Abstract
The present study examined the genetic and environmental etiology of decision-making (Iowa Gambling Task; Bechara et al., 1994), in a sample of twins at ages 11-13, 14-15, and 16-18 years. The variance across five 20-trial blocks could be explained by a latent “decision-making” factor within each of the three times of IGT administration. This latent factor was modestly influenced by genetic factors, explaining 35%, 20% and 46% of the variance within each of the three times of IGT administration. The remaining variance was explained by the non-shared environment (65%, 80% and 54%, respectively). Block-specific non-shared environmental influences were also observed. The stability of decision-making was modest across development. Youth showed a trend to choose less risky decks at later ages, suggesting some improvement in task performance across development. These findings contribute to our understanding of decision-making by highlighting the particular importance of each person's unique experiences on individual differences.
Keywords: Decision-making, Iowa Gambling Task, Genetics, Twins
The Iowa Gambling Task (IGT) was developed to characterize abnormalities in social and emotional decision-making using standardized laboratory procedures (Bechara et al., 1994). This task was specifically designed to detect decision-making deficits in patients with dysfunctional ventromedial prefrontal cortex (vmPFC) (Bechara et al., 1994), although recent brain imaging research has suggested that both ventral and dorsal prefrontal cortices are involved in the task (Lawrence et al., 2009). Based on the somatic marker hypothesis, emotion-based biasing signals arising from the body are integrated in higher brain regions, in particular the vmPFC, which in turn regulates decision-making (Damasio, 1994). To date, no study has examined to what extent genetic and environmental factors influence decision-making as measured by IGT performance using data collected on twins.
The IGT resembles real life decisions in terms of reward, punishment, and uncertainty of outcomes. The instrument requires participants to select 100 cards freely from any of four decks. Two of the decks are “risky” since they are associated with high immediate gains but larger occasional losses that result in net loss over time. The other two decks are “safe”, and produce smaller wins but negligible losses such that there is a net profit over repeated choosing. The participant is expected to adopt an advantageous strategy, and select cards from the “safe” decks. However, individuals with vmPFC dysfunction continue to perseverate with the “risky” decks, sometimes even though they know that they are losing money overall (Bechara, Damasio & Damasio, 2000).
Apart from individuals with vmPFC dysfunction, others that perform poorly on the IGT include those with substance abuse problems (Grant, Contoreggi & London, 2000), obsessive-compulsive disorder (Cavedini et al., 2002), pathological gambling disorder (Linnet et al., 2006), and antisocial and criminal individuals (Yechiam et al., 2007). Children with attention deficits/hyperactive disorder (ADHD) (Garon, Moore & Waschbusch, 2006), conduct disorder (Ernst et al., 2003), early-onset schizophrenia (Kester et al., 2006), and psychopathic tendencies (Blair, Colledge & Mitchell, 2001) show IGT impairments as well.
There is much evidence to suggest that risk taking is related to low self-regulation and high impulsivity (Freeman & Muraven, 2010; Zuckerman & Kuhlman, 2000). Exerting self-regulation requires correctly computing the value of a better long-term decision over a worse short-term decision. In Gottfredson and Hirschi's General Theory of Crime they hypothesize that poor self-regulation is the sole cause of crime and other related problem behaviors including drug use, school failures, and having delinquent friends that are all manifestations of an underlying tendency to pursue short-term immediate pleasure at the expense of long-term consequences. Individual self-regulation improves with age as a result of many factors including changing biology through hormonal development, socialization, and increasing opportunity costs of losing control (Gottfredson & Hirschi, 1990). Low self-control has been found to be related to gambling behavior, binge-drinking, antisocial behavior and traffic violations (Delisi & Vaughn, 2008; Gibson, 2010). Recently low self-control was shown to be the strongest predictor of career criminality; it was found to even exceed the impact of age, race, ethnicity, gender, socioeconomic status, mental illness, ADHD, and trauma experiences (Delisi & Vaughn, 2008). Therefore, understanding the etiology of decision-making may shed light on our further knowledge of the trait of self-control and related behavior problems.
The classical twin design, which compares the similarity of monozygotic (MZ) and dizygotic (DZ) twins, is a powerful method for estimating the relative contribution of genetic and environmental effects on human traits. MZ twins reared together share part of their environment and are assumed to share 100% of their genes. Resemblance between them is therefore due to genetic and shared environmental effects. The non-shared environmental factors are the extent to which MZ twins do not resemble each other. Resemblance between DZ twins reared together is due to shared environment and to shared genes. DZ twins are assumed to on average share 50% of their genes. Consequently, resemblance between them due to genetic effects will be lower for DZ pairs than for MZ pairs. The extent to which DZ twins do not resemble each other is due to non-shared environmental factors and to non-shared genetic effects (Evans, Gillespie & Martin, 2002; Plomin et al., 2001). To understand the etiology of decision-making includes understanding its genetic and environmental underpinnings as well. There are different implications for ways in which decision-making may relate to other maladaptive outcomes, for example, depending on whether individual differences in decision-making are influenced by genetic factors, by environmental factors, or a combination of both. It is therefore of great interest to examine the genetic and environmental bases of decision-making, which may in turn give clues as to how the genetic predisposition to psychopathological behaviors is ultimately manifested. A clear understanding of the etiology of decision-making needs first to be established in order to proceed with future studies of the genetic and environmental covariation among related behaviors, such as antisocial or gambling behaviors.
Although there are no twin-studies examining genetic and environmental influences on decision-making as measured by the IGT, there are several twin studies examining genetic and environmental influences on other forms of risky behaviors, including gambling behavior, sensation seeking and delay discounting. A recent cross-sectional twin study used data from the National Longitudinal Study of Adolescent Health (Add Health) to examined age and sex differences in heritability of self-reported gambling behaviors. For males, genetic factors explained 85% of the variance in gambling behavior, with remainder being explained by the non-shared environment. For females, shared environment explained 45% of the variance and the non-shared environment explained the remaining 55% of the variance (Beaver et al., 2010). Another study of adult twins aged 32-43 years reported no sex differences in the genetic and environmental components, roughly half of the variance in gambling behavior (based on lifetime DSM-IV pathological gambling symptom counts) was due to genetic influences with the other half being due to non-shared environmental influences, with no effect of shared environment between the twins (Slutske et al., 2010). Similarly, symptoms of disordered gambling were explained by genetic (83%) and non-shared environmental factors (17%) in both males and females, based on a web-based data collection in a sample of twin and non-twin siblings (mean age 25.4 years) (Blanco, Myers & Kendler, 2012). Sensation seeking and delay discounting have also been found to be heritable (Miles et al., 2001; Stoel, De Geus & Boomsma, 2006). Delay discounting refers to the preference for smaller immediate rewards over larger but delayed rewards. In a recent study, participants were asked to choose between a smaller ($7) reward available immediately and a larger ($10) reward to be received in 7 days. At ages 12 and 14 years, 30 and 51% of the variance in ‘delay discounting’ was due to genetic influences, respectively, and the rest of the variance was due to non-shared environment (Anokhin et al., 2010b). Further, executive functioning is a broad domain encompassing many dimensions, including response inhibition, organizing and planning, updating, as well as decision-making (Miyake et al., 2000). Several studies have examined the extent to which genetic and environmental factors influence executive functioning as measured by performance on the Wisconsin Card Sorting Test (WCST) (Heaton et al., 1993). These studies have found conflicting evidence, with some studies reporting that the majority of the variance in WCST performance is due to non-shared environmental factors, i.e., environmental factors not shared by twins (Kremen et al., 2007; Taylor, 2007). Whereas others report significant genetic influences, explaining 37-46% of variance in young adult females (Anokhin, Heath & Ralano, 2003), and 19% and 49% in females aged 12 and 14 years, respectively (Anokhin et al., 2010a). Yet others have found significant shared environmental influences, i.e., non-genetic influences that contribute to co-twin similarity, explaining 30-38% of the variance in WCST performance in male and female twins aged 12-16 years old (Chou et al., 2010). Further, the variance in nine tasks of executive functions (inhibiting dominant responses, updating working memory representations, and shifting between task sets) could be summarized with a common latent factor, and 99% of the total variance in this latent “executive function” factor was explained by genetic influences (Friedman et al., 2008). Taken together, previous research is inconclusive regarding the genetic and environmental architecture of executive functioning, and twin studies specifically examining decision-making as measured with the IGT are lacking.
