Preface
The ‘Seed and Soil’ hypothesis for metastasis sets forth the concept that a nutritive microenvironment, or niche, is required for disseminating tumour cells to engraft distant sites. This Opinion presents emerging data that support this concept and outlines the potential mechanism and temporal sequence by which changes in tissues distant from the primary tumour occur. To enable improvements in the prognosis of advanced malignancy, early interventions that target both the disseminating seed and the metastatic soil are likely to be required.
Introduction
Steven Paget's “Seed and Soil” hypothesis for metastasis was a pivotal milestone in the study of malignant disease, introducing the concept that a receptive microenvironment is required for malignant cells to engraft distant tissues and form metastases1, 2. Prior to this, the prevailing theory of the time was that the pattern of metastatic tumor dissemination was purely determined by the lodgement of tumour cell emboli in the vasculature3. However, from his analysis of 735 cases of fatal breast cancer, Paget deduced that certain organs such as the liver appeared to be particularly susceptible to metastases, and that this was not explicable by blood flow alone. He concluded that the “soil” or local tissue microenvironment of these organs must be more conducive for disseminating tumour cells to “seed” than that of other organs, such as the spleen, promoting the development of metastases in these sites. Forty years later, Paget's theory was challenged by James Ewing, who again proposed that metastasis was determined by the anatomy of the vascular and lymphatic channels that drain the primary tumour4. His view then prevailed until seminal studies by Isaiah Josh Fidler conclusively demonstrated that while tumour cells reached the vasculature of all organs, the development of metastases occurred selectively in certain organs but not others5, 6.
Attention on the metastatic soil was revived, and a wealth of research ensued exploring the pathophysiology of the local tissue microenvironment, or ‘niche’, of cells of the primary tumor and that of tumour cells at metastatic sites. The ‘metastatic niche model’ presented here incorporates new data regarding the metastatic microenvironment and outlines the cellular and molecular components that are thought to collaborate to form the conducive soil of the metastatic microenvironment (Figure 1). Furthermore, the model proposes the temporal sequence of events involved and the emerging concept that changes in future metastatic tissues may occur earlier during carcinogenesis than was previously thought and play an instigating role in tumour metastasis. Despite substantial advances in the treatment of localized malignancies, metastatic disease remains the primary cause of morbidity and mortality in cancer. The implications of the metastatic niche model are that in order to improve the prognosis for patients with advanced malignancy, early therapeutic targeting of both the disseminating seed and the evolving metastatic soil are likely to be required. Moreover, therapies may need to be tailored to specific stages of the metastatic cascade.
Figure 1. A model of the evolution of a metastatic niche.
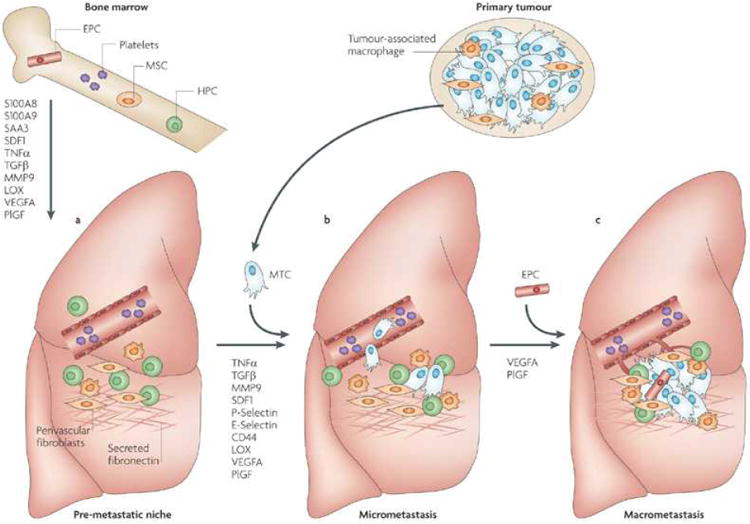
This figure depicts the premetastatic, micrometastatic to macrometastatic transition. (A) In response to growth factors secreted by the primary tumour including vascular endothelial growth factor (VEGF)17, placental growth factor (PlGF)17 and transforming growth factor-b (TGF- b)18, inflammatory S100 chemokines and serum amyloid A (SAA) 318, 19 are upregulated in premetastatic sites leading to clustering of bone marrow-derived haematopoietic progenitor cells (HPCs)17. Platelet-deployed stromal-derived growth factor (SDF)-1 is also chemotactic for CXCR4+ HPCs and metastatic tumour cells (MTCs)39. HPCs secrete a variety of premetastatic factors including TNF-a, matrix metalloproteinase (MMP)-9 and TGF-β17, 28, 29 Activated fibroblasts secrete fibronectin, an important adhesion protein in the niche, and lysoyl oxidase (LOX) expression is increased, modifying the local extracellular matrix45. (B) MTCs engraft the niche to populate micrometastases. The site specific expression of adhesion integrins on activated endothelial cells such as P-selectin and E-selectin may enhance MTC adhesion and extravasation at these sites89, and cell-cell interactions such as CD44 ligation in the metastatic niche may promote MTC survival and enable proliferation. (C) Recruitment of endothelial progenitor cells (EPCs) to the early metastatic niche mediates the angiogenic switch and enables progression to macrometastases17, 59.
