward circuitry, including frontostriatal and limbic areas, particularly the NAcc and ACC, may form the neurobiological basis of atypical motivational functioning in ASD (Neuhaus et al., 2010).
It must be noted that all three studies investigated neural reward processing in the context of different experimental tasks, each tapping into another cognitive domain. Because the subjective value of a reward (and respective brain responsivity) varies inversely with the effort required to obtain it (Botvinick et al., 2009), the findings of aberrant reward system activity should be considered in the context of the cognitive demands associated with the performance of each type of task.
A paradigm that is widely used to assess reward anticipation (initiated by cue signals) followed by goal-directed behaviour (e.g. button press or inhibitory response) and a potentially rewarding outcome is the cued incentive go/no-go task (Schultz et al., 1992). Recently, we employed such a task with social and monetary reward contingencies while measuring event-related brain potentials in children with ASD and healthy controls (Kohls et al., 2011). We found attenuated brain reactivity (i.e. compromised P3 effect) in patients in response to go cues associated with a timely reaction to obtain a reward, irrespective of reward type. This finding indicates an atypical motivation-related brain response to incentive cues, which may disrupt the capacity to modulate response initiation in the service of higher-ranking goals such as rewards (Rinehart et al., 2006).
Research has demonstrated that, in particular, a unique neural circuitry, comprising the NAcc, amygdala and ventral prefrontal cortex/ACC, promotes cue-evoked reward-seeking behaviour (Sesack and Grace, 2010). Thus, in the fMRI study, we aimed to investigate the blood-oxygenation-level-dependent (BOLD) effect of social and monetary reward in the context of the cued incentive go/no-go paradigm in children with ASD relative to matched typically developing controls (TDC). Specifically, we explored the differential effects of both incentive types on reward circuitry activation, primarily in the NAcc, ACC and amygdala. In contrast to previous studies (Scott-Van Zeeland et al., 2010a; Dichter et al., 2012a), we only included comorbid-free and medication-naive ASD participants, since it has been repeatedly shown that drugs such as psychostimulants up- or down-regulate mesocorticolimbic reward system activity and, thus, may substantially bias study results (e.g. Rubia et al., 2009).
Based on our previous findings (Kohls et al. 2011), we predicted abnormal neural activation patterns in participants with ASD (in particular, in NAcc, ACC and amygdala) to both social and monetary rewards when an active response was required to obtain a reward. The most prominent neural dysfunction was expected in the reward system under social reward conditions (Scott-Van Zeeland et al., 2010a). Additionally, we ran exploratory correlational analyses and tested whether the magnitudes of brain activation in response to social reward would be related to the severity of social dysfunction in ASD.
MATERIALS AND METHODS
Participants
Our initial sample consisted of 18 right-handed boys with ASD (diagnosed with Asperger syndrome) and 18 matched TDC. Subsequently, three participants with ASD were excluded because of excessive head movements during the fMRI scan (i.e. >3 mm of translational motion in the x, y and z direction throughout the course of the scan). One control participant was excluded because he scored over the cut-off on both the Social Communication Questionnaire (SCQ; Rutter et al., 2003) and the Social Responsiveness Scale (SRS; Constantino and Gruber, 2002). Included participants (ASD n = 15, TDC n = 17) ranged in age from 9 to 18 years and had a full-scale IQ ≥ 80 (WISC-III; Gleissner et al., 2003). The groups did not differ with respect to age or IQ (all P > 0.5; Table 1).
Table 1.
Demographic and clinical characteristics of the participant sample
| Measure | ASD (n = 15) | TDC (n = 17) | P values |
|---|---|---|---|
| M (s.d.) | M (s.d.) | ||
| Age (Years) | 14.6 (3.3) | 13.9 (3.0) | 0.58 |
| Handedness (Edinburgh) | 72.1 (23.9) | 74.3 (17.8) | 0.76 |
| IQ (WISC-III) | 109.8 (12.1) | 112.9 (12.6) | 0.49 |
| ADOS-G social | 8.3 (2.6) | NA | |
| ADOS-G communication | 4.1 (1.7) | NA | |
| ADOS-G stereotypy | 1.5 (1.0) | NA | |
| ADI-R social | 16.2 (4.4) | NA | |
| ADI-R communication | 16.8 (4.6) | NA | |
| ADI-R stereotypy | 5.5 (2.6) | NA | |
| SCQ (total) | 23.3 (6.6) | 3.6 (2.0) | <0.001 |
| SRS (total) | 108.9 (33.9) | 14.4 (11.1) | <0.001 |
Note: ASD = autism spectrum disorders, TDC = typically developing children, WISC-III = Wechsler Intelligence Scale for Children, ADOS-G = Autism Diagnostic Observation Schedule-Generic, ADI-R = Autism Diagnostic Interview-Revised, SCQ = Social Communication Questionnaire, SRS = Social Responsiveness Scale. P-values based on two-sample t-tests.
Individuals with ASD were recruited from the Departments of Child and Adolescent Psychiatry and Psychotherapy in Aachen and Marburg, Germany. All participants were diagnosed by experienced clinicians according to ICD-10 and DSM-IV criteria. Diagnoses were confirmed using the Autism Diagnostic Observation Schedule (ADOS-G; Lord et al., 2000) and the Autism Diagnostic Interview-Revised (ADI-R; Lord et al., 1994) conducted by a trained examiner (M.S.-R., I.K.-B.). Additionally, all parents were asked to complete the SCQ and the SRS. None of the included ASD participants had a history of comorbid psychiatric disorders or was taking psychotropic medication.
The TDC were recruited from local schools and underwent an extensive psychiatric examination using a standardized, semi-structured interview to assess current and past episodes of psychopathology according to DSM-IV criteria (K-SADS-PL; Kaufman et al., 1997). In addition, parents evaluated the behaviour of their children with regard to psychopathology using the Child Behaviour Checklist (CBCL 4–18; Achenbach, 1991). None of the TDC had a history of psychiatric or neurological disorders or was taking any medication. All participants had normal or corrected-to-normal vision.
Participants were compensated for their participation in this study. Informed consent was obtained from all participants and their parents. This study was approved by the Ethics Committee of the RWTH Aachen University Hospital.