Another gap in the literature concerns the lack of stability data on the IGT performance. Several studies have found a pattern of decreased risky selection at repeated testing sessions (Ernst et al., 2003). For example, decreased risky selection on the IGT over the course of three administrations during one testing session was found among smokers and nonsmokers (Lejuez et al., 2003). A longitudinal study including 192 Chinese adolescents ages 15-17 years found a one-year phenotypic stability of r=.36 (Xiao et al., 2010). However, no study has directly examined the stability of the IGT over a longer time period, although it would be extremely helpful in assessing the performance improvement in the pre-and post-intervention situations (Buelow & Suhr, 2009), which usually spans in months or even years.
Few studies have examined the age effects on IGT performance. Cross-sectional studies show that performance on IGT significantly improves with age among children and adolescents (Overman, 2004). For example, a study including children and adolescents aged 8-10, 12-14, and 16-18 years using a child version of the IGT found that adolescents aged 16-18 learned to make advantageous choices over task blocks faster than the two younger age groups (Crone & van der Molen, 2007). Thus, it is possible that decision-making is related to prefrontal cortex maturation and cognitive development.
In the present study, we investigated the genetic and environmental sources of decision-making as measured by IGT in a longitudinal study at ages 11-13, 14-15 and 16-18 years, with data from a sample of male and female twins. Our aims were (1) to examine the genetic and environmental influences on decision-making within each measurement time point and the genetic and environmental overlap across time, and (2) to assess the stability of decision-making across development.
Method
Participants
The sample was drawn from participants in the University of Southern California Risk Factors for Antisocial Behavior (RFAB) twin study, which is a prospective study of the interplay of genetic, environmental, social, and biological factors on the development of antisocial behavior from childhood to emerging adulthood. The twins were recruited from the Los Angeles community and the sample is representative of the ethnic and socio-economic diversity of the greater Los Angeles area. Zygosity was determined based on DNA microsatellite analysis. Four waves of data have so far been collected. The total study sample consists of 1,573 subjects, complete details on the procedures and measures can be found elsewhere (Baker et al., 2006; Tuvblad et al., 2011). The present study used data from Waves 2, 3 and 4 as data on IGT were not collected during Wave 1. There were IGT data on n=343 subjects from the first IGT administration (i.e., Wave 2, twins age 11-13 years), n=831 subjects from the second IGT administration (i.e., Wave 3, twins age 14-15 years) and n=451 subjects from the third IGT administration (i.e., Wave 4, twins age 16-18 years). The large variation in N's across Waves occurred in part because not all subjects came to the lab to participate in the study, but instead participated via mail surveys and thus did not complete the IGT.
Iowa Gambling Task (IGT)
Computerized IGT was administered and the participants were asked to draw a card from each of the four (A, B, C, D) decks presented on a computer screen. They were told that some decks were better than the others and they could win more money by avoiding the bad decks. There were 100 trials in total. The number of card selection from risky decks (A or B) was computed for each of the five blocks, with 20 trials in each block (Mitchell et al., 2002). A total net score was calculated by subtracting the total number of cards selected in advantageous decks from the disadvantageous decks, e.g., (C+D)-(A+B). Prior to genetic analyses, the IGT scores were ranked and normalized using PROC RANK, the option normal=blom and PROC STANDARD to reduce the positive skewness in their distributions (van den Oord et al., 2000) in the statistical software SAS 9.1.3 (SAS, 2005). There was no significant mean difference in IGT performance between those that only participated during the first IGT administration and those that participated in the second IGT administration (t(821)=-.38, p=.71), and there was no significant mean difference in IGT performance between those that only participated during the second IGT administration and those that participated in the third IGT administration (t(449)=-1.19, p=.24),
Validity of the Iowa Gambling Task (IGT)
The construct validity of the IGT was carefully examined in a recent review (Buelow & Suhr, 2009) in which the authors concluded that there is preponderance of evidence to support the use of the IGT to detect poor decision-making. Using the current sample, we recently found a significant bio-social interaction whereby decision-making measured at ages 11-13 years (first IGT administration) was associated with psychopathic tendencies, but only in children from benign (i.e. high socioeconomic status) but not adverse home environments (Gao et al., 2009). Decision-making measured at ages 11-13 years was also significantly correlated with motor impulsivity (r=.07, p<.05), inattention-impulsivity (r=.11, p<.05), and non-planning impulsivity (r=.09, p<.05) as assessed by the Barratt Impulsiveness Scale (Barratt, 1959). We also found that in the present sample IGT performance measured at ages 14-15 years (second IGT administration) and at ages 16-18 years (third IGT administration) was each modestly but significantly correlated with concurrent self-reported psychopathic personality (14-15 years, r=.10, p<.05; Wave 4, r=.10, p<.05), and externalizing behavior (14-15 years, r=.10, p<.05; Wave 4, r=.07, p<.05). These findings together suggest that the construct validity of the IGT is modest in the present sample.
Statistical Analyses
First, potential differences in the mean and variance across zygosity group and sex were examined with t-test and F-test for decision-making using the total net score. To get a first indication of the underlying sources of variance in decision-making, comparisons were made among monozygotic (MZ) and dizygotic (DZ) intraclass correlations for the total net score. For example, a higher MZ than DZ correlation would indicate the influence of genetic factors.
Next, a 5×3 repeated measures analysis of variance (ANOVA) with Block (five blocks of 20 trials each) and Time of IGT administration (ages 11-13, 14-15, and 16-18 years) as within-subject factors was conducted on the number of risky selections (A or B) to examine whether the participants were able to avoid the risky decks during the course of 100 selections and if the change in selection was a function of age.
