Abstract
Melatonin is increasingly used for the treatment of sleep disorders. Surge-sustained formulations consisting of combined immediate release and controlled release dosing may mimic the endogenous melatonin physiologic profile. However, relatively little is known about the pharmacokinetic properties of low dose (<0.5 mg) and high dose (>2 mg) melatonin in a combined immediate release/controlled release dose, especially in older adults who may also exhibit altered melatonin disposition. To assess this, we conducted a randomized, double-blind, placebo-controlled study of low (0.4 mg) and high (4.0 mg) dose melatonin (25% immediate release+75% controlled release) in 27 older adults with insomnia complaints and low endogenous melatonin levels to determine if melatonin pharmacokinetic properties differ between these two doses. The time to maximum level (1.3 hrs vs 1.5 hrs), elimination half-life (1.8 hrs vs 2.1 hrs), and apparent total clearance (379 l/hr vs 478 l/hr) did not differ significantly between the low and high dose arms, respectively. The maximum concentration was 405±93 pg/ml for the low dose arm and 3999±700 pg/ml for the high dose arm, both of which are substantially higher than physiologic melatonin levels for this age group. In addition, subjects in the high dose arm maintained melatonin levels >50 pg/ml for an average of 10 hours, which could result in elevated melatonin levels beyond the typical sleep period. Renal and liver function parameters remained stable after 6 weeks of treatment. The linear pharmacokinetic behavior of melatonin observed in the elderly can form the basis for future studies exploring a wider range of dosing scenarios to establish exposure-response relationships for melatonin-mediated sleep outcomes.
Keywords: Melatonin, pharmacokinetics, aged, insomnia, polysomnography
Introduction
Melatonin is a secretory product produced in the pineal gland and in several extrapineal sites, which has a wide variety of functions [1, 2]. Exogenous melatonin has been used as a chronobiotic (to shift circadian phase) [3], as an antioxidant [4, 5], and as an oncostatic agent [6]. There has been a particular interest in the potential role of melatonin for the treatment of insomnia in older adults because, unlike most hypnotic medications, melatonin has minimal effects on psychomotor function [7]. Furthermore, melatonin deficiency may contribute to insomnia because levels generally decrease with age [8] while sleep fragmentation increases with age [9], and sleep latency increases with melatonin suppression [10] or delayed melatonin onset [11]. Research examining the effects of exogenous melatonin on sleep in older adults has had variable findings, however [12–15].
One challenge in interpreting the existing literature is the paucity of pharmacokinetic (PK) data on exogenous melatonin in older adults with different delivery methods or dosages. Several different oral melatonin formulations have been developed, including immediate release, controlled (sustained) release and surge-sustained release [14–19]. A large range of doses have been used in clinical trials, with considerable debate regarding the role of low dose (0.1–0.5 mg) and high dose (2–10 mg) melatonin. Past studies have found bioavailability of exogenous melatonin to be highly variable, ranging from 1 to 74% [20–24], though this broad range may indicate formulation and/or dose input dependence.
Endogenous melatonin is primarily metabolized in the liver by hydroxylation (approximately 90%) to 6-hydroxymelatonin and excreted in the urine following conjugation with sulfuric or glucuronic acid [25, 26]. Research in mice using radiolabelled melatonin found that after 48 hours, 70% of the tracer was in urine and 15% in the feces [26]. Older adults, because of age-related alterations in hepatic and renal clearance as well as changes in body composition (e.g. fat, water), may have different melatonin PK properties or be at increased risk of adverse melatonin effects, such as next-day sleepiness [27].
To clarify the PK properties of low and high dose melatonin in older adults, we conducted a placebo-controlled, double-blind, randomized study using two different melatonin doses (0.4 mg and 4.0 mg) in a surge-sustained release formulation [25, 28, 29]. The study sample consisted of 27 community-dwelling older adults with insomnia complaints that did not have elevated endogenous melatonin levels (urine 6-sulfatoxymelatonin <10,000 pg/ml) that were part of a larger study (55 subjects) of melatonin and agreed to participate in the PK study. Our specific hypothesis was that melatonin levels from the higher dose formulation would result in supraphysiologic levels for older adult subjects and that melatonin would exhibit linear PK over the studied dose range. The findings from this study have implications for melatonin administration in older patients, a population that may have unique melatonin PK properties due to age-related changes in metabolism and clearance.
Materials and Methods
Study sample
Research study participants were recruited via telephone from the Penn Partners in Healthy Living patient database (a registry of over 100,000 older adults in the greater Philadelphia metropolitan area), advertisements in print and radio media, and from the waiting areas of primary care clinics and senior citizens’ centers. Fifty-six study participants enrolled in the melatonin randomized trial, of whom 27 agreed to participate in the PK sub-study presented herein.
Informed consent was obtained on all study participants prior to data collection and enrollment. The study protocol was reviewed and approved by the University of Pennsylvania Institutional Review Board (IRB) and the University of Pennsylvania Clinical and Translational Research Center (CTRC, funded by the University of Pennsylvania Clinical and Translational Science Award). It was audited yearly by the University of Pennsylvania Office of Human Research (OHR) and an independent Data and Safety Monitoring Board (DSMB), and conducted under Food and Drug Administration (FDA) Investigational New Drug Application (IND) 70,234 (IND holder: Nalaka S. Gooneratne).
Inclusion/exclusion criteria
Inclusion criteria were 1) objective insomnia (<80% sleep efficiency on polysomnography), 2) the absence of high endogenous melatonin levels (urine melatonin <10,000 pg/ml; to minimize the effects of endogenous melatonin and assess if melatonin has a therapeutic effect in patients with low endogenous levels) [28], 3) the ability to provide consent, and 4) an age greater than 65. Study participants with the following criteria were excluded from this study: 1) moderate to severe sleep apnea as determined by polysomnography (apnea-hypopnea index of >20) [30], 2) anemia (Hb<10 gm/dl), 3) active alcohol or drug use, 4) concurrent sedative treatment for insomnia (diphenhydramine, zolpidem, etc.), 5) liver disease, 6) autoimmune disease [31], 7) leukemia/lymphoma [32], 8) restless legs syndrome/periodic limb movement disorder, and 9) asthma [33]. There were no weight restrictions, other comorbid medical exclusions or functional requirements.