The majority of participants (ASD n = 14, TDC n = 15) also participated in a parallel EEG study (Kohls et al., 2011). The order of the fMRI and EEG sessions was counterbalanced among participants and groups, with most participants attending both test sessions on 2 successive days.
fMRI task
We used an incentive go/no-go task in a blocked-design previously introduced by Kohls et al. (2011; Figure 1). Altogether, 18 go blocks and 18 no-go blocks were presented pseudorandomly (counterbalanced across participants), including three different incentive conditions: non-reward (NR), social reward (SR) and monetary reward (MR). Each reward condition comprised six go and six no-go blocks. Every block consisted of five trials, which were either go or no-go trails. In go blocks, all five trials were go trials. In no-go blocks, on average, 65% were go trials and 35% were no-go trials. Blocks started with an individual block cue (for 2950 ms) signalling the reward type that could be obtained in the ongoing block for correct performance. Each trial started with an instruction cue (for 250 ms), indicating a go trial (downward arrow) or a no-go trial (upward arrow). One second after the cue, the target stimulus (black square) was presented for 500 ms. The pre-target period, showing a fixation cross, served as an anticipation phase. Participants were instructed to respond with their index finger of the right hand on a MR-compatible response console (LumitouchTM, Photon Control Inc., BC, Canada) as quickly and accurately as possible upon seeing the target after the go cue and to refrain from responding upon seeing the target after the no-go cue. Feedback was presented for 1500 ms immediately after the target disappearance, followed by an intertrial interval of 1000 ms. Altogether, each trial had a length of 4250 ms, and the block length was 24.2 s.
Fig. 1.
Illustration of the cued incentive go/no-go task including three different incentive conditions: non-reward (NR), social reward (SR) and monetary reward (MR). Each reward condition comprised six go and six no-go blocks. Every block consisted of five trials, which were either go or no-go trails. In go blocks, all five trials were go trials. In no-go blocks, on average 65% were go trials and 35% were no-go trials.
Depending on the reward condition, participants were rewarded for successful task performance (i.e. an accurate button press in go trials within a response time window of 500 ms and a correct inhibitory response in no-go trials) with a probability of 80% in order to strongly drive neural reward circuitry, particularly the NAcc (Bjork and Hommer, 2007). In the SR condition, positive facial expressions served as rewards and neutral faces were shown after errors (for more details, see Kohls et al., 2009a; 2011). Correct task performance in the MR condition was rewarded with money, symbolized by different wallets, each filled with a 50 Eurocent coin. Empty wallets were shown after errors. All participants won an additional 10 Euros, irrespective of their performance, although they were told that better performance would result in a larger amount of money paid after the experimental session. Before scanning, we confirmed that each individual fully understood the concept and value of money as a tangible reward that could be gained during the experiment and later exchanged for other goods. In the NR condition, meaningless feedback (represented by mosaic pictures) was given for both successful and failed task performance. Mosaic pictures were produced to resemble the social and monetary feedback pictures in complexity, size and luminance. Visual stimulation was displayed on a rear projection LCD screen and viewed by the participant through a mirror attached to the head coil. Behavioural data collection and stimulus presentation were controlled by the Presentation 12.0 software (Neurobehavioral Systems, Albany, CA, USA).
To ensure that all participants understood the task instructions, the experimental procedure was preceded by 10 practice trials in each reward condition outside the magnet. After the experimental procedure, participants were asked to complete a rating questionnaire to assess their insight into aspects of task manipulations.
Image acquisition
T2*-weighted BOLD images were obtained with echoplanar imaging using a Siemens Trio 3.0 T scanner (Erlangen, Germany) and a multichannel head coil. Whole brain volumes of 36, 3-mm thick axial images (TR = 2200 ms, TE = 30 ms, gap = 0.6 mm, flip angle = 90 degrees, 64 × 64 matrix, voxel size = 3.1 × 3.1 × 3 mm3 and field of view = 200 × 200 mm2) were obtained continuously through one 15 min functional run. Altogether, 403 volumes were acquired per participant preceded by four ‘dummy’ scans allowing for T1 magnetic saturation. For each participant, high-resolution T1-weighted MPRAGE images of the entire brain were obtained by the following functional run (TR = 2250 ms, TE = 3.93 ms, 256 × 256 matrix, voxel size = 1 × 1 × 1 mm3, field of view = 256 × 256 mm2, 176 1-mm thick sagittal images).
Image analysis
Image processing and statistical analyses were carried out using FEAT v5.98, part of the FSL analysis package v4.1.4. Prior to image analysis, the first four images of the functional data set were discarded because of the non-equilibrium state of magnetization. For pre-processing, functional volumes for each participant were skull-stripped, motion-corrected, temporally high-pass filtered and spatially smoothed using a Gaussian kernel (FWHM = 5 mm).
Regression analysis was carried out on the pre-processed functional time series of each participant using FMRIB’s Improved Linear Model with autocorrelation correction. Eight ‘block’ regressors (i.e. GoNR, NogoNR, GoSR, NogoSR, GoMR, NogoMR, GoR and NogoR) were included in the regression model and convolved with a double-gamma hemodynamic response function, along with its temporal derivatives. Error trials and motion parameters were entered as ‘regressors of no interest’. The groups did not differ with respect to the amount of head movement during the scan (ASD: absolute displacement = 0.37 mm ± 0.18 mm; TDC: absolute displacement = 0.36 mm ± 0.19 mm; P = 0.87). Each regressor resulted in a voxelwise effect-size parameter estimate (β-values) image reflecting the magnitude of brain activation associated with that regressor. In order to create comparisons of interest, β-value images were contrasted. Note that we did not use a low-level fixation baseline; thus, the non-reward conditions served as our high-level baselines. Functional data were registered to the corresponding high-resolution structural image via an affine transformation with six degrees of freedom and warped into MNI space using an affine transformation with 12 degrees of freedom.
Group inferential statistical analyses were carried out using FMRIB's Linear Analysis of Mixed Effects 1 + 2. Within-group mixed effects, models were run for each contrast of interest, followed by two-sample t-tests (TDC vs ASD). We analysed go and no-go blocks separately because instrumental, more active conditions like the go blocks have been shown to activate the reward system significantly more strongly than more passive conditions, like the no-go blocks (Bjork and Hommer, 2007). All Z (Gaussianized T) statistical maps were cluster-corrected with a mean cluster threshold of Z > 2.3 and a cluster-corrected significance threshold of P ≤ 0.05 (Worsley, 2001).