To estimate the relative contributions of additive genetic factors (A), shared environmental factors (C), and non-shared environmental factors (E) to decision-making within each of three times of IGT administration a series of multivariate genetic models were fit (Neale et al., 2003). All multivariate analyses were conducted across the five blocks within each of the three times of IGT administration. A longitudinal Cholesky decomposition was fit as a baseline model to risky selections across the five blocks within each of three times of IGT administration. A Cholesky model decomposes the variance of each phenotype (in this case the five blocks), as well as the co-variances among the five blocks into genetic (A), shared environmental (C) and non-shared (E) environmental factors. Cholesky decomposition model has the same number of factors in each of the A, C, and E components as the number of observed variables. That is, the first genetic factor loads on all five blocks, the second genetic factor loads on all blocks (Block II-V) except the first block (Block I) and so on, until the last genetic factor only loads on the final remaining block (Block V); this same procedure repeats for the shared environmental (C) and non-shared (E) environmental components. Next, a Common Pathway model was fit to the data across the five blocks within each of three times of IGT administration. In this model common genetic (Ac), common shared environmental (Cc), and common non-shared environmental (Ec) factors are mediated through a shared latent factor that represents the variance shared among the risky selections within each of the five blocks (Blocks I-V). The Common Pathway modelestimates fewer parameters than the multivariate Cholesky decomposition model and therefore, is more parsimonious. In addition to the genetic and environmental effects on the shared latent factor, As, Cs, and Es (which includes measurement error) parameters that are specific to each measure are also estimated (McArdle & Goldsmith, 1990; Neale & Cardon, 1992).
Genetic correlations (rg) and non-shared environmental correlations (re) among latent common factors (Ac, Ec) among the three times of IGT administration were also estimated using a Cholesky decomposition model. The genetic (or environmental) correlation indicates the extent to which individual differences in decision-making at two time points reflect overlapping (i.e., correlated) genetic (or environmental) influences. This statistic varies from -1.0 to +1.0, and is independent of the extent to which each trait is influenced by genetic (or environmental) factors (Posthuma et al., 2003).
Models were fit with the structural equation program Mx (Neale et al., 2003), using a maximum likelihood estimation procedure for raw data. Raw maximum likelihood yields a goodness of fit index called log-likelihood. The adequacy of fit was assessed by computing twice the difference between the log-likelihood of a full model and that of a submodel. This difference follows a χ2 distribution and a significant χ2 indicates that the model with fewer parameters to be estimated fits the data worse. The suitability of the models was also determined by comparing the model's Akaike Information Criterion (AIC) (Akaike, 1987) and Bayesian Information Criterion (BIC), where increasingly negative values correspond to increasingly better fitting models (Raftery, 1995).
Results
Descriptive Statistics and Correlations
No significant mean (based on t-tests) or variance differences (based on F-tests) were found between MZ and DZ male twins for decision-making (total net score) (Time 1 of IGT administration, 11-13 years: t(165)=.31, p=.76, F(87,78)=1.05, p=.83; Time 2 of IGT administration, 14-15 years, t(394)=-1.52, p=.13, F(158,236)=1.09, p=.54; Time 3 of IGT administration, 16-18 years, t(219)=-.22, p=.82, F(91,128)=1.02, p=.93), nor between MZ and DZ female twins (Time 1 of IGT administration, 11-13 years, t(174)=-.30, p=.77, F(88,86)=1.14, p=.54; Time 2 of IGT administration, 14-15 years, t(423)=1.28, p=.20, F(235,188)=1.21, p=.16; Time 3 of IGT administration, 16-18 years, t(226)=-.52, p=.61, F(121,105)=1.13, p=.51). No mean differences were found between males and females during Time 1 of IGT administration (t(341)=.40, p=.69) or Time 3 of IGT administration (t(341)=.40, p=.69), but during Time 2 of IGT administration males showed significantly higher mean scores (t(821)=2.31, p=.02).
Correlations
Phenotypic stability correlations across the three Times of IGT administration, as well as intraclass twin correlations for MZ and DZ pairs are shown in Table 1. The phenotypic stability in decision-making (total net score) across the three time points were moderate, Times 1–2 of IGT administration r=.32, p<.01; Times 1–3 of IGT administration r=.19, p<.01; Times 2–3 of IGT administration r=.39, p<.01.
Table 1.
Correlations for Decision-making on Three Occasions at Ages 11-13, 14-15 and 16-18 Years
| Phenotypic Stability | Twin Correlations | ||||
|---|---|---|---|---|---|
| Time 1 | Time 2 | Time 3 | MZ | DZ | |
| Time 1 (11-13 years) | 1 | .32* | .19* | .26* | .14 |
| Time 2 (14-15 years) | 1 | .39* | .07 | .12* | |
| Time 3 (16-18 years) | 1 | .33* | .16* | ||
Note.
p<.05
At Times 2 and 4, the MZ intraclass correlations were higher than the DZ intraclass correlations, suggesting genetic influences on decision-making (total net score).
Repeated Measures ANOVA
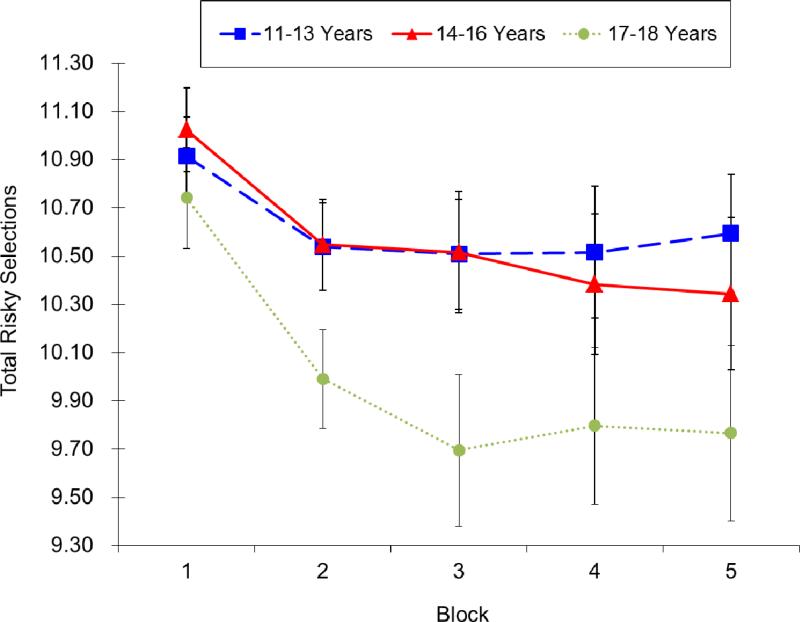
Among the subjects with complete IGT data at all three times (n=128), a significant main effect of Block was found, F(4, 124)=3.23, p<.05, indicating that the individuals learned to avoid risky decks across trials. The main effect of Time was also significant, F(2, 126)= 4.36, p<.05. Subjects made fewer risky selections at Time 3 of IGT administration than during Times 1 and 2 of IGT administration, F (1, 127) > 8.78, p < .01, but there was no significant difference between Times 1 and 2 of IGT administration, F (1, 127) < 1, n.s. The Block ×Time interaction was not significant, F(8, 120)< 1, n.s. The number of risky selections across blocks at three times of IGT administration is displayed in Figure 1. Although no significant Block×Time interaction effect was found, further probing showed that compared to Times 1 and 2 of IGT administration, subjects made fewer risky selections at Time 3 at Block 2, F(1, 127)>4.59, p <.05 and Block 3, F(1, 127)>4.76, p < .05, but not at Blocks 1, 4, and 5, F(2, 126)< 1, n.s. This finding suggests that subjects performed significantly better at ages 16-18 years (Time 3 of IGT administration) than at the two earlier ages of assessment.
