Abstract
Background
This study examined the relationship between cognitive function and sleep onset/maintenance difficulties (SO/MD) in nondemented older adults. We hypothesized that SO/MD negatively impacts cognition and that older adults with lower education would be especially vulnerable to its effects.
Methods
The sample comprised 549 older adults from the Einstein Aging Study (EAS), a community-based sample. Participants completed neuropsychological assessment and a sleep questionnaire. Univariate ANCOVAs were performed with cognitive performance as a dependent variable, SO/MD (present or absent) and education (lower:≤12 years; higher:>12 years) as between-subjects factors, and age, ethnicity, gender, depression, and cardiovascular comorbidies as covariates.
Results
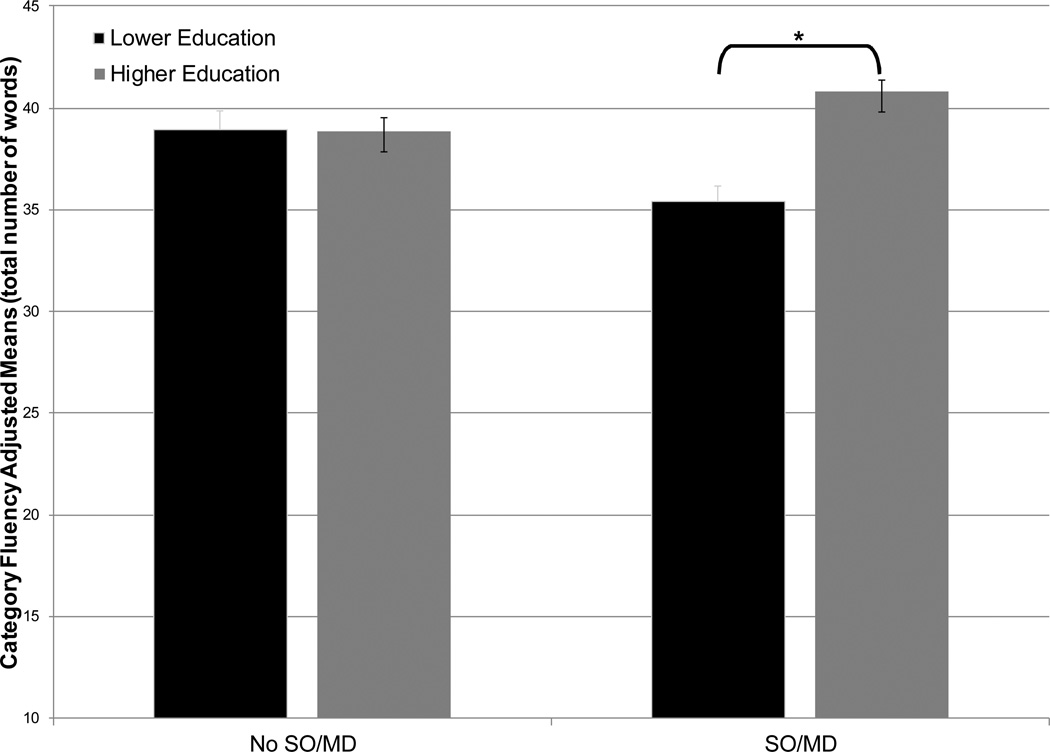
Participants were an average age of 79.7±5.0 years (range=71–97). Fifty-seven percent (n=314) of the sample met criteria for SO/MD. Among participants with SO/MD, those with lower education performed more poorly on a test of category fluency than participants with higher education (means: 35.2 vs. 41.0, p<0.001); among older adults without SO/MD, educational attainment had no measurable effect on cognition (SO/MD × education interaction (F(1,536)=14.5, p=0.00)).
Conclusions
Consistent with the cognitive reserve hypothesis, older adults with lower education appear selectively vulnerable to the negative effects of sleep onset/maintenance difficulties on tests of verbal fluency.
Keywords: Sleep, Neuropsychology, Cognitive Reserve, Elderly, Education, Depression
Introduction
The rising worldwide population of older adults necessitates an urgent focus on the unique health care needs of elderly individuals. It is well-established that older adults experience decline across a variety of cognitive abilities, including processing speed, attention, memory, language, and higher-order executive processes (Anstey & Low, 2004; Park, 2000; Zimmerman & Brickman, 2009). In addition, cognitive abilities are highly variable in healthy older adults, with substantial evidence of both intra- and inter-individual variability in test performance (Christensen, 2001). Variability in cognitive function may be caused by a multitude of environmental and biological factors that include disruptions in the sleep/wake cycle, among other factors. With advancing age, healthy adults experience alterations in sleep architecture, more frequent waking after sleep onset, poorer sleep efficiency, reduced total sleep time, daytime sleepiness, and increased napping (Ancoli-Israel & Lieberman, 2004; Hoyt, 2005; McCrae et al., 2003; Ohayon, 2002). Sleep complaints consistent with insomnia, such as difficulty initiating and maintaining sleep, are reported in up to 50% of older adults (D. J. Foley et al., 1995). Age-related changes in both cognitive function and the sleep/wake cycle are associated with compromised activities of daily living, decreased quality of life, and comorbid medical conditions such as depression and anxiety (Kryger, Monjan, Bliwise, & Ancoli-Israel, 2004; Perlis et al., 2006).
Several cross-sectional studies of older adults have reported associations between poorer cognitive performance and sleep disturbances, particularly in the domains of attention, executive function, and memory (Haimov, Hanuka, & Horowitz, 2008; Nebes, Buysse, Halligan, Houck, & Monk, 2009; Ohayon, 2002; Schmutte et al., 2007). However, other cross-sectional (Merlino et al., 2010) and longitudinal (D. Foley et al., 2001) reports failed to find associations between insomnia and cognitive decline and dementia. Another longitudinal study found that insomnia was associated with the development of cognitive decline among older men, but not older women (Cricco, Simonsick, & Foley, 2001).
Mixed findings of relationships between sleep quality and cognitive function among elderly adults may reflect a wide range of methodological designs that include differences in sample demographic characteristics (age, gender, education), eligibility criteria (e.g., inclusion of participants with dementia or preclinical dementia), and measurement strategies for both insomnia and cognitive function. In addition, the concept of cognitive reserve may have an unexamined effect on these relationships whereby individuals with lower premorbid cognitive abilities are less able to compensate for insomnia-induced neural dysfunction than individuals with higher premorbid cognitive abilities (Stern, 2009). Although the cognitive reserve hypothesis is widely applied to investigations of dementia, it is not commonly considered in studies of sleep disorders.
