Abstract
Primary malignant mesothelioma (MM) of spermatic cord is extremely rare. We presented two malignant mesotheliomas involving the spermatic cords; one was primary, one secondary. The secondary one represented the direct involvement by a peritoneal MM. No occupational exposure to asbestos was identified in either patient. Both of them presented with a painless inguinal mass. Microscopically the primary MM was epithelioid type with tumor nests infiltrating adjacent adipose tissue, while the secondary MM grew in mixed type. No tumor necrosis was seen in the primary MM, while extensive necrosis was seen in the secondary one. Rare mitotic figure was seen in the primary MM while the mitosis in the secondary tumor was brisk, and with atypical mitosis. Immunohistochemically the tumor cells were positive for calretinin and CK5/6 and negative for BER-EP4 and BRST3 in both cases. The reported cases of primary MM from spermatic cord in English literature were briefly reviewed.
Key words: malignant mesothelioma, spermatic cord.
Introduction
Malignant mesothelioma (MM) is a tumor of mesothelial cell origin, mostly arising from the pleura, peritoneum and pericardium. Only 0.3% to 1.4% of MM is presented as a paratesticular mass arising from the mesothelial lining that surrounds the testicle, the tunica vaginalis.1–4 In most cases of malignant mesothelioma, occupational exposure to asbestos is thought to be the cause, but environmental exposure can also cause it. For example, the family member of an asbestos worker can expose to asbestos fibers from close contact. Primary MM arising from the spermatic cord is extremely rare; to our knowledge, only 9 cases were reported in the English literature. Here we reported a primary MM arising from spermatic cord in a 45 year old Caucasian patient (case 1) and a secondary MM involving spermatic cord from a 68 years-old man (case 2).
Method
The database of the Department of Pathology and Laboratory Medicine of the University of Pennsylvania was searched for malignant mesothelioma and spermatic cord. Two cases of malignant mesothelioma involving the spermatic cords were identified. This study was approved by the University of Pennsylvania Institutional Review Board.
Immunohistochemistry
Imunohistochemistry of formalin fixed paraffin embedded tissue was performed using antibodies against calretinin (Invitrogen 18-0211; 1:75 dilution), cytokeratin 5/6 (Dako M7237; 1:10 dilution), BRST-3 (Signet 3612-1000; 1:10 dilution) and epithelial antigen Ber-Ep4 (Dako M0804; 1:50 dilution). Immunohistochemistry was done on a Leica Bond™ instrument using the Novocastra Bond Polymer Refine Detection System. Heat induced epitope retrieval was done for 20 minutes with ER2 solution (Leica Microsystems AR9640) for Calretinin, Cytokeratin and ER1 solution (Leica Microsystems AR9961) for BRST3 and BerEp4.
Case Report #1
The patient was a 45 year-old Caucasian man who presented with a painless left inguinal mass for 4 months. His urology history was notable only for a previous vasectomy. An ultrasound of scrotum revealed thickening of the soft tissue just deep to the subcutaneous fat in the left inguinal region, measured 2.5 cm in thickness. No bowel or bladder symptoms were present. The laboratory tests were normal including normal complete blood count. Imaging studies of chest and abdomen were negative. Both of testicles and epididymis were of normal, no intra-testicular mass was seen. The patient underwent resection of the left inguinal mass as well as part of the spermatic cord via an inguinal incision. He had no known history of exposure to asbestos. The patient did not received any radiation or chemotherapy and was alive without evidence of disease 6 month after surgery.
Case Report #2
The patient was a 68-year-old man with symptomatic right inguinal hernia, which had been present for many years. Physical examination showed a very firm mass within the hernia sac. Laboratory blood tests showed only mild anemia. The patient also had history of intraabdominal malignant mesothelioma. The patient underwent right inguinal hernia repair and resection of the inguinal lesion and orchiectomy. The patient was lost to follow up after surgery.