Based on our a priori hypotheses, we examined group differences in three regions of interest (ROI), including the NAcc, ACC and amygdala, which were structurally defined areas from the Harvard–Oxford structural probabilistic atlases. For the ROI analyses, we applied a FWE corrected threshold of P ≤ 0.05 across each particular region using the Randomise v2.1 program. We also extracted individual mean parameter estimates from those ROIs separately from the left and right hemisphere for both go and no-go conditions in order to explore the Pearson product–moment correlations between social reward-based brain activation magnitudes and clinical symptoms as measured by the ADOS-G and ADI-R subscales. Bonferroni corrections were applied to adjust the alpha level for multiple comparisons.
Subjective rating questionnaire
Following the experimental procedure, participants were asked separately for the three reward conditions: (i) how motivating they found the condition, (ii) how important it was for them to perform well and (iii) how rewarding they found the feedback stimuli. The participants were also asked how much they were motivated with regards to doing the task prior to the scan. Participants indicated their answers by marking a 10-cm, horizontal visual-analogue scale min = 0, max = 100).
Behavioural data analysis
The three scales of the subjective rating questionnaire were analysed within a MANOVA, with incentive type as a within-participants repeated factor (NR, SR and MR) and group (ASD and TDC) as the between-participants factor, followed by univariate ANOVAs. Reaction times (RT) for hits (in ms) on the go/no-go task were analysed using a 3 × 2 (reward × group) ANCOVA with IQ as a covariate, since IQ (but not age) was significantly correlated with RT. As IQ and age were not correlated with the remaining dependent measures, these variables were not included as covariates in data analyses. Task accuracy (go trials: hit rate in %; no-go trials: rejection rate in percentage) was analysed within a 2 × 3 × 2 (trial × reward × group) ANOVA model, followed by planned contrasts. The α-level was set at 0.05. Effect sizes were calculated using partial eta squared (η2p).
RESULTS
Subjective pre- and post-test ratings
Both groups started the experimental procedure equally motivated according to self-ratings (Table 2). The post-test questions revealed a significant main effect of reward on the subjective rating scales [F(6,25) = 14.89, P < 0.001, η2p = 0.78], which was related to all three rating scales (motivation: P < 0.001, η2p = 0.61; importance: P < 0.001, η2p = 0.29; and reward value: P < 0.001, η2p = 0.67), with the highest ratings for the MR condition, the lowest ratings for the NR condition and with the SR condition intermediate (all P ≤ 0.017). These data demonstrate that reward manipulation within the experimental paradigm was successful across all participants. The group by reward interaction effect was non-significant [F(6,25) = 2.13, ns, η2p = 0.34]. However, a significant group effect was found [F(3,28) = 3.55, P = 0.027, η2p = 0.28], related to lower importance ratings in the ASD group (P = 0.044).
Table 2.
Main performance variables of the incentive go/no-go task and subjective motivation ratings by group and incentive condition
| Measures | ASD (n = 15) | TDC (n = 17) | P-values |
|---|---|---|---|
| M (s.d.) | M (s.d.) | ||
| Motivation rating (max. 100) | |||
| Start | 67.0 (24.7) | 75.9 (11.8) | 0.20 |
| Non-reward | 41.3 (28.9) | 45.8 (30.2) | 0.67 |
| Social reward | 82.0 (19.9) | 80.6 (15.6) | 0.82 |
| Monetary reward | 86.7 (13.9) | 94.1 (7.9) | 0.07 |
| RT for hits (in ms): | |||
| Non-reward | 244.8 (26.5) | 236.6 (28.7) | 0.41 |
| Social reward | 240.9 (23.7) | 234.4 (33.9) | 0.54 |
| Monetary reward | 243.4 (28.7) | 231.4 (29.2) | 0.25 |
| Go hit rate (accuracy in %): | |||
| Non-reward | 87.2 (10.3) | 91.3 (8.0) | 0.22 |
| Social reward | 89.9 (7.7) | 91.1 (7.9) | 0.67 |
| Monetary reward | 88.7 (8.7) | 92.5 (6.1) | 0.16 |
| No-go rejection rate (accuracy in %): | |||
| Non-reward | 95.8 (4.3) | 95.5 (7.2) | 0.87 |
| Social reward | 97.8 (3.5) | 97.5 (3.4) | 0.81 |
| Monetary reward | 98.4 (2.3) | 97.8 (3.8) | 057 |
Note: ASD = autism spectrum disorders, TDC = typically developing children. P-values based on two-sample t-tests.
Task performance
The analysis of reaction time for hits revealed a main effect of reward [F(2,28) = 5.53, P = 0.009, η2p = 0.28)], with faster reaction times observed for both reward conditions relative to the non-reward condition (SR = MR < NR, all significant P ≤ 0.027). On the other hand, we did not find a main effect of group [F(1,29) = 0.40, ns, η2p = 0.01] or a significant group by reward interaction effect [F(2,28) = 0.49, ns, η2p = 0.03]. Taken together, these data indicate that response speed in both groups changed similarly under the conditions of reinforcement, irrespective of reward type.
The analysis of performance accuracy revealed a significant main effect of trial [F(1,30) = 48.77, P < 0.001, η2p = 0.62], with higher accuracy for no-go than go trials. The main effect of reward approached significance [F(2,29) = 2.83, P = 0.075, η2p = 0.16]; rewards did not substantially enhance performance accuracy under no-go or go conditions across groups. The main effect of group was not significant (P = 0.45), indicating that both groups performed equally well under conditions of response initiation and motor inhibition. All other interaction effects (e.g. reward by group) were also found to be non-significant (all P’s > 0.1; Table 2).
Reward circuitry and social brain activation
Go blocks
The reward vs non-reward contrast across both groups (GoR > GoNR) revealed the expected reward circuitry activation in the NAcc (right: 8, 8, −6; Z = 5.97, left: −10, 8, −6; Z = 5.68), caudate (right: 10, 14, 0; Z = 6.22, left: −10, 10, −2; Z = 5.74), putamen (right: 24, 8, −4; Z = 6.06, left: −26, 0, −8; Z = 6.19), amygdala (right: 24, −2, −14; Z = 5.01, left: −22, −2, −12; Z = 5.66), thalamus (right: 4, −18, 10; Z = 7.08, left: −2, −20, 8; Z = 5.61), insula (right: 32, 16, −10; Z = 3.95, left: −34, 8, −14; Z = 3.79), and OFC (right: 34, 30, −8; Z = 3.36, left: −28, 12, −16; Z = 3.1; Figure 2). Except for the amygdala, NAcc and OFC, all of these areas were more strongly active in the monetary reward condition relative to the social reward condition (GoMR > GoSR). Additionally, the (ventral) ACC, as well as a number of regions outside of the ‘classical’ reward circuitry such as the cerebellum, supramarginal gyrus and frontal pole, showed stronger brain activation during monetary reward condition than during social reward processing (Table 3). The reverse contrast (GoSR > GoMR) revealed stronger activity in parts of the social brain network, including the amygdala, fusiform gyrus, superior temporal sulcus, temporal pole and ventromedial prefrontal cortex (Table 3).