In the current study, we administered a standardized questionnaire of sleep quality and a comprehensive neuropsychological battery to a large, ethnically-diverse systematically recruited community-based sample that was screened for both dementia and mild cognitive impairment. We hypothesized that sleep difficulties consistent with insomnia in healthy older adults would be associated with poorer performance on neuropsychological tests of attention, executive function, and memory. Furthermore, we hypothesized that older adults with lower education, a common proxy of cognitive reserve (Hall et al., 2007; Lane, Paul, Moser, Fletcher, & Cohen, 2011), would be especially vulnerable to the negative effects of sleep onset/maintenance difficulties on neuropsychological tests of attention, executive function, and memory.
Methods
Study Participants
The sample consisted of 549 participants from the Einstein Aging Study (EAS), an ongoing longitudinal community-based study of ethnically-diverse individuals over the age of 70 residing in the Bronx, New York. The study was approved by the Albert Einstein College of Medicine Institutional Review Board. EAS study design and methods are described in more detail elsewhere (Lipton et al., 2003). In brief, the cohort was launched in 1993 using systematic sampling recruitment methods that utilized Health Care Financing Administration (HCFA) and voter registration lists for Bronx County. To be eligible for EAS enrollment, participants must be: over the age of 70, Bronx residents, non-institutionalized, be fluent in English, and have a telephone. Exclusion criteria include visual or auditory impairments that preclude neuropsychological testing, active psychiatric symptomatology that interferes with the ability to complete assessments, or nonambulatory status. Individuals eligible for study participation are identified through a phone screen and invited to an in-clinic visit where final eligibility is confirmed and medical, neuropsychological, and psychosocial assessments are conducted.
For the current study, global cognitive function was assessed with the Blessed Information-Memory-Concentration test (BIMC (Blessed, Tomlinson, & Roth, 1968)). The BIMC contains 22 items (score range 0–33; better performance denoted by lower scores) that assess general mental status. It has been shown to be strongly correlated (0.73 to 0.83) with the Mini-Mental State Examination (MMSE) (Thal, Grundman, & Golden, 1986). Cognitive status was determined by a study neurologist and neuropsychologist at a diagnostic case conference. As there is evidence (Beaulieu-Bonneau & Hudon, 2009; Naismith et al., 2010) (McCurry & Ancoli-Israel, 2003; Neikrug & Ancoli-Israel, 2010) that older adults with dementia and mild cognitive impairment (MCI) (Petersen, 2004) experience a disproportionate amount of day and nighttime sleep disturbances that suggest shared biological underpinnings, we excluded individuals with dementia and amnestic MCI (n=75; 12% of sample) because our goal was to examine the effect of sleep disturbance on cognition in the elderly rather than the effect of neurodegeneration on sleep function. Individuals with non-amnestic MCI were not excluded. Diagnosis of dementia was based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV)(American, 1994). Amnestic MCI was determined using performance on the Free and Cued Selective Reminding Test (FCSRT), described below.
Sleep Questionnaire
Sleep quality was assessed using the Medical Outcomes Study sleep scale (MOS-SS;(Cole, Dubois, & Kosinski, 2007; Hays, Martin, Sesti, & Spritzer, 2005). The MOS-SS was introduced in the EAS in 2005. MOS-SS data from each participant’s initial administration of the scale were utilized in the current report. The MOS-SS self-report questionnaire consists of 12 sleep function questions that require recall over the preceding 4 weeks. Responses are indicated on a 6-point scale that ranges from 1=”all of the time” to 6=”none of the time.” Responses are recoded and scored to generate measures of sleep quantity (average hours of sleep per night) and six subscale scores (sleep disturbance, snoring, wakening with shortness of breath or headache, adequacy of sleep, daytime somnolence, and a global sleep problem index score). MOS-SS subscales and the index score range from 0–100, with higher scores reflecting more problems with the concept indicated. The sleep onset/maintenance difficulties (SO/MD) case definition was defined using criteria established by Katz and McHorney (D. A. Katz & McHorney, 2002) using two questions from the MOS-SS that indicate difficulties with sleep initiation and/or maintenance. The sleep onset question was, “How often during the past four weeks did you have trouble falling asleep?” while the sleep maintenance question was, “How often during the past four weeks did you awaken during your sleep time and have trouble falling asleep again?” Participants reporting difficulty with sleep onset or maintenance “a good bit”, “some”, “most”, or “all” of the time were designated SO/MD.
Neuropsychological Evaluation
Verbal Memory
The Free and Cued Selective Reminding Test (FCSRT) is a measure of verbal cued learning and memory that controls attention and strategy use in an encoding phase to maximize learning and ensure deep semantic processing (Buschke, 1984; Grober & Buschke, 1987). In the first part of the task, participants name 16 objects that are pictorially displayed. They are then presented with the same 16 objects (four at a time in a 2 × 2 grid) and asked to identify each object following a cue that is a categorical prompt. The grid is then removed and the participants are again asked to recall each object following provision of the same categorical cue. In the free recall condition, a measure of self-organized retrieval, the participant is immediately asked to recall the 16 objects. If the participant fails to correctly recall an object, they are provided with a category cue to test cued recall. There are a total of three free and cued recall trials and each trial was preceded by 20 seconds of counting backwards as a distractor task. The primary variable of interest was the score for the free recall condition (range: 0–48). This measure has been shown to be a sensitive marker of preclinical dementia as well as a strong predictor of the development of dementia in community-based samples of older adults (Grober & Kawas, 1997; Grober, Lipton, Hall, & Crystal, 2000; Ivnik et al., 1997; Sarazin et al., 2007). In addition, performance on this measure has been shown to be unrelated to race in older adults (Grober, Lipton, Katz, & Sliwinski, 1998).
Attention
The Digit Span subtest from the Wechsler Adult Intelligence Scale – 3rd edition (WAIS-III;(Wechsler, 1997)) is a measure of auditory attention that consists of two conditions; digits forward and digits backward. In both conditions, the examiner reads aloud up to eight (forward) or seven (backward) pairs of random digits at a rate of one digit per minute and the participant is asked to repeat the digits in order. The primary variables of interest were the total correct scores for Digit Span Forward (range: 0–16) and Digit Span Backward (range: 0–14).
Trail Making Test part A (TMTA) (Battery, 1944) is a measure of attention requiring complex visual scanning and sequencing with a motor component. The participant is presented with an 8×11.5 inch sheet of paper with an array of circled numbers ranging from 1–25 that appear to be randomly positioned. Participants are asked to draw a line connecting the numbers in order as quickly as possible. Scores are provided as seconds to task completion.