Figure 1.
The number of risky cards selection (A or B) across blocks at three occasions of IGT administration.
Genetic Modeling
We first tested for quantitative sex differences using total net score within each of the three times of IGT administration. The magnitude of genetic and environmental influences were the same in boys and in girls (Time 1 of IGT administration, 11-13 years, χ2=22.39, df=14; p=.07; Time 2 of IGT administration, 14-15 years, χ2=23.70, df=14. p=.05; Time 3 of IGT administration, 16-18 years, χ2=18.31, df=14. p=.19), analyses available upon request from the first author. All subsequent analyses were conducted using two groups. One group comprised of all male and female MZ twins and the other group comprised of all male, female, and opposite sex DZ twins (Time 1 of IGT administration, 11-13 years: n=177 MZ twins, n=166 DZ twins; Time 2 of IGT administration, 14-15 years: n=348 MZ twins, n=473 DZ twins; Time 3 of IGT administration, 16-18 years: n=198 MZ twins, n=251 DZ twins).
Next, a series of multivariate genetic models were next fit to the five blocks within each of the three times of IGT administration, see Table 2. A Common Pathway model provided a better fit of the data than the Cholesky decomposition model based on the AIC and BIC criteria (e.g., Time 1 of IGT administration, 11-13 years, Model # 2, AIC = 1307.76, BIC = -2017.20). This model could be further reduced by dropping all (common and unique) shared environmental influences (e.g., 11-13 years, Model 2a, χ2=.33; df=6; p=.99) and all unique genetic influences (e.g., 11-13 years, Model 2b, χ2=.26; df=5; p=.99). Model # 2b also had the lowest AIC and BIC (e.g., 11-13 years, AIC=1286.356, BIC=-205.31) compared to the other models. Figures 2, 3 and 4 display standardized parameter estimates from these reduced Common Pathway models.
Table 2.
Fit Indices for Multivariate Genetic Models of Decision-making within Each of the Five Blocks at Ages 11-13, 14-15 and 16-18 Years
| Overall fit | Chi-square difference test | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Models | -2LL | Df | AIC | BIC | χ 2 | df | P | Compared to # | χ 2 | Δdf | p | |
| Time 1: 11-13 years | ||||||||||||
| 1 | Multivariate Cholesky | 4661.40 | 1665 | 1331.40 | -1968.98 | |||||||
| 2 | Common Pathway | 4683.76 | 1688 | 1307.76 | -2017.20 | 22.36 | 23 | .50 | ||||
| 2a | Common Pathway Drop all C | 4684.10 | 1694 | 1296.10 | -2032.53 | 22.69 | 29 | .79 | 2 | .33 | 6 | .99 |
| 2b | Common Pathway Drop all C Drop all unique A | 4684.36 | 1699 | 1286.36 | -2045.31 | 22.95 | 34 | .93 | 2a | .26 | 5 | .99 |
| Time 2: 14-15 years | ||||||||||||
| 1 | Multivariate Cholesky | 10923.99 | 4055 | 2813.99 | -6803.84 | |||||||
| 2 | Common Pathway | 10974.75 | 4078 | 2818.75 | -6848.03 | 50.76 | 23 | .01 | ||||
| 2a | Common Pathway Drop all C | 10975.44 | 4084 | 2807.44 | -6865.84 | 51.45 | 29 | .01 | 2 | .69 | 6 | .99 |
| 2b | Common Pathway Drop all C Drop all unique A | 10979.14 | 4089 | 2801.14 | -6879.11 | 55.15 | 34 | .01 | 2a | 3.70 | 5 | .59 |
| Time 3: 16-18 years | ||||||||||||
| 1 | Multivariate Cholesky | 5779.91 | 2195 | 1389.91 | -3078.34 | |||||||
| 2 | Common Pathway | 5829.43 | 2218 | 1393.43 | -3116.12 | 49.52 | 23 | .01 | ||||
| 2a | Common Pathway Drop all C | 5831.45 | 2234 | 1383.45 | -3131.42 | 51.54 | 29 | .01 | 2 | 2.03 | 6 | .91 |
| 2b | Common Pathway Drop all C Drop all unique A | 5832.33 | 2229 | 1374.33 | -3144.57 | 52.42 | 34 | .02 | 2a | .88 | 5 | .97 |
Note. -2LL=-2(log-likelihood), AIC=Akaike's Information Criterion, BIC=Bayesian Information Criterion, χ2 =difference in log-likelihoods between models, df= degrees of freedom. Best-fitting model within each Time shown in bold.
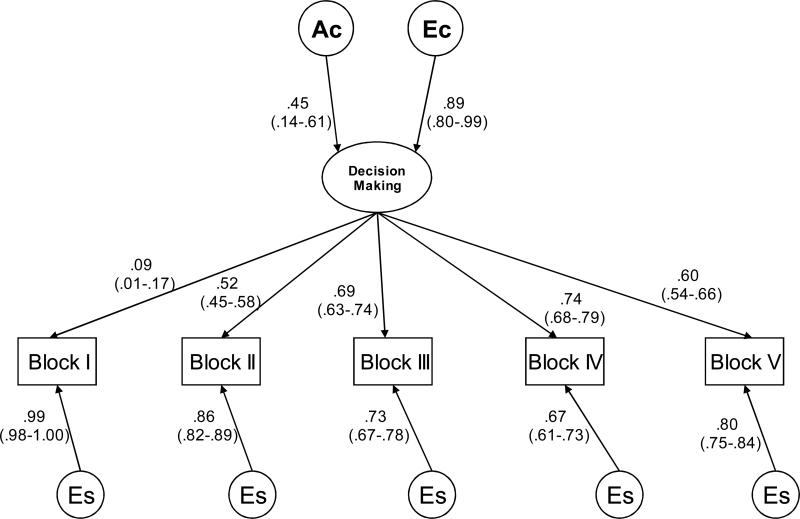
Figure 2. Decision-making at ages 11-13 years (Time 1).
