Abstract
Purpose of Review
2008 marks the 20th anniversary of the first use of umbilical cord blood (UCB) as a source of donor cells for hematopoietic stem cell transplantation. In those early days, there was great doubt and skepticism about the utility of UCB as a source of hematopoietic stem cells. Doubts about whether UCB, containing 10-20x fewer cells than bone marrow, had sufficient cells to durably engraft a myeloablated patient and, after demonstration that engraftment occurred with less graft-versus-host disease (GvHD), whether it would confer graft versus leukemia (GvL) activity were raised.
Recent Findings
Transplantation with UCB is effective in the treatment of children with hematological malignancies, marrow failure, immunodeficiencies, hemoglobinopathies and inherited metabolic diseases. Transplantation without full HLA matching is possible and despite a lower incidence of GvHD, GvL is preserved. The number of cells in a single UCB can be limiting, but the use of 2 UCBs for a single transplant shows promise to overcome this obstacle.
Summary
Cord blood transplantation is now an established field with enormous potential. UCB increases access to transplantation therapy for many patients unable to indentify a fully matched adult donor. In the future, it may emerge as a source of cells for cellular therapies focused on tissue repair and regeneration.
Keywords: Umbilical Cord Blood, Unrelated Donors, Hematopoietic Stem Cell Transplantation, Inborn Errors of Metabolism
Introduction
The 20th anniversary of the first umbilical cord blood transplant (UCBT), performed in a 6 year old boy with Fanconi Anemia from his HLA-matched sibling, was celebrated in the US and France in 2008. The patient, now 26 years old, durably engrafted and well, attended the session and spoke eloquently about the decision his parents made to allow him to undergo this first-in-man clinical experiment. Despite the ultimate success of this transplant, at the time it was considered highly unlikely that cord blood would emerge as a standard source of stem and progenitor cells for hematopoietic stem cell transplantation (HSCT). Yet, today, cord blood is donated and banked for unrelated donor transplantation on a routine basis. There are >400,000 units banked in inventory in >100 unrelated donor banks, facilitating >14,000 unrelated donor cord blood transplants worldwide.
Background and history
Since the first allogeneic transplant for a child with severe combined immunodeficiency syndrome in 1968, thousands of patients have been cured of life-threatening malignant and non-malignant hematologic and genetic disorders, establishing the field of marrow transplantation. Over the years, the National Marrow Donor Program (NMDP) in the United States and other international registries have recruited, typed, and maintained databases of >13 million volunteer unrelated adult donors. Despite this large number of donors, ~50-70% of the patients in need of a transplant are still unable to find a suitable adult donor in a timely manner. This problem is more prominent in patients belonging to ethnic and racial minorities who, because of larger HLA diversity and some reluctance to volunteer as adult donors, have an even lower probability of identifying an unrelated adult donor.