Executive Function
Trail Making Test part B (TMTB)(Battery, 1944) is a measure of executive function involving mental flexibility, set-shifting, and concept formation. The participant is presented with an 8×11.5 inch sheet of paper with an array of circled numbers and letters ranging from 1–13 and A to L. Participants are asked to draw a line alternating between numbers and letters in order as quickly as possible. Scores are provided as seconds to task completion.
Verbal fluency was measured using the Category Fluency and Letter Fluency tests (Benton & Hamsher, 1989). Category Fluency is a measure of verbal semantic production in which the participant is asked to name as many words as possible that belong in a designated category within a one minute time period. The primary variable of interest was the total number of words produced across three categories (animals, fruits, and vegetables). Letter Fluency is a measure of verbal fluency in which the participant is asked to name as many words as possible that begin with a designated letter within a one minute period. The primary variable of interest was the total number of words produced across three letters (F, A, S). For both Category and Letter fluency, repetitions and intrusion errors were not included in the total score.
Medical and Psychological History
Participants completed a medical and psychosocial history interview and report of current use of medications during the study visit of the initial MOS-SS administration. Medications of interest in the current report that are used to treat or may contribute to sleep disturbance in older adults include benzodiazepines, benzodiazepine receptor agonists (nonbenzodiazepines), melatonin receptor agonists, tricyclic/sedating antidepressants (TCA), selective serotonin reuptake inhibitors (SSRI), serotonin and norepinephrine reuptake inhibitor (SNRI), beta-blockers/anti-hypertensives, antipsychotics, and mood stabilizers. Information was also obtained regarding present or past history of myocardial infarction, stroke, coronary artery bypass graft (CABG), diabetes, hypertension, arrhythmia, angina, heart failure, Parkinson’s disease, cancer, or pace maker. A cumulative dichotomous variable indicated whether the participant reported the presence or absence of any of these medical conditions at either the study visit of the initial MOS-SS administration or any previous study visit. A cardiovascular summary score was calculated to indicate any current or past history of either myocardial infarction, stroke, or CABG (Zimmerman et al., 2011). Body Mass Index (BMI) was calculated for each participant using weight in kilograms divided by height in meters squared. Using published guidelines (Flegal, Carroll, Ogden, & Curtin, 2010), an individual was designated as overweight with a BMI between 25.0 and 29.2. Individuals with a BMI of 30.0 or higher were designated obese.
Depressive symptoms at the initial MOS-SS administration were assessed using the 15-item Geriatric Depression Scale (GDS)(Yesavage et al., 1982). This inventory consists of 15 questions that assess mood disturbance symptoms that are commonly associated with depression experienced among older adults. Respondents are asked to select either “yes” or “no” for each symptom experienced over the past week with a total range of scores from 0 to 15. A higher score is indicative of more depressive symptoms. A cut score of 5 or higher is associated with a greater risk for clinically significant symptoms of depression (Julian et al., 2009). Anxiety symptoms were assessed using the Beck Anxiety Inventory (BAI)(Beck, Epstein, Brown, & Steer, 1988). The BAI is a 21-item self-report inventory that measures the severity of anxiety symptoms that are experienced over the past week. Response options range from 0 (“Not at all”) to 3 (“Severely-It bothered me a lot”). Scores range from 0 to 63, with higher scores indicative of more anxiety symptoms. A cut score of 11 or higher is associated with a greater risk of clinically significant symptoms of anxiety (Karsten, Nolen, Penninx, & Hartman, 2010). BAI data were available for a subsample of 174 participants.
Statistical Analysis
All analyses were conducted using SPSS 17 (SPSS for Windows, Released 8.23.2008. Chicago: SPSS Inc.). To characterize the sample, Pearson correlations were used to examine relationships among demographic variables and neuropsychological test performance. Independent sample t-tests were used to examine gender and ethnicity group differences on neuropsychological test performance. Independent sample t-tests were used to examine SO/MD group differences on demographics (age, education, BMI, depression, anxiety) and neuropsychological function. Chi-square analyses were used to examine SO/MD group differences on gender, ethnicity, and cardiovascular history. Due to non-normality of BIMC data, a nonparametric Mann-Whitney U test was used to examine SO/MD group differences on global cognitive function. For hypothesis testing, a series of separate general linear models were used to examine SO/MD (present or absent) and education (lower or higher) differences (between-subjects factors) on neuropsychological variables (dependent variables) with age, ethnicity, gender, depression, and a cardiovascular summary score serving as covariates. To correct for multiple comparisons, a Bonferroni correction was applied where the significance level was set at alpha = 0.007 (0.05/7 comparisons).
Results
Sample Demographic Characteristics
Sample demographics as a function of SO/MD and educational status are presented in Table 1. Mean age was 79.7±5.0 years (range=71–97) and mean education was 14.5±3.3 years (range=3–22). A total of 314 (57.2%) participants met criteria for SO/MD. To create ecologically valid education groups based on United States high school graduation attainment, individuals with 12 or fewer years of education were designated “lower education” (n=197; 35.9%) and individuals with 13 or more years of education were designated “higher education” (n=352; 64.1%). The sample comprised 341 women (62.1%) and 208 men (37.9%). Sample ethnicity was 70.5% Caucasian, 24.2% African American, 3.5% Hispanic/White, 0.9% Hispanic/Black, 0.5% Asian, and 2 participants reported other ethnicities. For remaining analyses, ethnicity was collapsed into two groups that comprised Caucasians (70.5%) and African American/Other Ethnicity (29.5%). English was reported as the first acquired language for 449 (81.8%) participants. The average age of English language acquisition of those for whom English was not their first language was 10.4±8.2 (range=1–42) years. Average BMI was 27.7±4.8 (range=15.6–46.3). Using BMI cut scores, 38.8% of the sample was overweight (BMI=25.0 – 29.9), while 24.2% of the sample was obese (BMI≥30.0). These prevalence rates are lower than previously reported overweight rates in men (78.4%) and women (68.6%) and obese rates in men (37.1%) and women (33.6%) over the age of 60 in the Unites States (Flegal et al., 2010). The mean scores on the Geriatric Depression Scale and Beck Anxiety Inventory were not consistent with clinically significant depression or anxiety. Using a cut score of 5 or higher on the GDS (Julian et al., 2009), 10.9% of the sample reported clinically significant symptoms of depression. BAI data were available for 174 participants; 3.3% of this subsample reported clinically significant symptoms of anxiety using a cut score of 11 or higher (Karsten et al., 2010). A screening index of cognitive function (BIMC) confirmed that the sample was not globally cognitively impaired at the time of the sleep assessment (Grober et al., 2000). A minority of participants used medication that may be used to treat insomnia (benzodiazepines (1.6%), nonbenzodiazepines (3.3%), melatonin receptor agonists (0%), TCA (2.2%), antipsychotics (0.2%)) or medications that may exacerbate sleep difficulties (SSRI (3.8%), SNRI (0.5%), beta-blockers (29.7%), and mood stabilizers (1.8%)). Due to relatively low usage rates, medication use was not considered further in analyses. Participants reported the following medical histories: myocardial infarction (7.8%), stroke (8.9%), CABG (8.7%), diabetes (18.0%), hypertension (62.5%), arrhythmia (26.4%), angina (8.7%), heart failure (3.5%), Parkinson’s disease (0.2%), cancer (34.8%), and use of a pace maker (2.7%). These prevalence rates are consistent with those from our larger cohort (M. J. Katz et al., In Press) as well as a national U.S. survey of adults over the age of 65 (Pleiss, Ward, & Lucas, 2010). Participants reported the following for a cardiovascular summary score (any current or past history of either myocardial infarction, stroke, or CABG): 79.2% reported none of these cardiovascular conditions, 16.2% reported one, 4.4% reported two, and 0.2% reported three.
