Abstract
The glial cell line-derived neurotrophic factor (GDNF) is a secreted protein, best known for its role in the development of the central and peripheral nervous systems and the survival of adult dopaminergic neurons. More recently, accumulating evidence suggests that GDNF plays a unique role in negatively regulating the actions of drugs of abuse. In this article, we review these data and highlight the possibility that the GDNF pathway may be a promising target for the treatment of addiction.
Keywords: GDNF, Growth factor, Addiction, Alcohol, Psychostimulants, Opioids
1. Introduction
GDNF is a distant member of the transforming growth factor β superfamily that was originally isolated from the rat B49 glial cell line (Lin et al., 1993). GDNF is expressed throughout the central nervous system during development (Schaar et al., 1993; Stromberg et al., 1993; Choi-Lundberg & Bohn, 1995; Nosrat et al., 1996), and is also expressed in the adult brain, albeit in more restricted areas. High levels of the growth factor are found in the striatum (dorsal striatum and nucleus accumbens), thalamus, cortex and hippocampus (Springer et al., 1994; Choi-Lundberg & Bohn, 1995; Nosrat et al., 1996; Pochon et al., 1997; Trupp et al., 1997; Golden et al., 1998; Golden et al., 1999; Barroso-Chinea et al., 2005). Although GDNF is expressed in astrocytes, in the adult brain GDNF is mainly expressed and detected in neurons (Pochon et al., 1997; Barroso-Chinea et al., 2005). As described below, a major site of action of GDNF is the midbrain in which the growth factor is expressed at low levels under basal conditions (Golden et al., 1998; Semba et al., 2004; He et al., 2005). The major source of GDNF to the midbrain is the striatum where GDNF is retrogradely transported by dopaminergic neurons of the substantia nigra pars compacta (SNc) and the ventral tegmental area (VTA) (Tomac et al., 1995b; Lapchak et al., 1997; Kordower et al., 2000; Ai et al., 2003; Barroso-Chinea et al., 2005).
2. GDNF-mediated signaling
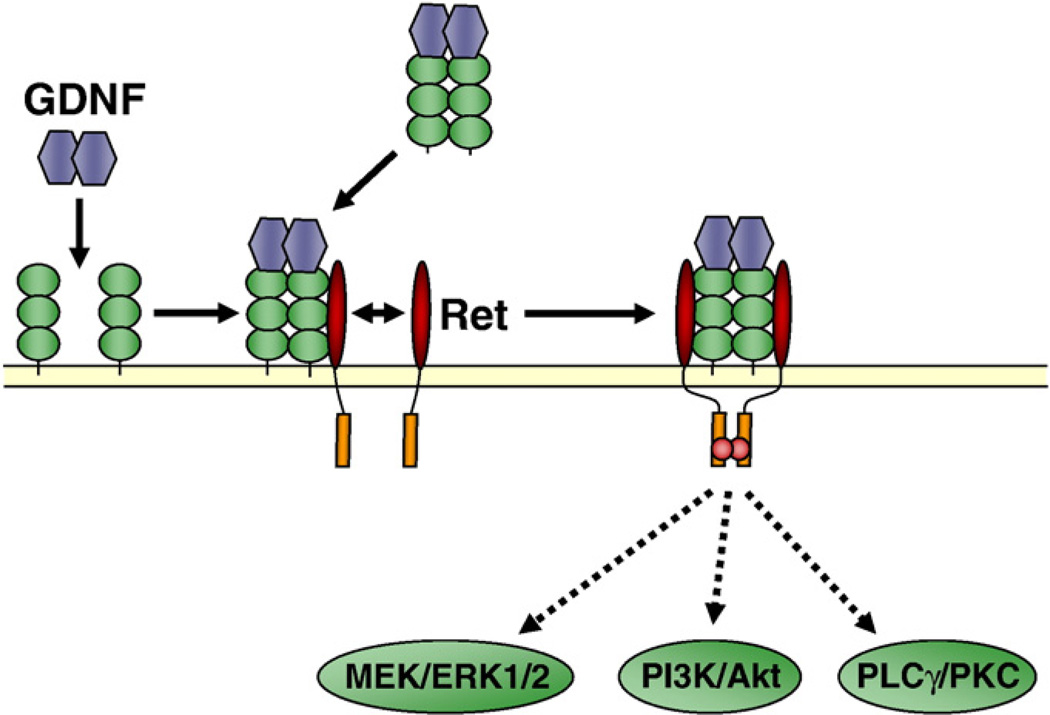
The main pathway by which GDNF transduces its signal is via the activation of the Rearranged during transfection receptor (Ret) (Jing et al., 1996; Treanor et al., 1996; Trupp et al., 1996; Eketjall et al., 1999). Ret is the product of the c-ret proto-oncogene and is a receptor tyrosine kinase (Tsui-Pierchala et al., 2002a). Activation of the GDNF pathway is initiated via the ligation of GDNF to its co-receptor, GDNF family receptor α1 (GFRα1), which is a glycosylphosphatidylinositol (GPI)-linked membrane-associated protein (Jing et al., 1996; Treanor et al., 1996; Eketjall et al., 1999), and the recruitment of Ret to the GFRα1-GDNF complex (Jing et al., 1996) (Fig. 1). GFRα1-Ret association can occur when both are expressed on the same cell (cis signaling), or when GFRα1 is presented in a soluble form (trans signaling) (Paratcha et al., 2001) (Fig. 1). Upon complex formation, Ret is activated by autophosphorylation (Durbec et al., 1996; Coulpier et al., 2002), leading to the activation of the mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinase 1/2 (ERK1/2), as well as the phosphatidylinositol 3 kinase (PI3K) and phospholipase Cγ (PLCγ) cascades (Airaksinen & Saarma, 2002) (Fig. 1).
Fig. 1.
GFRα1 and Ret-mediated GDNF signaling pathways. Ligation of GDNF to GPI-anchored GFRα1 (cis signaling) or soluble GFRα1 (trans signaling) increases the affinity of GFRα1 for Ret, inducing its recruitment and dimerization. Ret is activated by autophosphorylation of tyrosine residues shown in red, leading to the activation (in green) of several signaling pathways, such as the MAPK (ERK1/2), PI3K and PLCγ pathways. MEK: MAPK/ERK kinase. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
GFRα1 and Ret are expressed in several brain regions in the developing and adult brain, including the cerebellum, hypothalamic nuclei, the amygdala and the hippocampus (Trupp et al., 1997; Glazner et al., 1998; Golden et al., 1998; Burazin & Gundlach, 1999; Golden et al., 1999; Matsuo et al., 2000). Very low or negligible levels of GFRα1 and Ret are detected in the striatum, however the receptors are particularly abundant in the SNc and the VTA (Trupp et al., 1997; Glazner et al., 1998; Golden et al., 1998; Burazin & Gundlach, 1999; Golden et al., 1999; Matsuo et al., 2000; Sarabi et al., 2001; Jain et al., 2006). Interestingly, the distribution of GFRα1 and Ret expression does not necessarily overlap. For example, GFRα1, but not Ret, is expressed in the cortex and adult hippocampus (Trupp et al., 1997; Glazner et al., 1998; Golden et al., 1998; Burazin & Gundlach, 1999; Golden et al., 1999). The absence of Ret in these brain regions suggests the existence of GDNF signaling pathways that are independent of Ret. To this effect, Paratcha et al. (2003) reported that GDNF also signals via a direct interaction of GDNF with the neural cell adhesion protein, NCAM. This mode of activation results from the association of GFRα1 with NCAM, which promotes high-affinity binding of GDNF to NCAM (Paratcha et al., 2003). The association of GDNF to the adhesion receptor leads to the activation of the Src family of tyrosine kinases including Fyn, and the subsequent stimulation of Schwann cell migration and axonal outgrowth in hippocampal and cortical neurons (Paratcha et al., 2003). Interestingly, Chao et al. reported that the functional blockade of NCAM with anti-NACM inhibitory antibodies antagonizes the effects of GDNF on midbrain dopaminergic neurons in vitro and in vivo (Chao et al., 2003), suggesting a role for this pathway even in brain regions that express Ret.
An important factor in the regulation of the GDNF pathway is the compartmentalization of its receptors within or outside of lipid rafts. Lipid rafts are membranal microdomains that contain high levels of cholesterol, sphingolipids and GPI-anchored membranal proteins, and are thought to function as platforms for signaling cascades (Harding & Hancock, 2008; Kinoshita et al., 2008). In neurons, lipid rafts play an important role in growth factor signaling, cell adhesion, axon guidance and synaptic transmission (Tsui-Pierchala et al., 2002b). The GPI-linked GFRα1 is localized, at least in part, to lipid rafts, and upon GDNF binding to GFRα1, the complex recruits Ret into the rafts, leading to the activation of the receptor and to the association of Ret with the downstream kinase, Src (Tansey et al., 2000; Encinas et al., 2001). Soluble GFRα1 (trans activation) can also recruit Ret into the lipid rafts, however at a much slower rate (Paratcha et al., 2001), and interestingly, Pierchala et al. (2006), recently showed that lipid rafts play an important role in protecting the Ret receptor from proteo-some-dependent degradation.
The most well-documented consequence of the activation of GDNF-mediated signaling cascades is an increase in gene transcription (Trupp et al., 1999; Hayashi et al., 2000; Jongen et al., 2005). For example, GDNF was found to increase the expression of the calcium binding protein frequenin (Wang et al., 2001), and the expression of the bone marrow zinc finger 3 (BMZF3) was increased in neuroblastoma cells upon incubation with GDNF (Suzuki et al., 2008). Activation of the PI3K signaling pathway in substantia nigra neurons by GDNF leads to increased expression of yet another calcium binding protein, Calbindin (Wang et al., 2008). We showed that the activation of the MAPK pathway by GDNF leads to the initiation of a positive autoregulatory loop in which the growth factor upregulates its own expression resulting in a sustained activation of Ret (He & Ron, 2006). Interestingly, a recent study identified a transmembrane protein, Lrig1, that acts to negatively regulate the GDNF-mediated pathway by interacting with Ret, resulting in the inhibition of GDNF binding and the activation of the MAPK (Ledda et al., 2008). It would be of great interest to determine possible interaction between the positive and negative mechanisms of activation of the GDNF signaling pathways.