The metastatic niche model
In ecological systems, the ‘niche’ describes the interactive position of a species or population within a specific ecosystem. In the niche, the organism responds to the distribution of available resources and pressures of competitors, and in turn modulates the biological and physical components of its microenvironment by limiting access to other species and other actions. The place, status or activity for which a person is most suited is also commonly referred to as a ‘niche’. Similarly, in stem cell biology the niche describes the specialized microenvironment that supports stem cell maintenance and actively regulates cell function and proliferation7-9. A similar model has been suggested to delineate the interactions of malignant cells with their microenvironment at the primary tumour and at metastatic sites10-12.
The ‘soil’ of the primary tumour has been better characterized than that of metastatic sites. This microenvironment comprises supportive (non malignant) stromal cells, soluble factors, vascular networks, nutrients and metabolic components and the structural extracellular matrix architecture13-15. While the precise genetic make-up of a cell is undoubtedly pivotal in determining its malignant phenotype (cell autonomous activities), the metastatic niche model stipulates that microenvironmental (non-cell-autonomous) factors are also important in permitting malignant cells to realize their metastatic potential. Moreover, adaption of the microenvironment is an important prerequisite for the initiation and progression of metastasis.
The metastatic niche model (Figure 1) suggests that a suitably conducive microenvironment (premetastatic niche) must evolve in order for tumor cells to be able to engraft (metastatic niche) and proliferate at secondary sites (micro- to macro- metastatic transition). This hypothesis builds on Paget's seed and soil hypothesis by suggesting a temporal evolution for the development of the soil, and incorporates novel data suggesting the key cellular and molecular components of the metastatic microenvironment. The evidence for this model is primarily drawn from mouse models and largely focused on the lung as a target organ, although other organs and pathological samples from patients have also been examined suggesting that this model may be widely applicable to solid tumour metastasis in general.
Much of the data supporting this model is novel, and this hypothesis remains controversial. An alternative school of thought would argue that the intrinsic properties of the metastatic seed are more crucial determinants of metastasis than any contribution of the host microenvironment. Both these theories are compatible with the generally accepted step-wise progression of metastasis, in which a tumour cell must first detach from the primary tumour, invade and intravasate into the vasculature, arrest in local capillaries, extravasate and invade the local tissue of the secondary site where it must survive and proliferate. The significant distinction between more traditional concepts of metastasis as compared with the seed and soil or metastatic niche models is whether the tumour cell dictates its own fate, or alternatively whether formation of a hospitable microenvironment is not just permissive but essential to enable a disseminating tumour cell to spawn a secondary tumour growth. Whether changes to the tissue parenchyma at target sites of metastasis begin prior to the arrival of the first tumor cells as a result of systemic effects of factors secreted by the primary tumour, or whether tumour cells condition their own metastatic microenvironments thereby creating metastatic niches in a paracrine fashion is also controversial.
The premetastatic niche
Mechanical forces of the vascular channels govern the initial delivery of cells from the primary tumour to distant tissues. The anatomical route of vascular drainage from the primary tumour, vessel lumen diameter, blood flow and pressure, and the physical characteristics of the tumour cells all influence where the tumour cells are likely to arrest as they transit through the vasculature. Following adhesion and extravasation, efficient survival and proliferation of tumour cells is required for successful metastatic growth, and these processes require a receptive microenvironment at the destination site16. In recent years, evidence has emerged that growth factors secreted by the primary tumour prime certain tissues for tumour cell engraftment17-19. In response to these soluble factors, tumour-associated cells such as haematopoietic progenitor cells and macrophages cluster at ‘premetastatic niches’, creating an environment that is conducive for tumour cell adhesion and invasion (Figure 1)17, 18. Indeed, in premetastatic organs, similar pathways may constitute ‘homing signals’ for both tumour cells and tumour-associated cells such as haematopoietic cells18. Specific sites within organs that are ‘primed’ in this fashion may be considered ‘premetastatic niches’, evolving into ‘metastatic niches’ following tumour cell engraftment. It appears that these niches preferentially develop at certain locations within an organ, such as around the terminal bronchioles and bronchiole veins in the lung17, although this has not been definitively shown. In addition, differences between tumours in their pattern of metastatic dissemination appear to be a result of specific soluble factors secreted by the primary tumour, in that administration of media conditioned by tumour cells is able to specifically direct the target organs for premetastatic niche initiation17.