Best-fitting Common Pathway models, showing one twin in a pair: Circles indicate variance components, and rectangles indicate observed variables, decision-making measured by the Iowa Gambling Task. Ac=common additive genetic component; Ec=common non-shared environmental component, Es=measurement specific non-shared environmental component.
Figure 3. Decision-making at ages 14-15 years (Time 2).
Best-fitting Common Pathway models, showing one twin in a pair: Circles indicate variance components, and rectangles indicate observed variables, decision-making measured by the Iowa Gambling Task. Ac=common additive genetic component; Ec=common non-shared environmental component, Es=measurement specific non-shared environmental component.
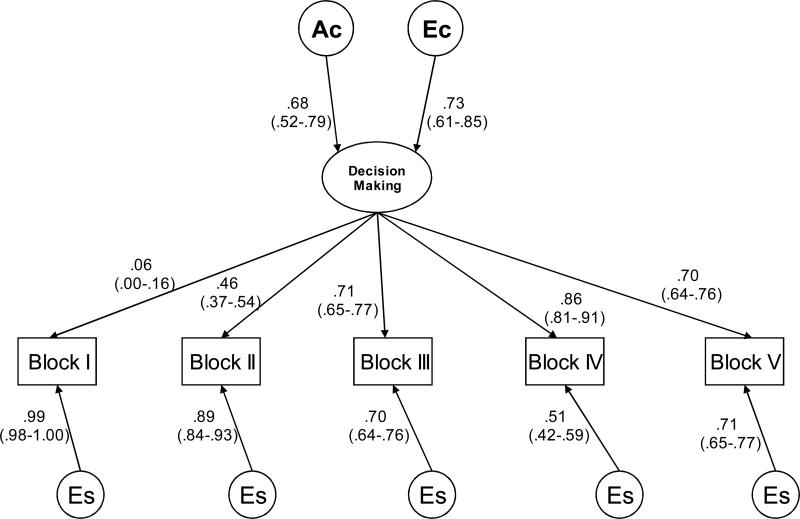
Figure 4. Decision-making at ages 16-18 years (Time 3).
Best-fitting Common Pathway models, showing one twin in a pair: Circles indicate variance components, and rectangles indicate observed variables, decision-making measured by the Iowa Gambling Task. Ac=common additive genetic component; Ec=common non-shared environmental component, Es=measurement specific non-shared environmental component.
Squaring the standardized parameter estimates presented in Figures 2, 3 and 4 provides the relative contributions to the phenotypic variance. For Time 1 of IGT administration, 11-13 years, 35% of the variance in the latent factor (labeled “decision-making”) was due to genetic factors, and 65% was due to non-shared environmental factors. For Time 2 of IGT administration, 14-15 years, 20% of the variance of the latent factor was due to genetic factors, and 80% was due to non-shared environmental factors, see Table 3. Despite the pattern of the intraclass correlations during Time 2 of IGT administration, 14-15 years, a small but significant genetic variance was found in these data. This is probably explained by the variance and covariance structure across twin 1 and twin 2 and across the five blocks. For Time 3 of IGT administration, 16-18 years, 46% (p<.05) of the variance of the latent factor was due to genetic factors, and 54% (p<.05) was due to non-shared environmental factors. There were also significant block-specific non-shared environmental influences, which include measurement error, explaining between 58% and 98% of the variance for each block at Time 1 of IGT administration, between 45% and 98% of the variance at Time 2 of IGT administration, and between 26% and 98% of the variance at Time 3 of IGT administration.
Table 3.
Squared Standardized Parameter Estimates, Genetic and Non-shared Environmental Correlations and 95% Confidence Intervals from Best-Fitting Common Pathway Models
| Time 1 11-13 years | Time 2 14-15 years | Time 3 16-18 years | Genetic Correlations (rg) | Non-shared Environmental Correlations (re) | |
|---|---|---|---|---|---|
| Ac (Latent ‘Decision-making’ Factor)* | 35% (6-58) | 20% (2-37) | 46% (27-62) | ||
| Ec (Latent ‘Decision-making’ Factor)* | 65% (42-94) | 80% (64-98) | 54% (37-72) | ||
| Es (unique to Block I) | 98% (92-1.00) | 98% (96-1.00) | 98% (96-1.00) | ||
| Es (unique to Block II) | 71% (59-83) | 74% (67-79) | 79% (71-86) | ||
| Es (unique to Block III) | 67% (53-79) | 53% (45-61) | 49% (41-58) | ||
| Es (unique to Block IV) | 58% (44-72) | 45% (37-53) | 26% (18-35) | ||
| Es (unique to Block V) | 76% (64-86) | 64% (56-71) | 50% (42-59) | ||
| 11-13 years to 14-15 years | 21 (.04-.51) | .24 (.02-.54) | |||
| 14-15 years to 16-18 years | .23 (.00-86) | .29 (.06-.73) | |||
| 11-13 years to 16-18 years | .13 (.00-52) | .11 (.00-47) |
Lastly, genetic correlations were calculated based on factor scores that were derived from the best-fitting Common Pathway models, see Table 3. The genetic correlations (rg) for decision-making are: rg=.21 (95% CI, .04-.51) between Times 1 and 2 of IGT administration, rg=.23 (95% CI, .00-86) between Times 2 and 3 of IGT administration, and rg=.13 (95% CI, .00-52) between Times 1 and 3 of IGT administration. This indicates a modest genetic stability for decision-making from late childhood (11-13 years) to early adolescence (14-15 years). The non-shared environmental correlations (re) for decision-making are: re=.24 (95% CI, .02-.54) between Times 1 and 2 of IGT administration, re=.29 (95% CI, .06-.73) between Times 2 and 3 of IGT administration, and re=.11 (95% CI, .00-47) Times 1 and 3 of IGT administration. This indicates a moderate non-shared environmental stability for decision-making from late childhood through adolescence.
Discussion
To our knowledge, this is the first study to investigate how genetic and environmental factors contribute to decision-making as measured by IGT performance in a community sample of twins. Genetic factors explained 35%, 20%, and 46% of the variance in decision-making as measured by IGT performance when the twins were 11-13 years, 14-15 years, and 16-18 years old, respectively. The remaining variance in this latent factor was explained by the non-shared environment (65%, 80% and 54%, respectively). Block-specific non-shared environmental influences (including uncorrelated measurement error) were observed for all blocks within each of the three times of IGT administration. The stability of decision-making was moderate across the three times of IGT administration, with correlations ranging from r=.19 to .39. These youth also showed a trend towards performing much better at ages 16-18 years than the two earlier assessments at younger ages.