3. GDNF’s functions
GDNF promotes survival of mesencephalic dopamine neurons in culture (Lin et al., 1993) and plays an essential role in the development and survival of motor neurons, the development of sympathetic and sensory neurons, and kidneys (Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996), as well as in hippocampal synaptogenesis (Ledda et al., 2007). GDNF was also shown to promote survival and re-growth of dopaminergic neurons in the adult brain following injury (Beck et al., 1995; Tomac et al., 1995a; Hou et al., 1996), and is an essential factor for the maintenance and survival of adult dopamine neurons (Pascual et al., 2008). For example, repeated injections of GDNF adjacent to the SN prevented axotomy-induced loss of tyrosine hydroxylase (TH)-expressing neurons in that brain region (Beck et al., 1995), and adenoviral delivery of GDNF into the SN protected against degeneration of dopamine neurons following injection of 6-hydroxydopamine in the striatum (Choi-Lundberg et al., 1997). Injection (Tomac et al., 1995a) or lentiviral delivery (Kordower et al., 2000) of GDNF into the SN and striatum protected against nigrostriatal degeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which is toxic to dopaminergic neurons. GDNF has also been shown to selectively protect dopaminergic neurons, as compared to serotonergic neurons, against the neurotoxic effects of metham-phetamine (Cass,1996). As such, its potential as a therapeutic agent for the treatment of pathologies such as Parkinson's disease has been widely explored (Georgievska et al., 2002; Eslamboli et al., 2003; Azzouz et al., 2004; Kirik et al., 2004; Eslamboli et al., 2005).
Several studies suggested that GDNF also regulates neuronal excitability and transmitter release (Wang et al., 2001; Yang et al., 2001; Wang et al., 2003; Kobori et al., 2004). For example, GDNF treatment of mesencephalic dopaminergic neurons in culture, rapidly activates the MAPK pathway, resulting, within minutes, in an increase in the excitability of the neurons by inhibition of A-type potassium channels (Yang et al., 2001). GDNF exposure in the striatum increases dopamine neuronal sprouting (Hudson et al., 1995), and dopamine levels in adult rats (Hebert et al., 1996). In cultured VTA neurons, chronic treatment with GDNF is reported to increase the number of synapses onto dopaminergic, but not GABAergic, neurons, and to enhance dopamine and glutamate release (Bourque & Trudeau, 2000). GDNF signaling also mediates neuronal migration and chemotaxis (Tang et al., 1998), and was shown to produce analgesia in models of neuropathic pain (Sakai et al., 2008). Finally, the growth factor was reported to contribute to learning and memory, as mice with a deletion of one copy of the GDNF gene are impaired in the acquisition of a spatial memory task (Gerlai et al., 2001).
Taken together, these findings indicate that GDNF can alter the function and activity of neurons through a combination of short-term effects on ion channels and longer-term effects on gene regulation and synaptic remodeling.
4. GDNF and addiction
As described above, GFRα1 and Ret are highly expressed in VTA dopaminergic neurons. Dopaminergic neurons within the VTA are a critical component of the neural circuitry implicated in addictive behaviors. All drugs of abuse increase the level of extracellular dopamine in the NAc of rodents (Di Chiara & Imperato, 1988) and humans (Volkow et al., 2007), and alteration of the dopaminergic transmission modulates self-administration of drug of abuse and relapse (Koob et al., 1998; Hyman et al., 2006). Over the past several years, accumulating evidence suggests that GDNF plays a regulatory role in the actions of drugs of abuse, including psychostimulants, morphine and alcohol (ethanol). Interestingly, most of the studies described below suggest that activation of the GDNF pathway results in the attenuation of biochemical and behavioral changes observed upon exposure of rodents to drugs of abuse. The studies described in this chapter are also summarized in Table 1.
Table 1.
| Reinforcers | Behavioral procedures | GDNF modulation | Species/straina | Effects | References |
|---|---|---|---|---|---|
| A) Effects of upregulation of GDNF levels on behavior | |||||
| Cocaine | CPP | Infusion, VTA | Sprague–Dawley | ↓↓ | Messer et al., 2000 |
| SA | Infusion, striatum | Sprague–Dawley | ↓↓↓ | Green-Sadan et al., 2003 | |
| SA | Overexpression, striatum (embryonic cells) | Sprague–Dawley | ↓↓↓ | Green-Sadan et al., 2003 | |
| SA | Overexpression, striatum (nanoparticles) | Sprague–Dawley | ↓↓↓ | Green-Sadan et al., 2005 | |
| Ethanol | SA | Microinjection, VTA | Long–Evans | ↓↓ | Carnicella et al., 2008 |
| SA — reacquisition | Microinjection, VTA | Long–Evans | ↓↓↓ | Carnicella et al., 2008 | |
| TBC | Microinjection, VTA | Long–Evans | ↓↓↓ | Carnicella et al., (2009b) | |
| Water | SA | Overexpression, striatum (embryonic cells) | Sprague–Dawley | – | Green-Sadan et al., 2003 |
| SA | Overexpression, striatum (nanoparticles) | Sprague–Dawley | – | Green-Sadan et al., 2005 | |
| Sucrose | SA | Microinjection, VTA | Long–Evans | – | Carnicella et al., 2008 |
| Reinforcers | Procedures | GDNF modulation | Species/strainb | Effects | References |
| B) Effects of downregulation of GDNF levels on behavior | |||||
| Cocaine | Locomotor sensitization | GDNF HET mice | C57BL/6J | ↑↑ | Messer et al., 2000 |
| Locomotor sensitization | GDNF HET mice | C57BL/6J | – | Airavaara et al., 2004 | |
| CPP | GDNF HET mice | C57BL/6J | TT | Messer et al., 2000 | |
| CPP | Neutralizing Ab, VTA | Sprague–Dawley | ↑↑ | Messer et al., 2000 | |
| Ethanol | Locomotor sensitization | GDNF HET mice | DBH/CD1 | – | Carnicella et al., (submitted) |
| TBC | GDNF HET mice GFRα1 HET mice | DBH/CD1 C57/CD1 | – | Carnicella et al., (submitted) | |
| TBC — deprivation effect | GDNF HET mice GFRα1 HET mice | DBH/CD1 C57/CD1 | ↑↑ | Carnicella et al., (submitted) | |
| Methamphetamine | Locomotor sensitization | GDNF HET mice | C57BL/6J | – | Yan et al., 2007 |
| CPP | GDNF HET mice | C57BL/6J | ↑↑↑ | Niwa et al., 2007c | |
| SA | GDNF HET mice | C57BL/6J | ↑↑ | Yan et al., 2007 | |
| SA — reinstatement of seeking | GDNF HET mice | C57BL/6J | ↑↑↑ | Yan et al., 2007 | |
| Morphine | Locomotor sensitization | GDNF HET mice | C57BL/6J | ↑↑ | Airavaara et al., 2007 |
| CPP | GDNF HET mice | C57BL/6J | ↑↑↑ | Niwa et al., 2007a | |
| CPP | GDNF HET mice | C57BL/6J | – | Airavaara et al., 2007 | |
| Food | SA | GDNF HET mice | C57BL/6J | – | Yan et al., 2007 |
| SA — reinstatement of seeking | GDNF HET mice | C57BL/6J | – | Yan et al., 2007 | |
| Saccharin | TBC | GDNF HET mice GFRα1 HET mice | DBH/CD1 C57/CD1 | ↑ – | Carnicella et al., (submitted) |
| Saccharin | TBC — deprivation effect | GDNF HET mice GFRα1 HET mice | DBH/CD1 C57/CD1 | – | Carnicella et al., (submitted) |
| Sucrose | SA | GDNF HET mice | C57BL/6J | ↑ | Griffin et al., 2006 |
CPP: conditioned place preference; SA: operant self-administration; TBC: two-bottle choice.
4.1. GDNF and psychostimulants
The first report on the intriguing function of GDNF in addiction came out of Nestler's group who showed that infusion of GDNF into the VTA blocked or reversed increases in the NR1 subunit of the NMDA receptor and the level of TH in the VTA, as well as increases in ΔFosB and the catalytic subunit of protein kinase A in the NAc induced by repeated exposure to cocaine (Messer et al., 2000). Importantly, they found that intra-VTA infusion of GDNF was also effective in reducing conditioned place preference (CPP) to cocaine (Messer et al., 2000). In line with these data, transplantation of GDNF overexpressing cells, delivery of nanoparticles conjugated to GDNF, as well as continuous infusion of GDNF via a micro-osmotic pump into the striatum, was shown to impede acquisition of cocaine self-administration in rats (Green-Sadan et al., 2003; Green-Sadan et al., 2005).
Interestingly, several studies suggest that the endogenous GDNF system is altered in response to exposure to stimulants. Specifically, GDNF expression in the NAc was found to be increased 24 h after repeated exposure to methamphetamine (Niwa et al., 2007c), as well as in the VTA after repeated phencyclidine (PCP) exposure (Semba et al., 2004). In addition, 12 days of cocaine self-administration decreased GDNF expression in the dorsal striatum, but not in the NAc (Green-Sadan et al., 2003). Finally, the basal phosphorylation (i.e., activation) level of Ret was reduced in the midbrain of mice after repeated systemic administration of cocaine (Messer et al., 2000). Since exogenous administration of GDNF inhibits the biochemical and behavioral adaptation to cocaine, and repeated exposure to psychostimulants changes GDNF levels and the GDNF signaling pathway, it may be possible that endogenous GDNF plays a role in the regulation of the effects of psychostimulants. In agreement with this hypothesis, Messer et al. showed that intra-VTA infusion of anti-GDNF neutralizing antibodies, which prevent GDNF from binding and thus activating its receptors, resulted in increased sensitivity of mice to the rewarding effect of cocaine (Messer et al., 2000). Furthermore, GDNF heterozygote (HET) mice, which have significantly lower GDNF levels compared to wild-type (WT) (Airavaara et al., 2004; Griffin et al., 2006; Niwa et al., 2007c; Yan et al., 2007), exhibit increased sensitivity to the rewarding effect of cocaine and methamphetamine (Messer et al., 2000; Niwa et al., 2007c). In addition, motivation to self-administer and to seek methamphetamine is potentiatedin GDNF HET mice (Yan et al., 2007). In this elegant study, Yan et al. (2007) showed a faster acquisition of methamphetamine self-administration, a dramatic upward shift in the dose–response curve for methamphetamine self-administration, and an increased breaking point under a progressive-ratio reinforcement schedule in the HET mice compared to their WT littermates. An increase in methamphetamine-priming- and cue-induced reinstatement of methamphetamine seeking after prolonged withdrawal was also evidencedinthese mice (Yan et al., 2007). Messer et al. (2000) also found that GDNF HET mice were more vulnerable to cocaine-induced psychomotor sensitization than their WT littermates. However, using the same dose of cocaine and the same protocol of sensitization, Airavaara et al. (2004) observed a similar degree of sensitization in the GDNF HET and WT mice, and recently Yan et al. (2007), showed that the level of psychomotor sensitization to methamphetamine is the same in both genotypes. These results suggest that GDNF may not significantly contribute to the mechanisms that underlie the enhancement in locomotor activity in response to psychostimulants. Nevertheless the data presented above provide strong evidence that GDNF is a negative regulator of some biochemical and behavioral adaptations to psychostimulants.