Table 1.
Sample demographics by sleep onset/maintenance difficulties (SO/MD) and education status.
| SO/MD n=314 |
No SO/MD n=235 |
Test Statistic 1. main effect SO/MD 2. main effect education 3. interaction |
||||
|---|---|---|---|---|---|---|
| Low Education n=119 |
High Education n=195 |
Low Education n=78 |
High Education n=157 |
|||
| Age, years, mean (SD) | 79.7 (4.9) | 79.5 (4.9) | 80.6 (5.2) | 79.5 (5.2) | 1. F=0.94, p=0.33 2. F=2.13, p=0.15 3. F=0.93, p=0.33 |
|
| Education, years, mean (SD) | 11.4 (1.3) | 16.3 (2.4) | 11.0 (1.7) | 16.5 (2.5) | 1. F=0.11, p=0.75 2. F=721.57, p<0.001 3. F=2.27, p=0.13 |
|
| Gender, %, women | 67.2 | 60.0 | 59.0 | 62.4 | 1. X2=0.12, p=0.79 2. X2=0.45, p=0.52 |
|
| Ethnicity, %, Caucasian | 65.5 | 74.9 | 66.7 | 70.7 | 1. X2=0.25, p=0.64 2. X2=3.00, p=0.10 |
|
| BMI, total, mean (SD) | 27.6 (5.4) | 27.5 (4.8) | 27.3 (4.9) | 27.0 (4.7) | 1. F=1.01, p=0.32 2. F=0.19, p=0.66 3. F=0.05, p=0.83 |
|
| BIMC, total errors, median | 2 | 1 | 1 | 1 | 1. F=0.05, p=0.82 2. F=10.59, p<0.001 3. F=0.95, p=0.33 |
|
| GDS, total score, mean (SD) | 2.5 (2.2) | 2.2 (2.3) | 2.0 (2.0) | 1.8 (1.9) |
1. F=4.90, p=0.03 2. F=1.82, p=0.18 3. F=0.20, p=0.65 |
|
| BAI, total score, mean (SD) | 4.3 (5.0) | 5.7 (6.5) | 2.3 (3.0) | 2.8 (3.6) |
1. F=10.23, p=0.00 2. F=1.42, p=0.24 3. F=0.30, p=0.59 |
|
| Cardiovascular Summary Score, % | 0 | 77.3 | 74.4 | 80.8 | 86.0 | 1. X2=7.01, p=0.07 2. X2=2.19, p=0.53 |
| 1 | 17.6 | 20.5 | 12.8 | 11.5 | ||
| 2 | 4.2 | 5.1 | 6.4 | 2.5 | ||
| 3 | 0.9 | 0 | 0 | 0 | ||
Test statistics obtained using univariate analysis of variance with the exception of chi-square analyses for gender, ethnicity, and cardiovascular history. Tests statistics significant at the p≤0.05 level are denoted in bold.
Abbreviations: SD=Standard Deviation, BMI=Body Mass Index, BIMC=Blessed Information Memory Concentration Test; GDS=15-item Geriatric Depression Scale, BAI=Beck Anxiety Inventory
Note: BAI data available for n=174.
Correlations between Demographics and Neuropsychological Function
Age was associated with Category Fluency (r=−0.14, p=0.00), TMTA (r=0.15, p=0.00), TMTB (r=0.21, p<0.001), and FCSRT (r=−0.11, p=0.01). Education was associated with all neuropsychological tests (range: r=0.32 to 0.08, p<0.001 to =0.05). There were gender differences on Category Fluency (t(545)=3.04, p=0.00) and FCSRT (t(547)=3.24, p=0.00), with women performing better than men. There were ethnicity differences on all neuropsychological tests except FCSRT (range: t=3.22 to 7.95, p<0.001 all tests), with Caucasians performing better than other ethnicities. Depression was associated with Category Fluency (r=−0.10, p=0.02), TMTA (r=0.10, p=0.03), TMTB (r=0.13, p=0.00), and FCSRT (r=−0.11, p=0.00). Anxiety was not associated with any of the neuropsychological scores. Due to the relationships between demographic variables and neuropsychological test performance, age, gender, ethnicity, and depression were used as covariates in all subsequent analyses. The cardiovascular summary score was also included as a covariate because it demonstrated a trend toward a significant relationship with insomnia (X2(3)=7.01, p=0.07).