Our finding that the majority of the variance in decision-making is explained by the non-shared environment is in parallel with Gottfredson and Hirschi's theory. As mentioned above, risky decision-making is closely related to low self-control. According to Gottfredson and Hirschi, poor self-regulation is the most important factor explaining antisocial and criminal behaviors and the development of poor self-regulation is strongly related to ineffective parenting, including insufficient monitoring, failure to recognize that a behavior is deviant or unacceptable, and failure of parents to appropriately correct a behavior (Gottfredson & Hirschi, 1990). Specifically during mid-adolescence when the twins were 14-15 years, the non-shared environment has been found to explain as much as 80% of the variance. These non-shared environmental experiences contain experiences that are unique to each twin in a pair and may directly affect the decision-making strategies an individual adopts. Given that it is around this age that adolescent-limited risky behaviors increases (Moffitt, 1993), this finding suggests that these non-shared environmental influences may reflect a growing influence of peers. In other words, this could reflect peer pressure at that age which influences what decisions an adolescent makes. During mid-adolescence individuals are probably less supervised by their parents and experience more autonomy compared to younger children. It is also likely that parents are not aware of all the activities their teenage children are engaged in (Rutter, Giller & Hagell, 1998) and this could contribute to the development of poor self-regulation and in turn poor decision-making. It should be noted too, that the best-fitting model (i.e., a Common Pathway model) in the present study partitions measurement error from variance in the latent “decision-making” factor. When doing this, the non-shared environment for latent “decision-making” factor was found to account for a large portion of the variance at ages 11-13 years, 14-15 years and 16-18 years, thus contributing to a significant amount of variance independent of measurement error.
Our findings of a modest genetic influence and a larger non-shared environmental influence on decision-making is consistent with studies on WCST performance reporting little or no influence of familial (genetic and shared environment) influences, and a large non-shared environmental component (Kremen et al., 2007; Taylor, 2007). Our result may also be compared to twin studies on risky behaviors, including studies on gambling behavior that have found that the variance in gambling behavior is primarily explained by genetic and non-shared environmental influences, with the shared environment being of minimal importance (Blanco et al., 2012; Slutske et al., 2009; Slutske et al., 2010). Furthermore, delay discounting which refers to the preference for smaller immediate rewards over larger but delayed rewards has been found to have a moderate genetic influence (Anokhin et al., 2010b).
Significant measurement-specific non-shared environmental effects were also found within each of the three times of IGT administration, across the five blocks. In this type of modeling that we were using, i.e., a Common Pathway model, measurement-specific non-shared environmental effects also include measurement error.
The genetic correlations between any two time points were low, and only significant between late childhood (11-13 years) and early adolescence (14-15 years), indicating some continuity of the same genetic influences on decision-making in this time frame. The importance of stable genetic influences has previously been reported in studies on child psychopathology (Eley, Lichtenstein & Moffitt, 2003; Haberstick et al., 2005; O'Connor et al., 1998). We also found a significant non-shared environmental overlap between ages 11-13 years and 14-15 years, and between ages14-15 years and 16-18 years. This indicates a moderate non-shared environmental stability for decision-making from late childhood through adolescence. There was a moderate phenotypic stability in decision-making across development, ranging between .19 and .39. This finding is comparable to the phenotypic stability reported in a longitudinal study including 192 Chinese adolescents aged 15-17 years. They found a one-year phenotypic stability of rp=.36 (Xiao et al., 2010). We also found that when the youths were 16-18 years old they showed a trend to perform better in the task compared to measurement time points, 11-13 years and 14-15 years (Figure 1), suggesting learning effects or vmPFC maturation and cognitive development across time. This finding is in line with previous cross-sectional research showing that performance on IGT significantly improves with age (Crone & van der Molen, 2007; Overman, 2004). For example, a study using a sample of 145 adolescents aged 9-17 years found that 14-17 years olds made more advantageous selections compared to 9-10 and 11-13 year olds (Hooper et al., 2004). Although we were not able to statistically examine how much of the change in performance is due to learning effects and how much due to natural maturation, failure to avoid the risky decks in the first block at ages 11-13 and 14-15 years suggests that the participants did not learn from their experiences at previous times (Figure 1). Therefore, it is hypothesized that the changes in performance may be mainly due to natural maturation of nervous system. Future studies assessing decision-making at multiple time points within each session and at multiple sessions are needed to further test this hypothesis.
Interestingly, the factor loading from Block I (the first 20 trials) was very low at each of the three times of IGT administration (Figures 2-4, .15, .09, and .06 respectively) compared to the factor loadings for the last 4 blocks (Blocks II-V, last 80 trials). During the IGT, a participant must first learn by experience which decks are advantageous before he/she can go on to try to maximize his/her winnings (Bechara et al., 1997). As such, performance on the IGT consists of two phases. Phase I relates to decision-making under ambiguity, as in these early trials, the participant does not have knowledge of which decks are advantageous and which are disadvantageous. Phase II relates to decision-making without ambiguity as the participant has now consciously or unconsciously learnt which decks are advantageous. This suggests that more errors are committed during the first 20 trials and this may explain why we found that the factor loadings were lower for Block I within each of the three measurement time points. It has also been shown that decision-making under ambiguity involves a different genetic make-up compared to decision-making under risk (Stoltenberg & Vandever, 2010). In a recent study including a sample of 477 college students researchers found that the genetic variation at the 5-HTTLPR polymorphism was associated with decision-making under ambiguity (trials1–20), but not under risk (trials 21–100) (Stoltenberg et al., 2011).
We did not find any significant sex differences in genetic and environmental variance components in decision-making and we could equate these to be equal across boys and girls. This finding is in line with other twin studies on gambling behavior which report finding no significant sex differences (Blanco et al., 2012; Slutske et al., 2010). Nor did we find any mean difference on IGT performance between males and females at ages 11-13 and 16-18 years, which is in line with other studies (Hooper et al., 2004). However, at ages 14-15 years males made more risky decisions than the females. This is consistent with the notion that males are generally more sensation-seeking and impulsive than females and that there may be sex differences in the genetic structure of executive functioning in children and adolescents (Anokhin et al., 2010a). The fact that sex differences were found at 14-15 years, but not at ages 11-13 and 16-18 years may be partly due to the age-related sex differences in brain maturational processes (Campbell et al., 2005; De Bellis et al., 2001). Future neuroimaging studies with IGT performance in both sex groups are needed to further investigate whether the better decision-making in girls are partly due to their early brain maturation at this age.
A few limitations of the present study should be acknowledged. Even though the test re-test reliability was moderate (ages 11-13 to 14-15 years r=.33; 11-13 to 16-18 years r=.19; 14-15 to 16-18 years r=.39), suggesting that the IGT task has a reasonable reliability across development, the construct validity of the IGT task was modest in the current sample, which may have led to an underestimation of familial (genetic and shared environmental) influences. Second, the pattern of the intraclass correlations during the second IGT data collection suggested limited influence of genetic factors. Regardless, a small but significant genetic influence was found. Also, the sample size at each time of IGT administration was relatively small. Failure to find significant performance differences across occasions may be partly due to the low statistical power. Therefore it is important to replicate our findings in larger samples. As mentioned above, we are not able to answer the question of how much of the performance change across ages is due to maturation and how much due to learning effects as the same version of the IGT was administered to the participants at each of the three measurement occasions (ages 11-13, 14-15 and 16-18 years). Future studies with multi-time within each session and multi-session assessments are needed to address this issue. Finally, there are several assumptions related to the classical twin design, which possibly may not have been met. For example, the heritability statistic is population and time specific. A more detailed discussion of these and other limitations of the assumption in the classical twin design can be found elsewhere (Plomin et al., 2001).