4.2. GDNF and opioids
Similar to psychostimulants, some data suggest that GDNF negatively regulates opioid-related phenotypes. Specifically, intra-VTA infusion of GDNF was shown to inhibit increases in TH levels in the VTA induced by chronic exposure to morphine (Messer et al., 2000). Conversely, repeated morphine administration increases GDNF expression in the NAc (Niwa et al., 2007a). GDNF transgenic mice were also used to test the contribution of endogenous GDNF to mechanisms that reduce sensitivity of mice to morphine. Compared to WT mice, GDNF HET mice were more sensitive to the psychomotor effects of a challenge injection of morphine after repeated morphine administration (Airavaara et al., 2007), and exhibited greater place preference to morphine than the WT mice (Niwa et al., 2007a; but see Airavaara et al., 2007). Acute and challenge injection of morphine have also been shown to produce a greater increase in accumbal extracellular dopamine levels in the GDNF HET than in the WT mice (Airavaara et al., 2006, 2007), suggesting an important role of GDNF in regulating the striatal dopamine response to opioids. Interestingly, this effect was not observed with an acute administration of cocaine (Airavaara et al., 2004). Cocaine increases dopamine extracellular concentration by blocking its reuptake at the synaptic level, whereas morphine does so by stimulating VTA dopaminergic neurons (Koob et al., 1998). Therefore, it is plausible that GDNF regulates dopamine response by acting directly on the excitability of the dopaminergic neurons within the VTA, but not on dopamine release mechanisms.
4.3. GDNF and ethanol
Over the past several years, we have accumulated data that implicate GDNF in the negating the mechanism that underlies ethanol-drinking behaviors. We found that a single administration of GDNF into the VTA of rats results in a rapid reduction of operant self-administration of ethanol (He et al., 2005; Carnicella et al., 2008). Importantly, we found that administration of GDNF into the VTA reduced consumption of moderate levels of ethanol in a paradigm that resembles social drinking, as well as in models of excessive and binge drinking of ethanol (He et al., 2005; Carnicella et al., 2008; Carnicella et al., 2009b). Specifically, GDNF in the VTA attenuated rat operant self-administration of a solution of 10% ethanol, which result in a moderate amount of ethanol intake (0.4–0.6 g/kg/1 h) (He et al., 2005; Carnicella et al., 2008). GDNF infusion in the VTA also reduced lever presses for a 20% solution of ethanol that resulted in a much higher level of ethanol consumption (0.8 g/kg/30 min) (Carnicella et al., 2008). In addition, intra-VTA administration of GDNF was effective in decreasing ethanol consumption in rats that were trained to drink high levels of a 20% ethanol solution in an intermittent-access 2-bottle choice drinking paradigm (Carnicella et al., 2008). This paradigm produces a “binge-like” drinking behavior, as a high level of ethanol is consumed in a very short period of time at the beginning of the session (1.4 g/kg/30 min), leading to an average blood ethanol concentration of 80 mg% (Carnicella et al., 2009b). Together, these results suggest that intra-VTA administration of a single dose of GDNF leads to a marked decrease in ethanol drinking behaviors. Interestingly, the decrease in ethanol self-administration was long-lasting and was observed 3 h (Carnicella et al., 2008), 12 h, and even 24 h after a single injection of GDNF (Fig. 2). GDNF–s actions to reduce ethanol self-administration are likely to be localized to the VTA, as infusion of the growth factor into the neighboring midbrain region, the SNc, did not alter ethanol self-administration (Carnicella et al., 2008). Importantly, the actions of GDNF to reduce ethanol consumption are specific for ethanol and are not due to a reduction of general reward or changes in locomotor activity, as the growth factor had no effect on operant self-administration of sucrose (Carnicella et al., 2008). Importantly, we found that the activation of the GDNF pathway in the VTA reduces rat self-administration of ethanol in a paradigm that models relapse. Intra-VTA infusion of GDNF 10 min before the beginning of the session blocked the reacquisition of operant responding for ethanol after a period of extinction (Carnicella et al., 2008). In summary, our data suggest that GDNF is a potent, rapid inhibitor of excessive consumption of, and relapse to, ethanol.
Fig. 2.
Persistent decrease of operant ethanol self-administration after intra-VTA GDNF infusion. Long–Evans rats were trained for 2 months to press on a lever to obtain 0.1 ml of a 10% ethanol solution during 1 h sessions. Upon establishing a baseline of ethanol intake, cannulae were implantedinthe VTA, and rats were microinjected with GDNF (10 µg/side) or vehicle (PBS), 12 or 24 h before the beginning of the operant self-administration session. A two-way ANOVA showed a significant effect of treatment (F(1, 10)=12.74, p<0.01), no effect of time (F(1, 10)=0.01, p=0.93), and no interaction between both factors (F(1, 10)= 0.17, p=0.69). n=11. Data are shown as mean±SEM. *p<0.05, **p<0.01.
We also determined the signaling pathway by which GDNF mediates its rapid actions to reduce ethanol-drinking behaviors. As described above, the main signaling pathways activated via GDNF-mediated Ret activation are the MAPK, PI3K and PLCγ (Fig. 1). We found that GDNF in the VTA activates the MAP kinase ERK1/2, and that a specific inhibitor to the upstream kinase that phophorylates and activates MAPK, MEK (Sweatt, 2001), but not an inhibition to PI3K, blocked GDNF-mediated reduction in ethanol self-administration (Carnicella et al., 2008). We were unable to test the contribution of the PLCγ pathway since the inhibitor of this pathway reduced ethanol self-administration (Carnicella et al., 2008). This could be due to the fact that the kinase downstream of PLCγ, protein kinase C (PKC) (Fig. 1), is activated under basal conditions and may be directly involved in ethanol-drinking behaviors (Newton & Ron, 2007).
The effector proteins downstream of GDNF-mediated ERK1/2 activation that mediate the reduction in ethanol consumption are unknown. However, since the effect of GDNF is so rapid, the initial action of the growth factor is likely to be via a non-genomic mechanism. GDNF was shown to rapidly inhibit the activity of A-type potassium channel currents in primary midbrain neurons via a mechanism that requires the activation of the MAPK pathway (Yang et al., 2001). Therefore, it is possible that GDNF, via the inhibition of an A-type potassium channel, alters the excitability of the neurons in the VTA, leading to the decrease in ethanol self-administration.
Like other growth factors, GDNF induces several transcriptional changes that could underlie the long-lasting actions of the growth factor (Fig. 2). For example, GDNF was reported to increase the expression of the dopamine transporter (Consales et al., 2007), inducing modifications of the mesolimbic system that persist beyond the activation and termination of GDNF signaling. Another possible mechanism is the sustained activation of the GDNF pathway, as we recently showed that GDNF upregulates its own expression, leading to a prolonged activation of the GDNF signaling pathway (He & Ron, 2006). In addition, several studies suggest a role for GDNF in the regulation of tyrosine hydroxylase, the rate-limiting enzyme in the biosynthesis of dopamine (Nagatsu et al., 1964; Levitt et al., 1965), in the midbrain (Messer et al., 2000; Rosenblad et al., 2003; Georgievska et al., 2004). For example, GDNF overexpression by lentiviral delivery in the striatum reduced mRNA and/or protein levels of TH in the SN (Rosenblad et al., 2003; Georgievska et al., 2004) and the VTA (Rosenblad et al., 2003). Increased levels of GDNF were also associated with a decrease in TH enzyme activity and dopamine levels in striatum (Sajadi et al., 2005). Furthermore, infusion of GDNF into the VTA reverses chronic cocaine-or morphine-induced increase in TH protein levels in this brain region (Messer et al., 2000). Upregulation of TH levels has been reported as one of the hallmarks of biochemical adaptations to in vivo chronic exposure to drugs of abuse, including ethanol (Beitner-Johnson & Nestler, 1991; Ortiz et al., 1995; Self et al., 1995; Ortiz et al., 1996; Messer et al., 2000; Belin et al., 2007), and we recently showed that in a dopaminergic-like cell line, an ethanol-mediated increase in TH protein levels is reversed by GDNF (He & Ron, 2008). Interestingly, we observed that ethanol treatment resulted in an increase in TH association with the chaperone heat shock protein 90 (HSP90), and this biochemical adaptation was reversed by GDNF, leading to the degradation of TH (He & Ron, 2008). Taken together, these data suggest that prolonged ethanol exposure leads to increased association of TH and HSP90, resulting in the stabilization and subsequent accumulation of TH, and that GDNF reverses this ethanol-mediated adaptation by inhibiting the interaction of TH with HSP90 (Fig. 3). At this point we cannot directly tie the effects of GDNF on ethanol drinking behaviors to this GDNF-mediated action on the protein stability of TH, however, our present study suggests that GDNF may act as a negative regulator of various adaptations to prolonged in vivo exposure to ethanol.
Fig. 3.
GDNF attenuates ethanol-mediated induction of TH protein levels. (A) Exposure to ethanol leads to the formation of a complex between HSP90 and TH via the activation of the cyclic AMP-dependent protein kinase A (cAMP/PKA) pathway, resulting in the stabilization and accumulation of TH protein. (B) GDNF reverses this ethanol-mediated adaptation by inhibition of the association between TH and HSP90, leading to the reduction in TH protein stability.