Melatonin formulation and manufacture
For both the 0.4 mg and 4.0 mg dosing levels, we simultaneously administered immediate release (25% of the total dose) and controlled release (75% of the total dose) tablets to create a surge-sustained release effect that would most closely replicate the physiologic melatonin profile. For the low dose arm, the total immediate release dose was 0.1 mg and the controlled release dose was 0.3 mg (total of 0.4 mg), and was intended to create melatonin levels that approximated normal melatonin peak levels based on prior research in older subjects where endogenous levels may be as low as 30–40 pg/ml [25, 28, 29]. Ten-fold higher of each of the formulation (i.e., immediate release dose of 1.0 mg, controlled release dose of 3.0 mg for a total of 4.0 mg) was administered to the high dose group and was intended to produce supraphysiologic levels of melatonin; this reflects doses that are more commonly used in clinical settings [25].
Analytical-grade melatonin was obtained from Regis Technologies (Morton Grove, IL). Both immediate release and controlled release melatonin tablets were formulated by ABCO Labs, Inc (Fairfield, California, USA). The controlled release melatonin used an ethyl cellulose suspension to provide prolonged release of 0.3 mg or 3.0 mg of melatonin over 3 to 5 hours. The dissolution characteristics and purity were independently tested by ConsumerLabs.com, Inc., using high-performance liquid chromatography (HPLC) following USP (United States Pharmacopedia) 2040 guidelines entitled “Disintegration and Dissolution of Nutritional Supplements.” Both formulations of melatonin were manufactured in accordance with FDA Good Manufacturing Practices (GMP). Dissolution characteristics of the 0.3 mg controlled release melatonin were as follows: 1 hour, 0.1 mg; 3 hour, 0.3 mg; 5 hour, 0.3 mg; 7 hour, 0.3 mg; total amount released, 0.3 mg. For the 3.0 mg controlled release melatonin, dissolution characteristics were: 1 hour, 1.4 mg; 3 hour, 2.5 mg; 5 hour, 3.1 mg; 7 hour, 3.1 mg; total amount released, 3.1 mg.
The placebo was identical in appearance to the melatonin tablets. All active and placebo tablets were placed in blister packs under the supervision of the Investigational Drug Service at the University of Pennsylvania. Study participant randomization was also performed centrally by the Investigational Drug Service using a computer-generated randomization allocation. The study investigators, all research staff, and the study participants were blinded to study randomization.
Study methods
Eligible study participants first underwent pre-treatment studies at the CTRC. The study participants received a medical examination by a physician (N.S.G.), a complete blood count, and an electrocardiogram.
Subjective assessment of sleep was obtained using the Pittsburgh Sleep Quality Index (PSQI), which provides a global score of sleep quality and is a recommended insomnia research instrument [34, 35]; this was an a priori primary study outcome measure. Study participants also underwent two nights of in-lab polysomnography on study days −10 and −9 (i.e., 10 and 9 days prior to randomization). Sleep studies were performed using 16-channel polysomnography which included electroencephalogram, electrocardiogram, electrooculogram, snoring monitor, chin and limb electromyelogram, chest and abdominal respiratory belts, finger oximetry, and airflow monitoring with nasal and oral thermistors. Sleep records were manually scored in 30-second epochs according to standard criteria by a sleep technician [36]. The first sleep study was used to habituate the study participant to the sleep lab to minimize any first night effects that may lead to artificial changes in insomnia severity, and the second night was then used for objective assessment of sleep parameters (sleep efficiency, sleep latency, wakefulness after sleep onset); the polysomnography-derived sleep efficiency was also an a priori primary study outcome measure [37].
On study day −8, study participants underwent serum melatonin sampling, in which an intravenous line was used to collect 28 separate 4 cc specimens over a 24 hour period. Dim light conditions were maintained for melatonin collection by turning the room lights to <50 lux, and the study participants wore sunglasses so that retina light exposure was <10 lux. This prevented artificial light from affecting intrinsic circadian pacemaker and melatonin levels during the collection [38]. Study participants were asked to lie in a supine position, and refrain from caffeine or smoking for the entire 24 hour period [39, 40]. The specific timing of the specimen collections was as follows: Starting at 12:00 (noon) to 18:00 on day −8, melatonin specimens were collected at two hour intervals to determine daytime melatonin levels. From 18:00 to 24:00, specimens were collected at 30 min intervals. From 01:00 to 12:00 noon, specimens were collected at one hour intervals to determine melatonin peak levels. The intravenous line used for melatonin collection was connected to a double stopcock assembly so that a waste specimen could be drawn along with the sample to prevent dilution effects. After each sample was collected, the stopcock also allowed for the sterile re-injection of waste specimen to minimize blood loss. The blood sample was centrifuged to collect the serum. Specimens were screened for hemolysis, and redrawn if present. Collected serum was frozen at −70 degrees Celsius until analyses were conducted within the span of six months. After specimen collection stopped at noon, the study participants were returned to normal light levels. Study participants were screened for orthostatic hypotension by the research study nurse and anemia as determined by a complete blood count.
For the treatment phase, study participants were randomized into three arms to receive either low dose oral melatonin (consisting of both 0.1 mg immediate release and 0.3 mg controlled release tablets), high dose oral melatonin (consisting of both 1.0 mg immediate release and 3.0 mg controlled release tablets), or a placebo. The tablets were identical in appearance. The study participants were not stratified by any variable for the randomization. The medication was taken 30 minutes before bedtime for 42 days. Study participants were monitored for adverse effects on day 7 and 21 by history and physical exam (performed by an MD), routine blood tests, and electrocardiogram. On study day 40, study participants completed the PSQI, and on study days 40 and 41, they again underwent two nights of polysomnography. Study participants were admitted for melatonin sampling similar to the pre-treatment studies to determine the effects of the exogenous melatonin on study days 42 to 43. In the event that on-intervention serum melatonin levels were above assay thresholds of >300 pg/ml, serial dilution was performed as per assay kit recommendations. On day 43 of the study, the study participants ceased taking melatonin or placebo, and they received an exit clinical exam, blood work, and electrocardiogram.