In conclusion, our results show that decision-making as measured by the IGT performance was moderately stable between late childhood and adolescence. Importantly, however, individual differences in decision-making in the IGT was modestly influenced by genetic factors and more strongly influenced by non-shared environmental factors. Although genetic predispositions are of some importance, unique experiences appear to be of greater significance in guiding decision-making in youth throughout adolescence.
Acknowledgment
This study was funded by NIMH (R01 MH58354). Adrian Raine was supported by NIMH (Independent Scientist Award K02 MH01114-08).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akaike AC. Factor analysis and AIC. Psychometrika. 1987;52:317–32. [Google Scholar]
- Anokhin AP, Golosheykin S, Grant JD, Heath AC. Developmental and genetic influences on prefrontal function in adolescents: A longitudinal twin study of WCST performance. Neuroscience Letters. 2010a;472(2):119–22. doi: 10.1016/j.neulet.2010.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Grant JD, Heath AC. Heritability of delay discounting in adolescence: a longitudinal twin study. Behavior Genetics. 2010b;41(2):175–83. doi: 10.1007/s10519-010-9384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Heath AC, Ralano A. Genetic influences on frontal brain function: WCST performance in twins. Neuroreport. 2003;14(15):1975–8. doi: 10.1097/00001756-200310270-00019. [DOI] [PubMed] [Google Scholar]
- Baker LA, Barton M, Lozano DI, Raine A, Fowler JH. The Southern California Twin Register at the University of Southern California: II. Twin Research and Human Genetics. 2006;9:933–40. doi: 10.1375/183242706779462912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt ES. Anxiety and impulsiveness related to psychomotor efficiency. Perceptual and Motor Skills. 1959;9:191–8. [Google Scholar]
- Beaver KM, Hoffman T, Shields RT, Vaughn MG, DeLisi M, Wright JP. Gender differences in genetic and environmental influences on gambling: results from a sample of twins from the National Longitudinal Study of Adolescent Health. Addiction. 2010;105(3):536–42. doi: 10.1111/j.1360-0443.2009.02803.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damásio AR, Damásio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision-making and the orbitofrontal cortex. Cereb Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;28(275(5304)):1293–5. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Mitchell DG. Somatic markers and response reversal: is there orbitofrontal cortex dysfunction in boys with psychopathic tendencies? Journal of Abnormal Child Psychology. 2001;29(6):499–511. doi: 10.1023/a:1012277125119. [DOI] [PubMed] [Google Scholar]
- Blanco C, Myers J, Kendler KS. Gambling, disordered gambling and their association with major depression and substance use: a web-based cohort and twin-sibling study. Psychological Medicine. 2012;42(3):497–508. doi: 10.1017/S0033291711001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelow MT, Suhr JA. Construct validity of the Iowa Gambling Task. Neuropsychology Review. 2009;19:102–14. doi: 10.1007/s11065-009-9083-4. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Darchia N, Khaw WY, Higgins LM, Feinberg I. Sleep EEG evidence of sex differences in adolescent brain maturation. Sleep. 2005;28(5):637–43. doi: 10.1093/sleep/28.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavedini P, Riboldi G, D'Annucci A, Belotti P, Cisima M, Bellodi L. Decision-making heterogeneity in obsessive-compulsive disorder: ventromedial prefrontal cortex function predicts different treatment outcomes. Neuropsychologia. 2002;40(2):205–11. doi: 10.1016/s0028-3932(01)00077-x. [DOI] [PubMed] [Google Scholar]
- Chou LN, Kuo PH, Lin CC, Chen WJ. Genetic and environmental influences on the Wisconsin Card Sorting Test performance in healthy adolescents: a twin/sibling study. Behavior Genetics. 2010;40(1):22–30. doi: 10.1007/s10519-009-9299-3. [DOI] [PubMed] [Google Scholar]
- Crone EA, van der Molen MW. Development of decision-making in school-aged children and adolescents: evidence from heart rate and skin conductance analysis. Child Development. 2007;78(4):1288–301. doi: 10.1111/j.1467-8624.2007.01066.x. [DOI] [PubMed] [Google Scholar]
- Damasio A. Descartes’ error: Emotion, reason, and the human brain. GP Putnam's Sons; New York: 1994. [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cerebral cortex. 2001;1(6):552–7. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- Delisi M, Vaughn MG. The Gottfredson-Hirschi critiques revisited: reconciling self-control theory, criminal careers, and career criminals. International Journal of Offender Therapy and Comparative Criminology. 2008;52(5):520–37. doi: 10.1177/0306624X07308553. [DOI] [PubMed] [Google Scholar]
- Eley TC, Lichtenstein P, Moffitt TE. A longitudinal behavioral genetic analysis of the etiology of aggressive and non-aggressive antisocial behavior. Development and Psychopathology. 2003;15(2):383–402. doi: 10.1017/s095457940300021x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Grant SJ, London ED, Contoreggi CS, Kimes AS, Spurgeon L. Decision-making in adolescents with behavior disorders and adults with substance abuse. American Journal of Psychiatry. 2003;160(1):33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- Evans DM, Gillespie NA, Martin NG. Biometrical genetics. Biological Psychology. 2002;61:33–51. doi: 10.1016/s0301-0511(02)00051-0. [DOI] [PubMed] [Google Scholar]
- Freeman N, Muraven M. Self-control depletion leads to increased risk taking. Social Psychological and Personality Science. 2010;1:175–81. [Google Scholar]
- Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology. 2008;137(2):201–25. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Baker LA, Raine A, Wu H, Bezdjian S. Brief Report: Interaction between social class and risky decision-making in children with psychopathic tendencies. Journal of Adolescence. 2009;32(2):409–14. doi: 10.1016/j.adolescence.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N, Moore C, Waschbusch DA. Decision-making in children with ADHD only, ADHD-anxious/depressed, and control children using a child version of the Iowa Gambling Task. Journal of Attention Disorders. 2006;9(4):607–19. doi: 10.1177/1087054705284501. [DOI] [PubMed] [Google Scholar]
- Gibson CL. Gottfredson and Travis: Self-Control Theory. In: Cullen FT, Wilcox P, editors. Encyclopedia of Criminological Theory. SAGE; CA: Thousand Oaks: 2010. [Google Scholar]