Finally, using the transgenic GDNF HET mice and theirWT littermate controls, we tested whether endogenous GDNF contributes to the regulation of ethanol-drinking behaviors. We found that the reduction in the level of endogenous GDNF resulted in increased ethanol consumption after a period of abstinence (Carnicella et al., submitted). Increased ethanol intake after a period of deprivation was also observed in mice with a reduced level of the GDNF receptor, GFRα1, as compared to their WT littermates (Carnicella et al., submitted). These results, together with our previous observation that application of GDNF into the VTA prevented relapse to ethanol self-administration in rats(Carnicella et al., 2008), suggest that GDNF plays an important role in controlling ethanol-drinking behaviors after a period of abstinence, and put forward the possible use of agents that activate the GDNF pathway as potential drugs to prevent relapse. The potential role of GDNF as a novel therapeutic approach to treat addiction to alcohol and drugs of abuse is discussed below.
4.4. GDNF and non-drug reinforcers
In contrast to its actions on drugs of abuse, GDNF does not affect intake of non-drug rewarding and/or reinforcing substances. For example, intra-VTA administration of GDNF did not alter operant sucrose self-administration (Carnicella et al., 2008), and transplantation of GDNF overexpressing cells or delivery of nanoparticles conjugated to GDNF did not alter operant water self-administration in water-restricted rats (Green-Sadan et al., 2003; Green-Sadan et al., 2005). In addition, although intra-VTA administration decreased excessive ethanol intake in a 20% ethanol intermittent-access 2-bottle choice drinking paradigm, the total fluid intake was unchanged as the reduction of fluid consumption due to the decrease in ethanol intake was compensated for by an increase in water intake (Carnicella et al., 2009b). Similarly, no differences were found in operant food self-administration and reinstatement of food-seeking between GDNF HET and WT mice (Yan et al., 2007). A small but significant increase in sucrose and saccharin self-administration was seen in GDNF HET mice compare to WT littermates (Griffin et al., 2006; Carnicella et al., submitted). However, this change was not observed in GFRα1 mice, and one week of abstinence resulted in similar levels of saccharin intake in the GDNF HET and WT mice (Carnicella et al., submitted), suggesting that the changes in the intake of sweet substances observed in the GDNF HET mice might be due to compensatory mechanisms resulting from the global reduction of the gene throughout development.
5. GDNF as a possible target to treat addiction
Taken together, the data described above support a selective role for GDNF in the regulation of drugs of abuse-related behaviors and suggest that upregulation of the GDNF pathway may be a valuable strategy to combat several forms of addiction. However, it is unlikely that GDNF itself could be used as a therapeutic agent. GDNF is a 24 kDa protein that cannot readily cross the blood-brain barrier (Kastin et al., 2003; Kilic et al., 2003), and its delivery to the brain requires intracerebral or intracerebroventricular surgical interventions that may be not desirable or applicable for the treatment of drug and alcohol abuse and dependence. There are at least two alternative approaches to overcome this major caveat: enable the penetration of GDNF by its conjugation or fusion to peptides or protein transduction domains that undergo transport across the blood-brain barrier (Albeck et al., 1997; Kilic et al., 2003; Boado et al., 2008), or the use of small molecules that can be applied systemically to increase the production of GDNF or activate its receptors. Studies suggesting the possible feasibility of the second approach are summarized below and in Table 2.
Table 2.
Potential GDNF expression inducers and mimetics.
| Generic or chemical name | Trade name/company | Development phase | Drugs of abuse tested | References |
|---|---|---|---|---|
| Cabergoline | Cabaser, Dostinex/Pfizer | FDA-approved | Alcohol, cocaine | Carnicella et al. (2009b); Shoptaw et al., 2005 |
| 1,25-Dihydroxyvitamin | N/A | In vivo studiesa | Not tested | Sanchez et al., 2002 |
| Leu-Ile | N/A | In vivo studies | Methamphetamine, morphine | Niwa et al., 2007a,b,c |
| Ladostigil | Teva Pharmaceuticals | Clinical Phase IIa | Not tested | Weinreb et al., 2007 |
| 18-Methoxycoronaridine | N/A | In vivo studies | Alcohol, opiates, psychostimulants, nicotine | Maisonneuve and Glick, 2003, Rezvani et al., 1997 |
| PYM50028/smilagenin | Cogane/Phytopharm | In vivo studies | Not tested | Visanji et al., 2008 |
| Rasagiline | Azilect/Teva Pharmaceuticals | FDA-approved | Not tested | Weinreb et al., 2007, Maruyama et al., 2004 |
| XIB4035 | N/A | In vitro studies | Not tested | Tokugawa et al., 2003 |
Non human animals studies.
5.1. Ibogaine-mimetics
Ibogaine is a natural alkaloid reported to reverse the adverse actions of multiple drugs of abuse including opiates, psychostimulants, nicotine and alcohol in humans, as well as in rodent models (Popik et al., 1995; Mash et al., 1998; Glick & Maisonneuve, 2000; Alper et al., 2008; Maciulaitis et al., 2008). Despite its attractive properties, Ibogaine can induce severe side-effects such as hallucinations, whole-body tremors and ataxia that may be related to neurotoxicity in the cerebellum and dysregulation of the cardiovascular system (O'Hearn & Molliver, 1993; Popik et al., 1995; Maas & Strubelt, 2006; Maciulaitis et al., 2008). We previously demonstrated that systemic administration of low non-toxic doses of Ibogaine in rats reduces ethanol self-administration and relapse (He et al., 2005). We were therefore interested in identifying the molecular pathway mediating the beneficial actions of Ibogaine on ethanol-drinking behaviors. We found that Ibogaine increased GDNF expression resulting in the activation of the GDNF pathway (He et al., 2005). Importantly, we showed that the actions of Ibogaine to reduce ethanol in take were localized to the VTA, and that infusion of anti-GDNF neutralizing antibodies into the VTA attenuated the Ibogaine-mediated decrease in ethanol self-administration (He et al., 2005). Together, these results suggest that the desirable actions of this drug are mediated, as least partially, by GDNF. A potential strategy to overcome these undesirable actions of Ibogaine is the use of derivatives that share only its valuable actions. In this regard, the main metabolite of Ibogaine, noribogaine, and 18-methoxycoronaridine (18-MC) a synthetic congener, may have promising profiles. Noribogaine has been shown to induce a long-lasting reduction in morphine and cocaine, but not water, self-administration in rats (Glick et al., 1996b), and 18-MC was found to reduce morphine, psychostimulant and nicotine self-administration, as well as ethanol intake in rodents, without affecting water consumption (Rezvani et al., 1997; Maisonneuve & Glick, 2003). Importantly, in contrast to Ibogaine, noribogaine and 18-MC have no tremorigenic effects (Glick et al., 1996b; Baumann et al., 2001; Maisonneuve & Glick, 2003), and no evidence of cerebellar toxicity was found for 18-MC in rats, even after administration of a high dose (Glick et al., 1996a). Taken together, these data suggest that Ibogaine derivatives may be an effective treatment of addiction and safer than the parent compound. However, it should be noted that it is currently unknown whether the desirable actions of noribogaine and 18-MC are mediated by GDNF.
5.2. Leucine-isoleucine (Leu-Ile)
The hydrophobic dipeptide Leu-Ile was designed from a part of the immunophilin-binding site of the immunosuppressant drug FK506 (Kaminska et al., 2004). Leu-Ile was shown to increase GDNF mRNA and protein levels in cultured hippocampal neurons (Cen et al., 2006; Niwa et al., 2007c) through Akt/cAMP response element binding protein (CREB) signaling (Cen et al., 2006). Importantly, repeated systemic administration of Leu-Ile was also shown to upregulate GDNF protein levels in the striatum in vivo (Nitta et al., 2004), and to potentiate the increase in striatal GDNF observed after repeated exposure to methamphetamine and morphine (Niwa et al., 2007a; Niwa et al., 2007c). Niwa and collaborators further showed that systemic injections of Leu-Ile inhibited the development and expression of methampheta-mine- and morphine-induced place preference, and locomotor sensitization in mice (Niwa et al., 2007a; Niwa et al., 2007b; Niwa et al., 2007c). In contrast, Leu-Ile did not prevent naloxone-precipitated withdrawal from morphine (Niwa et al., 2007b). No significant reduction of place preference for morphine or methamphetamine was observed in GDNF HET mice after Leu-Ile treatment (Niwa et al., 2007a; Niwa et al., 2007c), however, the authors did not show the efficacy of Leu-Ile in the WT littermates, and therefore the interpretation of the results is difficult. Nevertheless, these data suggest that GDNF-inducing agents such as Leu-Ile can be used to attenuate psychomotor sensitization and rewarding properties of methamphetamine and morphine.
5.3. Cabergoline
Cabergoline (Cabaser, Dostinex) is an FDA-approved drug for the treatment of hyperprolactinemia (Webster et al., 1994; Colao et al., 2006) that has also been used as an adjunctive or mono-therapy for Parkinson's disease (Bonuccelli, 2003; Jankovic & Stacy, 2007). Cabergoline is a dopamine D2-like receptors agonist which was reported to also act as an agonist of the dopamine D1 and several serotoninergic subtypes receptors, and to inhibit the α2-adrenoceptors (Millan et al., 2002; Newman-Tancredi et al., 2002a,b). Interestingly, cabergoline was also shown to increase GDNF expression and secretion in cultured astrocytes (Ohta et al., 2003; Ohta et al., 2004). We recently tested the potential use of cabergoline for the treatment of alcohol abuse in preclinical rodent models. We found that cabergoline also increased GDNF expression and the subsequent activation of the GDNF pathway in the dopaminergic-like SH-SY5Y cell line (Carnicella et al., 2009a). Systematic administration of cabergoline resulted in an increase inGDNF expression in the VTA (Carnicella et al., 2009a), the site of action of GDNF to reduce ethanol consumption and relapse (He et al., 2005; Carnicella et al., 2008; Carnicella et al., 2009a). Importantly, we found that systemic administration of cabergoline significantly reduced operant ethanol, but not sucrose, self-administration in rats (Carnicella et al., 2009a). This action of cabergoline is likely to be mediated by the VTA, as microinjection of cabergoline into this brain area was highly effective in reducing operant ethanol self-administration (Carnicella et al., 2009a). Moreover, systemic administration of cabergoline reduced both the reacquisition of operant responding for ethanol after a period of extinction and cue-induced ethanol-seeking after abstinence, two different models of relapse (Carnicella et al., 2009a). Finally, cabergoline was also effective in selectively decreasing ethanol intake in C57BL/6J and GDNF WT mice, but not in the GDNF HET mice (Carnicella et al., 2009a). Together, these results suggest that cabergoline decreases ethanol-drinking and -seeking behaviors, and that these effects are mediated via upregulation of the GDNF pathway in the mesolimbic system. Interestingly, a pilot study was conducted on the efficacy of cabergoline in reducing cocaine use in addicts. Cabergoline significantly reduced cocaine use over the 8 weeks of treatment, as evaluated by analysis of cocaine metabolite levels in urine samples and self-report of substance use (Shoptaw et al., 2005). Importantly, the beneficial effect of cabergoline on cocaine use was shown with a weekly dose of 0.5 mg, a low, safe and well-tolerated dose (Webster et al., 1994; Colao et al., 2006) that circumvents the increasing prevalence of cardiac valvulopathy induced by high-doses of cabergoline (2–6 mg/day) used in Parkinsonian patients (Delgrange, 2006; Zanettini et al., 2007). Together, these data suggest that cabergoline could be a potentially safe and effective treatment against alcoholism and other drug addictions.