Analytical method
Melatonin Direct I-125 radioimmunoassay (RIA), manufactured by IBL Laboratories, Hamburg, Germany, was used to determine melatonin levels in serum [41]. Specimens were assayed as singleton. Pharmasan Laboratories (Osceola, Wisconsin) performed the assays. The intra-assay coefficient of variation was 8.1 to 8.5% and the limit of detection was <3.5 pg/ml.
Pharmacokinetic and statistical analysis
PK parameters of melatonin were derived from standard noncompartmental linear trapezoid linear interpolation method using Phoenix™ WinNonlin® software (Version 6.1., Pharsight, Mountain View, California, USA). Data points for inclusion in the terminal phase were chosen based on visual inspection of individual concentration time curves. Subjects were excluded from the noncompartmental analysis if a terminal elimination phase was not observed. The same dataset were fitted with an extravascular one-compartmental model using Phoenix™ to determine the oral first order absorption rate constant. Graphs of predicted concentration versus observed data were visually examined and subjects with poor fit were excluded from the analysis.
Comparisons of baseline melatonin secretions among the three study arms were performed by one-way ANOVA using SAS (Version 9.2, SAS Institute Inc., Cary, NC). Comparisons of time-matched melatonin levels between baseline and pre-dosing period in the treatment groups or between baseline and dosing period in the placebo group were performed by paired student’s t-test using SAS. Changes in pre- and post-treatment serum creatinine and liver enzyme levels, as well as sleep parameters (PSG sleep efficiency, PSG sleep onset latency, PSQI sleep quality), were analyzed by paired student’s t-test using SAS. Statistical significance was accepted at P < 0.05 for all tests.
Results
Twenty-seven subjects (placebo = 8, low dose 0.4 mg = 9, high dose 4 mg = 10) completed the PK study. Study sample characteristics are presented in table 1. No significant differences were noted between the study arms on any of these measures. The study sample demonstrated evidence of insomnia both by subjective self-report (PSQI) and polysomnography measures as suggested by a PSQI global score >=5, and PSG sleep efficiency <80% or sleep onset latency >30 min.
Table 1.
Subject demographics by study arm
| Parameters | Placebo | Low dose | High dose | Statistic* | p-value |
|---|---|---|---|---|---|
| Age | 75.1 (6.2) | 73.0 (5.9) | 75.7 (4.7) | 0.6 | 0.55 |
| Gender (percent female) | 75 | 78 | 70 | 0.15 (X2) | 0.93 |
| Race (percent Caucasian) | 100 | 100 | 89 | 1.74 (X2) | 0.41 |
| Medications | 5.6 (2.9) | 7.1 (3.7) | 4.5 (2.9) | 1.29 | 0.3 |
| Medical diagnoses | 2.3 (1.2) | 3.2 (2.3) | 2.9 (1.3) | 0.38 | 0.69 |
| BMI (kg/m2) | 29.0 (7.8) | 28.6 (5.4) | 28.0 (4.7) | 0.05 | 0.95 |
| AHI (events/hr) | 8.1 (3.9) | 7.6 (5.1) | 5.5 (2.6) | 1.10 | 0.35 |
| Sleep onset latency, PSG (min) | 17.7 (16.1) | 44.1 (36.4) | 24.3 (22.4) | 2.33 | 0.12 |
| Sleep efficiency, PSG (percent) | 70.2 (9.0) | 67.1 (14.9) | 69.0 (9.2) | 0.16 | 0.85 |
| PSQI | 10.6 (5.5) | 11.6 (3.2) | 11.2 (4.6) | 0.09 | 0.91 |
AHI: apnea/hypopnea index; BMI: body-mass index; PSG: polysomnography; PSQI: Pittsburg Sleep Quality Index
F-statistics are presented unless otherwise noted.
The 24 hour endogenous melatonin secretion followed a typical circadian pattern with the rise in melatonin levels at around 20:00 h, reaching peak concentration at 01:00 h, and declines at approximately 05:00 h based on pooled data average and confirmed by visual inspection (Fig. 1). The median pre-onset melatonin concentration was 5.8 pg/mL (range: 2 to 19.5 pg/mL). The median peak concentrations pre-treatment was 46.7 pg/ml (range: 23.6 to 118 pg/mL), representing approximately a 7-fold increase from baseline values. No circadian rhythm pattern was observed in six patients (two from each study arm, Fig. 1 insert). Their melatonin secretion remained constant through the twenty-four hour cycle with median concentrations ranged from 3.15 to 9.4 pg/mL. There were no significant differences in pre-treatment endogenous melatonin serum levels among the study arms across all sampling times (ANOVA p-values range: 0.20 to 0.99).
Figure 1.