Initiating the premetastatic niche
Haematopoietic cells derived from the bone marrow (Box 1) that express the VEGF receptor 1 (VEGFR-1) have been described localizing at premetastatic sites prior to the arrival of tumour cells, and are key components of the premetastatic niche17. These cells were identified as of myeloid lineage, and appeared not to differentiate but maintained their expression of immature surface markers including c-Kit and Sca-1 within the tissue parenchyma. The VEGFR-1+ cells also expressed the fibronectin receptor VLA-4, and fibronectin expression was also noted to be increased17. The hypothesis that these localized accumulations of myeloid cells and stromal fibronectin were attractive docking sites for disseminating tumour cells set forth the concept that the induction of premetastatic niches within specific organs was a vital and permissive step for metastasis.
Box 1. Subtypes of bone marrow-derived cells and their postulated roles in tumourigenesis and metastasis.
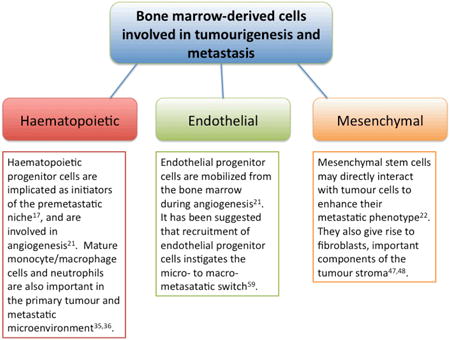
Previously, mobilization of VEGFR-1+ myeloid cells from the bone marrow and their recruitment to premetastatic sites was thought to result mainly from the angiogenic cytokines VEGF and placental growth factor (PlGF, a VEGF family member that binds specifically to VEGFR-1) secreted by the primary tumour17. More recently, it was shown that inflammatory chemokines also recruit haematopoietic cells and tumour cells to premetastatic sites18. In examining the premetastatic lung in a mice with syngeneic Lewis Lung or B16 melanoma tumours implanted intradermally in the flank, Hiratsuka et al reported that VEGF-A, TGF-β and TNF-α released by the primary tumour induced the expression of S100A8 and S100A9 inflammatory proteins specifically within the parenchyma of the lung, the target site of metastasis but not in other organs, such as liver or kidney. This triggered infiltration by Mac-1 (CD11b/CD18) + myeloid cells18. S100A8-stimulated lung was strongly chemoattractive for tumour cells in addition to Mac-1+ myeloid cells, and activation of the p38 signaling pathway was required for the recruitment of both cell types. Remarkably, treatment with S100A8 and S100A9 antibodies inhibited the infiltration of Mac-1+-myeloid cells and resulted in 80-90% reduction in tumour cell colonization of the lung, indicating that tumour cells and tumour-associated myeloid cells may respond to ‘guidance signals’ via similar molecular mechanisms.
In a recent extension of this work, serum amyloid A (SAA) 3 was shown to mediate S100A8- and A100A9-induced chemoattraction, acting via Toll-like receptor 4 on macrophages and tumour cells19, 20. Moreover, the induction of the S100 chemokines and SAA3 occurred primarily in the lung, with minimal expression in liver or kidneys10. These data suggest that the selective upregulation of migration-stimulating factors in certain organs may contribute to the site-specificity of metastasis that is characteristic of many tumour types
Simultaneously, cell–niche interactions occurring within the bone marrow enable mobilization of bone marrow-derived cells to the circulation in response to tumour-derived factors21, 22 (Box 1). The cellular kinetics of bone marrow cells are regulated by a variety of stromal cells, including osteoblasts, osteoclasts and vascular endothelial cells23-25. Whereas osteoblast-derived signals normally inhibit stem cell proliferation, it is thought that osteoclast and vascular signals promote proliferation and mobilization26. It is possible that in the setting of metastatic progression, the balance alters in favour of endothelial cell/osteoclast-driven stem cell mobilization from the bone marrow over osteoblast-mediated cell quiescence, although this has yet to be directly studied. The cell-microenvironment interactions occurring in the bone marrow are analogous to those between tumour cells and their stromal microenvironment at the primary tumour site and within premetastatic/metastatic niches. Indeed, it is possible that the bone marrow niches may be already well adapted to serve as metastatic niches, which may explain the high survival rate of tumour cells within the bone marrow as compared to other organs in patients with malignancy27.