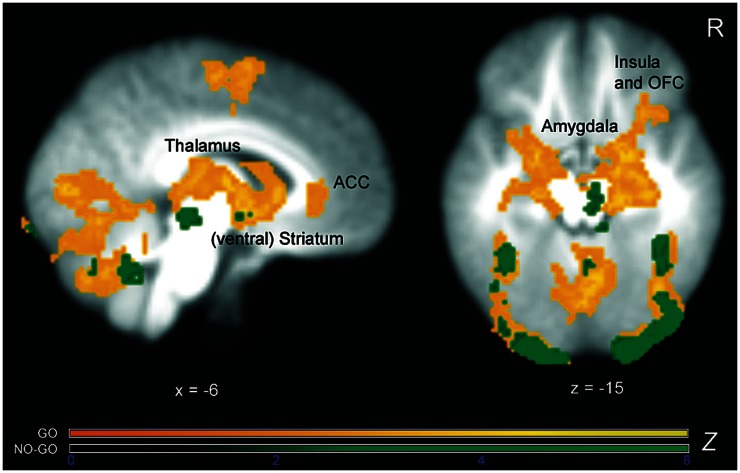
Fig. 2.
Z-statistic activation maps of the combined reward > non-reward contrast separately for go and no-go condition across the whole sample overlaid on the averaged group T1 image (cluster-corrected for multiple comparisons with a mean cluster threshold of Z > 2.3 and a cluster-corrected significance threshold of P ≤ 0.05). Color bars indicate Z-statistics. As expected, and in line with prior imaging research, reward obtained during go blocks led to strong and widespread activation within the motivation circuitry, comprising striatum, amygdala, thalamus, insula and prefrontal areas. In contrast, reward obtained during no-go blocks activated only a small number of reward areas such as midbrain and striatum.
Table 3.
Peak activation coordinates (MNI) for monetary versus social reward contrasts separately for the two trial conditions across the whole sample (N = 32)
| Anatomical region | Side | x | y | z | Maximum Z-score |
|---|---|---|---|---|---|
| GoMR > GoSR | |||||
| Caudate | L | −18 | 4 | 22 | 4.32 |
| R | 18 | 0 | 18 | 4.58 | |
| Cerebellum | 2 | −62 | −20 | 4.12 | |
| Cingulate cortex (anterior) | L | −8 | 34 | 18 | 4.33 |
| R | 10 | 34 | 18 | 4.91 | |
| Frontal pole | L | −34 | 50 | 18 | 3.91 |
| R | 42 | 38 | 30 | 5.00 | |
| Fusiform gyrus | L | −28 | −48 | −14 | 5.53 |
| R | 24 | −50 | −18 | 5.47 | |
| Insula | R | 40 | 18 | −6 | 3.81 |
| Lingual gyrus | L | −4 | −82 | −6 | 4.06 |
| Occipital cortex (lateral) | L | −50 | −64 | 0 | 4.41 |
| R | 52 | −62 | −8 | 4.50 | |
| Parietal lobule (superior) | L | −24 | −52 | 54 | 4.61 |
| R | 36 | −50 | 56 | 5.48 | |
| Precentral gyrus | R | 26 | −6 | 48 | 4.33 |
| Putamen | R | −24 | 8 | −2 | 3.38 |
| Supramarginal gyrus | L | −50 | −36 | 48 | 4.69 |
| R | 52 | −38 | 46 | 5.51 | |
| Temporal gyrus (inferior) | R | 60 | −56 | −10 | 4.83 |
| Thalamus | L | −4 | −14 | 12 | 3.20 |
| R | 6 | −14 | 14 | 4.10 | |
| GoSR > GoMR | |||||
| Amygdala | L | −20 | −8 | −18 | 4.40 |
| R | −28 | −6 | −18 | 4.38 | |
| Fusiform gyrus | R | 46 | −50 | −22 | 5.14 |
| Medial prefrontal cortex | L | −4 | 42 | −18 | 4.78 |
| R | 2 | 50 | −14 | 4.97 | |
| Precuneus | R | 2 | −54 | 24 | 4.12 |
| Temporal sulcus (superior) | L | −56 | −10 | −14 | 4.00 |
| R | 54 | −10 | −10 | 5.13 | |
| Temporal pole | L | −40 | 18 | −30 | 4.77 |
| R | 36 | 18 | −30 | 5.33 | |
| NogoMR > NogoSR | |||||
| Caudate | L | −10 | 6 | 8 | 4.12 |
| Cerebellum | −34 | −64 | −34 | 4.30 | |
| Cingulate cortex (anterior) | L | −8 | 38 | 0 | 3.80 |
| R | 2 | 44 | −2 | 5.08 | |
| Frontal pole | L | −34 | 56 | −8 | 4.59 |
| R | 30 | 52 | −12 | 4.30 | |
| Fusiform gyrus | L | −26 | −66 | −14 | 5.20 |
| R | 28 | −50 | −18 | 5.36 | |
| Insula | R | 42 | 18 | −8 | 4.47 |
| Lingual gyrus | R | 2 | −82 | 0 | 4.30 |
| Occipital cortex (lateral) | L | −28 | −84 | 20 | 5.13 |
| R | 36 | −82 | 12 | 4.69 | |
| Supramarginal gyrus | L | 48 | −42 | 52 | 5.10 |
| R | −50 | −42 | 52 | 4.19 | |
| Temporal gyrus (inferior) | L | −52 | −44 | −12 | 3.65 |
| R | 56 | −54 | −10 | 4.39 | |
| NogoSR > NogoMR | |||||
| Amygdala | L | −18 | −8 | −18 | 4.80 |
| R | 20 | −8 | −18 | 4.65 | |
| Fusiform gyrus | R | 42 | −46 | −22 | 6.82 |
| Medial prefrontal cortex | L | −4 | 48 | −14 | 4.55 |
| R | 6 | 50 | −16 | 4.16 | |
| Temporal pole | L | −36 | 12 | −26 | 4.40 |
Note: x, y, z refers to axis in MNI (Montreal Neurological Institute) space; L = left hemisphere; R = right hemisphere; GoMR = monetary reward go-bocks; GoSR = social reward go-blocks; GoNR = non-reward go-blocks; NogoMR = monetary reward no-go-blocks; NogoSR = social reward no-go-blocks; NogoNR = non-reward no-go-blocks.