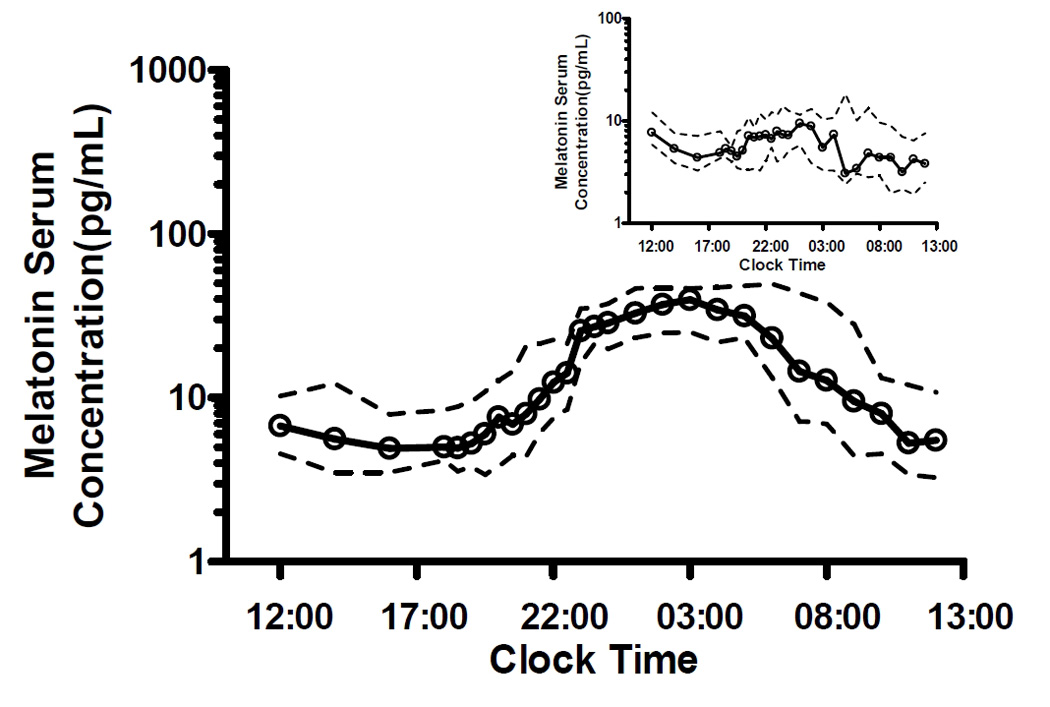
Baseline endogenous melatonin secretion pattern in elderly subjects. Serum melatonin levels were measured in all subjects pre-treatment for 24 hours. Insert: Serum melatonin levels from six patients who did not exhibit circadian rhythm secretion pattern. Points represent medians and dashed lines represent 25th and 75th percentile.
Oral melatonin was taken between 19:15 h to 23:15 h per the individual subject’s bedtime schedule. Melatonin was rapidly absorbed following oral ingestion in all subjects (Fig. 2). Maximal concentration was reached in 1.3±0.19 hours in the low dose arm and 1.5±0.24 hours in the high dose arm (Tmax), with no significant difference between the two arms (P = 0.6). The average maximal serum concentration (Cmax) was 405 pg/mL and 3999 pg/mL in the low dose and high dose group, respectively (table 2). This represents approximately a seven-fold and sixty-five-fold increase from their respective endogenous maximal levels (Fig. 3 top). The average maximal serum concentration was significantly greater in the high dose arm (P < 0.001). Elimination followed a first-order exponential decay pattern with a mean half-life (t1/2) of 1.8 hours and 2.1 hours in the low dose and high dose arms, respectively (P = 0.4). Using a non-compartmental analysis approach, both the mean apparent clearance (CL/F: low dose 379 L/hr; high dose 478 L/hr) and mean apparent volume of distribution (V/F: low dose 1035 L; high dose 1602 L) were similar between the two treatment groups (P = 0.5 and 0.4, respectively). Total melatonin exposure or area under the concentration-time curve (AUC), either estimated to the last sampling time (AUC 0-tlast) or extrapolated to infinity (AUC 0-∞) was approximately 10-fold higher for the high dose group than the low dose group, which is consistent with linear pharmacokinetics (Figure 3 bottom) (P = 0.002 for both). The relative contribution of endogenous melatonin to total exposure was estimated to be about 22% and 3.6% for the low and high dose group, respectively, based on pre-treatment levels. Elevated melatonin levels (> 50 pg/mL) were present for significantly longer periods in the high dose arm: they were sustained for 6.4 hours in the low dose arm and 10 hours in the high dose arm following oral administration (P = 0.006). Of note, two subjects were excluded from the noncompartmental analysis due to lack of absorption phase data and two subjects were excluded from Ka determination due to poor pharmacokinetic modeling fit of observed data.
Figure 2.

Melatonin concentration time profiles in treatment groups on day 42. Serum melatonin levels were measured at the end of the study period for 24 hours from noon to noon next day. Graph depicts melatonin levels after ingestion. Points represent mean ± SD.
Table 2.
Comparison of pharmacokinetic parameters of exogenous melatonin in low dose and high dose groups.
| Parameters | Low Dose Mean (SE) |
High Dose Mean (SE) |
||
|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 1 | Visit 2 | |
| CL/F, L/hr | -- | 379 (89.4) | -- | 478 (116) |
| V/F, L | -- | 1035 (304) | -- | 1602 (578) |
| Ka, hr−1 | -- | 2.6 (0.89) | -- | 1.3 (0.30) |
| t1/2, hr | 1.7 (0.16) | 1.8 (0.19) | 1.6 (0.16) | 2.1 (0.34) |
| Tmax, hr | 4.7 (0.96) | 1.3 (0.19) | 5.1 (1.4) | 1.5 (0.24) |
| Cmax, pg/mL | 52 (8.1) | 405 (93.0) | 61 (11) | 3999 (700) |
| T>50pg/mL, hr | 2.0 (1.1) | 6.4 (0.65) | 2.5 (1.5) | 10.0 (0.89) |
|
AUC0-tlast, pg/mL•hr |
337 (64) | 1577 (373) | 418 (73) | 12039 (2268) |
|
AUC0-∞, pg/mL•hr |
350 (65) | 1595 (374) | 438 (79) | 12123 (2277) |
CL/F, apparent total clearance; V/F, apparent volume of distribution; Ka, first-order absorption constant; t1/2, elimination half-life; Tmax, time to reach maximum concentration; Cmax, maximum concentration; T>50pg/mL, time duration above 50 pg/mL; AUC0-last, area under the concentration-time curve up to last observed data; AUC0-∞, area under the concentration-time curve extrapolated to infinity.
Two subjects were excluded from the noncompartmental analysis due to lack of absorption phase and two subjects were excluded from Ka determination due to poor PK modeling fit of observed data.
Figure 3.

Box-and-whisker plot of melatonin (A) Cmax and (B) AUC0-∞ for the treatment groups. Pharmacokinetic parameters were derived from noncompartmental analysis based on melatonin serum levels before treatment (baseline) and treatment day 42.