- Gottfredson MR, Hirschi T. A general theory of crime. Stanford University Press; Stanford, CA: 1990. [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision-making. Neuropsychologia. 2000;38(8):1180–7. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Haberstick BC, Schmitz S, Young SE, J.K. H. Contributions of genes and environments to stability and change in externalizing and internalizing problems during elementary and middle school. Behavior Genetics. 2005;35(4):381–96. doi: 10.1007/s10519-004-1747-5. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL. Wisconsin Card Sorting Test manual, Psychological Assessment Research Resources. Odessa, Florida: 1993. al., e. [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents’ performance on the Iowa Gambling Task: implications for the development of decision-making and ventromedial prefrontal cortex. Developmental Psychology. 2004;40(6):1148–58. doi: 10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Kester HM, Sevy S, Yechiam E, Burdick KE, Cervellione KL, Kumra S. Decision-making impairments in adolescents with early-onset schizophrenia. Schizophrenia Research. 2006;85(1-3):113–23. doi: 10.1016/j.schres.2006.02.028. Epub 2006 Jun 2. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Eisen SA, Tsuang MT, Lyons MJ. Is the Wisconsin Card Sorting Test a useful neurocognitive endophenotype? American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;5(144B(4)):403–6. doi: 10.1002/ajmg.b.30527. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Jollant F, O'Daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortical subregions in the Iowa gambling task. Cerebral Cortex. 2009;19:1134–43. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Jones HA, Strong DR, Richards JB, Kahler CW. The balloon analogue risk task (BART) differentiates smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2003;11:26–33. doi: 10.1037//1064-1297.11.1.26. al., e. [DOI] [PubMed] [Google Scholar]
- Linnet J, Røjskjaer S, Nygaard J, Maher BA. Episodic chasing in pathological gamblers using the Iowa gambling task. Scandinavian Journal of Psychology. 2006;47(1):43–9. doi: 10.1111/j.1467-9450.2006.00491.x. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Goldsmith HH. Alternative common factor models for multivariate biometric analyses. Behavior Genetics. 1990;20:569–608. doi: 10.1007/BF01065873. [DOI] [PubMed] [Google Scholar]
- Miles DR, van den Bree MB, Gupman AE, Newlin DB, Glantz MD, Pickens RW. A twin study on sensation seeking, risk taking behavior and marijuana use. Drug and Alcohol Dependence. 2001;62(1):57–68. doi: 10.1016/s0376-8716(00)00165-4. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Colledge E, Leonard A, Blair RJ. Risky decisions and response reversal: is there evidence of orbitofrontal cortex dysfunction in psychopathic individuals? Neuropsychologia. 2002;40(12):2013–22. doi: 10.1016/s0028-3932(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognitive Psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychological Review. 1993;100(4):674–701. [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes H. Mx: Statistical modeling. Department of Psychiatry, Medical College of Virginia; Richmond, VA: 2003. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Kluwer Academic Publications; Dordrecht, The Netherlands: 1992. [Google Scholar]
- O'Connor TG, Neiderhiser JM, Reiss D, Hetherington EM, Plomin R. Genetic contributions to continuity, chnage, and the co-occurence of antisocial and depressive symptoms in adolescence. Journal of Child Psychology and Psychiatry. 1998;39:323–36. [PubMed] [Google Scholar]
- Overman WH. Sex differences in early childhood, adolescence, and adulthood on cognitive tasks that rely on orbital prefrontal cortex. Brain and Cognition. 2004;55(1):134–47. doi: 10.1016/S0278-2626(03)00279-3. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. Worth Publisher; United States of America: 2001. [Google Scholar]
- Posthuma D, Beem AL, de Geus EJC, van Baal CM, von Hjelmborg JB, Iachine I, Boomsma DI. Theory and practice in quantitative genetics. Twin Research and Human Genetics. 2003;6(5):361–76. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- Raftery AE. Bayesian model selection in social research. Sociological Methodology. 1995;25:111–63. [Google Scholar]
- Rutter M, Giller H, Hagell A. Antisocial Behavior by Young People. Cambridge University Press; Cambridge, UK: 1998. [Google Scholar]
- SAS . SAS/STAT software: changes and enhancements through release 9.2. SAS Institute Inc.; Cary, NC: 2005. [Google Scholar]
- Slutske WS, Meier MH, Zhu G, Statham DJ, Blaszczynski A, Martin NG. The Australian Twin Study of Gambling (OZ-GAM): rationale, sample description, predictors of participation, and a first look at sources of individual differences in gambling involvement. Twin Research and Human Genetics. 2009;12(1):63–78. doi: 10.1375/twin.12.1.63. [DOI] [PubMed] [Google Scholar]
- Slutske WS, Zhu G, Meier MH, Martin NG. Genetic and environmental influences on disordered gambling in men and women. Archives of General Psychiatry. 2010;67(6):624–30. doi: 10.1001/archgenpsychiatry.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoel RD, De Geus EJ, Boomsma DI. Genetic analysis of sensation seeking with an extended twin design. Behavior Genetics. 2006;36(2):229–37. doi: 10.1007/s10519-005-9028-5. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF, Lehmann MK, Anderson C, Nag P, Anagnopoulos C. Serotonin transporter (5-HTTLPR) genotype and childhood trauma are associated with individual differences in decision-making. Frontiers In Genetics. 2011;2:1–9. doi: 10.3389/fgene.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltenberg SF, Vandever JM. Gender moderates the association between 5-HTTLPR and decision-making under ambiguity but not under risk. Neuropharmacology. 2010;58:423–8. doi: 10.1016/j.neuropharm.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. Heritability of Wisconsin Card Sorting Test (WCST) and Stroop Color-Word Test performance in normal individuals: implications for the search for endophenotypes. Twin Research and Human Genetics. 2007;10(6):829–34. doi: 10.1375/twin.10.6.829. [DOI] [PubMed] [Google Scholar]
- Tuvblad C, Gao Y, Isen J, Botwick T, Raine A, Baker LA. The Heritability of the Skin Conductance Orienting Response: A Longitudinal Twin Study. Biological Psychology. 2011 doi: 10.1016/j.biopsycho.2011.09.003. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Oord EJ, Simonoff E, Eaves L, Pickles A, Silberg J, Maes H. An evaluation of different approaches for behavior genetic analyses with psychiatric symptom scores. Behavior Genetics. 2000;30:1–18. doi: 10.1023/a:1002095608946. [DOI] [PubMed] [Google Scholar]
- Xiao L, Bechara A, Palmer PH, Trinidad DR, Wei Y, Jia Y. Parent-child engagement in desicion-making and the development of adolescent affective decision capacity and binge-drinking. Personality and Individual Differences. 2010 doi: 10.1016/j.paid.2010.04.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechiam E, Kanz JE, Bechara A, Stout JC, Busemeyer JR, Altmaier EM, Paulsen JS. Neurocognitive deficits related to poor decision-making in people behind bars. Psychonomic Bulletin & Review. 2007;15(1):44–51. doi: 10.3758/pbr.15.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM. Personality and risk-taking: Common biosocial factors. Journal of Personality. 2000;68:999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]