Banked umbilical cord blood provides access to transplantation
After the success of the initial related cord blood transplant (1), approximately 60 others were performed in the matched sibling setting and results were reported to a volunteer international registry demonstrating that cord blood contained sufficient numbers of hematopoietic stem and progenitor cells to reconstitute a pediatric patient and also that there was a lower incidence of acute and chronic GvHD when cord blood, as compared to bone marrow, was used as the source of donor cells.(2) This led to the hypothesis that cord blood could be banked and used as a source of unrelated donor cells, possibly without full HLA matching.(3) In 1992, Dr. Pablo Rubinstein established a pilot unrelated donor umbilical cord blood bank at the New York Blood Center. (4) In 1993, the first unrelated donor UCBT was performed with a unit from this bank in a child with refractory leukemia at Duke University Medical Center. (5)
The idea of using cord blood for hematopoietic transplantation arose from interactions among a group of physicians and scientists in the early 1980s. Using blood from near-term mouse donors, Dr. Ted Boyse at Memorial Sloan Kettering Cancer Center demonstrated reconstitution of hematopoiesis in lethally irradiated mice. Concomitantly, Dr. Hal Broxmeyer in New York and Indiana performed early experiments characterizing the hematopoietic stem and progenitor cells in human UCB demonstrating that cord blood derived HPC had higher proliferative capacity and higher replating potential as compared to similar cells in bone marrow, and also that HPC from cord blood could be readily cryopreserved. (3,6) Furthermore, in-vivo studies in non--obese diabetic (NOD)/SCID repopulating cell assays have shown that the SCID-repopulating cell (SRC) frequency in UCB is three- fold higher than in marrow and six fold higher than in mobilized peripheral blood, suggesting that UCB contains a higher proportion of primitive hematopoietic stem and progenitor cells. (6)
Unrelated-Donor UCB transplantation
In 1993, the first unrelated UCB transplant was performed in a 3-year-old child with refractory T-cell acute lymphoblastic leukemia utilizing a 4/6 matching cord blood unit from the New York Blood Center. While this child engrafted, he later died of interstitial pneumonitis. A second child, also with refractory leukemia, transplanted with a 4/6 matched cord blood survived more than 15 years later in remission with full donor chimerism, normal immune function and no evidence of graft-versus-host disease (GvHD). Subsequently, the outcomes of the first 25 successive transplants with unrelated UCB banked at the New York Blood Center, and transplanted at Duke University, were reported in 1996. (5) Important observations in these patients, which were confirmed in subsequent reports from other centers and transplant registries including the New York Blood Center and the European Cord Blood Registry (Eurocord), (7-9) confirmed that banked unrelated donor cord blood contained sufficient numbers of stem and progenitor cells to reconstitute the marrow of children undergoing myeloablative therapy for leukemias, congenital marrow failure and genetic diseases without full HLA matching between the donor and recipient. These patients experienced a lower incidence and severity of acute and chronic GvHD as compared to that seen with matched unrelated marrow transplants without loss of graft-versus-leukemia effects.(9) In these patients, cell dose strongly correlated with clinical outcomes including time to and probability of engraftment and probability of overall survival. (5-9) Engraftment times were observed to be slower than those seen when marrow or mobilized peripheral blood were used as the source of donor stem cells.
Donor recipient matching strategies for cord blood transplantation established in the days of these early transplants, were much less stringent than those used for adult donors. Low resolution (antigen level) matching was used at HLA Class I A and B loci with high resolution matching for HLA Class II DRB1. Matching at HLA C, DQ or DP was not employed. This approach continues to be used for donor cord blood unit selection although the question of whether allele level matching at Class I, as well as Class II or HLA C matching would improve outcomes, currently remains unanswered.
1st Prospective study: COBLT
The first prospective, multi-institutional study of cord blood banking and transplantation in the United States was sponsored by the National Heart, Lung, and Blood Institute, which funded a prospective multicenter clinical trial of unrelated cord blood banking and transplantation—the Cord Blood Transplantation Study (COBLT)—from 1997 to 2004. As part of this study, 3 additional unrelated cord blood banks were established. A steering committee of bankers, obstetricians, ethicists and transplanters created common quality standards and standard operating procedures (SOPs) addressing donor recruiting and screening, cord blood collection, processing, testing, cryopreservation, storage and thawing for transplantation.(10,11) Maternal donors were screened for events in their medical history that would exclude them as donors (e.g., multiple pregnancy, congenital anomalies, prematurity, placental deformity, a first degree relative of the baby donor diagnosed with cancer, prior receipt of a transplant, or demonstration of high risk behaviors likely to increase the chance of infection with blood-borne infectious diseases). SOPs were also established and validated for obtaining donor consent; obtaining medical histories; obtaining blood samples from maternal donors; cord blood collecting, processing, testing, cryopreservation, and storage; as well as searching for and releasing cord blood units for transplantation. Twenty-six transplant centers participated in the COBLT transplant study, a prospective clinical trial designed to examine the safety and unrelated cord blood transplantation in infants, children, and adults with malignancies; children with congenital immunodeficiency disorders; and children with inborn errors of metabolism. Definitions of engraftment, grading of GvHD, toxicity scoring and algorithms for causes of death were developed. The study used common preparative regimens, prophylaxis against GVHD, and supportive care measures.