Z scores are based on cluster-level correction for multiple comparisons across the whole brain with a mean cluster threshold of Z > 2.3 and a cluster-corrected significance threshold of P ≤ 0.05.
No-go blocks
The reward vs non-reward contrast within the no-go blocks (NogoR > NogoNR) revealed smaller patterns of significant brain activation across both groups, including midbrain (right: 8, −16, −12; Z = 3.39), thalamus (right: 22, −30, −12; Z = 3.75), putamen (left: −22, 6, −2; Z = 3.17) and NAcc (left: −10, 6, −6; Z = 3.28; Figure 2). The NogoMR > NogoSR contrast showed that parts of the motivation circuitry were particularly activated under monetary compared to the social reward condition (Table 3). The simple NogoSR > NogoMR contrast revealed activation in the social brain network, comparable to the findings for the go trials, including the amygdala, fusiform gyrus, temporal pole and ventromedial prefrontal cortex (Table 3).
Atypical brain responses in ASD
Go blocks
Between-group comparisons revealed that during monetary reward processing (GoMR > GoNR), the ASD group showed significantly less brain activation than the TDC in numerous reward-related brain areas, including the midbrain, thalamus, amygdala, dorsal and ventral striatum/NAcc. Moreover, several clusters within the cingulate cortex, including the ventral, pregenual ACC, anterior dorsal ACC, as well as the posterior dorsal ACC, were found to be less active in ASD participants than TDC when money was at stake (Table 4).
Table 4.
Peak activation coordinates (MNI) from whole-brain and ROI analyses for the between-group contrast TDC > ASD separately for reward and trial conditions
| Anatomical region | Side | x | y | z | Maximum Z (or t) score |
|---|---|---|---|---|---|
| GoMR > GoNR | |||||
| Amygdala | L | −16 | −4 | −14 | t = 5.03* |
| R | 18 | −10 | −12 | Z = 3.29 | |
| Caudate | R | 10 | 10 | 0 | Z = 3.10 |
| Cerebellum | 2 | −64 | −34 | Z = 3.57 | |
| Cingulate cortex | |||||
| Anterior (dorsal) | L | 0 | 38 | 20 | Z = 3.62 |
| Anterior (ventral) | R | 0 | 42 | −4 | t = 4.58* |
| L | −4 | −24 | −12 | Z = 3.88 | |
| Posterior (dorsal) | L | −2 | −36 | 32 | Z = 3.54 |
| Hippocampus | L | −28 | −22 | −12 | Z = 3.46 |
| Lingual gyrus | L | −2 | −76 | −2 | Z = 3.75 |
| R | 6 | −68 | 0 | Z = 3.43 | |
| Midbrain | R | 8 | −18 | −14 | Z = 3.32 |
| Nucleus accumbens | L | −6 | 8 | −4 | t = 3.35* |
| R | 12 | 12 | −4 | Z = 3.17 | |
| Pallidum | L | −24 | −12 | −4 | Z = 3.53 |
| Parietal lobule (superior) | L | −36 | −44 | 54 | Z = 3.32 |
| Precentral gyrus | L | −54 | 8 | 36 | Z = 3.49 |
| Putamen | L | −28 | 0 | −8 | Z = 3.22 |
| Temporal gyrus (inferior) | L | −46 | −50 | −8 | Z = 3.42 |
| Thalamus | L | −14 | −24 | 10 | Z = 3.13 |
| R | 12 | −14 | 10 | Z = 3.28 | |
| GoSR > GoNR | |||||
| Amygdala | L | −16 | −4 | −14 | t = 4.13** |
| Cingulate cortex | |||||
| Anterior (ventral) | L | −2 | 40 | −4 | t = 3.34** |
| NogoMR > NogoNR | No significant activation differences | ||||
| NogoSR > NogoNR | within whole-brain or ROI analyses | ||||
Note: The reverse group contrasts ASD > TDC did not reveal significant activation differences. The MR > SR and SR > MR contrasts for the go and no-go conditions did not reveal group differences. TDC = typically developing children; ASD = autism spectrum disorders; x, y and z refers to axis in MNI (Montreal Neurological Institute) space; L = left hemisphere; R = right hemisphere; GoMR = monetary reward go-bocks; GoSR = social reward go-blocks; GoNR = non-reward go-blocks; NogoMR = monetary reward no-go-blocks; NogoSR = social reward no-go-blocks; NogoNR = non-reward no-go-blocks; ROI = region of interest.
Z-scores are based on cluster-level correction for multiple comparisons across the whole brain with a mean cluster threshold of Z > 2.3 and a cluster-corrected significance threshold of P ≤ 0.05.
*ROI results are family wise error (FWE) corrected at P ≤ 0.05 across this particular region. Please note that group activation differences were also found in this region using cluster correction for multiple comparisons across the whole brain at P ≤ 0.05.