The pre-dose melatonin secretion profiles were similar between the pre-treatment period and the treatment period for both dose groups, suggesting no apparent accumulation of exogenous melatonin or alteration in endogenous melatonin levels: There was no significant difference in time-matched melatonin serum levels in 6 out of 7 (paired t-test P-values range: 0.1685 to 0.7518) or 4 out of 6 (P-values range: 0.0895 to 0.3889) time points for the low dose or high dose group, respectively for the time points that preceded melatonin dosing. Furthermore, no change in melatonin profile was observed in the placebo group: No significant differences were found for 27 out of 28 sampling times (paired t-test P-values range: 0.13 to 0.97).
Additional exploratory analyses were conducted to evaluate the effects of short-term melatonin therapy on sleep outcome measures and whether there was any correlation between melatonin PK and efficacy. Table 3 summarizes the descriptive statistics on five sleep outcome metrics among control, low dose, and high dose groups. Large variability was observed for all sleep measures. Paired t-test was used to compare pre- and post-treatment measures within each group. Positive improvement in Pittsburg Sleep Quality Index (PSQI) global scores with marginal statistical significance were observed for both low dose (−2.0 ± 3.2; P = 0.0978) and high dose (−3.3 ± 5.1; P = 0.0690) melatonin groups. However, none of the subjects were classified as “insomnia-free” at study conclusion based on their PSQI global score (threshold < 5). Objective (polysomnographic) sleep onset latency and sleep efficiency were not significantly changed following melatonin treatment. Discrepancies in the same sleep measures were noticed between polysomnography (objective) and PSQI questionnaire (subjective) results.
Table 3.
Comparisons of sleep outcome measures before and after melatonin treatment.
| Measure | Group | Mean (SD) | |||
|---|---|---|---|---|---|
| Pre- treatment |
Post- treatment |
Difference | P-value | ||
| Apnea/hypopnea index |
Control | 8.1 (3.9) | 10.7 (5.8) | 2.5 (9.1) | 0.456 |
| Low dose | 7.6 (5.1) | 9.6 (8.8) | 2.1 (8.5) | 0.491 | |
| High dose | 5.5 (2.6) | 6.5 (3.9) | 0.95 (4.3) | 0.503 | |
| Periodic limb movement index |
Control | 23.3 (49.3) | 19.2 (38.6) | −4.2 (13.9) | 0.427 |
| Low dose | 2.8 (7.9) | 2.2 (4.3) | −0.61 (9.8) | 0.856 | |
| High dose | 16.5 (29.3) | 14.3 (25.3) | −2.2 (14.2) | 0.632 | |
| Sleep onset latency (PSG) |
Control | 17.7 (16.1) | 31.9 (29.6) | 14.2 (22.9) | 0.124 |
| Low dose | 44.1 (36.4) | 38.2 (31.1) | −5.9 (50.0) | 0.733 | |
| High dose | 24.4 (22.4) | 43.2 (42.4) | 18.8 (26.5) | 0.051 | |
| Sleep efficiency (PSG) |
Control | 0.70 (0.09) | 0.74 (0.08) | 0.043 (0.12) | 0.349 |
| Low dose | 0.67 (0.15) | 0.71 (0.15) | 0.042 (0.19) | 0.524 | |
| High dose | 0.69 (0.09) | 0.69 (0.11) | 0.0043 (0.16) | 0.933 | |
| PSQI global score | Control | 10.6 (5.5) | 10.8 (4.1) | 0.13 (4.3) | 0.937 |
| Low dose | 11.6 (3.2) | 9.6 (3.3) | −2.0 (3.2) | 0.098 | |
| High dose | 11.2 (4.6) | 7.9 (2.6) | −3.3 (5.1) | 0.069 | |
PSG: polysomnography; PSQI: Pittsburg Sleep Quality Index
Linear regression analysis was used to investigate correlation between melatonin PK and sleep outcome measures. Total melatonin exposures (AUC0-∞) and peak melatonin serum concentrations (Cmax) were negatively correlated to PSQI global score, indicating a higher melatonin exposure or level was associated with a lower (improved) PSQI global score (Fig. 4)
Figure 4.

Correlation between PSQI global score and melatonin PK parameters in the treatment groups.
The effects of exogenous melatonin on renal and hepatic functions were examined for the six week therapy. No significant changes in serum creatinine or liver enzymes were observed before and after treatment in either the low dose or high dose group (Figure 5, P-values: 0.16 to 0.6).
Figure 5.
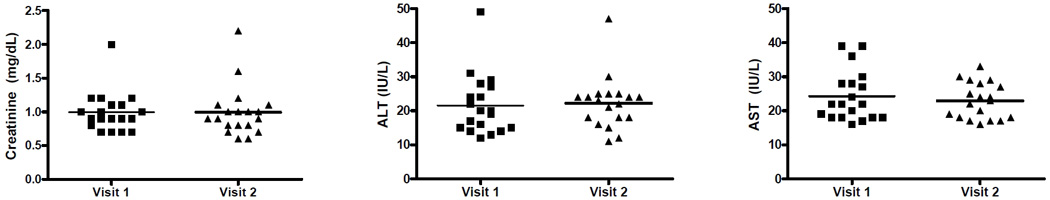
Effects of exogenous melatonin on renal and hepatic functions. (A) Serum creatinine, (B) ALT, and (C) AST levels were measured before (visit 1) and on day 42 (visit 2) of melatonin treatment.
