Abstract
Small cell carcinoma of the gastrointestinal tract is a rare and aggressive neuroendocrine tumor. This study aims to analyze the clinical characteristics and potential prognostic factors for patients with limited stage small cell carcinoma of the gastrointestinal tract. The records of 27 patients with limited stage small cell carcinoma of the gastrointestinal tract, who all received surgery with lymphadenectomy, were retrieved and analyzed retrospectively. The median age of patients was 60 years old (range 38–79). The primary locations of tumor were the esophagus (74.1%) and stomach (14.8%). The rate of preoperative accurate diagnosis (16.7%) was low for small cell carcinoma of the esophagus and stomach. 40.7% of all the patients had regional lymph node metastases. Five patients underwent surgery alone, and the other 22 were treated with surgery + postoperative chemotherapy. All patients had disease progression or recurrence. The overall median survival time was 10 months and the 1-year survival rate was 37.0%. Patients who received postoperative chemotherapy had a median survival time of 12 months, which was superior to the 5-month survival of for those who only had surgery (P<0.0001). TNM stage (P=0.02) and postoperative chemotherapy (P<0.0001) were considered as two prognostic factors in uni-variate analysis. Postoperative chemotherapy was a significant independent prognostic factor in multivariate analysis (P=0.01). The prognosis for patients with limited stage small cell carcinoma of the gastrointestinal tract remains dismal, however, postoperative chemotherapy may have the potential to improve the outcome for these patients.
Key words: carcinoma, small cell, gastrointestinal tract, pathology, clinical.
Introduction
Small cell carcinoma (SCC) is a rare aggressive malignant neuroendocrine tumor composed of small round or egg-shaped cells with little cytoplasm. The most common site of SCC is lung. In 1930, extrapulmonary SCC was first described by Duguid and Kennedy.1 Since then, cases of SCC have been reported in almost all sites of the body, including the gastrointestinal tract (GIT),2 head and neck,3 urinary tract,4 and genital system.5
SCC of the GIT occurs infrequently. Approximately 1000 cases have been reported in the English literature with the estimated prevalence of 0.1% to 1% of all gastrointestinal tumors.6 SCC can originate throughout the GIT, with the esophagus frequently involved. Early sporadic data have indicated that SCC arising from different digestive locations has similar biological behavior, including clinical presentation and pattern of metastasis, which suggests that SCC of the GIT could be regarded and treated as one clinical entity.2 Regrettably, overall information about SCC of the GIT is limited due to its rarity and the fact that most reports focused on a particular location within the GIT. Currently, its clinicopathological characteristics and standard treatment are far from being well established. Especially in the setting of limited stage SCC of the GIT, early accurate diagnosis is challenging and the role of surgery is still controversial. Some reports suggested that surgical resection of limited stage SCC could result not only in a significant locoregional control but also in long-term disease-free survival.7,8 However, other data indicated that patients treated with surgery alone had rapid systemic recurrence.9,10
Therefore, this retrospective study was performed to analyze the clinical characteristics and potential prognostic factors for patients with clinically limited stage SCC of the GIT.
Materials and Methods
We reviewed the records of 27 consecutive patients with limited stage SCC of the GIT diagnosed histologically, who underwent intended surgery with regional lymphadenectomy in the Affiliated Drum Tower Hospital of Nanjing University Medical School (Nanjing, China) from March 2006 to August 2011. The following data were collected for each patient: demographic data, presenting symptoms, methods of tumor diagnosis, staging procedures, pathologic findings, types of treatment, and survival time. Differential diagnoses were made immuohistochemically by using antibodies chromogranin A, synaptophysin, neuron-specific enolase (NSE) and cluster differentiation 56 (CD56). The histological and immunochemical diagnosis of SCC was confirmed by two independent pathologists. Staging work-up included computed tomography (CT) scan of the chest, abdomen and pelvis. Other modalities included endoscopic ultrasound (EUS) and magnetic resonance imaging. Tumors were staged according to the 2002 American Joint Committee on Cancer (AJCC) TNM staging system for each affected organ and Veterans' Administration Lung Study Group (VALSG) criteria. The latter consists of two staging categories: limited and extensive disease. Limited disease is defined as a tumor confined to a localized anatomic region such as any single organ (e.g. the esophagus, stomach, colon, gallbladder, and pancreas), with or without regional lymph node involvement. Extensive disease is defined as a tumor spread beyond any localized anatomic region. Survival time was defined as the time from the date of the end of treatment to death or the last follow-up visit. All patients gave informed consents prior to gastroscopy, surgery, or chemotherapy. This study was approved by the Ethics Committee of Drum Tower Hospital.