**ROI results are family wise error (FWE) corrected at P ≤ 0.05 across this particular region.
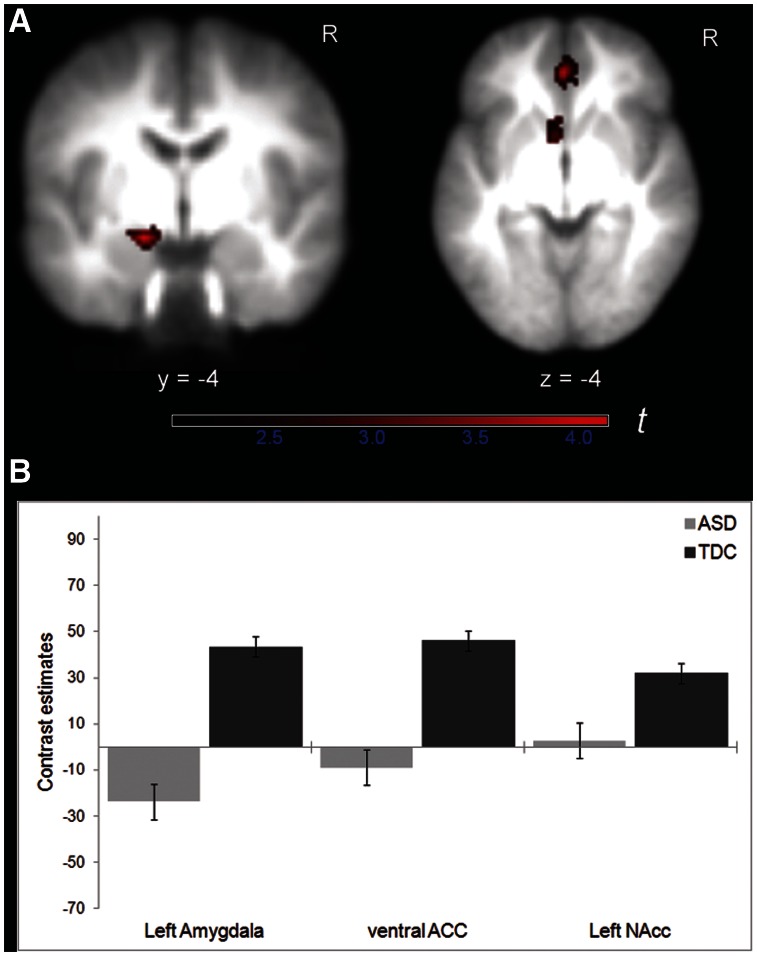
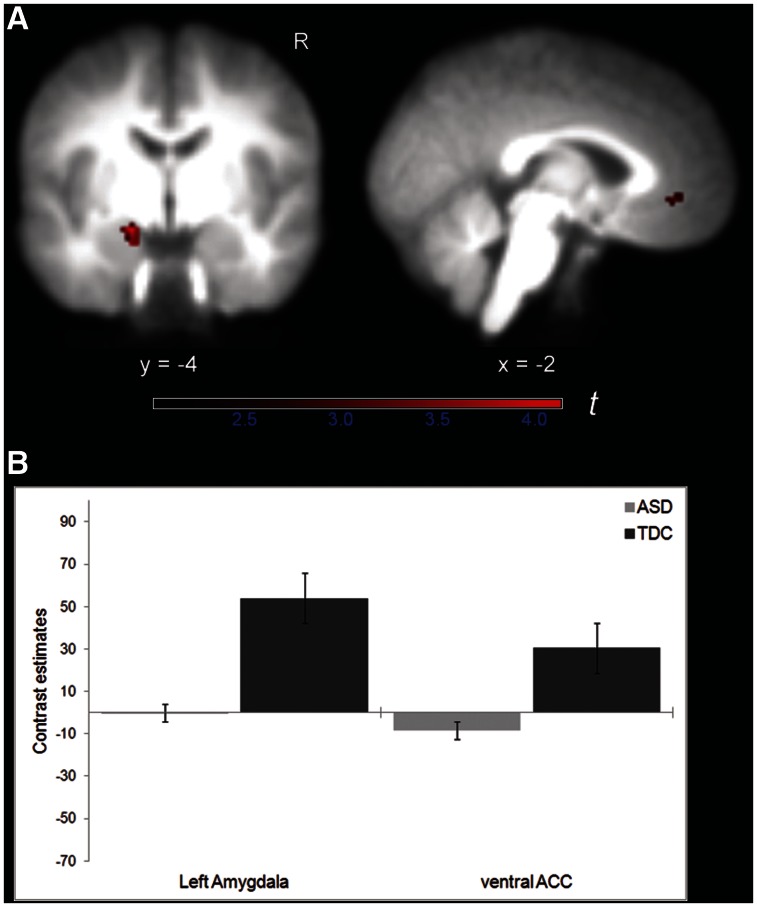
ROI analyses confirmed that the left NAcc, left amygdala and ventral ACC showed reduced brain activation in the ASD group relative to controls when money could be actively obtained (GoMR > GoNR; Table 4 and Figure 3). Although the GoSR > GoNR contrast did not reveal significant group activation differences using whole-brain cluster thresholding, the additional ROI analyses demonstrated that the left amygdala and ventral ACC were less activated in children with ASD than TDC when social reward was at stake (Table 4 and Figure 4). None of the other group comparisons (e.g. ASD > TDC) revealed significant brain activation differences.
Fig. 3.
(A) Decreased activation in left amygdala (coronal slice), and left NAcc as well as ventral ACC (axial slice) in children with ASD compared to TDC for the contrast GoMR > GoNR, overlaid on the averaged group T1 image (FWE corrected at P ≤ 0.05 for structurally defined ROI). Color bar indicates t-statistics. (B) Contrast estimates for the same contrast for each group in regions shown in (A). Error bars indicate ± 1.0 SEM.
Fig. 4.
(A) Decreased activation in left amygdala (coronal slice) and ventral ACC (sagittal slice) in children with ASD compared to TDC for the contrast GoSR > GoNR, overlaid on the averaged group T1 image (FWE corrected at P ≤ 0.05 for structurally defined ROI). Color bar indicates t-statistics. (B) Contrast estimates for the same contrast for each group in regions shown in (A). Error bars indicate ± 1.0 SEM.
No-go blocks
None of the group contrasts showed significant activation differences.
Brain-behaviour correlational analyses
Exploratory correlational analyses between the NAcc, ACC or amygdala activation magnitudes during social reward processing and clinical symptoms as assessed by the ADOS-G and ADI-R subscales did not reveal significant associations within the ASD group.
DISCUSSION
In the study, we applied a cued incentive go/no-go task to explore the differential effects of social and monetary reward on motivation circuitry activation in children with and without ASD. With regard to reward-based brain responsivity in children with ASD, our fMRI analyses revealed the expected hypoactivation in the mesocorticolimbic circuitry in response to both social and monetary reward. In particular, diminished reward system activations were found in ASD under monetary reward conditions that required an active response to gain a reward, demonstrated in the midbrain, thalamus, amygdala, striatum (including NAcc) and ACC. Moreover, in addition to lower NAcc responsivity to monetary reward, ROI analyses revealed that the ventral ACC and amygdala were significantly less activated within the ASD group in response to both social and monetary incentives.
The current imaging findings are in line with our previous event-related brain potential (ERP) results using the same task in an almost identical sample of children with and without ASD (Kohls et al., 2011). Our prior study revealed attenuated brain reactivity (i.e. compromised P3 activity) in participants with ASD in response to go signals associated with a timely reaction to achieve a social or a monetary reward. Taken together, both data sets indicate atypical motivation-related brain responses associated with active goal-directed behaviour in individuals with ASD, irrespective of reward type. Such a deficiency may severely affect the initiation of properly motivated behaviour by disrupting the ability to seek and approach environmental incentives.