Discussion
This study examined the pharmacokinetic profile of low (0.4 mg) and high (4.0 mg) dose surge-sustained release oral melatonin tablets in older adult patients. While several studies have examined melatonin PK in older adults [28, 29, 42–44], to our knowledge none have directly compared low (<0.5 mg) and high (>2 mg) dose formulations and no studies have described surge-sustained release melatonin PK in older adults [25]. We observed that the low dose melatonin arm was associated with a seven-fold increase in melatonin peak levels, while the high dose melatonin arm had a sixty-five fold increase. The mean apparent clearance (CL/F), apparent volume of distribution (V/F), time to reach maximal concentration (Tmax) and elimination half-life (t1/2) were similar between the two doses. These data suggest that there was no significant departure from linear kinetic behavior in older subjects receiving oral melatonin across the studied dose range. There were no significant hepatic/renal toxicity effects noted after six weeks of therapy in either arm. However, subjects in the high dose arm had sustained high levels of melatonin (>50 pg/ml) that could extend to post-awakening hours.
The melatonin half-life noted in our study is comparable to that observed in other PK studies. In a study by Markantonis et al. [43] using 6 mg oral immediate release melatonin, the half-life was similar between the younger and older patients: 45.6 minutes for pre-menopausal females and 51.6 minutes for post-menopausal females. In critically ill intensive care unit patients, Mistraeletti et al. [44] found the elimination half-life to be even longer at 1 hour and 34 min despite a Tmax that occurred only 16 min after administrating 3 mg of melatonin through a nasogastric tube. In regards to elimination half-life, DeMuro et al. [22] also found no statistical difference in half-life between 2 mg oral, 2 mg intravenous, and 4 mg oral melatonin, with half-life values ranging from 59 to 65 minutes.
In general, our Cmax values using a surge-sustained dosing were lower than those noted from studies that used immediate release tablets only. For example, 6.0 mg immediate release melatonin had a Cmax of 16,438 pg/ml in a study by Mistraletti et al. [44], approximately four times the value we noted with our 4.0 mg surge-sustained dose. Hughes and colleagues [29] also noted lower Cmax levels with the controlled release formulation (394.5 pg/ml) relative to the immediate release formulation (703.8–838.7 pg/ml) despite the identical 0.5 mg melatonin dose in both formulations. Most likely, the controlled release component resulted in a more gradual release of melatonin, thus avoiding a large surge effect that can occur with immediate release only tablets. Even in our low dose melatonin arm, however, the melatonin levels increased markedly and exceeded physiologic values for young/middle aged subjects, which can be approximately 100–150 pg/ml [28]. This is consistent with findings from other studies in older adults, where Zhdanova and colleagues, using doses of 0.3 mg immediate release melatonin, observed a mean Cmax of 254.9 pg/ml [28]. Of note, they also noticed that the Cmax had a tendency to be higher in the older adults relative to the younger adults (170.2 pg/ml) despite the same dose, suggesting potential differences in absorption kinetics between the two age groups [28]. [24, 43]
Time to reach maximal concentration (Tmax) in our study showed similar values at both dose levels. As expected, the Tmax (1.3–1.5 hours) for the surge-sustained formulation was longer than the Tmax of an immediate release formulation (approximately 50 minutes) [16, 28, 43]. In addition to Tmax, another important physiologic parameter is the duration of elevated melatonin levels. At higher doses or with controlled release formulations, there is an increased risk of prolonged periods of supraphysiologic melatonin levels [29, 45]. For example, in one study of critically ill older adults, although serum melatonin levels decreased considerably within 4 hours, supraphysiologic levels were maintained for up to the next 6 hours when using a 3.0 mg immediate release dose [44]. Some have suggested that low melatonin doses (0.1–0.3 mg) may more closely replicate normal physiological levels [45]. Other research using low dose melatonin (0.5 mg, with 0.4 mg as controlled release), however, has noted melatonin levels above 100 pg/ml for over 8 hours in younger subjects [16].
One concern with these prolonged high melatonin levels is the risk of daytime sleepiness. However, other studies using prolonged release melatonin at 2 mg doses have not observed any next-day carryover effect [14, 15]. Another concern with long-term use of supraphysiologic melatonin doses is the potential for increased risk of melatonin adverse effects[31][46]. Higher doses may also be counterproductive to promoting circadian phase changes because high melatonin levels can persist into the delay zone, thus reducing the otherwise greater phase advance from the higher dose [47].
Melatonin had no significant effects on renal or hepatic function after six weeks of therapy. Research examining melatonin profiles in patients with renal or liver impairment have found that melatonin levels of patients with renal disease under chronic hemodialysis are similar to healthy subjects [48]. In patients with liver damage, the onset and peak of melatonin were delayed for several hours at night and daytime levels can be significantly higher than normal [48].
Our study has several limitations that merit comment. We did not gather melatonin profiles using intravenous melatonin, thus bioavailability estimates are not possible. We could not draw conclusions regarding effects on sleep because our small sample size carried a risk of type 2 error. It is possible that a more rapid dissolution rate for the controlled release tablet may have resulted in a shorter period of elevated melatonin levels. However, this would have resulted in a shorter “plateau” period and been less likely to recreate a more physiologic profile. Melatonin was custom manufactured for this study; commercial melatonin immediate and controlled release products can have significant variability in their dissolution rates due to variable levels of purity, thus the findings from this study may not necessarily be directly applicable to those obtained from commercially available melatonin [49].
In conclusion, the findings from this study suggest that melatonin half-life, Tmax, apparent total clearance and apparent volume of distribution are similar for both low and high dose surge-sustained melatonin formulations in older adults, consistent with linear pharmacokinetic behavior. However, the use of high doses of melatonin resulted in exposures that are markedly higher than physiologic levels. In addition, surge-sustained release high dose tablets carried a greater risk of prolonged elevation in melatonin level that could persist into daytime periods. Future work will involve developing a melatonin sleep pharmacokinetic/pharmacodynamic (PK/PD) model which will allow rational dose projection via simulation to achieve clinically meaningful benefits.
Acknowledgements
Funding support for this project has been provided by the following: 1) National Center for Complementary and Alternative Medicine (NCCAM) R01-AT001521 (Gooneratne), 2) National Institute on Aging (NIA) K23-AG01021 (Gooneratne), and 3) the Clinical and Translation Science Award UL1-RR-024134.
Footnotes
Conflicts of Interest: There are no author conflicts of interest related to this manuscript
Author Contributions
Nalaka Gooneratne: concept/design, acquisition of data, data analysis/interpretation, drafting of the manuscript, critical revision of the manuscript.