Results
Pathology: first patient
The gross specimen showed red-tan rubbery tissue with small amount of attached fat. Cut surface showed pale-tan to red rubbery tissue. Microscopically, epithelioid cells grew in nested patterns. The tumor infiltrated into the surrounding adipose tissue of the vas deferens (Figure 1A). The nuclei of tumor nuclei were centrally located with small yet distinct nucleoli (Figure 1B). No tumor necrosis was identified. No lymphovascular invasion was seen. Occasional mitotic figures were present. The diagnosis was malignant mesothelioma, epithelial type, infiltrating into the surrounding soft tissue and encircling the vas deferens.
Figure 1.
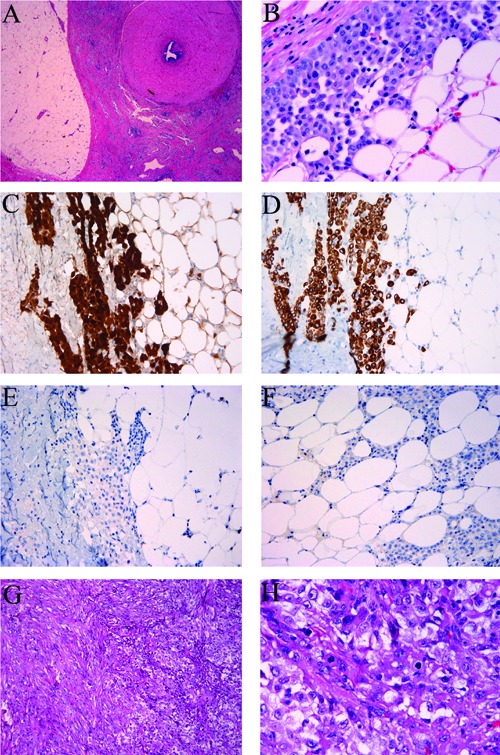
Malignant mesothelioma involving spermatic cord. A-F) Case 1. A-B) Hematoxylin and Eosin, malignant mesothelioma cells infiltrate into the surrounding connective tissue of vas deferens. A) x25; B) x400; C-F) immunohistochemical stains, x200. Malignant mesothelioma cells are positive for Calretinin (C), cytokeratin CK5/6 (D), and negative for BER-EP4 (E) and BRST3 (F). G-H) Case 2. G) The tumor grew in a mixed pattern, Hematoxylin and Eosin, x100. H) The tumor showed brisk mitosis, Hematoxylin and Eosin, x400.
Pathology: second patient
Grossly the specimen was composed of right spermatic cord and testicle, measured 16x6x3.5 cm overall. A white, firm and lobulated mass was located within the spermatic cord, 0.1 cm away from the spermatic cord margin. The testicle was unremarkable. Microscopically, there was a malignant mesothelioma growing in a mixed pattern (Figure 1G). The tumor infiltrated the soft tissue around the spermatic cord. Tumor necrosis was extensive. The epithelioid component grew either in tubulopapillary or nested pattern. Mitosis was brisk (∼ 19/10 HPF) with atypical mitosis (Figure 1H). The diagnosis was malignant mesothelioma, mixed type.
Immunohistochemistry
Immunohistochemical stains for calretinin (Figure 1C) and CK5/6 (Figure 1D) with adequate control were performed on both cases and showed that the tumor cells from both cases were positive for these two markers, and negative for Ber-EP4 (Figure 1E) and BRST 3 (Figure 1F).
Discussion
MM can involve spermatic cord, either as a primary or secondary tumor. Primary MM arising from the processes vaginalis around the spermatic cord is extremely rare. To our knowledge, only 10 cases are available including 9 cases from the literature review and one of our cases (Table 1).5–11 The patient age ranges from 26–74 years with an average age of 47. Eight cases are from left side and two right. The patients most commonly present with painless inguinal mass. Six cases have clinical information of asbestos exposure available and none of them is positive.12 Seven indicate tumor histologic types with epithelioid type in 5 of them and mixed in 2 of them.