Results of the individual strata of the COBLT transplant study have now been published. (12-15). Overall outcomes in children with malignant and non-malignant conditions were favorable, with 2 year, event-free survivals of 55% in children with high risk malignancies (15) and 78% survival in children with non-malignant conditions. (14 The cumulative incidence of engraftment by Day 42 after transplantation was approximately 80% in all study strata including adults and children as well as children with malignant diseases, inborn errors of metabolism, and immunodeficiency syndromes. Factors adversely affecting engraftment or survival included lower cell doses, pre-transplant cytomegalovirus seropositivity in the recipient, non-European ancestry, and greater HLA mismatching. In the strata of patients with pediatric malignancies, there was no difference in outcomes between those patients receiving 4/6 or 5/6 matched grafts. The number of patients receiving 6/6 matched grafts was very small, but these patients appeared to have superior survival as compared to those transplanted with HLA mismatched grafts.
The COBLT study results in a very high-risk group of adults were inferior to those seen in children and in individuals receiving marrow from an unrelated donor. Subsequent studies in adults, reported by single centers or registries, revealed more encouraging results. (13, 16-17) Most recently, the use of 2 cord blood units for a single transplant, pioneered in adults by Drs. John Wagner and Juliet Barker at the University of Minnesota, has improved rates of both engraftment and survival increasing the use of cord blood donors in adults around the world. (18) In these pilot studies, the proportion of patients engrafting with their cord blood donor(s) increase to ≥90% and overall one year survivals increased to 60-80% depending upon patient diagnosis, comorbidities and disease status at the time of transplant. Interestingly, when 2 cord blood units are administered, 1 generally engrafts and the other is rejection. This typically occurs in the first month post transplant. The engrafting unit cannot be predicted by traditional parameters like cell dose, timing of administration, HLA match or CD34 or CFU dosing. The exact role of the non-engrafting cord blood unit remains unclear but is the subject of current preclinical and clinical studies. To further determine the role of 2 versus 1 cord blood units, the Blood and Marrow Transplant Clinical Trials Network (BMT-CTN), a NIH sponsored cooperative group for multi-institutional transplant clinical trials is currently sponsoring a study in pediatric patients with hematological malignancies asking in a randomized, prospective phase III clinical trial, whether outcomes are better when 2 or 1 cord blood unit is used to deliver donor hematopoietic stem cells. This study will help determine whether 2 cord bloods are better than 1.
Retrospective and registry studies