Neuropsychological Performance, Sleep Onset/Maintenance Difficulties, and Education
Table 2 depicts sample neuropsychological performance raw scores as a function of SO/MD and education status. Consistent with the simple correlations, fully adjusted models examining performance on Digit Span Backward, TMTB, and Letter Fluency revealed that older adults with lower education performed more poorly than those with higher education (main effect of education: range (F=7.29 to 31.32, p=0.00 to p<0.001)). Fully adjusted models examining Category Fluency performance revealed that individuals with lower education performed more poorly than those with higher education (main effect of education (F(1,536)=13.44, p<0.001). There was no main effect of SO/MD status. There was a significant interaction (SO/MD × education interaction (F(1,536)=14.5, p<0.001); see Figure 1) such that among participants with SO/MD, those with lower education performed more poorly than participants with higher education (means: 35.2 vs. 41.0, pairwise comparison p<0.001); among older adults without SO/MD, education level had no measurable impact on cognition (means: 38.4 vs. 39.1, pairwise comparison p=0.76). A similar trend was revealed on a test of attention (Trail Making Test part A; SO/MD × education interaction (F(1,535)=3.7, p=0.05); among participants with SO/MD, those with lower education performed more poorly than participants with higher education (means: 23.5 vs. 17.9, pairwise comparison p=0.04); among older adults without SO/MD, education level had no measurable impact on cognition (means: 15.6 vs. 18.5, pairwise comparison p=0.50). However, this relationship did not survive Bonferroni correction for statistical significance. There were no other significant models for the prediction of neuropsychological test performance by SO/MD and education or their interaction.
Table 2.
Sample neuropsychological function raw scores in mean (SD) by sleep onset/maintenance difficulties (SO/MD) and education status.
| SO/MD n=314 |
No SO/MD n=235 |
Test Statistic 1. main effect SO/MD 2. main effect education 3. interaction |
|||
|---|---|---|---|---|---|
| Low Education n=119 |
High Education n=195 |
Low Education n=78 |
High Education n=157 |
||
| WAIS-III Digit Span, total forward (0–16) | 9.0 (1.6) | 9.3 (1.9) | 8.9 (1.9) | 9.3 (2.1) | 1. F=0.00, p=1.0 2. F=2.63, p=0.11 3. F=0.20, p=0.66 |
| WAIS-III Digit Span, total backward (0–14) | 5.7 (2.0) | 6.2 (2.0) | 5.5 (1.9) | 6.1 (1.9) | 1. F=0.31, p=0.58 2. F=7.29, p=0.00 3. F=0.18, p=0.67 |
| Trail Making Test part A, seconds | 56.5 (23.4) | 50.5 (17.9) | 51.4 (15.6) | 51.7 (18.5) | 1. F=1.47, p=0.23 2. F=1.05, p=0.31 3. F=3.73, p=0.05 |
| Trail Making Test part B, seconds | 140.7 (56.3) | 111.3 (45.7) | 136.9 (62.2) | 118.3 (48.3) | 1. F=0.04, p=0.83 2. F=19.73, p<0.001 3. F=1.59. p=0.21 |
| Letter Fluency, total score | 34.2 (10.8) | 41.3 (13.2) | 34.6 (11.1) | 40.6 (12.3) | 1. F=0.00, p=0.95 2. F=31.32, p<0.001 3. F=0.13, p=0.72 |
| Category Fluency, total score | 35.2 (6.9) | 41.0 (8.9) | 38.4 (8.8) | 39.1 (8.6) | 1. F=1.23, p=0.27 2. F=13.44, p<0.001 3. F=14.53, p<0.001 |
| FCSRT, free recall (0–48) | 32.2 (4.2) | 33.0 (4.0) | 33.2 (5.0) | 33.3 (4.5) | 1. F=2.26, p=0.13 2. F=0.69, p=0.41 3. F=1.36, p=0.24 |
All test statistics obtained using univariate analysis of covariance covarying for age, gender, ethnicity, depression, and cardiovascular history.
Test statistics significant at the p≤0.007 level are denoted in bold.
Abbreviations: SD=Standard Deviation; WAIS-III=Wechsler Adult Intelligence Scale – 3rd edition; FCSRT=Free and Cued Selective Reminding Test
Figure 1.
Category fluency adjusted means and standard errors by sleep onset/maintenance difficulties (SO/MD) and education level in nondemented older adults.
* p<0.001 in pairwise comparisons; means adjusted for age, gender, ethnicity, depression, and cardiovascular disease.
Supplementary Analyses
The model that examined Category Fluency was repeated using another common proxy of cognitive reserve, performance on the Reading subtest of the Wide Range Achievement Test (WRAT;(Wilkinson, 1993)). Data on this measure were available for 396 participants. This model retained a significant main effect of SO/MD (F(1,387)=270.53, p=0.04), main effect of WRAT (F(1,387)=22.23, p<0.001), and their interaction (F(1,387)=3.91, p=0.05).
Education was examined as a dichotomous variable in this study with the intention of creating ecologically valid group distinctions that vary as a function of high school graduation attainment. In supplementary analyses, we also examined education as a continuous variable and education categorized by quartiles. The model that examined Category Fluency demonstrated the same statistically significant pattern of findings (continuous education and SO/MD interaction (F(1,536)=10.83, p=0.00); quartile education and SO/MD interaction (F(3,532)=5.53, p=0.00).
To examine the effect of English language acquisition, the model that examined Category Fluency was repeated by splitting the sample by those for whom English was their first language (n=449; 81.8%) and those for whom it was not (n=100; 18.2%). The pattern of primary findings remained unchanged in models for each group.
To examine the specificity of our findings, we repeated the model that examined Category Fluency with a general sleep problems index from the MOS-SS. This general sleep problems index comprises items assessing daytime somnolence and snoring, as well as sleep disturbance, sleep adequacy, and awakening with shortness of breath and headache. We found that the interaction of the general sleep problems index and education was not significant (F(1,536)=2.26, p=0.13).
Discussion
This study examined relationships between late-life sleep onset/maintenance difficulties and cognitive function in a large community-based sample of healthy older adults who were free of dementia and amnestic mild cognitive impairment. We also sought to determine whether those relationships varied as a function of educational attainment, a proxy for cognitive reserve. Sleep difficulties were common in our sample of older adults, with 57% endorsing problems initiating or maintaining sleep, complaints that are consistent with insomnia. Our primary finding was that older adults with SO/MD and lower education performed more poorly on a test of language fluency than older adults with SO/MD and higher education, even after controlling for demographic factors and medical comorbidities. There were no education level differences in language fluency among older adults without SO/MD, consistent with the cognitive reserve hypothesis.