Premetastatic niches: primed for tumour engraftment
At the premetastatic niche, newly recruited myeloid cells collaborate with other cell types including stromal cells and endothelial cells residing in the tissue parenchyma. Together, these cells provide a platform of chemokines, growth factors, matrix degrading factors and adhesion molecules accelerating assembly of the metastatic lesion18.
For example, TNF-α is a pro-metastatic cytokine produced by host myeloid cells. TNF-α mediates a variety of processes, including enhanced tumour cell proliferation, increased vascular permeability, and recruitment of other host cells, thereby contributing to the development of a supportive microenvironment for metastatic growth28. Very recent studies indicated that tumour-secreted factors directly induced myeloid cells to secrete tumour-promoting cytokines including TNF-α28. Of particular interest in this report was the exploration of the molecular pathway underlying the interaction between tumours and macrophages. The tumour-secreted matrix protein versican was found to interact with TLR2 on host bone marrow-derived macrophages, leading to their activation and secretion of pro-metastatic inflammatory cytokines such as TNF-α28. Metastasis was severely abrogated in the absence of either TLR2 or TNF-α in this study, with very few metastatic clusters observed in the lungs of TLR2-deficicent mice inoculated with syngeneic Lewis Lung carcinoma cells in a tail vein metastasis model28.
Local tissue remodeling is essential to enable tumour cell invasion and metastatic outgrowth and the expression of matrix metalloproteinases (MMPs) is also upregulated at the premetastatic niche17, 29. MMPs are instrumental in degrading extracellular matrix components during inflammatory responses and tissue repair as well as in primary tumour growth30, 31. MMP9 expression is specifically increased in endothelial cells and Mac-1+ and VEGFR1+ myeloid cells in the premetastatic lung, in a VEGF-dependent fashion17, 29. MMP9 expression at premetastatic sites can serve both to facilitate tumour cell invasion and also to release growth factors and chemokines including soluble kit ligand that further recruit bone marrow-derived progenitor cells and tumour cells that express the c-Kit receptor17.
It is hypothesized that a major function of tumour-associated myeloid cells at the primary tumour site is to orchestrate other cells of the immune response to promote an immunosuppressive, anti-inflammatory phenotype and allow the tumour to escape immune detection32. For example, TGF-β production by Gr1+/CD11b+ myeloid cells directly interferes with CD8+ cytotoxic T lymphocyte function and these cells also inhibit natural killer cells, B cells and the functional maturation of dendritic cells32. It is possible that myeloid cells recruited to premetastatic sites have a similar function: to create immune sanctuary sites where malignant cells are able to survive and proliferate without detection. Expression of osteopontin by myeloid cells, a protein implicated in tumour cell adhesion and survival and in regulating MMP activity, also inhibits the host immune defence33, 34.
Many details remain unknown regarding the interactions between tumour cells, bone marrow-derived myeloid cells and resident stromal populations at metastatic sites. The molecular and functional phenotype of the myeloid cells that are recruited to premetastatic sites has yet to be fully characterized; the variation between laboratories in surface markers used to identify the cells compounds this challenge. In studies of the primary tumour, other groups have distinguished between Gr1+/CD11b+ immature myeloid cells and terminally differentiated, Mac-1+, F4/80+ tumour-associated macrophages35, 36. Both Mac-1 and VEGFR1 are expressed on a wide variety of myeloid cells including progenitor cells and it is likely that both fully differentiated cells and immature cells are involved at the premetastatic/metastatic niche.
In addition to myeloid cells, other cell types also play a role in establishing the premetastatic niche. Stromal-derived factor 1 (SDF-1) is an important chemokine for directing the site-specific localization of bone marrow-derived cells via the CXC-chemokine receptor CXCR4 in haematopoiesis and angiogenesis37, 38. VEGFR1+ haematopoietic progenitor cells also express CXCR4, and the homing of VEGFR1+ CXCR4+ cells to sites of neovascularization in ischaemic tissues and growing tumours was shown to be dependent on SDF-1 released from platelet granules39. Although the role of platelets in the premetastatic niche has yet to be examined, it is possible that they play a similar role in this context, delivering chemokines and angiogenic regulatory factors in a site-specific fashion40, 41. Several tumours also express CXCR4 and may therefore be influenced by platelet-derived SDF-1 gradients. The platelet surface adhesion receptor glycoprotein Ib-IX also appears to be important in mediating colonization of the lung by metastatic melanoma cells in mouse models42. Similarly, other host cells resident at the premetastatic niche such as fibroblasts and endothelial cells may express chemokines and adhesive proteins that attract circulating tumour cells to bind to these specific sites43, 44.