Over the nearly 15 years since the first unrelated-donor cord blood transplantation, more than 14,000 such procedures have been performed at more than 150 centers located throughout the world. Retrospective analyses performed by transplant registries in the US and Europe supported the conclusions reached in the early studies of UCBT. Efficacy of unrelated cord blood transplantation has been demonstrated in children and adults with hematological malignancies and children with a variety of nonmalignant disorders including hemoglobinopathies, (19) immunodeficiencies, (20, 21) and inborn errors of metabolism.(14, 22-25) A majority of transplants have been mismatched at one or two HLA loci. Yet, a recent retrospective comparative analysis from the Center for International Blood and Marrow Transplant Research (CIBMTR) of unrelated donor graft sources—cord blood vs marrow—showed a comparable 5-year leukemia-free survival in children with acute leukemia who received allele-matched marrow vs one- or two-antigen mismatched cord blood transplants. The analysis suggests that 6/6 matched cord blood may yield superior survival, even though the comparison fell short of statistical significance likely due to the small numbers of patients receiving 6/6 matched graft available for this analysis. (26) A minimum cell dose threshold of 2.5 to 3.0 X 10e7 nucleated cells/kg in the cryopreserved cord blood has been associated with superior clinical outcomes. (5,7-9.15,22)
UCBT for children with inherited metabolic disorders
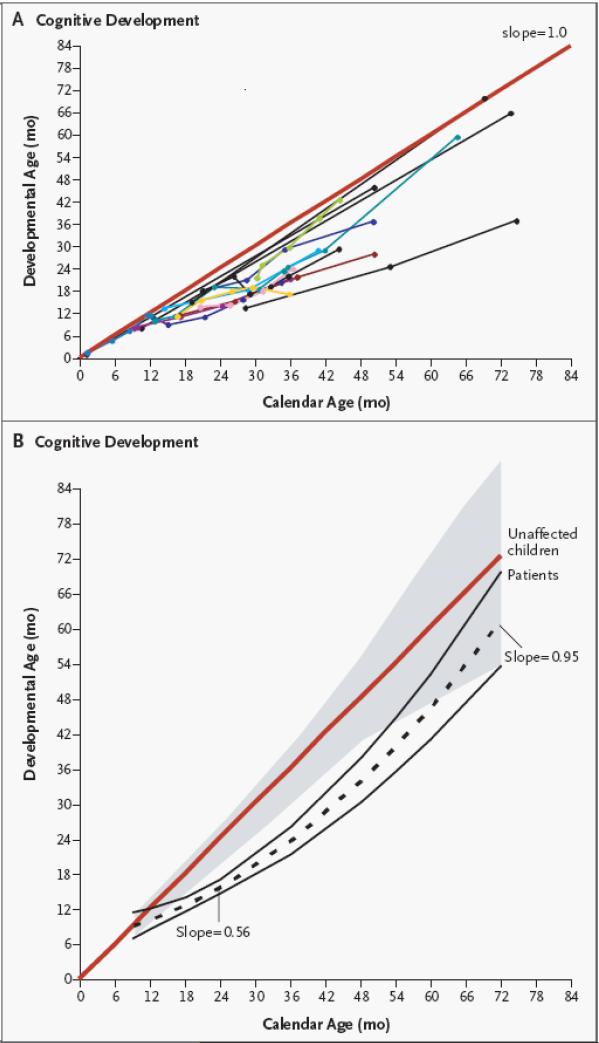
Cord blood transplantation has been particularly effective in the treatment of young infants and children with certain inborn errors of metabolism, e.g., mucopolysaccharidoses such as Hurler syndrome and leukodystrophies such as Krabbe disease. (22-25) These diseases are examples of lysosomal and peroxisomal storage diseases, which result from single gene defects leading to specific enzyme deficiencies. These deficiencies cause defective lysosomal breakdown and accumulation of a toxic substrate, which in turn leads to progressive involvement of brain and other organs and premature death. In these patients, durably engrafted cord blood cells of donor origin provides a form of cell-based enzyme replacement therapy. Donor cells provide continuous, life long production of the missing or defective enzyme. For example, most patients with untreated severe phenotype Hurler syndrome (MPS I) die between 5-10 years of age from progressive cardiac and pulmonary involvement. They also suffer from severe bony abnormalities, corneal clouding, massive hepatosplenomegaly, and severe neurocognitive impairment. Engrafted cord blood cells have the ability to cross the blood-brain barrier and have been shown to prevent neurological deterioration and to even cause cognitive improvement (Figure 1), a beneficial outcome that is not expected with the alternative approach of enzyme replacement therapy. (22,23,25) Unrelated cord blood transplantation, when performed before the age of ~2 years, leads to correction of cardiac, pulmonary, liver, and neurologic damage and improves survival. In fact, a recent risk factor analysis of 146 patients from the European Group for Blood and Marrow Transplantation (EBMT) suggests that cord blood could be the preferred stem cell source in this disease. (25)
Figure 1.
Neurocognitive improvement of 20 consecutive patients with severe phenotype Hurler Syndrome transplanted with unrelated donor cord blood at a median of 16 months of age and followed for 5-7 years. Reprinted from the NEJM, reference 23.