Studies that have examined the relationship between late-life subjective sleep quality and cognitive function in healthy adults have reported somewhat inconsistent findings. Several cross-sectional studies reported associations between poorer cognitive performance and insomnia (Haimov et al., 2008), longer sleep onset latencies (Schmutte et al., 2007), daytime sleepiness (Ohayon, 2002), and being a “poor sleeper” (Nebes et al., 2009). Neuropsychological domains most affected by poor sleep quality include attention, executive function, and memory, although working memory, visuospatial ability, and time estimation also demonstrated significant associations. However, a recent population-based study found that daytime sleepiness, but not insomnia, was related to dementia in older adults (Merlino et al., 2010). Similarly, a longitudinal study found that daytime sleepiness, but not insomnia, was associated with cognitive decline and incident dementia over a 3-year time period using a brief cognitive screening instrument (D. Foley et al., 2001). Another longitudinal study found that insomnia was associated with the development of cognitive decline over 3 years in men, but not in women (Cricco et al., 2001). Finally, general subjective sleep complaints were associated with cognitive decline among older adults on a brief global cognition measure in a large prospective cohort study (Jelicic et al., 2002). Contrary to our hypothesis, we did not find that sleep difficulties suggestive of insomnia conferred a cognitive disadvantage among older adults after controlling for age, gender, ethnicity, depression, and medical comorbidities. Although consistent with some studies (D. Foley et al., 2001; Merlino et al., 2010), these findings are incongruent with others (Cricco et al., 2001; Haimov et al., 2008). Our sample differed in that our participants were generally older and were rigorously screened for dementia and preclinical dementia that may contribute to both lower cognitive scores and disruptions in the sleep/wake cycle. In addition, we controlled in analyses for a wide range of moderators that may have independent effects on both sleep and cognition, including age, depression, and medical comorbidities.
The wide-range of educational achievement in our sample allowed us to examine the cognitive reserve hypothesis, a model that is frequently applied to studies of dementia, but not commonly examined in reports of cognition associated with insomnia. Cognitive reserve is a theory that postulates that, in the context of deleterious disruptions in normal brain function, there are differences in an individual’s premorbid cognitive ability or lifetime experience that allow them to compensate when processing information (Stern, 2009). Such brain-based disruptions are commonly conceptualized as age-related neuropathology, but in this study, the effects of sleep disturbance on brain function were considered. Thus, an individual with low cognitive reserve may be less able to cognitively compensate for a disruption in brain function than an individual with high cognitive reserve. Educational attainment and reading achievement are common indices of cognitive reserve (Stern, 2009). Cognitive reserve previously has not been examined in the study of SO/MD and cognition. However, several studies have reported findings attributed to cognitive reserve among middle-aged adults with obstructive sleep apnea (Alchanatis et al., 2005; Yaouhi et al., 2009). Contrary to the hypothesis of the current study, we found that older adults with SO/MD and lower education performed more poorly on a test of category fluency than those with SO/MD and higher education. Older adults without SO/MD performed the same on this task regardless of educational attainment. These findings suggest that sleep onset/maintenance difficulties may have a negative impact on language fluency among healthy older adults, but those with higher education are better able to engage active cognitive compensation than their peers with lower educational attainment. It is important to note that these findings remained significant when performance on a reading measure, another common proxy of cognitive reserve, was examined.
Age-related decline in frontal lobe structure and function (Brickman et al., 2006; Zimmerman et al., 2006) may play a role in both sleep disturbance and cognitive difficulties experienced in healthy older adults. The frontal lobes have been shown to be involved in non-rapid eye movement (NREM) sleep and insomnia, in particular, has been associated with lesions in the dorsomedial frontal lobe (Koenigs, Holliday, Solomon, & Grafman, 2010) and smaller volumes of orbitofrontal cortex (Altena, Vrenken, Van Der Werf, van den Heuvel, & Van Someren, 2010). A functional neuroimaging study found that performance on a verbal fluency task, similar to the test employed in the current study, was associated with prefrontal hypoactivation in adults with insomnia (Altena et al., 2008). Category fluency is a test of verbal fluency that is commonly included in clinical neuropsychological evaluations of individuals across the lifespan in both normal and clinical samples (Lezak, Howieson, & Loring, 2004). Typically, performance on this measure is thought to require both semantic and executive function cognitive components, although recent studies have emphasized the role of speed of processing in task execution (McDowd et al., 2011). Among healthy adults, category fluency declines linearly with age at a greater rate than that of a related test of verbal fluency, letter fluency (Brickman et al., 2005; Tomer & Levin, 1993). Impaired performance on category fluency has been shown to be a diagnostic predictor of age-related cognitive disorders, such as Alzheimer’s disease (Barr & Brandt, 1996; Monsch et al., 1992). In addition, individuals with sleep deprivation have been shown to perform more poorly on a test of category fluency (Harrison & Horne, 1997). Considered together, these findings suggest that the observed relationship between verbal fluency and SO/MD in our sample may be supported by age-related changes in frontal lobe function.
Strengths of the current study include the comprehensive examination of cognition and sleep function in a large sample of older adults systematically recruited from the community who were carefully screened for mild cognitive impairment and dementia. In addition, this study examined the cognitive reserve hypothesis to enhance understanding of relationships between symptoms of insomnia and cognitive function among elderly individuals. There are several limitations, however, that warrant further discussion. Determination of sleep onset/maintenance difficulties was based on a self-report questionnaire that may not have the sensitivity or specificity of a complete clinical evaluation for insomnia. Prevalence rates of cardiovascular illness were relatively low in our sample, which may have contributed to our lack of findings with the primary relationships of interest. In addition, we excluded individuals with amnestic mild cognitive impairment from our sample, which resulted in a relatively restricted range in performance on our memory test that may have affected our ability to detect relationships between SO/MD and memory. Finally, this study employed a cross-sectional design that does not allow for inferences regarding the directionality of the observed relationships. Longitudinal studies will provide important information regarding possible bi-directional associations as well as the course of both cognitive and sleep function over time in older adults.
The findings from this study support the cognitive reserve hypothesis and suggest that older adults with lower education appear uniquely vulnerable to the negative effects of sleep problems suggestive of insomnia on language fluency. The complex relationship among these variables should be considered when assessing sleep disruption and cognitive function in elderly individuals.
Acknowledgments
The Authors would like to thank Charlotte Magnotta for assistance with participant recruitment, Betty Forro, Alicia Gomez, Wendy Ramratan, and Mary Joan Sebastian for assistance with clinical and neuropsychological assessments, Michael Potenza for assistance with data management, and all of the study participants who generously gave their time in support of this research. The work presented in this paper was supported by National Institute on Aging Grant AG03949 and Merck Sharp & Dohme Corporation. A subset of the analyses presented in this manuscript was previously presented at the Alzheimer’s Association International Conference on Alzheimer’s Disease in July of 2011.