5.4. Other potential GDNF expression inducers and mimetics
Listed below are several small molecules that have been reported to increase the expression of GDNF or activate the GDNF signaling pathway. However, their potential to alter behaviors associated with exposure to drugs of abuse remains to be tested. XIB4035 is small molecule that was reported to bind GFRα1, leading to the recruitment and activation of Ret and to the neurotrophic effects of GDNF in neuron-2A cells (Tokugawa et al., 2003), suggesting that XIB4035 could be used as a GFRα1/Ret agonist. Smilagenin (also known as PYM50028 (Cogane)) is a small molecule that was shown to increase the GDNF message in cultured mesencephalic neurons (Zhang et al., 2008). Smilagenin, given orally to mice, was reported to increase the level of GDNF protein in the striatum (Visanji et al., 2008). In addition, an active metabolite of vitamin D, 1,25-Dihydroxyvitamin D3, was also shown to increase GDNF expression in a rat glioma cell-line (Naveilhan et al., 1996) and GDNF release in human glioblastoma cells (Verity et al., 1999). Moreover, systemic injection of this molecule increases striatal GDNF mRNA and protein expression in rats (Sanchez et al., 2002). Similarly, the monamine oxydase inhibitors developed for the treatment of neurodegenerative diseases, ladostigil (currently in Phase IIa clinical trials) and its parent compound rasagiline (FDA-approved) (Youdim & Van der Schyf, 2007), were shown to be potent upregulators of GDNF mRNA levels in human neuroblastoma cell lines (Maruyama et al., 2004; Weinreb et al., 2007), raising the possibility that these drugs may be used as therapeutics to treat addiction.
6. Summary
The present reviewed data suggest that GDNF negatively regulates the actions of drugs of abuse, as reducing endogenous GDNF levels or inhibition of the GDNF pathway increases several biochemical and behavioral adaptations to psychostimulants, opioids and ethanol, whereas GDNF administration in the mesolimbic system results in opposite effects. The mechanisms of action of GDNF to counter these adaptations associated with addiction are currently unknown. The rapid MAPK-mediated effect of GDNF on dopaminergic neurons (Yang et al., 2001) in combination with GDNF's long-term actions to alter the level of TH (He & Ron, 2008) are possible candidates. These changes mediated by GDNF could lead to synaptic remodeling and change the responsiveness of the mesolimbic dopaminergic system and by doing so, counter the incentive and/or rewarding value of, and the neuroadaptations induced by, drugs of abuse. For example, it is plausible that activation of the MAPK signaling pathway by GDNF reverses the decrease in VTA dopaminergic cell size resulting from the downregulation of the insulin receptor substrate 2 (IRS2)-Akt signaling pathway, observed after chronic morphine administration (Russo et al., 2007). Other growth factors such as the brain derived neurotrophic factor (BDNF) activate similar signaling pathways as GDNF (Papoutian & Reichardt, 2001). Interestingly, BDNF was shown to modulate biochemical and behavioral adaptation to drugs of abuse. For example, intra-VTA infusion of BDNF prevents the upregulation of TH levels induced by chronic morphine and cocaine administration (Berhow et al., 1995). BDNF has been shown to potentiate cocaine-seeking behaviors in the mesolimbic system (Lu et al., 2004; Graham et al., 2007), but to negatively regulate ethanol-drinking behaviors within the dorsal striatum (McGough et al., 2004; Jeanblanc et al., 2006). Interestingly, recently Esposito et al. (2008) found a direct crosstalk between the BDNF and the GDNF signaling pathway in neuroblastoma cell lines. It would therefore be of great interest to determine if such cross talk exists in the brain and whether it contributes to the action of these two growth factors.
Importantly, a growing number of studies support the possibility that GDNF-mimetics may be potent and selective agents to treat addiction. FDA-approved drugs such as cabergoline open new and promising avenues for the development of therapeutic approaches to treat addiction.
Abbreviations
- ERK1/2
extracellular signal-regulated kinase 1/2
- GDNF
glial cell line-derived neurotrophic factor
- GFRα1
GDNF family receptor α1
- HET
heterozygote
- HSP90
heat shock protein 90
- MAPK
mitogen-activated protein kinase
- Nac
Nucleus accumbens
- 18-MC
18-methoxycoronaridine
- MEK
MAPK/ERK kinase
- PI3K
phoshatidylinositol 3 kinase
- PLCγ
phospholipase Cγ
- Ret
Rearranged during transfection receptor
- SN
substantia nigra
- SNc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- VTA
ventral tegmental area
- WT
wild-type
Footnotes
This work was supported by NIH-NIAAA R01 AA014366-02 (D.R.) and the State of California for Medical Research on Alcohol and Substance Abuse through the University of California, San Francisco (D.R.)
References
- Ai Y, Markesbery W, Zhang Z, Grondin R, Elseberry D, Gerhardt GA, et al. Intraputamenal infusion of GDNF in aged rhesus monkeys: distribution and dopaminergic effects. J Comp Neurol. 2003;461:250–261. doi: 10.1002/cne.10689. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Airavaara M, Mijatovic J, Vihavainen T, Piepponen TP, Saarma M, Ahtee L. In heterozygous GDNF knockout mice the response of striatal dopaminergic system to acute morphine is altered. Synapse. 2006;59:321–329. doi: 10.1002/syn.20245. [DOI] [PubMed] [Google Scholar]
- Airavaara M, Planken A, Gaddnas H, Piepponen TP, Saarma M, Ahtee L. Increased extracellular dopamine concentrations and FosB/DeltaFosB expression in striatal brain areas of heterozygous GDNF knockout mice. Eur J Neurosci. 2004;20:2336–2344. doi: 10.1111/j.1460-9568.2004.03700.x. [DOI] [PubMed] [Google Scholar]
- Airavaara M, Tuomainen H, Piepponen TP, Saarma M, Ahtee L. Effects of repeated morphine on locomotion, place preference and dopamine in heterozygous glial cell line-derived neurotrophic factor knockout mice. Genes Brain Behav. 2007;6:287–298. doi: 10.1111/j.1601-183X.2006.00260.x. [DOI] [PubMed] [Google Scholar]
- Albeck DS, Hoffer BJ, Quissell D, Sanders LA, Zerbe G, Granholm AC. A non-invasive transport system for GDNF across the blood-brain barrier. Neuroreport. 1997;8:2293–2298. doi: 10.1097/00001756-199707070-00039. [DOI] [PubMed] [Google Scholar]
- Alper KR, Lotsof HS, Kaplan CD. The ibogaine medical subculture. J Ethnopharmacol. 2008;115:9–24. doi: 10.1016/j.jep.2007.08.034. [DOI] [PubMed] [Google Scholar]
- Azzouz M, Ralph S, Wong LF, Day D, Askham Z, Barber RD, et al. Neuroprotection in a rat Parkinson model by GDNF gene therapy using EIAV vector. Neuroreport. 2004;15:985–990. doi: 10.1097/00001756-200404290-00011. [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea P, Cruz-Muros I, Aymerich MS, Rodriguez-Diaz M, Afonso-Oramas D, Lanciego JL, et al. Striatal expression of GDNF and differential vulnerability of midbrain dopaminergic cells. Eur J Neurosci. 2005;21:1815–1827. doi: 10.1111/j.1460-9568.2005.04024.x. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Rothman RB, Pablo JP, Mash DC. In vivo neurobiological effects of ibogaine and its O-desmethyl metabolite, 12-hydroxyibogamine (noribogaine), in rats. J Pharmacol Exp Ther. 2001;297:531–539. [PubMed] [Google Scholar]
- Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, et al. Mesencephalic dopaminergic neurons protected by GDNF from axotomy- induced degeneration in the adult brain. Nature. 1995;373:339–341. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- Beitner-Johnson D, Nestler EJ. Morphine and cocaine exert common chronic actions on tyrosine hydroxylase in dopaminergic brain reward regions. J Neurochem. 1991;57:344–347. doi: 10.1111/j.1471-4159.1991.tb02133.x. [DOI] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V, Jaber M. Cocaine-induced sensitization is associated with altered dynamics of transcriptional responses of the dopamine transporter, tyrosine hydroxylase, and dopamine D2 receptors in C57Bl/6J mice. Psychopharmacology (Berl) 2007;193:567–578. doi: 10.1007/s00213-007-0790-3. [DOI] [PubMed] [Google Scholar]
- Berhow MT, Russell DS, Terwilliger RZ, Beinter-Johnson D, Self DW, Lindsay RM, Nestler EJ. Influence of neurotrophic factors on morphine- and cocaine-induced biochemical changes in the mesolimbic dopamine system. Neuroscience. 1995;68:969–979. doi: 10.1016/0306-4522(95)00207-y. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Zhang Y, Zhang Y, Wang Y, Pardridge WM. GDNF fusion protein for targeted-drug delivery across the human blood-brain barrier. Biotechnol Bioeng. 2008;100:387–396. doi: 10.1002/bit.21764. [DOI] [PubMed] [Google Scholar]
- Bonuccelli U. Comparing dopamine agonists in Parkinson's disease. Curr Opin Neurol 16 Suppl. 2003;1:S13–19. doi: 10.1097/00019052-200312001-00004. [DOI] [PubMed] [Google Scholar]