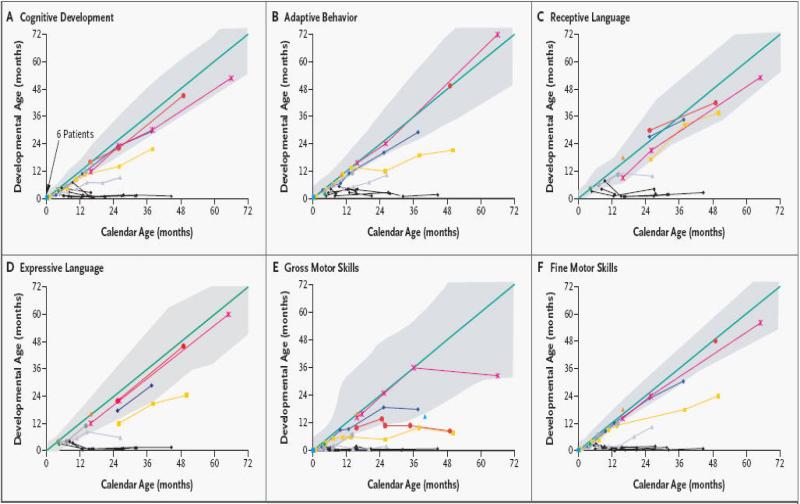
In patients with leukodystrophies, cord blood transplantation has been shown to be maximally effective when performed in patients with pre-symptomatic or minimally symptomatic disease. In these patients, UCBT can prevent demyelination in the central and, often, the peripheral nervous systems, extending life and improving the overall quality of life for these patients. (22,24,25) Neurocognitive function is preserved in all newborn patients while varying degrees of motor disability persist. In contrast, symptomatic infants undergoing UCBT fail to gain neurological skills post transplant. UCBT is no longer recommended for symptomatic infants with Krabbe Disease. (24, Figure 2). Successes using UCBT in newborns with infantile forms of Krabbe disease have led to the development of a pilot newborn screening program in New York State.
Figure 2.
Developmental Outcomes of UCBT for Newborns and Older Babies with Infantile Krabbe Disease. Colored lines represent individual patients transplanted as newborns while black lines show older infants transplanted after the development of neurological deterioration. Reprinted from Reference 24.
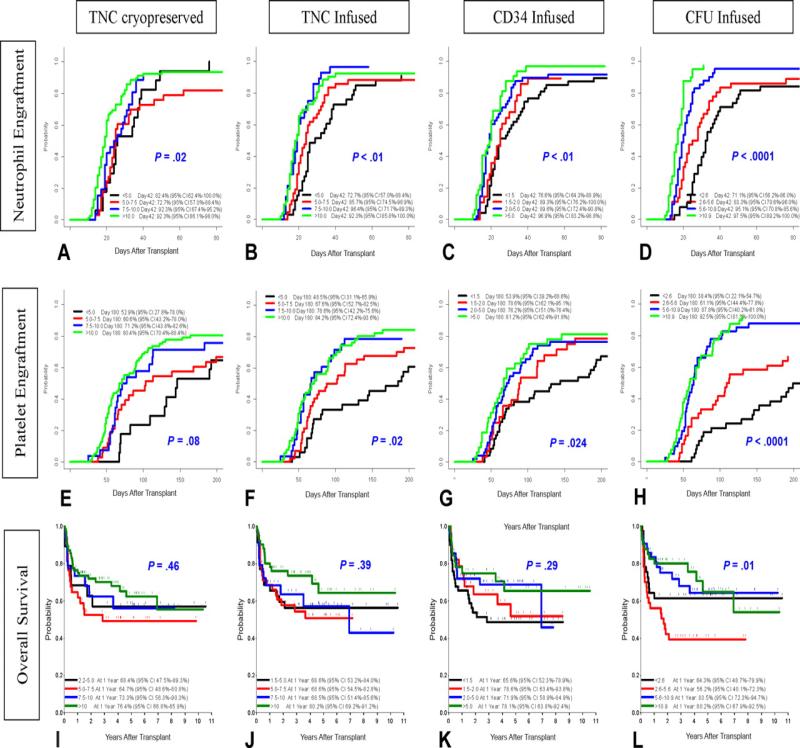
A recently published series of 159 consecutively transplanted pediatric patients with inherited metabolic diseases showed that performance status and various characteristics of the infused graft best predicted clinical outcomes. (25). Post thaw content of CFU-GM and CD34+ cell counts best predicted both engraftment and overall survival. (Figure 3).