Alena Z Edwards: data analysis/interpretation, drafting of the manuscript, critical revision of the manuscript.
Chen Zhou: data analysis/interpretation, drafting of the manuscript, critical revision of the manuscript.
Norma Cuellar: data interpretation, drafting of the manuscript, critical revision of the manuscript.
Michael Grandner: data interpretation, drafting of the manuscript, critical revision of the manuscript.
Jeffrey Barrett: data analysis/interpretation, drafting of the manuscript, critical revision of the manuscript.
References
- 1.Sanchez-Barcelo EJ, Mediavilla MD, Tan DX, et al. Clinical uses of melatonin: evaluation of human trials. Curr Med Chem. 2010;17:2070–2095. doi: 10.2174/092986710791233689. [DOI] [PubMed] [Google Scholar]
- 2.Slominski A, Tobin DJ, Zmijewski MA, et al. Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol Metab. 2008;19:17–24. doi: 10.1016/j.tem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Burgess HJ, Revell VL, Molina TA, et al. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocrinol Metab. 2010;95:1–7. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51:1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee D, Roy SG, Bandyopadhyay A, et al. Melatonin protects against isoproterenol-induced myocardial injury in the rat: antioxidative mechanisms. J Pineal Res. 2010;48:251–262. doi: 10.1111/j.1600-079X.2010.00749.x. [DOI] [PubMed] [Google Scholar]
- 6.Dauchy RT, Blask DE, Dauchy EM, et al. Antineoplastic effects of melatonin on a rare malignancy of mesenchymal origin: melatonin receptor-mediated inhibition of signal transduction, linoleic acid metabolism and growth in tissue-isolated human leiomyosarcoma xenografts. J Pineal Res. 2009;47:32–42. doi: 10.1111/j.1600-079X.2009.00686.x. [DOI] [PubMed] [Google Scholar]
- 7.Otmani S, Demazieres A, Staner C, et al. Effects of prolonged-release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteers. Hum Psychopharmacol. 2008;23:693–705. doi: 10.1002/hup.980. [DOI] [PubMed] [Google Scholar]
- 8.Wetterberg L, Bergiannaki JD, Paparrigopoulos T, et al. Normative melatonin excretion: a multinational study. Psychoneuroendocrinology. 1999;24:209–226. doi: 10.1016/s0306-4530(98)00076-6. [DOI] [PubMed] [Google Scholar]
- 9.Ohayon MM, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 10.Burgess HJ, Sletten T, Savic N, et al. Effects of bright light and melatonin on sleep propensity, temperature, and cardiac activity at night. J Appl Physiol. 2001;91:1214–1222. doi: 10.1152/jappl.2001.91.3.1214. [DOI] [PubMed] [Google Scholar]
- 11.Olbrich D, Dittmar M. Older Poor-Sleeping Women Display a Smaller Evening Increase in Melatonin Secretion and Lower Values of Melatonin and Core Body Temperature Than Good Sleepers. Chronobiol Int. 2011;28:681–689. doi: 10.3109/07420528.2011.599904. [DOI] [PubMed] [Google Scholar]
- 12.Singer C, Tractenberg RE, Kaye J, et al. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer's disease. Sleep. 2003;26:893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baskett JJ, Broad JB, Wood PC, et al. Does melatonin improve sleep in older people? A randomised crossover trial. Age Ageing. 2003;32:164–170. doi: 10.1093/ageing/32.2.164. [DOI] [PubMed] [Google Scholar]
- 14.Luthringer R, Muzet M, Zisapel N, et al. The effect of prolonged-release melatonin on sleep measures and psychomotor performance in elderly patients with insomnia. Int Clin Psychopharmacol. 2009;24:239–249. doi: 10.1097/YIC.0b013e32832e9b08. [DOI] [PubMed] [Google Scholar]
- 15.Wade AG, Ford I, Crawford G, et al. Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med. 2010;8:51. doi: 10.1186/1741-7015-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee BJ, Parrott KA, Ayres JW, et al. Design and evaluation of an oral controlled release delivery system for melatonin in human subjects. Int J Pharmaceutics. 1995;124:119–127. [Google Scholar]
- 17.Gehrman PR, Connor DJ, Martin JL, et al. Melatonin fails to improve sleep or agitation in double-blind randomized placebo-controlled trial of institutionalized patients with Alzheimer disease. Am J Geriatr Psychiatry. 2009;17:166–169. doi: 10.1097/JGP.0b013e318187de18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BJ, Ryu SG, Cui JH. Formulation and release characteristics of hydroxypropyl methylcellulose matrix tablet containing melatonin. Drug Dev Ind Pharm. 1999;25:493–501. doi: 10.1081/ddc-100102199. [DOI] [PubMed] [Google Scholar]
- 19.Paul MA, Miller JC, Gray GW, et al. Melatonin treatment for eastward and westward travel preparation. Psychopharmacology (Berl) 2010;208:377–386. doi: 10.1007/s00213-009-1737-7. [DOI] [PubMed] [Google Scholar]
- 20.Lane EA, Moss HB. Pharmacokinetics of melatonin in man: first pass hepatic metabolism. J Clin Endocrinol Metab. 1985;61:1214–1216. doi: 10.1210/jcem-61-6-1214. [DOI] [PubMed] [Google Scholar]
- 21.Di W, Kadva A, Johnston A, et al. Variable Bioavailability of Oral Melatonin (letter) N Engl J Med. 1997;336:1028–1029. doi: 10.1056/NEJM199704033361418. [DOI] [PubMed] [Google Scholar]
- 22.Demuro RL, Nafziger AN, Blask DE, et al. The absolute bioavailability of oral melatonin. J Clin Pharmacol. 2000;40:781–784. doi: 10.1177/00912700022009422. [DOI] [PubMed] [Google Scholar]