- Bourque MJ, Trudeau LE. GDNF enhances the synaptic efficacy of dopaminergic neurons in culture. Eur J Neurosci. 2000;12:3172–3180. doi: 10.1046/j.1460-9568.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- Burazin TC, Gundlach AL. Localization of GDNF/neurturin receptor (c-ret, GFRalpha-1 and alpha-2) mRNAs in postnatal rat brain: differential regional and temporal expression in hippocampus, cortex and cerebellum. Brain Res Mol Brain Res. 1999;73:151–171. doi: 10.1016/s0169-328x(99)00217-x. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Ahmadiantehrani S, He D-Y, Nielsen CK, Bartlett SE, Patricia H, Janak PH, Ron D. The FDA-approved drug cabergoline decreases alcohol drinking and seeking behaviors via glial cell line-derived neurotrophic factor. Biological Psychiatry in revision. 2009a doi: 10.1016/j.biopsych.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by GDNF. Alcohol in revision. 2009b doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA. GDNF selectively protects dopamine neurons over serotonin neurons against the neurotoxic effects of methamphetamine. J Neurosci. 1996;16:8132–8139. doi: 10.1523/JNEUROSCI.16-24-08132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen X, Nitta A, Ohya S, Zhao Y, Ozawa N, Mouri A, et al. An analog of a dipeptide-like structure of FK506 increases glial cell line-derived neurotrophic factor expression through cAMP response element-binding protein activated by heat shock protein 90/Akt signaling pathway. J Neurosci. 2006;26:3335–3344. doi: 10.1523/JNEUROSCI.5010-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Ma YL, Chu KY, Lee EH. Integrin alphav and NCAM mediate the effects of GDNF on DA neuron survival, outgrowth, DA turnover and motor activity in rats. Neurobiol Aging. 2003;24:105–116. doi: 10.1016/s0197-4580(02)00047-7. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Bohn MC. Ontogeny and distribution of glial cell line-derived neurotrophic factor (GDNF) mRNA in rat. Brain Res Dev Brain Res. 1995;85:80–88. doi: 10.1016/0165-3806(94)00197-8. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- Colao A, Di Sarno A, Guerra E, De Leo M, Mentone A, Lombardi G. Drug insight: cabergoline and bromocriptine in the treatment of hyperprolactinemia in men and women. Nat Clin Pract Endocrinol Metab. 2006;2:200–210. doi: 10.1038/ncpendmet0160. [DOI] [PubMed] [Google Scholar]
- Consales C, Volpicelli F, Greco D, Leone L, Colucci-D'Amato L, Perrone-Capano C, et al. GDNF signaling in embryonic midbrain neurons in vitro. Brain Res. 2007;115(9):28–39. doi: 10.1016/j.brainres.2007.04.071. [DOI] [PubMed] [Google Scholar]
- Coulpier M, Anders J, Ibanez CF. Coordinated activation of autopho-sphorylation sites in the RET receptor tyrosine kinase: importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. J Biol Chem. 2002;277:1991–1999. doi: 10.1074/jbc.M107992200. [DOI] [PubMed] [Google Scholar]
- Delgrange E. Cabergoline and mitral regurgitation. N Engl J Med. 2006;354:420. doi: 10.1056/NEJMc053329. author reply 420. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1998;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, et al. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996;381:789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- Eketjall S, Fainzilber M, Murray-Rust J, Ibanez CF. Distinct structural elements in GDNF mediate binding to GFRalpha1 and activation of the GFRalpha1-c-Ret receptor complex. EMBO J. 1999;18:5901–5910. doi: 10.1093/emboj/18.21.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas M, Tansey MG, Tsui-Pierchala BA, Comella JX, Milbrandt J, Johnson EM., Jr. c-Srcisrequired for glial cell line-derived neurotrophic factor (GDNF) family ligand-mediated neuronal survival via a phosphatidylinositol-3 kinase (PI-3K)-dependent pathway. J Neurosci. 2001;21:1464–1472. doi: 10.1523/JNEUROSCI.21-05-01464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslamboli A, Cummings RM, Ridley RM, Baker HF, Muzyczka N, Burger C, et al. Recombinant adeno-associated viral vector (rAAV) delivery of GDNF provides protection against 6-OHDA lesion in the common marmoset monkey (Callithrix jacchus) Exp Neurol. 2003;184:536–548. doi: 10.1016/j.expneurol.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Eslamboli A, Georgievska B, Ridley RM, Baker HF, Muzyczka N, Burger C, et al. Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson's disease. J Neurosci. 2005;25:769–777. doi: 10.1523/JNEUROSCI.4421-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito CL, D’Alessio A, de Franciscis V, Cerchia L. A cross-talk between TrkB and Ret tyrosine kinases receptors mediates neuroblastoma cells differentiation. PLoS One. 2008;3:e1643. doi: 10.1371/journal.pone.0001643. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Georgievska B, Kirik D, Rosenblad C, Lundberg C, Bjorklund A. Neuroprotection in the rat Parkinson model by intrastriatal GDNF gene transfer using a lentiviral vector. Neuroreport. 2002;13:75–82. doi: 10.1097/00001756-200201210-00019. [DOI] [PubMed] [Google Scholar]
- Georgievska B, Kirik D, Bjorklund A. Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system. J Neurosci. 2004;24:6437–6445. doi: 10.1523/JNEUROSCI.1122-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, McNamara A, Choi-Lundberg DL, Armanini M, Ross J, Powell-Braxton L, et al. Impaired water maze learning performance without altered dopaminergic function in mice heterozygous for the GDNF mutation. Eur J Neurosci. 2001;14:1153–1163. doi: 10.1046/j.0953-816x.2001.01724.x. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Mu X, Springer JE. Localization of glial cell line-derived neurotrophic factor receptor alpha and c-ret mRNA in rat central nervous system. J Comp Neurol. 1998;391:42–49. doi: 10.1002/(sici)1096-9861(19980202)391:1<42::aid-cne4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Glick SD, Kuehne ME, Maisonneuve IM, Bandarage UK, Molinari HH. 18-Methoxycoronaridine, a non-toxic iboga alkaloid congener: effects on morphine and cocaine self-administration and on mesolimbic dopamine release in rats. Brain Res. 1996;719:29–35. doi: 10.1016/0006-8993(96)00056-x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Pearl SM, Cai J, Maisonneuve IM. Ibogaine-like effects of noribogaine in rats. Brain Res. 1996;713:294–297. doi: 10.1016/0006-8993(95)01563-9. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM. Development of novel medications for drug addiction. The legacy of an African shrub. Ann N Y Acad Sci. 2000;909:88–103. doi: 10.1111/j.1749-6632.2000.tb06677.x. [DOI] [PubMed] [Google Scholar]
- Golden JP, Baloh RH, Kotzbauer PT, Lampe PA, Osborne PA, Milbrandt J, et al. Expression of neurturin, GDNF, and their receptors in the adult mouse CNS. J Comp Neurol. 1998;398:139–150. doi: 10.1002/(sici)1096-9861(19980817)398:1<139::aid-cne9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Golden JP, DeMaro JA, Osborne PA, Milbrandt J, Johnson EM., Jr. Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Green-Sadan T, Kinor N, Roth-Deri I, Geffen-Aricha R, Schindler CJ, Yadid G. Transplantation of glial cell line-derived neurotrophic factor-expressing cells into the striatum and nucleus accumbens attenuates acquisition of cocaine self-administration in rats. Eur J Neurosci. 2003;18:2093–2098. doi: 10.1046/j.1460-9568.2003.02943.x. [DOI] [PubMed] [Google Scholar]
- Green-Sadan T, Kuttner Y, Lublin-Tennenbaum T, Kinor N, Boguslavsky Y, Margel S, et al. Glial cell line-derived neurotrophic factor-conjugated nanoparticles suppress acquisition of cocaine self-administration in rats. Exp Neurol. 2005;194:97–105. doi: 10.1016/j.expneurol.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Griffin WC, III, Boger HA, Granholm AC, Middaugh LD. Partial deletion of glial cell line-derived neurotrophic factor (GDNF) in mice: effects on sucrose reward and striatal GDNF concentrations. Brain Res. 2006;1068:257–260. doi: 10.1016/j.brainres.2005.10.080. [DOI] [PubMed] [Google Scholar]
- Harding AS, Hancock JF. Using plasma membrane nanoclusters to build better signaling circuits. Trends Cell Biol. 2008;18:364–371. doi: 10.1016/j.tcb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Ichihara M, Iwashita T, Murakami H, Shimono Y, Kawai K, et al. Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene. 2000;19:4469–4475. doi: 10.1038/sj.onc.1203799. [DOI] [PubMed] [Google Scholar]
- He DY, McGough NN, Ravindranathan A, Jeanblanc J, Logrip ML, Phamluong K, et al. Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J Neurosci. 2005;25:619–628. doi: 10.1523/JNEUROSCI.3959-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DY, Ron D. Autoregulation of glial cell line-derived neurotrophic factor expression: implications for the long-lasting actions of the anti-addiction drug, Ibogaine. FASEB J. 2006;20:2420–2422. doi: 10.1096/fj.06-6394fje. [DOI] [PubMed] [Google Scholar]
- He DY, Ron D. Glial cell line-derived neurotrophic factor reverses ethanol-mediated increases in tyrosine hydroxylase immunoreactivity via altering the activity of heat shock protein 90. J Biol Chem. 2008;283:12811–12818. doi: 10.1074/jbc.M706216200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert MA, Van Horne CG, Hoffer BJ, Gerhardt GA. Functional effects of GDNF in normal rat striatum: presynaptic studies using in vivo electrochemistry and microdialysis. J Pharmacol Exp Ther. 1996;279:1181–1190. [PubMed] [Google Scholar]
- Hou JG, Lin LF, Mytilineou C. Glial cell line-derived neurotrophic factor exerts neurotrophic effects on dopaminergic neurons in vitro and promotes their survival and regrowth after damage by 1-methyl-4-phenylpyridinium. J Neurochem. 1996;66:74–82. doi: 10.1046/j.1471-4159.1996.66010074.x. [DOI] [PubMed] [Google Scholar]
- Hudson J, Granholm AC, Gerhardt GA, Henry MA, Hoffman A, Biddle P, et al. Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res Bull. 1995;36:425–432. doi: 10.1016/0361-9230(94)00224-o. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jain S, Golden JP, Wozniak D, Pehek E, Johnson EM, Jr., Milbrandt J. RET is dispensable for maintenance of midbrain dopaminergic neurons in adult mice. J Neurosci. 2006;26:11230–11238. doi: 10.1523/JNEUROSCI.1876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J, Stacy M. Medical management of levodopa-associated motor complications in patients with Parkinson's disease. CNS Drugs. 2007;21:677–692. doi: 10.2165/00023210-200721080-00005. [DOI] [PubMed] [Google Scholar]