Figure 3.
Influence of Graft Characteristics on Clinical Outcomes after UCBT. Reprinted from Reference 25
Advantages and disadvantages of cord blood transplantation
Cord blood transplantation has several advantages over marrow or peripheral blood stem cell transplantation, the most significant of which is that cord blood transplantation can be performed successfully from unrelated donor with one or two HLA mismatches. Banked cord blood is ready available, generally within 1-2 weeks. There is minimal donor attrition and easier recruitment of minority donors. Current obstacles to successful use of UCBT include smaller inventories with lower cell doses and lack of sufficient donors to provide 5/6 or 6/6 matches for all patients in need. Slower engraftment of neutrophils leads to longer hospitalizations and increased utilization of medical resources. There are also significant limitations dictated mainly by the kinetics of cord blood engraftment. The majority of single cord blood units do not contain sufficient numbers of nucleated cells to be of benefit for adult patients. Delays in immune reconstitution lead to an increased risk of viral infections, particularly in the first year post transplant. While it is possible, there is also a limited ability to perform post-transplant donor-derived cellular therapy such as donor lymphocyte infusions or re-transplantation in the case of poor graft function or graft failure.
Cord Blood Banking
Umbilical cord blood is the remainder of the newborn infant's blood contained in the placenta after birth. It used to be routinely discarded at birth. Cord blood can be harvested without physical risk to the mother or infant donor, from the delivered placenta (ex utero) or during the third stage of labor (in utero). In either case, after sterile preparation, the umbilical vein is punctured with a needle attached to a sterile, closed-system collection bag containing citrate-phosphate-dextrose or heparin anticoagulant which is positioned lower than the placenta. Blood flows from the placenta, through the cord into the bag by gravity over approximately 5 to 10 minutes. Experienced collectors harvest an average of 110 ml containing ~1×10e9 nucleated cells from a single placenta. The cord blood unit is labeled and subsequently shipped to the cord blood bank for processing, testing, cryopreservation, and storage. (11)
There are two types of cord blood banks—public and private. In public banks, cord blood units are donated on a volunteer basis after written informed consent by mothers delivering healthy infants at term. There is no cost to donate to a public bank. Private banks, which are for-profit entities, store “directed donations” collected by obstetricians or midwives from infants born into families who intend to use the cord blood for autologous use or for a biological sibling in need of future transplantation therapy. While the indications for directed donation are relatively clear (e.g. a biological sibling with a hematological malignancy, hemoglobinopathy, marrow failure, immunodeficiency or inherited metabolic disease), the indications for autologous use are predominantly speculative at this time. It is possible that autologous cord blood could provide a source of stem and progenitor cells for cellular therapies, tissue repair and regeneration, in the future, these therapies are not possible at the present time.
In 2004, after appropriation of $20,000,000 by the US Congress to increase the inventory of cord blood units in US public banks, the Health Resources Services Administration (HRSA) asked the Institute of Medicine (IOM) to perform a study to determine the best way to organize public cord blood banking and distribution to patients undergoing unrelated transplantation, the results of which were published in April 2005. (27) In brief, the IOM recommended that HRSA contract with eligible banks to procure approximately 150,000 new, ethnically diverse, high quality, unrelated donor cord blood units over the next 5 years. Subsequently, the US Congress passed legislation that established the CW “Bill” Young Stem Cell Transplantation Program, a federally funded program which established a network of public cord blood banks (the National Cord Blood Inventory [NCBI]}, a single point of access donor registry, separate bone marrow and cord blood coordinating centers and an outcomes database, the Stem Cell Transplant Outcomes Database (SCTOD) all administered through HRSA. Contracts were awarded to 9 public cord blood banks, the NMDP and the Center for International Blood and Marrow Transplant Research (CIBMTR) to administer these programs. In 2008, a sibling donation program, providing directed donor banking of cord blood from biological siblings of children with cancer, hemogloginopathies and other blood disorders treated with allogeneic transplantation at no cost to the family, was launched by HRSA.