Statistical analysis was performed using SPSS 11.5 software (SPSS Inc., Chicago, IL, USA). The impact of clinical and pathologic risk factors on survival was evaluated using Kaplan-Meier life table analyses and log-rank tests. The independent prognostic factors were evaluated using Cox's hazard regression model. All tests were two-tailed and a P value <0.05 was considered significant.
Results
Patient's characteristics
Twenty-seven patients (18 men and 9 women) with limited stage SCC of the GIT accounted for 0.2% of all patients with digestive malignancies treated at our hospital during the study period. The clinical characteristics are summarized in Table 1. The median age was 60 years (range 38–79). The common symptoms were dysphagia (51.9%), loss of appetite (40%) and jaundice (7.4%). Paraneoplastic syndromes were not observed in our series. The most common primary sites were the esophagus (74.1%), followed by the stomach (14.8%). The average tumor length was 4.5 cm (range 0.2–14).
Table 1. Clinical characteristics of patients with small cell carcinoma of the gastrointestinal tract (N=27).
| Characteristic | No. in group (% of entire group) |
|---|---|
| Sex | |
| Male | 18 (66.7%) |
| Female | 9 (33.3%) |
| Location | |
| Esophagus | 20 (74.1%) |
| Stomach | 4 (14.8%) |
| Colon | 1 (3.7%) |
| Gallbladder | 1 (3.7%) |
| Pancreas | 1 (3.7%) |
| Colon | 1 (3.7%) |
| Gallbladder | 1 (3.7%) |
| Pancreas | 1 (3.7%) |
| Histological homology | |
| Pure | 25 (92.6) |
| Mixed | 2 (7.4) |
| Pure | 25 (92.6%) |
| Mixed | 2 (7.4%) |
| TNM | |
| I | 10 (37.0%) |
| II | 10 (37.0%) |
| III | 7 (25.9%) |
| Lymph node involvement | |
| Yes | 11 (40.7%) |
| No | 16 (59.3%) |
| Lymphatic vessel invasion | |
| Yes | 17 (63.0%) |
| No | 10 (37.0%) |
| Therapy | |
| Surgery | 5 (18.5%) |
| Surgery + chemotherapy | 22(81.5%) |
According to the TNM classifications, 10 cases were stage I, 10 cases were stage II, and 7 cases were stage III. Of the 27 patients, 40.7% (11/27) postoperatively had lymph node metastasis and 63.0% (17/27) had lymphatic vessel invasion.
Diagnostic investigations
Primary SCCs of the lung were excluded in all the patients based on the CT scans of the chest. Twenty-four patients (20 esophagus, 4 stomach) underwent gastroscopic examinations and were biopsied. Among 20 esophagus SCC, 50% were located in the lower third of the esophagus, 40% were in the middle, and only 10% were in the upper third. In terms of endoscopic appearance, 15 were classified as ulcerative type, 3 were mushroom type, and 2 were submucosal protruded type (Figure 1A). Interestingly, in one of the submucosal SCCs of the esophagus, a patch-like erosion was observed at 24–28 cm (6:00-7:00 position) before staining, and a large irregular unstained area was present near the tumor after Lugol's iodine staining (Figure 1B and C). Three patients (2 esophagus, 1 stomach) preoperatively underwent endoscopic ultrasound (EUS) examinations that exhibited homogeneous hypoechoic or isoechoic masses with regular borders originating from submucosal layer or muscularis propria, which were initially mistaken as granular cell tumors (Figure 1D) or stromal tumors. Only 4 (16.7%) biopsy specimens yielded a positive diagnosis for SCC, 8 (33.3%) were misclassified as undifferentiated adenocarcinoma, 5 (20.8%) were interpreted as squamous carcinoma, 4 (16.7%) were displayed as epithelial tumors, and 3 (12.5%) were considered as reactive or nonneoplastic. The remaining 3 cases (1 pancreas, 1 colon, and 1 gallbladder), all of whom received contrast-enhanced CT for diagnosis, were not biopsied preoperatively. In the case of SCC in pancreas, CT displayed a large, heterogeneous, and marked enhancing mass at the pancreatic head (Figure 2A and B).