In contrast to our prediction, and in contrast to Scott-Van Zeeland’s results (2010a), we did not find greater malfunctions in the reward circuitry in response to social reward compared to monetary reward in ASD. Most recently, Dichter et al. (2012b) applied a face in comparison with a money anticipation paradigm and found autism-related hypoactivations in the NAcc only when money was at stake, but not in the social incentive condition; this finding is consistent with our data. However, Scott-Van Zeeland used approving faces in combination with verbal praise as social reinforcement, whereas in the current and in Dichter’s study, static faces without praise were applied. It seems likely that the combination of facial rewards with praise may represent a stronger social incentive with respective greater NAcc responsivity, primarily in TDC, making it more likely that activation differences will be detected between children with and without ASD within this key reward area. Thus, future imaging studies are needed that apply more ecologically valid social reward stimuli, such as short social video clips (Blatter and Schultz, 2006), which might be able to reveal a clearer picture about NAcc involvement in relation to diminished socially motivated behaviour in individuals with ASD.
Our findings of lower NAcc activation in response to monetary incentives in our ASD sample are consistent with data reported by Dichter et al. (2012a,b). The NAcc has been found to be preferentially activated in response to reward-predicting cues in typically developing children and adults, primarily reflecting reward ‘wanting’ and, thus, the incentive salience of the reward at stake with greater NAcc activity for more salient incentives (Berridge et al., 2009). Blunted NAcc responses indicate that the salience of a secondary reinforcer such as money is diminished in ASD and might compromise the ability of reward-predicting cues to elicit appropriate goal-directed actions to approach the reward.
However, the NAcc does not act in isolation to regulate motivated behaviours, but is part of a functional network, mainly including the midbrain, thalamus, dorsal striatum, amygdala and (ventral) prefrontal areas (Haber and Knutson, 2010). In particular, the NAcc together with the amygdala (particularly the basolateral portion) and the ventral prefrontal cortex (including ventral ACC) form a unique circuitry, which promotes cue-evoked reward-seeking behaviour (Sesack and Grace, 2010), and which is modulated by ‘motivational’ neuropeptides, such vasopressin and oxytocin (e.g. Zink et al., 2010). In the psychopathology of addiction, dysfunction within this circuitry, such as an insufficient communication between the amygdala and/or the ventral prefrontal cortex to the NAcc, has been suggested to underlie aberrant motivation to seek detrimental substances at the expense of natural rewards (Kalivas and Volkow, 2005). The current study revealed diminished brain activation within the amygdala/prefrontal/NAcc circuitry in response to reward in children with ASD. One could speculate that an aberrant pattern of brain activity within this circuitry in individuals with ASD may trigger an excessive seeking of salient, autism-specific objects and situations (Turner-Brown et al., 2011) at the cost of neglecting other essential environmental rewards. It will be important for future research to test this assumption by comparing BOLD responses to typical autism-specific objects of interest (e.g. Dichter et al., 2012a) relative to different types of tangible, non-tangible and even primary biological incentives. Furthermore, in order to better capture the brain activation dynamics within the motivational circuitry, and the possible disruption thereof in ASD, functional connectivity measures should be applied in follow-up studies (Camara et al., 2009). Research suggests that ASD symptoms could reflect a neurofunctional disconnection syndrome (Geschwind and Levitt, 2007; Schipul et al., 2011), perhaps mediated by genetic factors, which might affect essential information transfer within the frontolimbic reward network (Scott-Van Zeeland et al., 2010b).
In contrast to social motivation deficit theories of autism (Chevallier et al., 2012; Dawson et al., 2005; Schultz, 2005), we did not find specifically diminished brain responses to social incentives in ASD, at least with the social incentives used in this age group here. Our fMRI data indicate that the brain reactivity to monetary incentives is also compromised, which is in line with previous reports (Schmitz et al., 2008; Kohls et al., 2011; Dichter et al., 2012a,b). However, it should be acknowledged that monetary and social rewards generally differ with respect to properties such as collectability (i.e. money is collectable, but not social rewards) and immediacy of effect (Estle et al., 2007), making comparisons between reward types fraught with difficulty. It is also true that money is imbued with social meaning, as its concept and value are primarily mediated through social interactions (Marshall and Magruder, 1960). Thus, social impairments, the core feature of ASD, may have contributed to diminished brain responses to monetary incentives in the current and previous reward studies.
Consistent with prior reports, we did not find different behavioural responsiveness to reward in participants with ASD compared to controls (Kohls et al., 2011; Dichter et al., 2012a,b). Because performance differences can be considered a confounding factor, the absence of such effects underscores the uniqueness of neural data in uncovering abnormal reward mechanisms in patients with mental disorders. Moreover, undisturbed behavioural reward responsiveness in ASD is in line with current psychosocial intervention programs that are effective in diminishing dysfunctional behaviour in individuals with ASD by applying different types of reinforcement (Vismera and Rogers, 2010). It is also plausible that normal reward responsivity on the behavioural level in our ASD group reflects the positive impact of achievement motivation tendencies, i.e. the general eagerness to perform well or the fear of failure (South et al., 2011), which we were not able to capture properly with thee current study design.
Consistent with previous research, we did not find motor inhibition deficits in our ASD group (e.g. Hill, 2004). However, reported effect sizes for deficient response inhibition using go/no-go tasks within this population range from small to medium (Pennington and Ozonoff, 1996). Such effect sizes would require a much bigger sample in order to have the power to detect significant group differences.
The present study has some limitations that should be considered. Our ASD sample consisted of high-functioning boys with Asperger syndrome. Given the heterogeneity within the autistic spectrum, it seems possible that reward circuitry dysfunction manifests differently in individuals across the spectrum. Hence, any conclusions derived need replication with larger samples that include, for instance, males and females with lower functioning autism relative to other clinical comparison groups (e.g. ADHD; Kohls et al., 2009b). The selection of an unmedicated and comorbidity-free sample of children with ASD may likely bias the study findings toward less impaired individuals with the disorder, which makes it difficult to generalize the results to the broader spectrum of ASD. The composition of our ASD group might also be a reason why we did not detect the expected brain-behaviour correlations. The power to uncover robust associations in our group of children with ASD was likely weakened by the small range of low to moderate severity scores on the social impairment measures (Gotham et al. 2009).