Conflicts of Interest: Dr. Zimmerman has received research funding from Merck Sharp & Dohme Corporation. Drs. Zimmerman and Derby and Ms. Katz are co-investigators on NIH grant AG03949. Dr. Lipton is the principal investigator of NIH grant AG03949 and has served as a consultant for Merck, Inc. Dr. Bigal is a full-time employee of Merck, Inc. and owns stocks and stock options of Merck, Inc.
References
- Alchanatis M, Zias N, Deligiorgis N, Amfilochiou A, Dionellis G, Orphanidou D. Sleep apnea-related cognitive deficits and intelligence: an implication of cognitive reserve theory. Journal of Sleep Research. 2005;14(1):69–75. doi: 10.1111/j.1365-2869.2004.00436.x. [DOI] [PubMed] [Google Scholar]
- Altena E, Van Der Werf YD, Sanz-Arigita EJ, Voorn TA, Rombouts SA, Kuijer JP, Van Someren EJ. Prefrontal hypoactivation and recovery in insomnia. Sleep. 2008;31(9):1271–1276. [PMC free article] [PubMed] [Google Scholar]
- Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Someren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biological Psychiatry. 2010;67(2):182–185. doi: 10.1016/j.biopsych.2009.08.003. [DOI] [PubMed] [Google Scholar]
- American Psychological Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Ancoli-Israel S, Lieberman JA., 3rd Insomnia in primary care: overcoming diagnostic and treatment barriers. Introduction. Postgraduate Medicine. 2004;116(6 Suppl Insomnia):4–6. doi: 10.3810/pgm.12.2004.suppl38.256. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Low LF. Normal cognitive changes in aging. Australian Family Physician. 2004;33(10):783–787. [PubMed] [Google Scholar]
- Barr A, Brandt J. Word-list generation deficits in dementia. Journal of Clinical and Experimental Neuropsychology. 1996;18(6):810–822. doi: 10.1080/01688639608408304. [DOI] [PubMed] [Google Scholar]
- Battery, Army Inidivual Test. Manual of Directions and Scoring. Washington D.C.: War Department, Adjutant Generals Office; 1944. [Google Scholar]
- Beaulieu-Bonneau S, Hudon C. Sleep disturbances in older adults with mild cognitive impairment. International Psychogeriatrics. 2009;21(4):654–666. doi: 10.1017/S1041610209009120. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City, Iowa: AJA Associates; 1989. [Google Scholar]
- Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. British Journal of Psychiatry. 1968;114(512):797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Paul RH, Cohen RA, Williams LM, MacGregor KL, Jefferson AL, Gordon E. Category and letter verbal fluency across the adult lifespan: relationship to EEG theta power. Archives of Clinical Neuropsychology. 2005;20(5):561–573. doi: 10.1016/j.acn.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, Gordon E. Regional white matter and neuropsychological functioning across the adult lifespan. Biological Psychiatry. 2006;60(5):444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Buschke H. Cued recall in amnesia. Journal of Clinical Neuropsychology. 1984;6(4):433–440. doi: 10.1080/01688638408401233. [DOI] [PubMed] [Google Scholar]
- Christensen H. What cognitive changes can be expected with normal ageing? Australian and New Zealand Journal of Psychiatry. 2001;35(6):768–775. doi: 10.1046/j.1440-1614.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- Cole JC, Dubois D, Kosinski M. Use of patient-reported sleep measures in clinical trials of pain treatment: a literature review and synthesis of current sleep measures and a conceptual model of sleep disturbance in pain. [Research Support, Non-U.S. Gov't] Clinical Therapeutics. 2007;29(Suppl):2580–2588. doi: 10.1016/j.clinthera.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. Journal of the American Geriatrics Society. 2001;49(9):1185–1189. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. Journal of the American Medical Association. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Foley D, Monjan A, Masaki K, Ross W, Havlik R, White L, Launer L. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. Journal of the American Geriatrics Society. 2001;49(12):1628–1632. doi: 10.1046/j.1532-5415.2001.t01-1-49271.x. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- Grober E, Buschke H. Genuine memory deficits in dementia. Developmental Neuropsychology. 1987;3:13–36. [Google Scholar]
- Grober E, Kawas C. Learning and retention in preclinical and early Alzheimer's disease. [Research Support, Non-U.S. Gov't Research Support, U.S.Gov't, P.H.S.] Psychology and Aging. 1997;12(1):183–188. doi: 10.1037//0882-7974.12.1.183. [DOI] [PubMed] [Google Scholar]
- Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54(4):827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- Grober E, Lipton RB, Katz M, Sliwinski M. Demographic influences on free and cued selective reminding performance in older persons. [Research Support, U.S. Gov't, PHS.] Journal of Clinical and Experimental Neuropsychology. 1998;20(2):221–226. doi: 10.1076/jcen.20.2.221.1177. [DOI] [PubMed] [Google Scholar]
- Haimov I, Hanuka E, Horowitz Y. Chronic insomnia and cognitive functioning among older adults. Behavioral Sleep Medicine. 2008;6(1):32–54. doi: 10.1080/15402000701796080. [DOI] [PubMed] [Google Scholar]
- Hall CB, Derby C, LeValley A, Katz MJ, Verghese J, Lipton RB. Education delays accelerated decline on a memory test in persons who develop dementia. [Research Support, N.I.H. Extramural] Neurology. 2007;69(17):1657–1664. doi: 10.1212/01.wnl.0000278163.82636.30. [DOI] [PubMed] [Google Scholar]
- Harrison Y, Horne JA. Sleep deprivation affects speech. Sleep. 1997;20(10):871–877. doi: 10.1093/sleep/20.10.871. [DOI] [PubMed] [Google Scholar]
- Hays RD, Martin SA, Sesti AM, Spritzer KL. Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Medicine. 2005;6(1):41–44. doi: 10.1016/j.sleep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Hoyt BD. Sleep in patients with neurologic and psychiatric disorders. Primary Care. 2005;32(2):535–548. ix. doi: 10.1016/j.pop.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Smith GE, Lucas JA, Tangalos EG, Kokmen E, Petersen RC. Free and cued selective reminding test: MOANS norms. [Clinical Trial Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, PHS.] Journal of Clinical and Experimental Neuropsychology. 1997;19(5):676–691. doi: 10.1080/01688639708403753. [DOI] [PubMed] [Google Scholar]