Table 1. Clinical and pathologic features of primary malignant mesothelioma of spermatic cord.
| References | Age | Side/ symptoms | Asbestos exposure | Size (cm) | Histologic type | Tumor dissemination | Follow-up |
|---|---|---|---|---|---|---|---|
| Kozlowski & Zoltowska5 | 63 | L/mass in the inguinal region for 3 M | NS | NS | Mixed | NS | NS |
| Arlen et al.6 | 40 | L/ mass higher near groin | NS | 0.5 | NS | External iliac lymph node | ANED 18 yr |
| Pizzolato et al.7 | 57 | R/hernia | NS | NS | Epithelioid | Local recurrence at 1 yr; periaortic and iliac nodes at 34 M | DOD ∼42 M |
| Blitzer et al.8 | 74 | L/scrotal mass for 12 M | NS | 1 | Mixed | No | ANED 30 M |
| Carp et al.9 | 54 | L/groin mass | No | NS | Epithelial | Local recurrence at 11 month of first presentation | DOD 64 M |
| Schure et al.10 | 35 | L/ inguinal and scrotal mass for 3 wks | No | NS | NS | Thoracic cavity and peritoneum at 2 M | DOD 2 M |
| 45 | Left-sided scrotal swelling for 1 M | No | NS | NS | No at 48 M | ANED 48 M | |
| Hai et al.11 | 26 | L spermatic cord mass | No | NS | Epithelioid | No | ANED 2 yr |
| 57 | R spermatic cord mass | No | NS | Epithelioid | Lung met pretreatment | Local recurrence in 2 year follow-up | |
| Current case | 45 | L/inguinal mass for 4 M | No | 2.5 | Epithelioid | No | ANED 6 M |
ANED, alive with no evidence of disease; DOD, died of disease; L, left; M, month; NS, not stated; R, right; yr: year.
The differential diagnosis of a tumor involving the inguinal area is broad. First of all, the tumor needs to be put into the right histological category. This can be achieved by morphological examination with the aid of immunohistochemical stains such as broad spectrum cytokeratins, melanocytic markers such as HMB-45, melan A, lymphoma markers such as leukocyte common antigen, calretinin, and other markers relevant sarcoma markers.
If the tumor is determined to be a mesothelial lesion, the differential diagnosis in this site includes reactive mesothelial hyperplasia and benign mesothelial tumor such as adenomatoid tumor or MM. The mesothelial cells are positive for calretinin, cytokeratin CK5/6 and negative for BRST3 and Ber-EP4, like in our cases. Such immunostaining profile may help to determine the mesothelial origin; however, to determine whether the tumor is benign or malignant, careful morphological examination of the tumor, looking for cytological atypia and tumor infiltration into the surrounding soft tissue are critical.
If a MM is rendered, the next critical issue is to differentiate whether the tumor is a primary or part of peritoneal MM. In our first case, there were no evidences of tumors in thoracic cavity, pericardium, peritoneum or testicle; therefore it was a primary MM in spermatic cord, likely from the remnant mesothelium of patent processus vaginalis from the hernia repair. The second case, however, represented the involvement of spermatic cord by a peritoneal MM. It is imperative to exclude the primary tumors in peritoneal and thoracic cavity and other common sites before a diagnosis of primary MM in spermatic cord is rendered. The optimal treatment for malignant mesothelioma of spermatic cord is yet to be determined. Current first line of treatment appears to be inguinal radical orchiectomy, which in combination of early tumor stage at the time diagnosis may result in a slightly better prognosis.