Figure 1.
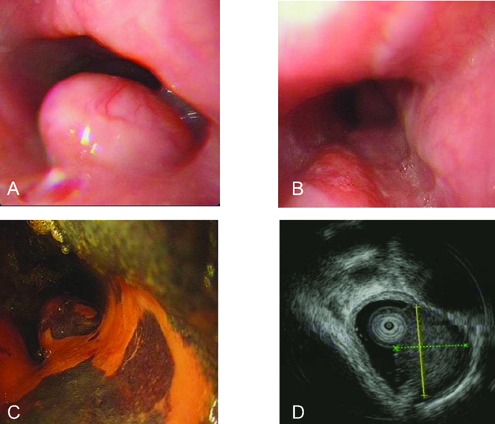
Endoscopic views of the small cell carcinoma of esophagus. A) A submucosal elevated lesion (1.5x1.5 cm) covered with normal esophageal mucosa at 25 cm in esophagus; B) a patch-like erosion is observed at 24-28 cm (6:00-7:00 position) in esophagus before staining; C) a large irregular unstained area at 24-28 cm in esophagus after Lugol's iodine staining; D) a homogeneous, isoechoic mass (1.0×1.1 cm2) with a regular border originating from submucosal layer in esophagus, which is diagnosed as a granular cell tumor by EUS.
Figure 2.
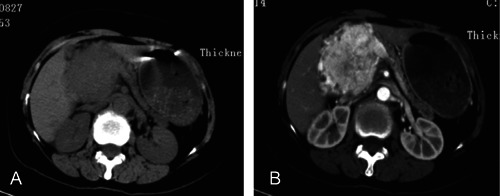
Small cell carcinoma of the pancreas in a 56-year-old man. A) Computed tomography (CT) scanning demonstrates a 5.0×3.8 cm mass at the head of the pancreas, with atrophic pancreatic parenchyma and main pancreatic duct dilation. B) Contrast-enhanced CT scanning shows a marked, heterogeneous enhancement in both peripheral and central portion of the tumor at 30s after administration of contrast medium.
Histology and immunohistochemistry
Histology in SCC of the GIT was similar to classical SCC: spindle-shaped cells with scanty cytoplasm and hyperchromatic nuclei. Twenty-five cases (92.6%) had pure SCC, 1 (3.7%) case of gallbladder had mixed type glandular adenocarcinoma differentiation (Figure 3A), and 1 (3.7%) case of esophagus had mixed type glandular squamous differentiation.
Figure 3.
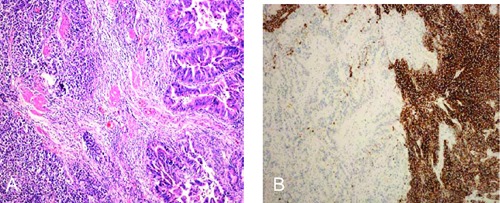
Mixed small cell adenocarcinoma of the gallbladder. A) Haematoxylin &Eosin stained sections shows small, round cells with scanty cytoplasm, granular nuclear chromatin and inconspicuous nucleoli (left), which is combined with components of adenocarcinoma (right) (Magnification x100); B) immunohistochemical staining for synaptophysin, which is positive in the small cell carcinoma area and negative in the adenocarcinoma area.
All surgical specimens were immunohistochemically stained for epithelial and neuroendocrine markers. Of all cases, 20 (74.1%) were synaptophysin positive (Figure 3B), 14 (51.9%) were chromogranin A positive, 12 (44.4%) were CD56 positive, and 10 (37.0%) were NSE positive.