- Jelicic M, Bosma H, Ponds RW, Van Boxtel MP, Houx PJ, Jolles J. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS) International Journal of Geriatric Psychiatry. 2002;17(1):73–77. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- Julian LJ, Gregorich SE, Earnest G, Eisner MD, Chen H, Blanc PD, Katz PP. Screening for depression in chronic obstructive pulmonary disease. Chronic Obstructive Pulmonary Disease. 2009;6(6):452–458. doi: 10.3109/15412550903341463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten J, Nolen WA, Penninx BW, Hartman CA. Subthreshold anxiety better defined by symptom self-report than by diagnostic interview. Journal of Affective Disorders. 2010 doi: 10.1016/j.jad.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. Journal of Family Practice. 2002;51(3):229–235. [PubMed] [Google Scholar]
- Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, Derby CA. Age and sex specific prevalence and incidence of mild cognitive impairment, dementia and Alzheimer’s disease in blacks and whites: A report from the Einstein Aging Study. Alzheimer's Disease and Associated Disorders. doi: 10.1097/WAD.0b013e31823dbcfc. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Holliday J, Solomon J, Grafman J. Left dorsomedial frontal brain damage is associated with insomnia. Journal of Neuroscience. 2010;30(47):16041–16043. doi: 10.1523/JNEUROSCI.3745-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryger M, Monjan A, Bliwise D, Ancoli-Israel S. Sleep, health, and aging. Bridging the gap between science and clinical practice. Geriatrics. 2004;59(1):24–26. 29–30. [PubMed] [Google Scholar]
- Lane EM, Paul RH, Moser DJ, Fletcher TD, Cohen RA. Influence of Education on Subcortical Hyperintensities and Global Cognitive Status in Vascular Dementia. Journal of the International Neuropsychological Society. 2011:1–6. doi: 10.1017/S1355617711000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DL. Neuropsychological Assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- Lipton RB, Katz MJ, Kuslansky G, Sliwinski MJ, Stewart WF, Verghese J, Buschke H. Screening for dementia by telephone using the memory impairment screen. Journal of the American Geriatrics Society. 2003;51(10):1382–1390. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- McCrae CS, Wilson NM, Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. 'Young old' and 'old old' poor sleepers with and without insomnia complaints. Journal of Psychosomatic Research. 2003;54(1):11–19. doi: 10.1016/s0022-3999(02)00543-3. [DOI] [PubMed] [Google Scholar]
- McCurry SM, Ancoli-Israel S. Sleep Dysfunction in Alzheimer's Disease and Other Dementias. Current Treament Options in Neurology. 2003;5(3):261–272. doi: 10.1007/s11940-003-0017-9. [DOI] [PubMed] [Google Scholar]
- McDowd J, Hoffman L, Rozek E, Lyons KE, Pahwa R, Burns J, Kemper S. Understanding verbal fluency in healthy aging, Alzheimer's disease, and Parkinson's disease. Neuropsychology. 2011;25(2):210–225. doi: 10.1037/a0021531. [DOI] [PubMed] [Google Scholar]
- Merlino G, Piani A, Gigli GL, Cancelli I, Rinaldi A, Baroselli A, Valente M. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: a population-based study. Sleep Medicine. 2010;11(4):372–377. doi: 10.1016/j.sleep.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal L. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Archives of Neurology. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Naismith SL, Rogers NL, Hickie IB, Mackenzie J, Norrie LM, Lewis SJ. Sleep well, think well: sleep-wake disturbance in mild cognitive impairment. Journal of Geriatric Psychiatry and Neurology. 2010;23(2):123–130. doi: 10.1177/0891988710363710. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. Journals of Gerontology Series B: Psychological Sciences and Socical Sciences. 2009;64(2):180–187. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult - a mini-review. Gerontology. 2010;56(2):181–189. doi: 10.1159/000236900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Medicine Reviews. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Park DC. Park DC, Schwarz N. Cognitive Aging: A Primer. Philadelphia: Psychology Press; 2000. The basic mechanisms accounting for age-related decline in cognition function; pp. 3–21. [Google Scholar]
- Perlis ML, Smith LJ, Lyness JM, Matteson SR, Pigeon WR, Jungquist CR, Tu X. Insomnia as a risk factor for onset of depression in the elderly. Behavioral Sleep Medicine. 2006;4(2):104–113. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Pleiss JR, Ward BW, Lucas J. Vital Health Statistics. Vol. 10. Washington, DC: National Center for Health Statistics; 2010. Summary health statistics for U.S. adults: National Health Interview Survey, 2009. [PubMed] [Google Scholar]
- Sarazin M, Berr C, De Rotrou J, Fabrigoule C, Pasquier F, Legrain S, Dubois B. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. [Comparative Study Research Support, Non-U.S. Gov't] Neurology. 2007;69(19):1859–1867. doi: 10.1212/01.wnl.0000279336.36610.f7. [DOI] [PubMed] [Google Scholar]
- Schmutte T, Harris S, Levin R, Zweig R, Katz M, Lipton R. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: results from the Bronx aging study. Behavioral Sleep Medicine. 2007;5(1):39–56. doi: 10.1207/s15402010bsm0501_3. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal LJ, Grundman M, Golden R. Alzheimer's disease: a correlational analysis of the Blessed Information-Memory-Concentration Test and the Mini-Mental State Exam. [Comparative Study] Neurology. 1986;36(2):262–264. doi: 10.1212/wnl.36.2.262. [DOI] [PubMed] [Google Scholar]
- Tomer R, Levin BE. Differential effects of aging on two verbal fluency tasks. Perceptual Motor Skills. 1993;76(2):465–466. doi: 10.2466/pms.1993.76.2.465. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test 3 Administration Manual. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- Yaouhi K, Bertran F, Clochon P, Mezenge F, Denise P, Foret JP, Desgranges B. A combined neuropsychological and brain imaging study of obstructive sleep apnea. Journal of Sleep Research. 2009;18(1):36–48. doi: 10.1111/j.1365-2869.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatry Research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Brickman AM. Neuropsychology of healthy aging. In: Paul RH, Sactor NC, Valcour V, Toshima KT, editors. HIV and the Brain: New Challenges in the Modern Era. Totowa, NJ: Humana Press; 2009. pp. 347–368. [Google Scholar]
- Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, Gordon E. The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. American Journal of Geriatric Psychiatry. 2006;14(10):823–833. doi: 10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Lipton RB, Santoro N, McConnell DS, Derby CA, Katz MJ, Saunders-Pullman R. Endogenous estradiol is associated with verbal memory in nondemented older men. [Research Support, N.I.H. Extramural] Brain and Cognition. 2011;76(1):158–165. doi: 10.1016/j.bandc.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]