The transformation of local fibroblasts is pathologically important in enhancing carcinomatous progression by providing growth factor support and modulating the extracellular matrix, and there is evidence that fibroblasts are important in forming premetastatic niches17, 45, 46. Cancer-associated fibroblasts (CAFs) are perpetually activated, proliferating faster and depositing higher amounts of extracellular matrix factors than resting fibroblasts in benign tissue47. CAFs play important roles both in the initiation of tumourigenesis and in malignant progression, facilitating proliferation, invasion and motility of malignant cells and constituting a source of MMPs for matrix degradation48. Activated fibroblasts have been shown to induce stromal remodeling required for the development of liver metastasis in a murine melanoma model49. A proliferation of stellate cells, the fibroblasts that surround the liver sinusoids, was observed in association with early melanoma micrometastases. These cells were highly activated, secreting MMPs and chemotactic factors that fostered a conducive early metastatic microenvironment49. Subsequently, hypoxic induction of angiogenic growth factors (primarily VEGF) in stellate cells recruited endothelial progenitors to the metastatic niche, facilitating the transition from micrometastases to angiogenic macrometastases50.
A subpopulation of CD45+ CD13+ mesenchymal cells referred to as fibrocytes has also been shown to contribute to the stromal changes in the premetastatic lung by upregulating MMP9 synthesis, which was functionally correlated with the tumour engraftment46. However, whether these cells were locally recruited or bone marrow derived was not determined in this study. Intriguingly, the setting of non-malignant kidney fibrosis, it has been reported that activated fibroblasts not only arise via epithelial-to-mesenchymal transition and recruitment from the bone marrow, but also may emerge via endothelial-to-mesenchymal transition51.
The extracellular matrix at the premetastatic niche
Alterations in tissue architecture is a hallmark of malignant disease52. As described above, myeloid cells and activated fibroblasts secrete factors such as MMPs that modulate the extracellular matrix. In addition, non-cellular factors such as local oxygen status may also play a role. Tissue hypoxia has been associated with several aspects of malignant progression including metastasis53. The expression of lysyl oxidase (LOX), an enzyme that cross-links collagens and elastins in the extracellular matrix, is upregulated in and secreted by hypoxic human tumour cells54. LOX secretion has been shown to substantially enhance the invasive migration of a human breast cancer cell line both in vitro and in vivo in murine studies55. Very recently, it was suggested that secreted LOX may be important for the formation of premetastatic niches in target organs45. LOX secreted by hypoxic breast cancer cells accumulated at premetastatic sites where it modified the extracellular matrix by cross-linking collagen fibrils to make it more receptive for myeloid cell infiltration45. Moreover, inhibition of LOX synthesis in human breast cancer cells reduced accumulation of CD11b+ myeloid cells in the premetastatic organs of mice with orthotopic flank tumours and prevented metastasis45.
Fibronectin, an extracellular matrix glycoprotein involved in numerous cellular processes including embryonic cell migration and vascular development56, also appears to be an important component of the premetastatic niche. Focal expression of fibronectin has been observed around the terminal bronchioles and bronchiolar veins in the lung, common sites for metastatic niches17, 45. Whether this fibronectin is derived from host stromal cells or from tumour cells is not yet clear. While expression of fibronectin at premetastatic niches in the murine lung appeared to occur prior to the arrival of the first metastatic tumour cells17, studies of human tumour cell lines in immunodeficient mice using antibodies specific to human fibronectin indicated that at least some of the fibronectin is tumour cell-derived45. Both LOX expression and the myeloid cell clusters colocalized with fibronectin, suggesting that fibronectin may be a key in initiating assembly of other constituents of the premetastatic niche. Whether the tumour cell-derived proteins LOX and fibronectin are deposited locally by disseminating cells transiting through the premetastatic/early metastatic lung or whether they are carried systemically from the primary tumour was not clarified.
The mechanical properties of the extracellular matrix such as tissue elasticity and matrix stiffness have been shown to have a direct impact on tumourigenesis, especially in the mammary gland57. Whether these properties also play a role at metastatic sites, and at what stage in its evolution they come into play (premetastatic, micrometastatic, or macrometastatic) has not yet been addressed. Similarly, little is known about the metabolic qualities of the metastatic niche. If these niches have a specific oxygen status, then the local availability of other metabolic nutrients such as glucose and calcium may also be compartmentalized and may affect the status of stromal cells and tumour cells at the niche58.