Treatment and prognosis
All of the 27 patients underwent radical surgery and in 2 cases (1 esophagus and 1 stomach) local resection was first performed by thoracoscopy or gastroscopy. Among all the patients undergoing operations, five were treated with surgery alone and the other 22 were treated with surgery + postoperative chemotherapy. Of the 5 patients undergoing surgery alone, three declined further treatment, and the other two were not candidates for chemotherapy because of poor performance status. Among those who received chemotherapy, twelve cases received etoposide combined with cisplatin (EP), and the other 10 cases received a combined regimen of cyclophosphamide + doxorubicin + cisplatin (CAP). All 27 patients died and none was lost to follow-up. The median survival was 10 months. The 6- and 12-month survival rates for the whole group were 70.4% and 37.0%, respectively (Figure 4A). In view of different primary sites, the median survival time for patients with SCC of the esophagus was 12 months followed by that of SCC of the stomach (median survival time, 11 months). There was no statistically significant difference in survival time among patients with SCC of these different locations (P=0.30). With regard to different therapeutic options, the median survival time for patients who received surgery alone was 5.0 months, with a 6-month survival rate of 0%. For patients undergoing surgery + postoperative chemotherapy, the median survival time was 12 months, with a 6-month survival rate of 86.4% and a 12-month survival rate of 50% (Figure 4B). Furthermore, there was a statistically significant difference in survival between surgery alone versus surgery + postoperative chemotherapy (P<0.0001). Also, there was no significant difference in the median survivals for the cases with EP and with CAP (12 vs 11 months, P=0.26). Univariate analyses revealed that TNM stage and postoperative chemotherapy correlated with the outcome of patients (Table 2). Multivariate analysis indicated that postoperative chemotherapy was a significant independent prognostic factor for overall survival [Hazard ratio, 0.43; 95% CI (0.004–0.49); P=0.01] (Table 3), which suggested that the patients who underwent postoperative chemotherapy had a better prognosis. In addition, all the patients had disease progression despite multimodal therapy during the follow-up, including 7 local recurrences and 20 distant metastasis. The most frequent sites of metastasis were as follows: lymph node (45%), liver (40%), lung (10%), and bone (5%).
Figure 4.
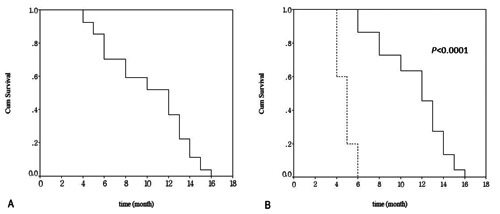
Kaplan-Meier curve of overall survival for the patients. A) Survival curve of patients with small cell carcinoma of the gastrointestinal tract with limited stage; B) survival curves for the patients with and without chemotherapy (Solid line: surgery + chemotherapy; dashed line: surgery; P<0.0001)
Table 2. Univariate analysis of survival on clinical and pathologic factors.
| Variables | Median survival (month) | Survival rate (%) | P value | |
|---|---|---|---|---|
| 6-month | 1-year | |||
| Gender | ||||
| Male | 9 | 66.7 | 27.8 | 0.09 |
| Female | 13 | 77.8 | 55.6 | |
| Age (year) | ||||
| <60 | 12 | 76.9 | 30.8 | 0.86 |
| ≥60 | 8 | 64.3 | 42.9 | |
| Location | ||||
| Esophagus | 12 | 75 | 40 | 0.30 |
| Stomach | 11 | 75 | 25 | |
| Colon | - | - | - | |
| Gallbladder | - | - | - | |
| Pancreas | - | - | - | |
| Tumor length (cm) | ||||
| <4.5 | 12 | 84.6 | 46.2 | 0.14 |
| ≥4.5 | 8 | 57.1 | 28.6 | |
| Histological homology | ||||
| Pure | 12 | 76 | 40 | 0.07 |
| Mixed | 6 | 0 | - | |
| TNM stage | ||||
| I | 13 | 90 | 60 | 0.02 |
| II | 12 | 70 | 40 | |
| III | 6 | 42.9 | 0 | |
| Lymph node involvement | ||||
| Yes | 8 | 54.6 | 27.3 | 0.41 |
| No | 12 | 81.3 | 43.8 | |
| Lymphatic vessel invasion | ||||
| Yes | 10 | 58.8 | 29.4 | 0.50 |
| No | 12.5 | 90 | 50 | |
| Therapy | ||||
| Surgery | 5 | 0 | 0 | <0.0001 |
| Surgery + chemotherapy | 12 | 86.4 | 45.5 | |
Table 3. Multivariate analysis of survival on clinical and pathologic factors.
| Variables | Hazard ratio (95% CI) | P |
|---|---|---|
| Gender | 1.44 (0.47-4.46) | 0.53 |
| Location | 0.96 (0.58-1.59) | 0.89 |
| Tumor length (≥4.5 cm) | 1.38 (0.18-1.77) | 0.56 |
| Pure histology | 0.39 (0.03-5.08) | 0.39 |
| TNM | 2.87 (0.87-9.47) | 0.08 |
| Lymph node involvement | 0.49 (0.10-2.51) | 0.39 |
| Lymphatic vessel invasion | 0.56 (0.18-1.77) | 0.33 |
| Chemotherapy | 0.43 (0.004-0.49) | 0.01 |
CI, confidence interval.