- Jeanblanc J, He DY, McGough NN, Logrip ML, Phamluong K, Janak PH, Ron D. The dopamine D3 receptor is part of a homeostatic pathway regulating ethanol consumption. J Neurosci. 2006;24:10542–10552. doi: 10.1523/JNEUROSCI.3786-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, et al. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- Jongen JL, Haasdijk ED, Sabel-Goedknegt H, van der Burg J, Vecht Ch J, Holstege JC. Intrathecal injection of GDNF and BDNF induces immediate early gene expression in rat spinal dorsal horn. Exp Neurol. 2005;194:255–266. doi: 10.1016/j.expneurol.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Kaminska B, Gaweda-Walerych K, Zawadzka M. Molecular mechanisms of neuroprotective action of immunosuppressants—facts and hypotheses. J Cell Mol Med. 2004;8:45–58. doi: 10.1111/j.1582-4934.2004.tb00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W. Glial cell line-derived neurotrophic factor does not enter normal mouse brain. Neurosci Lett. 2003;340:239–241. doi: 10.1016/s0304-3940(03)00007-7. [DOI] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Dietz GP, Bahr M. Intravenous TAT-GDNF is protective after focal cerebral ischemia in mice. Stroke. 2003;34:1304–1310. doi: 10.1161/01.STR.0000066869.45310.50. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Fujita M, Maeda Y. Biosynthesis, remodelling and functions of mammalian GPI-anchored proteins: recent progress. J Biochem. 2008;144:287–294. doi: 10.1093/jb/mvn090. [DOI] [PubMed] [Google Scholar]
- Kirik D, Georgievska B, Bjorklund A. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci. 2004;7:105–110. doi: 10.1038/nn1175. [DOI] [PubMed] [Google Scholar]
- Kobori N, Waymire JC, Haycock JW, Clifton GL, Dash PK. Enhancement of tyrosine hydroxylase phosphorylation and activity by glial cell line-derived neurotrophic factor. J Biol Chem. 2004;279:2182–2191. doi: 10.1074/jbc.M310734200. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Jiao S, Collins F, Miller PJ. Glial cell line-derived neurotrophic factor: distribution and pharmacology in the rat following a bolus intraventricular injection. Brain Res. 1997;747:92–102. doi: 10.1016/s0006-8993(96)01265-6. [DOI] [PubMed] [Google Scholar]
- Ledda F, Bieraugel O, Fard SS, Vilar M, Paratcha G. Lrig1 is an endogenous inhibitor of Ret receptor tyrosine kinase activation, downstream signaling, and biological responses to GDNF. J Neurosci. 2008;28:39–49. doi: 10.1523/JNEUROSCI.2196-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledda F, Paratcha G, Sandoval-Guzman T, Ibanez CF. GDNF and GFRalpha1 promote formation of neuronal synapses by ligand-induced cell adhesion. Nat Neurosci. 2007;10:293–300. doi: 10.1038/nn1855. [DOI] [PubMed] [Google Scholar]
- Levitt M, Spector S, Sjoerdsma A, Udenfriend S. Elucidation of the rate-limiting step in norepinephrine biosynthesis in the perfused guinea-pig heart. J Pharmacol Exp Ther. 1965;148:1–8. [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons [see comments] Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas U, Strubelt S. Fatalities after taking ibogaine in addiction treatment could be related to sudden cardiac death caused by autonomic dysfunction. Med Hypotheses. 2006;67:960–964. doi: 10.1016/j.mehy.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Maciulaitis R, Kontrimaviciute V, Bressolle FM, Briedis V. Ibogaine, an anti-addictive drug: pharmacology and time to go further in development. A narrative review. Hum Exp Toxicol. 2008;27:181–194. doi: 10.1177/0960327107087802. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Anti-addictive actions of an iboga alkaloid congener: a novel mechanism for a novel treatment. Pharmacol Biochem Behav. 2003;75:607–618. doi: 10.1016/s0091-3057(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Maruyama W, Nitta A, Shamoto-Nagai M, Hirata Y, Akao Y, Yodim M, et al. N-Propargyl-1 (R)-aminoindan, rasagiline, increases glial cell line-derived neurotrophic factor (GDNF) in neuroblastoma SH-SY5Y cells through activation of NF-kappaB transcription factor. Neurochem Int. 2004;44:393–400. doi: 10.1016/j.neuint.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Mash DC, Kovera CA, Buck BE, Norenberg MD, Shapshak P, Hearn WL, et al. Medication development of ibogaine as a pharmacotherapy for drug dependence. Ann N Y Acad Sci. 1998;844:274–292. [PubMed] [Google Scholar]
- Matsuo A, Nakamura S, Akiguchi I. Immunohistochemical localization of glial cell line-derived neurotrophic factor family receptor alpha-1 in the rat brain: confirmation of expression in various neuronal systems. Brain Res. 2000;859:57–71. doi: 10.1016/s0006-8993(99)02442-7. [DOI] [PubMed] [Google Scholar]
- McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, Kharazia V, Janak PH, Ron D. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. J Neurosci. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer CJ, Eisch AJ, Carlezon WA, Jr., Whisler K, Shen L, Wolf DH, et al. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron. 2000;26:247–257. doi: 10.1016/s0896-6273(00)81154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther. 2002;303:791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Wion D, Brachet P. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport. 1996;7:2171–2175. doi: 10.1097/00001756-199609020-00023. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Cussac D, Audinot V, Nicolas JP, De Ceuninck F, Boutin JA, Millan MJ. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D(2)-like receptor and alpha(1)/alpha(2)-adrenoceptor. J Pharmacol Exp Ther. 2002;303:805–814. doi: 10.1124/jpet.102.039875. [DOI] [PubMed] [Google Scholar]
- Newman-Tancredi A, Cussac D, Quentric Y, Touzard M, Verriele L, Carpentier N, Millan MJ. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. III. Agonist and antagonist properties at serotonin, 5-HT(1) and 5-HT(2), receptor subtypes. J Pharmacol Exp Ther. 2002;303:815–822. doi: 10.1124/jpet.102.039883. [DOI] [PubMed] [Google Scholar]
- Newton PM, Ron D. Protein kinase C and alcohol addiction. Pharmacol Res. 2007;55:570–577. doi: 10.1016/j.phrs.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Nitta A, Nishioka H, Fukumitsu H, Furukawa Y, Sugiura H, Shen L, et al. Hydrophobic dipeptide Leu-Ile protects against neuronal death by inducing brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor synthesis. J Neurosci Res. 2004;78:250–258. doi: 10.1002/jnr.20258. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Shen L, Noda Y, Nabeshima T. Involvement of glial cell line-derived neurotrophic factor in inhibitory effects of a hydrophobic dipeptide Leu-Ile on morphine-induced sensitization and rewarding effects. Behav Brain Res. 2007;179:167–171. doi: 10.1016/j.bbr.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Yamada Y, Nakajima A, Saito K, Seishima M, et al. Tumor necrosis factor-alpha and its inducer inhibit morphine-induced rewarding effects and sensitization. Biol Psychiatry. 2007;62:658–668. doi: 10.1016/j.biopsych.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Yamada Y, Nakajima A, Saito K, Seishima M, et al. An inducer for glial cell line-derived neurotrophic factor and tumor necrosis factor-alpha protects against methamphetamine-induced rewarding effects and sensitization. Biol Psychiatry. 2007;61:890–901. doi: 10.1016/j.biopsych.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Tomac A, Lindqvist E, Lindskog S, Humpel C, Stromberg I, et al. Cellular expression of GDNF mRNA suggests multiple functions inside and outside the nervous system. Cell Tissue Res. 1996;286:191–207. doi: 10.1007/s004410050688. [DOI] [PubMed] [Google Scholar]
- O'Hearn E, Molliver ME. Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience. 1993;55:303–310. doi: 10.1016/0306-4522(93)90500-f. [DOI] [PubMed] [Google Scholar]
- Ohta K, Fujinami A, Kuno S, Sakakimoto A, Matsui H, Kawahara Y, et al. Cabergoline stimulates synthesis and secretion of nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor by mouse astrocytes in primary culture. Pharmacology. 2004;71:162–168. doi: 10.1159/000077451. [DOI] [PubMed] [Google Scholar]
- Ohta K, Kuno S, Mizuta I, Fujinami A, Matsui H, Ohta M. Effects of dopamine agonists bromocriptine, pergolide, cabergoline, and SKF-38393 on GDNF, NGF, and BDNF synthesis in cultured mouse astrocytes. Life Sci. 2003;73:617–626. doi: 10.1016/s0024-3205(03)00321-7. [DOI] [PubMed] [Google Scholar]
- Ortiz J, DeCaprio JL, Kosten TA, Nestler EJ. Strain-selective effects of corticosterone on locomotor sensitization to cocaine and on levels of tyrosine hydroxylase and glucocorticoid receptor in the ventral tegmental area. Neuroscience. 1995;67:383–397. doi: 10.1016/0306-4522(95)00018-e. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology. 1996;14:443–452. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- Papoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F, Baars L, Coulpier M, Besset V, Anders J, et al. Released GFRalpha1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron. 2001;29:171–184. doi: 10.1016/s0896-6273(01)00188-x. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ledda F, Ibanez CF. The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands. Cell. 2003;113:867–879. doi: 10.1016/s0092-8674(03)00435-5. [DOI] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Pierchala BA, Milbrandt J, Johnson EM., Jr. Glial cell line-derived neurotrophic factor-dependent recruitment of Ret into lipid rafts enhances signaling by partitioning Ret from proteasome-dependent degradation. J Neurosci. 2006;26:2777–2787. doi: 10.1523/JNEUROSCI.3420-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon NA, Menoud A, Tseng JL, Zurn AD, Aebischer P. Neuronal GDNF expression in the adult rat nervous system identified by in situ hybridization. Eur J Neurosci. 1997;9:463–471. doi: 10.1111/j.1460-9568.1997.tb01623.x. [DOI] [PubMed] [Google Scholar]