Blood vessel integrity and lymphangiogenesis
The active vascularization of metastatic lesions is considered a hallmark of the micrometastatic to macrometastatic switch, as discussed below17, 41, 59. However, it is also possible that changes to the existing local microvasculature occur much earlier during the formation of the premetastatic niche, encouraging the clustering of tumour-associated myeloid cells, activated platelets and the first tumour cells. At the primary tumour, disruption of vascular integrity at the primary tumour site enables trafficking of extracellular proteins and inflammatory cells21 and is crucial for tumour cell invasion at metastatic sites60,61, 62. Many tumour-derived soluble factors have angiomodulatory effects, most notably VEGF. The endothelium of organs is heterogeneous63, and it is possible that vascular leakiness may not be a generalized phenomenon but could occur at specific sites – both organ-specific and site-specific within organs, perhaps influencing the formation of metastatic niches in these sites. Tissue-specific angiogenic factors have been identified, such as endocrine gland-derived VEGF (EG-VEGF)64, 65. EG-VEGF is only biologically active in specific cellular and tissue contexts: it is a potent mitogen, pro-survival and migration factor only for endothelium of the adrenal cortex and gonadal tissue but not aortic, umbilical or dermal microvasculature65. That tumours might secrete tissue-specific angiogenic molecules is appealing with respect to the formation of site-specific premetastatic niches, however none have yet been identified in the context of metastasis. Alternatively, tumour cells may produce tissue-specific inhibitors of angiogenesis and metastasis that prevent metastatic niche formation in certain sites.
Platelets act as delivery vehicles for a myriad of angiogenic regulatory molecules that alter local vasculature including pro- and anti-angiogenic growth factors such as VEGF and endostatin. Platelet-derived cytokines such as SDF-1 are chemoattractive, and the activated platelet surface provides a platform of adhesive ligands such as P-selectin to which circulating endothelial progenitor cells adhere in sites of angiogenesis41. Recent studies showed that the activation of specific proteinase-activated receptors (PARs) on the platelet surface may mediate selective deployment of pro-angiogenic vs. anti-angiogenic growth factors40, 66. Therefore, in addition to the existence of tissue-specific angiogenic factors, it is also possible that vascular permeability may be selectively modulated in certain organs by the site-specific, agonist-dependent deployment of growth factors by circulating platelets depending on the presence of certain agonists.
Differential expression of adhesion molecules by endothelial cells at certain sites may also influence the formation of premetastatic and/or metastatic niches67. For example, expression of P-selectin and E-selectin by endothelial cells is induced by inflammatory cytokines such as IL-1 and TNF-α promoting attachment of leukocytes to specific areas of endothelium. P-selectin and E-selectin have also been shown to mediate the attachment of cancer cells to activated endothelial cells. In one report, overexpression of E-selectin in multiple organs altered the organ distribution of metastasis in a transgenic mouse model68. However, the metastatic patterning did not correlate with the level of E-selectin expression in each organ, suggesting that other factors such as the haemodynamics of the blood supply in terms of the flow dynamics and sheer stress also influence tumour cell attachment.
While the number of studies focused on tumour angiogenesis has seemingly exploded over the last few decades, the importance of establishing lymph vessel supply in the context of solid organ metastasis remains relatively unexplored. In the majority of cancer types, malignant spread to local lymph nodes occurs prior to solid organ colonization. Overexpression of the VEGF family member VEGF-C, one of the most potent lymphangiogenic growth factors, has been correlated not only with accelerated lymph node metastasis but also with lung metastasis, despite having no effect on the rate of primary tumour growth in a murine model of chemically-induced squamous skin cancer69. Moreover, the onset of lymphangiogenesis within sentinel lymph nodes was demonstrated prior to tumour cell infiltration69, 70. These data suggest that the induction of lymphatic vascularization may be an important preparatory step for tumour metastasis 71. Whether lymphangiogenesis is important in the earliest stages of premetastatic niche formation in solid organs is not yet known.
The metastatic niche
In the metastatic niche model described here, significant changes occur in the local parenchyma at destination sites of future metastases that encourage subsequent homing and engraftment of circulating tumour cells (Figure 1). Tumour cells then extravasate into local tissues and lodge at the premetastatic niche, where they may seed micrometastases and eventually form metastatic outgrowths.