Conclusions and future directions
Despite limited enthusiasm in it's early days, the field of cord blood transplantation is now firmly established. Umbilical cord blood from related and unrelated donors, matched or mismatched at one or two antigens, is now widely regarded as an alternate donor source to matched marrow or peripheral blood for allogeneic transplantation in children as well as adults for a variety of malignant and non-malignant disorders. This use of cord blood dramatically increases access to transplantation therapy for patients lacking matched related or unrelated adult donors. Retrospective, registry and single center reports of UCBT outcomes show comparable results when cord blood or adult cells are used as donors. Yet, despite these successes, cord blood transplantation still faces many challenges. Delayed hematopoietic engraftment and immune reconstitution continue to limit its overall success. Results of UCBT in children with metabolic diseases suggest that cord blood contains cells capable of non-hematopoietic differentiation suggesting that cord blood may be a viable source of cells for cellular therapies as this field continues to mature. (28,29) For the present, cord blood has established itself as an important source of allogeneic donor cells increasing access to transplantation for many patients in need. Larger inventories, higher quality units, the development of potency assays and FDA licensure of cord blood should lead to increased usage and improved outcomes after UCBT in the next few years. Applications for cellular therapies, tissue repair and regeneration remain speculative at present, but it is highly likely that cord blood will emerge as a novel source of cells for cellular therapies within the next decade.
References
- 1.Gluckman E, Broxmeyer H, Auerbach A, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 2.Wagner J, Rosenthal J, Sweetman R, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: Analysis of engraftment and acute graft-versus- host disease. Blood. 1996;88:795–802. [PubMed] [Google Scholar]
- 3.Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryo-preservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 6.Mayani H, Lansdorp PM. Biology of human umbilical cord blood-derived stem/progenitor cells. Stem Cells. 1998;16:153–165. doi: 10.1002/stem.160153. [DOI] [PubMed] [Google Scholar]
- 7.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 8.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 9.Rocha V, Cornish J, Sievers EL, et al. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962–2971. doi: 10.1182/blood.v97.10.2962. [DOI] [PubMed] [Google Scholar]
- 10.Fraser J, Cairo M, Wagner E, et al. Cord Blood Transplantation Study (COBLT): Cord blood bank standard operating procedures. J Hematother. 1998;7:521–561. doi: 10.1089/scd.1.1998.7.521. [DOI] [PubMed] [Google Scholar]
- 11.Kurtzberg J, Cairo MS, Fraser JK, et al. Results of the Cord Blood Transplantation (COBLT) Study Unrelated Donor Banking Program. Transfusion. 2005;45:842–855. doi: 10.1111/j.1537-2995.2005.04428.x. [DOI] [PubMed] [Google Scholar]
- 12.Wall DA, Carter SL, Kernan NA, et al. Busulfan/melphalan/antithymocyte globulin Followed by Unrelated Donor Cord Blood Transplantation for Treatment of Infant Leukemia in Young Children: The Cord Blood Transplantation Study (COBLT) Experience. Bio Blood Marrow Transplant. 2005;11:637–646. doi: 10.1016/j.bbmt.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Chao NJ, Emerson SG, Weinberg KI. Stem Cell Transplantation (Cord Blood Transplants). Hematology Am Soc Hematol Educ Program. 2004:354–371. doi: 10.1182/asheducation-2004.1.354. [DOI] [PubMed] [Google Scholar]