- Popik P, Layer RT, Skolnick P. 100 years of ibogaine: neurochemical and pharmacological actions of a putative anti-addictive drug. Pharmacol Rev. 1995;47:235–253. [PubMed] [Google Scholar]
- Rezvani AH, Overstreet DH, Yang Y, Maisonneuve IM, Bandarage UK, Kuehne ME, et al. Attenuation of alcohol consumption by a novel nontoxic ibogaine analogue (18-methoxycoronaridine) in alcohol-preferring rats. Pharmacol Biochem Behav. 1997;58:615–619. doi: 10.1016/s0091-3057(97)10003-x. [DOI] [PubMed] [Google Scholar]
- Rosenblad C, Georgievska B, Kirik D. Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system. Eur J Neurosci. 2003;17:260–270. doi: 10.1046/j.1460-9568.2003.02456.x. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, et al. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Sajadi A, Bauer M, Thony B, Aebischer P. Long-term glial cell line-derived neurotrophic factor overexpression in the intact nigrostriatal system in rats leads to a decrease of dopamine and increase of tetrahydrobiopterin production. J Neurochem. 2005;93:1482–1486. doi: 10.1111/j.1471-4159.2005.03139.x. [DOI] [PubMed] [Google Scholar]
- Sakai A, Asada M, Seno N, Suzuki H. Involvement of neural cell adhesion molecule signaling in glial cell line-derived neurotrophic factor-induced analgesia in a rat model of neuropathic pain. Pain. 2008;137:378–388. doi: 10.1016/j.pain.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Sanchez B, Lopez-Martin E, Segura C, Labandeira-Garcia JL, Perez-Fernandez R. 1,25-Dihydroxyvitamin D(3) increases striatal GDNF mRNA and protein expression in adult rats. Brain Res Mol Brain Res. 2002;108:143–146. doi: 10.1016/s0169-328x(02)00545-4. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Sarabi A, Hoffer BJ, Olson L, Morales M. GFRalpha-1 mRNA in dopaminergic and nondopaminergic neurons in the substantia nigra and ventral tegmental area. J Comp Neurol. 2001;441:106–117. doi: 10.1002/cne.1400. [DOI] [PubMed] [Google Scholar]
- Schaar DG, Sieber BA, Dreyfus CF, Black IB. Regional and cell-specific expression of GDNF in rat brain. Exp Neurol. 1993;124:368–371. doi: 10.1006/exnr.1993.1207. [DOI] [PubMed] [Google Scholar]
- Self DW, McClenahan AW, Beitner-Johnson D, Terwilliger RZ, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to heroin self-administration. Synapse. 1995;21:312–318. doi: 10.1002/syn.890210405. [DOI] [PubMed] [Google Scholar]
- Semba J, Akanuma N, Wakuta M, Tanaka N, Suhara T. Alterations in the expressions of mRNA for GDNF and its receptors in the ventral midbrain of rats exposed to subchronic phencyclidine. Brain Res Mol Brain Res. 2004;124:88–95. doi: 10.1016/j.molbrainres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Watson DW, Reiber C, Rawson RA, Montgomery MA, Majewska MD, et al. Randomized controlled pilot trial of cabergoline, hydergine and levodopa/carbidopa: Los Angeles Cocaine Rapid Efficacy Screening Trial (CREST) Addiction. 2005;100(Suppl 1):78–90. doi: 10.1111/j.1360-0443.2005.00991.x. [DOI] [PubMed] [Google Scholar]
- Springer JE, Mu X, Bergmann LW, Trojanowski JQ. Expression of GDNF mRNA in rat and human nervous tissue. Exp Neurol. 1994;127:167–170. doi: 10.1006/exnr.1994.1091. [DOI] [PubMed] [Google Scholar]
- Stromberg I, Bjorklund L, Johansson M, Tomac A, Collins F, Olson L, et al. Glial cell line-derived neurotrophic factor is expressed in the developing but not adult striatum and stimulates developing dopamine neurons in vivo. Exp Neurol. 1993;124:401–412. doi: 10.1006/exnr.1993.1214. [DOI] [PubMed] [Google Scholar]
- Suzuki C, Murakumo Y, Kawase Y, Sato T, Morinaga T, Fukuda N, et al. A novel GDNF-inducible gene, BMZF3, encodes a transcriptional repressor associated with KAP-1. Biochem Biophys Res Commun. 2008;366:226–232. doi: 10.1016/j.bbrc.2007.11.118. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Tang MJ, Worley D, Sanicola M, Dressler GR. The RET-glial cell-derived neurotrophic factor (GDNF) pathway stimulates migration and chemoattraction of epithelial cells. J Cell Biol. 1998;142:1337–1345. doi: 10.1083/jcb.142.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey MG, Baloh RH, Milbrandt J, Johnson EM., Jr. GFRalpha-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron. 2000;25:611–623. doi: 10.1016/s0896-6273(00)81064-8. [DOI] [PubMed] [Google Scholar]
- Tokugawa K, Yamamoto K, Nishiguchi M, Sekine T, Sakai M, Ueki T, et al. XIB4035, a novel nonpeptidyl small molecule agonist for GFRalpha-1. Neurochem Int. 2003;42:81–86. doi: 10.1016/s0197-0186(02)00053-0. [DOI] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Tomac A, Widenfalk J, Lin LF, Kohno T, Ebendal T, Hoffer BJ, et al. Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc Natl Acad Sci U S A. 1995;92:8274–8278. doi: 10.1073/pnas.92.18.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treanor JJ, Goodman L, deSauvage F, Stone DM, Poulsen KT, Beck CD, et al. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- Trupp M, Arenas E, Fainzilber M, Nilsson AS, Sieber BA, Grigoriou M, et al. Functional receptor for GDNF encoded by the c-ret proto-oncogene. Nature. 1996;381:785–789. doi: 10.1038/381785a0. [DOI] [PubMed] [Google Scholar]
- Trupp M, Belluardo N, Funakoshi H, Ibanez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupp M, Scott R, Whittemore SR, Ibanez CF. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J Biol Chem. 1999;274:20885–20894. doi: 10.1074/jbc.274.30.20885. [DOI] [PubMed] [Google Scholar]
- Tsui-Pierchala BA, Ahrens RC, Crowder RJ, Milbrandt J, Johnson EM., Jr. The long and short isoforms of ret function as independent signaling complexes. J Biol Chem. 2002;277:34618–34625. doi: 10.1074/jbc.M203580200. [DOI] [PubMed] [Google Scholar]
- Tsui-Pierchala BA, Encinas M, Milbrandt J, Johnson EM., Jr. Lipid rafts in neuronal signaling and function. Trends Neurosci. 2002;25:412–417. doi: 10.1016/s0166-2236(02)02215-4. [DOI] [PubMed] [Google Scholar]
- Verity AN, Wyatt TL, Lee W, Hajos B, Baecker PA, Eglen RM, et al. Differential regulation of glial cell line-derived neurotrophic factor (GDNF) expression in human neuroblastoma and glioblastoma cell lines. J Neurosci Res. 1999;55:187–197. doi: 10.1002/(SICI)1097-4547(19990115)55:2<187::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Visanji NP, Orsi A, Johnston TH, Howson PA, Dixon K, Callizot N, et al. PYM50028, a novel, orally active, nonpeptide neurotrophic factor inducer, prevents and reverses neuronal damage induced by MPP+in mesencephalic neurons and by MPTP in a mouse model of Parkinson's disease. FASEB J. 2008;22:2488–2497. doi: 10.1096/fj.07-095398. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Wang CY, Yang F, He X, Chow A, Du J, Russell JT, et al. Ca(2+) binding protein frequenin mediates GDNF-induced potentiation of Ca(2+) channels and transmitter release. Neuron. 2001;32:99–112. doi: 10.1016/s0896-6273(01)00434-2. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Cao JP, Yu JK, Zhang LC, Jiang ZJ, Gao DS. Calbindin-D28K expression induced by glial cell line-derived neurotrophic factor in substantia nigra neurons dependent on PI3K/Akt/NF-kappaB signaling pathway. Eur J Pharmacol. 2008;595:7–12. doi: 10.1016/j.ejphar.2008.07.044. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen G, Lu B, Wu CP. GDNF acutely potentiates Ca2+ channels and excitatory synaptic transmission in midbrain dopaminergic neurons. Neurosignals. 2003;12:78–88. doi: 10.1159/000071817. [DOI] [PubMed] [Google Scholar]
- Webster J, Piscitelli G, Polli A, Ferrari CI, Ismail I, Scanlon MF. A comparison of cabergoline and bromocriptine in the treatment of hyperprolacti-nemic amenorrhea. Cabergoline Comparative Study Group. N Engl J Med. 1994;331:904–909. doi: 10.1056/NEJM199410063311403. [DOI] [PubMed] [Google Scholar]
- Weinreb O, Amit T, Bar-Am O, Youdim MB. Induction of neurotrophic factors GDNF and BDNF associated with the mechanism of neurorescue action of rasagiline and ladostigil: new insights and implications for therapy. Ann N Y Acad Sci. 2007;1122:155–168. doi: 10.1196/annals.1403.011. [DOI] [PubMed] [Google Scholar]
- Yan Y, Yamada K, Niwa M, Nagai T, Nitta A, Nabeshima T. Enduring vulnerability to reinstatement of methamphetamine-seeking behavior in glial-cell-line-derived neurotrophic factor mutant mice. Faseb J. 2007;21:1994–2004. doi: 10.1096/fj.06-7772com. [DOI] [PubMed] [Google Scholar]
- Yang F, Feng L, Zheng F, Johnson SW, Du J, Shen L, et al. GDNF acutely modulates excitability and A-type K(+) channels in midbrain dopaminergic neurons. Nat Neurosci. 2001;4:1071–1078. doi: 10.1038/nn734. [DOI] [PubMed] [Google Scholar]
- Youdim MBH, Van der Schyf C. Magic bullets or novel multimodal drugs with various CNS targets for Parkinson's disease? Nature Rev Drug Discov. 2007 [Google Scholar]
- Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson's disease. N Engl J Med. 2007;356:39–46. doi: 10.1056/NEJMoa054830. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xia Z, Hu Y, Orsi A, Rees D. Role of glial cell derived neurotrophic factor in the protective effect of smilagenin on rat mesencephalic dopaminergic neurons damaged by MPP+ FEBS Lett. 2008;582:956–960. doi: 10.1016/j.febslet.2008.02.039. [DOI] [PubMed] [Google Scholar]