Discussion
SCC of the GIT is a kind of rare and highly aggressive malignancy; however, the exact pathogenesis is remaining largely unknown. Ho et al. suggested that SCC might be of endodermal origin derived from a pluripotent stem cell, which might have differentiated into mucin or keratin producing cells.11 This suggestion is supported by the findings of Chen and Matsui.12,13 Similarly, our study also indicates that SCC of the esophagus and gallbladder are admixed with other histologic types of carcinoma such as adenocarcinoma or squamous cell carcinoma. Moreover, in one case with SCC of the esophagus, Lugol's iodine staining exhibited a clearly unstained area near the tumor, and biopsies revealed moderate dysplasia. Maitra et al. have described that dysplastic epithelium was present in gallbladder SCC.14 Burke et al. found that overlying adenomas were present in 45% of SCC of the large intestine.15 In addition, a few genetic and molecular alternations have been recorded, including the identification of high proliferative activity, prevalence of p53 overexpression, Rb loss, telomerase activation and k-ras mutations.14,16–18 However, these genetic and molecular data are only derived from SCC of the esophagus. To date, it is unclear whether such factors play a role in the pathogenesis of SCC of the GIT, but further investigations into their functions is warranted.
Demographically, the clinical features of our patients with limited stage SCC of the GIT are similar to those of patients with carcinomas in the corresponding affected organ of the GIT. Similar to the findings of Brenner et al,2 most of our patients were men with the median age of 60 years. The most common primary location of limited stage SCC of the GIT is the esophagus. Of note, several reports have addressed that SCC of the GIT rarely secretes various ectopic hormones such as vasoactive intestinal peptide, gastrin, calcitonin, adrenocorticotropic hormone and antidiuretic hormone,19–23 which can result in paraneoplastic syndromes and even dominate clinical presentation. Our series did not exhibit clinical signs of paraneoplastic syndromes, so we did not assess these hormone levels. In clinical practice, it is nevertheless necessary to evaluate paraneoplastic syndromes in the patients especially when there is a clinical suspicion of SCC of the GIT.
Grossly, most endoscopic and radiological features of limited stage SCC of the GIT are identical to those of other carcinomas of the corresponding sites. Our study reveals that two esophageal SCCs and one gastric SCC were submucosal tumors. Further EUS examinations found homogeneous hypoechoic or isoechoic solid masses with clear margins arising from the submucosal layer or muscularis propria. These masses were misclassified as granular cell tumors or stromal tumors by EUS. This phenomenon might be caused by rapid proliferation of the tumor into the submucosal layer. In our case of SCC of the pancreas, the mass was heterogeneously enhanced by contrast enhanced CT, as reported by Ichikawa et al.24 and Namieno T et al.25 Generally, the adenocarcinomas of the pancreas are hypovascular, and neuroendocrine tumors are hypervascular by contrast-enhanced CT, which suggests that contrast-enhanced CT is useful for the differential diagnosis of SCC of the pancreas. However, the diagnosis should be made carefully by contrast enhanced CT for large SCC of the pancreas with necrosis, which may present as a heterogeneously low-density mass on contrast-enhanced CT scanning. In addition, radio-labeled somatostatin analogue scanning is also used to detect metastatic diseases and evaluate the stage of SCC of the GIT,26 but its routine use in SCC of the GIT remains controversial.21
The diagnosis of SCC of the GIT primarily depends on histopathology, but it is extremely difficult to confirm the diagnosis preoperatively based on the biopsy. In our study, 83.3% cases were diagnosed as undifferentiated adenocarcinoma, squamous carcinoma or other types. The following reasons may account for the misdiagnosis based on the biopsy: first, biopsy specimen is difficult to be obtained because of the proliferations of tumor cells mainly in the submucosal layer; second, histological heterogeneity is common; third, microscopic features usually resemble other malignancies such as malignant lymphoma or undifferentiated carcinoma; and finally, SCC in biopsy material always becomes distorted and obscures the diagnosis.27,28 Consequently, more tissues and multipoint biopsies should be performed to establish a correct diagnosis. Moreover, electron-microscopical, immunohistochemical and molecular findings are useful for the differential diagnosis. Similar to previous reports,18,29 our results showed that most of operative specimens were positive for synaptophysin and chromogranin A. However, for the diagnosis of SCC of the GIT, it is unnecessary to make immunohistochemical assessment of neuroendocrine differentiation.30 Additionally, because of the paucity of SCC of the GIT, it is better to exclude the primary SCC of the lung first when establishing the diagnosis of SCC of the GIT.