- 14.Martin PL, Carter SL, Kernan NA, et al. Results of the Cord Blood Transplantation Study (COBLT): Outcomes of Unrelated Donor Umbilical Cord Blood Transplantation in Pediatric Patients with Lysosomal and Peroxisomal Storage Diseases. Bio Blood Marrow Transplant. 2006;12:184–194. doi: 10.1016/j.bbmt.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 15**.Kurtzberg J, Prasad VK, Carter SL, et al. Results of the Cord Blood Transplantation Study (COBLT): Clinical Outcomes of Unrelated Donor Umbilical Cord Blood Transplantation in Pediatric Patients with Hematological Malignancies. Blood. 2008 Aug; doi: 10.1182/blood-2007-06-098020. doi:10.1182/blood-2007-06-098020. [This article reports the results of the first prospective, multi-institutional study of the use of cord blood transplantation to treat children with hematological malignancies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–22. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 17.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–85. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 18.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 19.Hall J, Martin P, Wood S, et al. Unrelated umbilical cord blood transplantation for an infant with beta-thalassemia major. J Pediatr Hematol Oncol. 2004;26:382–385. doi: 10.1097/00043426-200406000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Parikh SH, Szabolcs P, Prasad VK, et al. Correction of chronic granulomatous disease after second unrelated-donor umbilical cord blood transplantation. Pediatr Blood Cancer. 2007;49:982–984. doi: 10.1002/pbc.21365. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharya A, Slatter MA, Chapman CE, et al. Single centre experience of umbilical cord stem cell transplantation for primary immunodeficiency. Bone Marrow Transplant. 2005;36:295–299. doi: 10.1038/sj.bmt.1705054. [DOI] [PubMed] [Google Scholar]
- 22**.Prasad VK, Kurtzberg J. Emerging trends in transplantation of inherited metabolic diseases. Bone Marrow Transplant. 2008;41:99–108. doi: 10.1038/sj.bmt.1705970. [This reference summarizes the use and success of cord blood transplantation in children with inborn errors of metabolism. It also analyzes the graft characteristics that best predict engraftment and survival in cord blood transplantation.] [DOI] [PubMed] [Google Scholar]
- 23.Staba SL, Escolar ML, Poe M, et al. Cord-blood transplants from unrelated donors in patients with Hurler's syndrome. N Engl J Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- 24.Escolar ML, Poe MD, Provenzale JM, et al. Transplantation ofumbilical-cord blood in babies with infantile Krabbe's disease. N Engl J Med. 2005;352:2069–81. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 25*.Boelens JJ, Wynn RF, O'Meara A, et al. Outcomes of hematopoietic stem cell transplantation for Hurler's syndrome in Europe: A risk factor analysis for graft failure. Bone Marrow Transplant. 2007;40:225–33. doi: 10.1038/sj.bmt.1705718. [This reference is the first to compare the use of cord blood or bone marrow as the donor source for transplantation to correct MPSI. Comparisons between these two cell sources as they impact engraftment, survival and graft versus host disease are presented.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: A comparison study. Lance. 2007;369:1947–54. doi: 10.1016/S0140-6736(07)60915-5. [This reference details the first comparison of cord blood versus bone marrow transplantation in the modern transplant error. Advantages and disadvantages of each stem cells source are discussed.] [DOI] [PubMed] [Google Scholar]
- 27.Meyer E, Hanna K, Gebbie K, et al., editors. Cord blood: Establishing a national hematopoietic stem cell bank program. National Academic Press; Washington, DC: 2005. p. 320. [Google Scholar]
- 28.Untying the Gordian knot: Policies, practices, and ethical issues related to banking of umbilical cord blood. J Clin Invest. 2005;115:2592–7. doi: 10.1172/JCI26690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Harris D, Rogers I. Umbilical cord blood: A unique source of pluripotent stem cells for regenerative medicine. Curr Stem Cell Res Ther. 2007;2:301–9. doi: 10.2174/157488807782793790. [Cord blood has great potential to emerge as a source of cells for cellular therapies in the future. This article discusses the potential for cord blood to provide the source material for therapies in tissue repair and regeneration.] [DOI] [PubMed] [Google Scholar]