- 23.Waldhauser F, Waldhauser M, Lieberman H, et al. Bioavailability of Oral Melatonin in Humans. Neuroendocrinology. 1984;39:307. doi: 10.1159/000123997. [DOI] [PubMed] [Google Scholar]
- 24.Fourtillan JB, Brisson AM, Gobin P, et al. Bioavailability of melatonin in humans after day-time administration of D(7) melatonin. Biopharm Drug Dispos. 2000;21:15–22. doi: 10.1002/1099-081x(200001)21:1<15::aid-bdd215>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186–195. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 26.Kopin IJ, Pare CM, Axelrod J, et al. The fate of melatonin in animals. J Biol Chem. 1961;236:3072–3075. [PubMed] [Google Scholar]
- 27.National Academies--Committee On The Framework For Evaluating The Safety Of Dietary Supplements. Dietary Supplements: A Framework for Evaluation Safety. Washington, D.C: The National Academies Press; 2004. Prototype Monograph on Melatonin; pp. D1–D71. [Google Scholar]
- 28.Zhdanova IV, Wurtman RJ, Balcioglu A, et al. Endogenous melatonin levels and the fate of exogenous melatonin: age effects. J Gerontol A Biol Sci Med Sci. 1998;53:B293–B298. doi: 10.1093/gerona/53a.4.b293. [DOI] [PubMed] [Google Scholar]
- 29.Hughes RJ, Sack RL, Lewy AJ. The role of melatonin and circadian phase in age-related sleep-maintenance insomnia: assessment in a clinical trial of melatonin replacement. Sleep. 1998;21:52–68. [PubMed] [Google Scholar]
- 30.Maksoud A, Moore CA, Harshkowitz M. The effect of melatonin administration on patients with sleep apnea. Sleep Res. 1997;26:114. [Google Scholar]
- 31.Maestroni GJ, Cardinali DP, Esquifino AI, et al. Does melatonin play a disease-promoting role in rheumatoid arthritis? J Neuroimmunol. 2005;158:106–111. doi: 10.1016/j.jneuroim.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 32.Conti A, Haran-Ghera N, Maestroni GJ. Role of pineal melatonin and melatonin-induced-immuno-opioids in murine leukemogenesis. Med Oncol Tumor Pharmacother. 1992;9:87–92. doi: 10.1007/BF02989659. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland ER, Ellison MC, Kraft M, et al. Elevated serum melatonin is associated with the nocturnal worsening of asthma. J Allergy Clin Immunol. 2003;112:513–517. doi: 10.1016/s0091-6749(03)01717-2. [DOI] [PubMed] [Google Scholar]
- 34.Buysse DJ, Ancoli-Israel S, Edinger JD, et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 35.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 36.Rechtschaffen A, Kales A. Brain Information Services/Brain Research Institute. Los Angeles, California: University of California at Los Angeles; 1968. A Manual of Standardization Terminology: Techniques and Scoring System for Sleep Stages of Human Subjects. [Google Scholar]
- 37.Riedel BW, Winfield CF, Lichstein KL. First night effect and reverse first night effect in older adults with primary insomnia: does anxiety play a role? Sleep Med. 2001;2:125–133. doi: 10.1016/s1389-9457(00)00054-x. [DOI] [PubMed] [Google Scholar]
- 38.Benloucif S, Burgess HJ, Klerman EB, et al. Measuring melatonin in humans. J Clin Sleep Med. 2008;4:66–69. [PMC free article] [PubMed] [Google Scholar]
- 39.Ursing C, Von Bahr C, Brismar K, et al. Influence of cigarette smoking on melatonin levels in man. Eur J Clin Pharmacol. 2005;61:197–201. doi: 10.1007/s00228-005-0908-7. [DOI] [PubMed] [Google Scholar]
- 40.Wright KJ, Badia P, Myers B, et al. Caffeine and light effects on nighttime melatonin and temperature levels in sleep-deprived humans. Brain Res. 1997;747:78–84. doi: 10.1016/s0006-8993(96)01268-1. [DOI] [PubMed] [Google Scholar]
- 41.IBL International GMBH. Melatonin direct RIA (serum/plasma) RE29301. Germany: Hamburg; 2010. [Google Scholar]
- 42.Shah J, Langmuir V, Gupta SK. Feasibility and functionality of OROS melatonin in healthy subjects. J Clin Pharmacol. 1999;39:606–612. doi: 10.1177/00912709922008218. [DOI] [PubMed] [Google Scholar]
- 43.Markantonis SL, Tsakalozou E, Paraskeva A, et al. Melatonin pharmacokinetics in premenopausal and postmenopausal healthy female volunteers. J Clin Pharmacol. 2008;48:240–245. doi: 10.1177/0091270007311112. [DOI] [PubMed] [Google Scholar]
- 44.Mistraletti G, Sabbatini G, Taverna M, et al. Pharmacokinetics of orally administered melatonin in critically ill patients. J Pineal Res. 2010;48:142–147. doi: 10.1111/j.1600-079X.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhdanova IV. Melatonin as a hypnotic: Pro. Sleep Med Rev. 2005;9:51–65. doi: 10.1016/j.smrv.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Riemersma-Van Der Lek RF, Swaab DF, Twisk J, et al. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. Jama. 2008;299:2642–2655. doi: 10.1001/jama.299.22.2642. [DOI] [PubMed] [Google Scholar]
- 47.Lewy AJ, Emens JS, Lefler BJ, et al. Melatonin entrains free-running blind people according to a physiological dose-response curve. Chronobiol Int. 2005;22:1093–1106. doi: 10.1080/07420520500398064. [DOI] [PubMed] [Google Scholar]
- 48.European Medicines Agency. Assessment Report for Circadin: Procedure No. EMEA/H/C/695. London, England: European Medicine Agency; 2007. p. 52. [Google Scholar]
- 49.Hahm H, Kujawa J, Augsburger L. Comparison of melatonin products against USP's nutritional supplements standards and other criteria. J Am Pharm Assoc (Wash) 1999;39:27–31. doi: 10.1016/s1086-5802(16)30412-0. [DOI] [PubMed] [Google Scholar]