SCC of the GIT has a very poor prognosis with a high metastatic potential. The literature reports that the median survival ranges from 6 to 12 months, and the 1-year survival rate varies from 30% to 50% for all stages of SCC of the GIT.2,6 Not surprisingly, patients with limited stage have a more favorable outcome than those with extensive stage SCC of the GIT. Brenner et al. found that the patients of limited stage had a better survival, with a median survival of 21.9 months and a 1-year survival rate of 72% as compared with those with extensive stage, who had a median survival of 5.8 months and a 1-year survival rate of 29% .2 Lv et al. reported a median survival of 14.0 months and a 1-year survival rate of 62.1% for limited stage in a series of esophageal SCC.10 In our study, the overall median survival and 1-year survival rate were 10 months and 37.0%, respectively. Compared with previous results, the patients in our series had a poorer prognosis due to the small sample size, a higher regional lymph node metastasis (40.7%), and lymphatic invasion (63.0%). In addition, Brenner et al. demonstrated that performance status, weight loss and the extent of disease were independent prognostic factors for all stages of SCC of the GIT.2 Casas et al. considered tumor size and additional chemotherapy as independent prognostic factors in esophageal SCC with limited stage.31 Chen et al. suggested that both surgery and chemotherapy strongly correlated with survival in esophageal SCC with limited stage.12 Our multivariate analysis found that postoperative chemotherapy significantly improved the outcome of patients with limited stage SCC of the GIT. Unfortunately, similar to other data,2,10,29 all patients in our group had disease progression or recurrence despite multimodal therapy. Consequently, it is of the utmost importance to perform a study of appropriate regimens for SCC of the GIT with limited stage.
Chemotherapy is now recognized as the cornerstone of treatment for SCC of the GIT because micrometastases are frequently present and the rate of recurrence is high,10,12,32 even in the setting of limited disease. In our series, postoperative chemotherapy significantly improved survival in patients with limited stage. However, current chemotherapeutic agents for SCC fail to eliminate tumor cells of adeno or squamous phenotype completely.2,33 Surgery therefore has a potential role in limited stage, given the high prevalence of mixed tumor histology. Yet, the exact role of surgical treatment remains controversial in limited stage. The most important explanation for this controversy is that the survival superiority found for different treatments may reflect a selection bias of more aggressive therapies for more suitable patients who are in better conditions before treatment. Our study could not respond to the question about the role of the surgery in limited stage, although surgical resection was frequently the first choice in our series. Therefore, without randomized controlled trials of treatment, it will remain difficult to draw conclusions about the most effective treatment. In practice, limited stage SCC of the GIT should be regarded as a systemic disease and treated by multimodal approaches.
Conclusions
In conclusion, SCC of the GIT is a relatively rare and aggressive tumor with a dismal prognosis. Our retrospective study shows a survival advantage favoring postoperative chemotherapy for limited stage SCC of the GIT. More prospective studies on a larger scale should be conducted to further illuminate the pathogenesis of limited stage SCC of the GIT and define the optimal therapeutic regimen.
Acknowledgements:
we would like to thank Dr. Jun Chen (Department of Pathology, the Affiliated Drum Tower Hospital of Nanjing University Medical School, China) for good advice on pathology of small cell carcinoma.