Metastasis is an early event
The dissemination of malignant cells from the primary tumour to secondary sites was traditionally considered to be a late-stage event. However, several lines of evidence indicate that the evolution of metastasis may begin earlier during tumourigenesis than was previously thought. Advanced immunocytochemical and molecular techniques able to detect even single tumour cells have demonstrated that tumour cells are frequently present circulating in the blood and bone marrow of cancer patients prior to clinical or histopathological metastasis27. Indeed, elegant studies using transgenic mice that conditionally express oncogenes in mammary epithelial cells demonstrated that even untransformed mammary cells may lodge at secondary sites, where they can assume malignant growth following oncogene activation even in the absence of detectable metastatic progression at the primary tumour site72. This suggests a novel hypothesis in which premalignant cells may disseminate during the very early stages of tumourigenesis, and that malignant transformation of these cells may occur in ectopic microenvironments such as the premetastatic lung. It is possible that these premalignant cells may in fact prime their own microenvironments, i.e. form the metastatic niche in situ73, collaborating with local stromal cells to recruit myeloid cells and initiate the formation of a metastatic niche. Nonetheless, if this is the case, certain signals directed by the primary tumour must lead them to home to specific sites over others.
Tumour cell engraftment
Tumor cells appear to preferentially localize to the clusters of myeloid cells, fibronectin, growth factors and matrix remodeling proteins that constitute the premetastatic niche17, 45. However, the molecular components that mediate the initial engraftment of tumour cells at these sites have yet to be fully characterized. Of the millions of cancer cells that enter the circulation, very few will successfully engraft, survive and proliferate at secondary sites74, 75. It is thought that during haematogenous dissemination, the initial localization and extravasation of cells at secondary sites occurs very efficiently, while the initiation and persistence of growth is highly inefficient16. This phenomenon may be determined by both the receptiveness of the local microenvironment where the tumour cells have sown76, and also by cell-intrinsic factors that may provide a survival advantage in specific environments. The work by Massague and colleagues identifying distinct genetic ‘signatures’ of tumour cell subpopulations that correlate with a propensity for metastasis to specific organs has been pivotal in understanding the dynamics of tumour dissemination66, 77 and these studies are likely to play a major role in diagnostics and individualization of clinical management in the near future. The majority of these genes encode proteins that influence the interaction of tumour cells with the microenvironment, emphasizing the importance of favourable interactions with the ‘soil’ of target sites for successful metastasis to occur66, 78. In addition, expression of the transcriptional inhibitor of differentiation (Id) genes Id1 and Id3, previously shown to be expressed in bone marrow progenitor cells mobilized for angiogenesis79, also appears to be pivotal for metastatic colonization of the lung by human breast tumour cells, by facilitating sustained cellular proliferation during the early stages of colonization80.
Other groups have investigated “metastasis suppressor genes”, which when re-expressed in malignant cells prevent metastasis without affecting their growth at the primary tumour site81. These genes may alter the cells' ability to respond to survival signals received from the local microenvironment and thereby determine whether a certain microenvironment is permissive or inhibitory for the establishment of metastases. For example, expression of breast cancer metastasis suppressor-1 (BRMS1) in human breast cancer cell lines was shown to selectively attenuate responses to the mitogenic factors: epidermal growth factor and platelet-derived growth factor, preventing colonization of distant tissues despite having no effect on primary tumour growth or haematogenous seeding of secondary sites in a mouse model82.
In order to found secondary tumour growth in a foreign organ, a malignant cell requires the capacity to migrate and self-renew, properties similar to those exhibited by physiological stem cells and proposed properties of cancer stem cells11, 43, 83, 84. The implication of this is that cancer stem cells may be more likely to successfully engraft in premetastatic niches. Indeed, recent evidence indicates that the process of epithelial-to-mesenchymal transition (EMT) during early cancer invasion induces stem cell-like properties in breast cancer cells85. Inducing EMT in non-tumourigenic mammary epithelial cells led to the expression of proposed cancer stem cell antigenic markers CD44high/CD24low and acquisition of self-renewal and differentiation capacities85. Recently, it has also been suggested that fusion of tumour cells with macrophages may confer a migratory phenotype86, 87. This intriguing hypothesis suggests that hybrids formed between tumour cells and primary tumour-associated macrophages may follow the same homing signals as the bone marrow-derived myeloid precursors to engraft premetastatic niches.
Metastatic tumour outgrowth
Following extravasation and invasion at the secondary site, tumour cell survival and proliferation may be influenced by cell-cell and cell-matrix interactions the metastatic niche. For a disseminated tumour cell to successfully spawn a metastatic lesion, it must evade the numerous cell death signals induced by loss of attachment to neighbouring cells (anoikis) and the extracellular matrix (amorphosis), survive in the circulation and then productively communicate with the stroma of the foreign site88. The hyaluronic acid receptor CD44 has been shown to be especially important in enabling tumour cells to evade apoptosis during micrometastasis formation89. In mice injected via the tail vein with syngeneic mammary carcinoma cells, while inhibition of the interaction between CD44-bearing tumour cells and the lung matrix did not interfere with initial adherence to pulmonary endothelium or penetration of the interstitial stroma, the vast majority of carcinoma cells underwent apoptosis and were unable to form micrometastases89. In addition to hyaluronic acid, other ligands for CD44 include fibronectin, collagen I, osteopontin and laminin. Therefore, it is likely that specific interactions between tumour cells and molecular components of the metastatic niche such as fibronectin may be important in the evasion of cell death within the foreign soil. The metastatic niche would also constitute a rich source of growth factors and cytokines, many of which (including VEGF) may directly regulate tumour cell proliferation in addition to survival.