Conclusions
Primary MMs in spermatic cord are aggressive tumors capable of local recurrent and lymphatic and hematogeneous metastases: 57% of the patients have the disease recurred within 2 years in the limited number of reported cases (Table 1), comparable to the 60% of patients of paratesticular MMs.13,14 The number of primary cases reported in spermatic cord is too small to decipher a meaningful mortality rate and prognostic factors; however, it has been shown that in paratesticular MMs 30% of patients die after a median survival of 24 months and age of the patient appears to be the most important prognostic factor.13,14 Patients younger than 60 years have better chance of survival in paratesticular MMs.2,13,14
References
- 1.Bisceglia M, Dor DB, Carosi I, et al. Paratesticular mesothelioma. Report of a case with comprehensive review of literature. Adv Anat Pathol. 2010;17:53–70. doi: 10.1097/PAP.0b013e3181c66fbc. [DOI] [PubMed] [Google Scholar]
- 2.Hassan R, Alexander R. Nonpleural mesotheliomas: mesothelioma of the peritoneum, tunica vaginalis, and pericardium. Hematol Oncol Clin North Am. 2005;19:1067–87. doi: 10.1016/j.hoc.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Janssen-Heijnen ML, Damhuis RA, Klinkhamer PJ, et al. Increased but low incidence and poor survival of malignant mesothelioma in the southeastern part of The Netherlands since 1970: a population-based study. Eur J Cancer Prev. 1999;8:311–4. doi: 10.1097/00008469-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Murai Y. Malignant mesothelioma in Japan: analysis of registered autopsy cases. Arch Environ Health. 2001;56:84–8. doi: 10.1080/00039890109604058. [DOI] [PubMed] [Google Scholar]
- 5.Kozlowski H, Zoltowska A. Mesothelioma of spermatic cord. Neoplasma. 1968;15:97–100. [PubMed] [Google Scholar]
- 6.Arlen M, Grabstald H, Whitmore WF., Jr Malignant tumors of the spermatic cord. Cancer. 1969;23:525–32. doi: 10.1002/1097-0142(196903)23:3<525::aid-cncr2820230302>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 7.Pizzolato P, Lamberty J. Mesothelioma of spermatic cord: electron microscopic and histochemical characteristics of its mucopolysaccharides. Urology. 1976;8: 403–8. doi: 10.1016/0090-4295(76)90502-1. [DOI] [PubMed] [Google Scholar]
- 8.Blitzer PH, Dosoretz DE, Proppe KH, et al. Treatment of malignant tumors of the spermatic cord: a study of 10 cases and a review of the literature. J Urol. 1981;126: 611–4. doi: 10.1016/s0022-5347(17)54650-9. [DOI] [PubMed] [Google Scholar]
- 9.Carp NZ, Petersen RO, Kusiak JF, et al. Malignant mesothelioma of the tunica vaginalis testis. J Urol. 1990;144:1475–8. doi: 10.1016/s0022-5347(17)39773-2. [DOI] [PubMed] [Google Scholar]
- 10.Schure PJ, van Dalen KC, Ruitenberg HM, et al. Mesothelioma of the tunica vaginalis testis: a rare malignancy mimicking more common inguino-scrotal masses. J Surg Oncol. 2006;94:162–4. doi: 10.1002/jso.20428. [DOI] [PubMed] [Google Scholar]
- 11.Hai B, Yang Y, Xiao Y, et al. Diagnosis and prognosis of malignant mesothelioma of the tunica vaginalis testis. Can Urol Assoc J. 2011:1–4. doi: 10.5489/cuaj.10200.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park YJ, Kong HJ, Jang HC, et al. Malignant mesothelioma of the spermatic cord. Korean J Urol. 2011;52:225–9. doi: 10.4111/kju.2011.52.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia de Jalon A, Gil P, Azua-Romeo J, et al. Malignant mesothelioma of the tunica vaginalis. Report of a case without risk factors and review of the literature. Int Urol Nephrol. 2003;35:59–62. doi: 10.1023/a:1025952129438. [DOI] [PubMed] [Google Scholar]
- 14.Plas E, Riedl CR, Pfluger H. Malignant mesothelioma of the tunica vaginalis testis: review of the literature and assessment of prognostic parameters. Cancer. 1998;83:2437–46. doi: 10.1002/(sici)1097-0142(19981215)83:12<2437::aid-cncr6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]