References
- 1.Duguid JB, Kennedy AM. Oat-cell tumors of mediastinal glands. J Pathol Bacteriol. 1930;33:93–9. [Google Scholar]
- 2.Brenner B, Shah MA, Gonen M, et al. Small-cell carcinoma of the gastrointestinal tract: a retrospective study of 64 cases. Br J Cancer. 2004;90:1720–6. doi: 10.1038/sj.bjc.6601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renner G. Small cell carcinoma of the head and neck: a review. Semin Oncol. 2007;34:3–14. doi: 10.1053/j.seminoncol.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Shahab N. Extrapulmonary small cell carcinoma of the bladder. Semin Oncol. 2007;34:15–21. doi: 10.1053/j.seminoncol.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Chan JK, Loizzi V, Burger RA, et al. Prognostic factors in neuroendocrine small cell cervical carcinoma: a multivariate analysis. Cancer. 2003;97:568–74. doi: 10.1002/cncr.11086. [DOI] [PubMed] [Google Scholar]
- 6.Brenner B, Tang LH, Shia J, et al. Small cell carcinomas of the gastrointestinal tract: clinicopathological features and treatment approach. Semin Oncol. 2007;34:43–50. doi: 10.1053/j.seminoncol.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Mitani M, Kuwabara Y, Shinoda N, et al. Long-term survivors after the resection of limited esophageal small cell carcinoma. Dis Esophagus. 2000;13:259–61. doi: 10.1046/j.1442-2050.2000.00124.x. [DOI] [PubMed] [Google Scholar]
- 8.Yachida S, Matsushita K, Usuki H, et al. Long-term survival after resection for small cell carcinoma of the esophagus. Ann Thorac Surg. 2001;72:596–7. doi: 10.1016/s0003-4975(00)02528-5. [DOI] [PubMed] [Google Scholar]
- 9.Takaku H, Oka K, Naoi Y, et al. Primary advanced gastric small cell carcinoma: a case report and review of the literature. Am J Gastroenterol. 1999;94:1402–4. doi: 10.1111/j.1572-0241.1999.01095.x. [DOI] [PubMed] [Google Scholar]
- 10.Lv J, Liang J, Wang J, et al. Primary small cell carcinoma of the esophagus. J Thorac Oncol. 2008;3:1460–5. doi: 10.1097/JTO.0b013e31818e1247. [DOI] [PubMed] [Google Scholar]
- 11.Ho KJ, Herrera GA, Jones JM, Alexander CB. Small cell carcinoma of the esophagus: evidence for a unified histogenesis. Hum Pathol. 1984;15:460–8. doi: 10.1016/s0046-8177(84)80081-7. [DOI] [PubMed] [Google Scholar]
- 12.Chen SB, Yang JS, Yang WP, et al. Treatment and prognosis of limited disease primary small cell carcinoma of esophagus. Dis Esophagus. 2011;24:114–9. doi: 10.1111/j.1442-2050.2010.01112.x. [DOI] [PubMed] [Google Scholar]
- 13.Matsui K, Kitagawa M, Miwa A, et al. Small cell carcinoma of the stomach: a clinico-pathologic study of 17 cases. Am J Gastroenterol. 1991;86:1167–75. [PubMed] [Google Scholar]
- 14.Maitra A, Tascilar M, Hruban RH, et al. Small cell carcinoma of the gallbladder: a clinicopathologic, immunohistochemical, and molecular pathology study of 12 cases. Am J Surg Pathol. 2001;25:595–601. doi: 10.1097/00000478-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Burke AB, Shekitka KM, Sobin LH. Small cell carcinomas of the large intestine. Am J Clin Pathol. 1991;95:315–21. doi: 10.1093/ajcp/95.3.315. [DOI] [PubMed] [Google Scholar]
- 16.Lam KY, Law S, Tung PH, Wong J. Esophageal small cell carcinomas: clinico-pathologic parameters, p53 overexpression, proliferation marker, and their impact on pathogenesis. Arch Pathol Lab Med. 2000;124:228–33. doi: 10.5858/2000-124-0228-ESCC. [DOI] [PubMed] [Google Scholar]
- 17.Takubo K, Nakamura K, Sawabe M, et al. Primary undifferentiated small cell carcinoma of the esophagus. Hum Pathol. 1999;30:216–21. doi: 10.1016/s0046-8177(99)90279-4. [DOI] [PubMed] [Google Scholar]