Despite these limitations, the present data indicate aberrant brain reactivity in response to social and monetary reward in an unmedicated and comorbidity-free sample of children with ASD compared to TDC. This neural dysfunction may cause maladaptive-motivated behaviour, such that essential environmental rewards are neglected in favour of salient, autism-specific objects and situations. An increased understanding of the biological mechanisms causing social deficits in ASD can be used to develop therapeutic interventions where, for instance, pharmacological agents (like oxytocin) support social learning via increased reward-based motivation (Bartz et al., 2011). If motivation deficits are indeed a fundamental cause of a cascade of events that support the development of ASD, then interventions that increase motivation could help individuals with ASD and their families.
Conflict of Interest
None declared.
Acknowledgments
This study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, IRTG 1328). We would like to thank all the young volunteers and their parents who participated in this research. We are grateful to Helmut Remschmidt, Ellen Greimel and Sarah Brieber for their help with participant recruitment, to Barbara Elghahwagi and Dorothe Krug for their help with data collection, and to Elinora Hunyadi, Alexander Foss, Vanessa Troiani and John Herrington for their support with the fMRI data analyses.
REFERENCES
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15:301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: “liking”, “wanting”, and learning. Current Opinion in Pharmacology. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW. Anticipating instrumentally obtained and passively-received rewards: a factorial fMRI investigation. Behavioural Brain Research. 2007;177(1):165–70. doi: 10.1016/j.bbr.2006.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter K, Schultz W. Rewarding properties of visual stimuli. Experimental Brain Research. 2006;168:541–6. doi: 10.1007/s00221-005-0114-y. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Huffstetler S, McGuire JT. Effort discounting in human nucleus accumbens. Cognitive, Affective, and Behavioral Neuroscience. 2009;9:16–27. doi: 10.3758/CABN.9.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. Trends in Cognitive Sciences. 2012. The social motivation theory of autism. [March 17 Epub ahead of print; doi:10.1016/j.tics.2012.02.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. Social Responsiveness Scale (SRS) Manual. Los Angeles: Western Psychological Services; 2002. [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27:403–24. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2012a;7:160–72. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. Journal of Autism and Developmental Disorders. 2012b;42:147–160. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estle SJ, Green L, Myerson J, Holt DD. Discounting of monetary and directly consumable rewards. Psychological Sciences. 2007;18:58–63. doi: 10.1111/j.1467-9280.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Current Opinion in Neurobiology. 2007;17:103–11. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gleissner U, von Ondarza G, Freitag H, Karlmeier A. Auswahl einer HAWIK-III-Kurzform für Kinder und Jugendliche mit Epilepsie. Zeitschrift für Neuropsychologie. 2003;14:3–11. [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL. Evaluating the theory of executive dysfunction in autism. Developmental Review. 2004;24:189–233. [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. The American Journal of Psychiatry. 2005;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy for Child and Adolescent Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Herpertz-Dahlmann B, Konrad K. Differential effects of social and non-social reward on response inhibition in children and adolescents. Developmental Science. 2009a;12:614–25. doi: 10.1111/j.1467-7687.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- Kohls G, Herpertz-Dahlmann B, Konrad K. Hyperresponsiveness to social rewards in children and adolescents with attention-deficit/hyperactivity disorder (ADHD) Behavioral and Brain Functions. 2009b;5:20. doi: 10.1186/1744-9081-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Schulte-Rüther M, et al. Atypical brain responses to reward cues in autism as revealed by event-related potentials. Journal of Autism and Developmental Disorders. 2011;41:1523–33. doi: 10.1007/s10803-011-1177-1. [DOI] [PubMed] [Google Scholar]
- Levy SE, Mandell DS, Schultz RT. Autism. The Lancet. 2009;374:1627–38. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorder. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord G, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorder. 2000;30:205–23. [PubMed] [Google Scholar]
- Marsgall HR, Magruder L. Relations between parent money education practices and children’s knowledge and use of money. Child Development. 1960;31:253–284. doi: 10.1111/j.1467-8624.1960.tb04964.x. [DOI] [PubMed] [Google Scholar]
- Neuhaus E, Beauchaine TP, Bernier R. Neurobiological correlates of social functioning in autism. Clinical Psychology Review. 2010;30(6):733–48. doi: 10.1016/j.cpr.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Rinehart NJ, Bellgrove MA, Tonge BJ, Brereton AV, Howells-Rankin D, Bradshaw JL. An examination of movement kinematics in young people with high-functioning autism and Asperger’s disorder: further evidence for a motor planning deficit. Journal of Autism and Developmental Disorder. 2006;36:757–767. doi: 10.1007/s10803-006-0118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad A-M, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57(7–8):640–52. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire. Manual for the SCQ. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Schmitz N, Rubia K, van Amelsvoort T, Daly E, Smith A, Murphy DGM. Neural correlates of reward in autism. The British Journal of Psychiatry. 2008;192:19–24. doi: 10.1192/bjp.bp.107.036921. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23:125–41. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1992;12(12):4595–610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer S. Reward processing in autism. Autism Research. 2010a;3:53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Abrahams BS, Alvarez-Retuerto AI, et al. Altered functional connectivity in frontal lobe circuits is associated with variation in the autism risk gene CNTNAP2. Science Translational Medicine. 2010b;2(56):56ra80. doi: 10.1126/scitranslmed.3001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35(1):27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M, Dana J, White SE, Crowley MJ. Failure is not an option: risk-taking is moderated by anxiety and also by cognitive ability in children and adolescents diagnosed with an autism spectrum disorder. Journal of Autism and Developmental Disorders. 2011;41(1):55–65. doi: 10.1007/s10803-010-1021-z. [DOI] [PubMed] [Google Scholar]
- Turner-Brown LM, Lam KSL, Holtzclaw TN, Dichter GS, Bodfish JW. Phenomenology and measurement of circumscribed interests in autism spectrum disorders. Autism. 2011;15:437–56. doi: 10.1177/1362361310386507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vismara LA, Rogers SJ. Behavioral treatments in autism spectrum disorder: what do we know? Annual Review of Clinical Psychology. 2010;6:447–468. doi: 10.1146/annurev.clinpsy.121208.131151. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. New York: Oxford University Press; 2001. [Google Scholar]
- Zink CF, Stein JL, Kempf L, Hakimi S, Meyer-Lindenberg A. Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. The Journal of Neuroscience. 2010;30(20):7017–22. doi: 10.1523/JNEUROSCI.4899-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]