- 18.Parwani AV, Geradts J, Caspers E, et al. Immunohistochemical and genetic analysis of non-small cell and small cell gallbladder carcinoma and their precursor lesions. Mod Pathol. 2003;16:299–308. doi: 10.1097/01.MP.0000062656.60581.AA. [DOI] [PubMed] [Google Scholar]
- 19.Watson KJ, Shulkes A, Smallwood RA, et al. Watery diarrhea-hypokalemia-achlorhydria syndrome and carcinoma of the esophagus. Gastroenterology. 1985;88:798–803. doi: 10.1016/0016-5085(85)90154-4. [DOI] [PubMed] [Google Scholar]
- 20.Nishimaki T, Suzuki T, Fukuda T, et al. Primary small cell carcinoma of the esophagus with ectopic gastrin production. Report of a case and review of the literature. Dig Dis Sci. 1993;38:767–71. doi: 10.1007/BF01316813. [DOI] [PubMed] [Google Scholar]
- 21.O'Byrne KJ, Cherukuri AK, Khan MI, et al. Extrapulmonary small cell gastric carcinoma. A case report and review of the literature. Acta Oncol. 1997;36:78–80. doi: 10.3109/02841869709100738. [DOI] [PubMed] [Google Scholar]
- 22.Corrin B, Gilby ED, Jones NF, Patrick J. Oat cell carcinoma of the pancreas with ectopic ACTH secretion. Cancer. 1973;31:1523–7. doi: 10.1002/1097-0142(197306)31:6<1523::aid-cncr2820310633>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Doherty MA, McIntyre M, Arnott SJ. Oat cell carcinoma of esophagus: a report of six British patients with a review of the literature. Int J Radiat Oncol Biol Phys. 1984;10:147–52. doi: 10.1016/0360-3016(84)90421-8. [DOI] [PubMed] [Google Scholar]
- 24.Ichikawa T, Federle MP, Ohba S, et al. Atypical exocrine and endocrine pancreatic tumors (anaplastic, small cell, and giant cell types): CT and pathologic features in 14 patients. Abdom Imag. 2000;25:409–19. doi: 10.1007/s002610000058. [DOI] [PubMed] [Google Scholar]
- 25.Namieno T, Koito K, Nagakawa T, et al. Diagnostic features on images in primary small cell carcinoma of the pancreas. Am J Gastroenterol. 1997;92:319–22. [PubMed] [Google Scholar]
- 26.Berkel S, Hummel F, Gaa J, et al. Poorly differentiated small cell carcinoma of the pancreas. A case report and review of the literature. Pancreatology. 2004;4:521–6. doi: 10.1159/000080526. [DOI] [PubMed] [Google Scholar]
- 27.Kusayanagi S, Konishi K, Miyasaka N, et al. Primary small cell carcinoma of the stomach. J Gastroenterol Hepatol. 2003;18:743–7. doi: 10.1046/j.1440-1746.2003.02822.x. [DOI] [PubMed] [Google Scholar]
- 28.Brenner B, Tang LH, Klimstra DS, Kelsen DP. Small-cell carcinomas of the gastrointestinal tract: a review. J Clin Oncol. 2004;22:2730–9. doi: 10.1200/JCO.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 29.Vos B, Rozema T, Miller RC, et al. Small cell carcinoma of the esophagus: a multi-centre Rare Cancer Network study. Dis Esophagus. 2011;24:258–64. doi: 10.1111/j.1442-2050.2010.01133.x. [DOI] [PubMed] [Google Scholar]
- 30.Gaffey MJ, Mills SE, Lack EE. Neuroendocrine carcinoma of the colon and rectum. A clinicopathologic, ultrastructural, and immunohistochemical study of 24 cases. Am J Surg Pathol. 1990;14:1010–23. doi: 10.1097/00000478-199011000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Casas F, Ferrer F, Farrús B, et al. Primary small cell carcinoma of the esophagus: a review of the literature with emphasis on therapy and prognosis. Cancer. 1997;80:1366–72. [PubMed] [Google Scholar]
- 32.Ku GY, Minsky BD, Rusch VW, Bains M, Kelsen DP, Ilson DH. Small-cell carcinoma of the esophagus and gastroesophageal junction: review of the Memorial Sloan-Kettering experience. Ann Oncol. 2008;19: 533–7. doi: 10.1093/annonc/mdm476. [DOI] [PubMed] [Google Scholar]
- 33.Medgyesy CD, Wolff RA, Putnam JB, Jr, Ajani JA. Small cell carcinoma of the esophagus: the University of Texas M. D. Anderson Cancer Center experience and literature review. Cancer. 2000;88:262–7. [PubMed] [Google Scholar]