The small proliferations of tumour cells at metastatic niches constitute micrometastases. Subsequently, the assembly of a functional vasculature is required to enable further cellular expansion and progression to macrometastases, a process for which activation of the angiogenic switch is required90, 91. Recent studies exploring the cellular and molecular pathways that mediate the micro- to macro- metastatic switch identified bone marrow-derived endothelial progenitor cells (EPCs) as critical regulators of this process59. The Id-1 transcription factor, previously shown to be involved in primary tumour angiogenesis21, 79, appears to be critical for mobilization of EPCs and their recruitment to micrometastases. While shRNA inhibition of Id1 did not affect initial colonization of the lung with tumour cells, angiogenesis and progression to macrometastases were prevented in the absence of EPC recruitment59. The functional contribution of the bone marrow-derived EPCs was particularly remarkable considering that they represented less than 15% of the total endothelial cells in the metastatic vasculature59. In addition to EPCs, haematopoietic and mesenchymal cells aid in macrometastatic progression. Tumour-associated macrophages potentiate the angiogenic stimulus by expression of VEGF and angiopoietins, accelerate recruitment of other inflammatory cells, and secrete proteases furthering matrix remodelling35.
The signals that initiate EPC recruitment and the angiogenic switch in the setting of dormant micrometastases and the molecular pathways underlying macrometastatic progression subsequent to EPC recruitment remain unclear. Further study is required to evaluate the role of the metastatic niche in tumour dormancy. Whether tumour cell dormancy results from ‘dormant niches’, or whether tumour cells may regulate the activation state of the niches that they inhabit is not known. In these scenarios, systemic factors such as tissue injury or ischaemia may be required to provide an angiogenic stimulus that ‘reactivates’ the niche.
Implications for the clinic
The metastatic niche model carries several implications for the clinical management of advanced malignancy. First, immunohistological features of the premetastatic niche such as myeloid cell clusters, activated fibroblasts, or stromal fibronectin may be used to identify a propensity to metastatic disease earlier than current prognostic techniques. In addition, examination of destination sites for metastasis may be used to distinguish those patients who present with seemingly localized disease but have evidence of premetastatic niche formation and may therefore benefit from anti-metastatic therapies such as specific inhibitors of VEGFR1+ myeloid cells, LOX or fibronectin.
Second, this model suggests that it may be beneficial for systemic therapies targeted to the metastatic microenvironment to be employed early, perhaps even as an adjunct to initial primary tumour treatment. If available, early interventions aimed at interfering with the formation of the premetastatic niche92 may be particularly important in the treatment of malignancies that have a tendency to exhibit metastatic dormancy such as breast carcinoma. Finally, there is the implication that treatments may need to be tailored to each stage of metastatic progression; premetastatic, micrometastatic and macrometastatic. Suggested targets for future therapies are suggested in Figure 2.
Figure 2. Stage-specific targeting of the metastatic microenvironment.
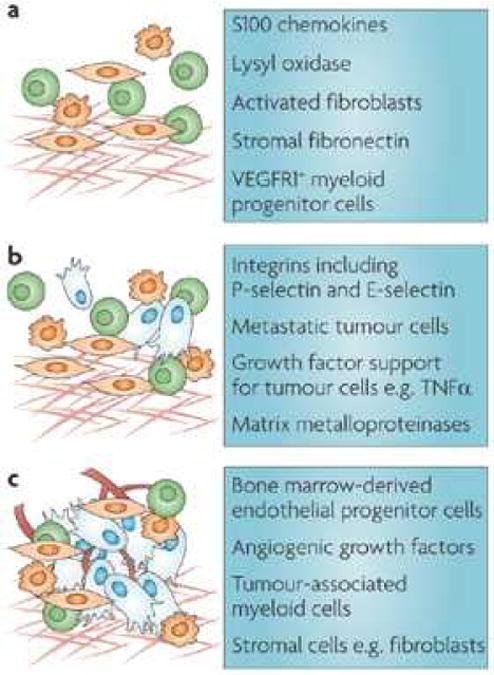
Cellular and molecular targets relevant to each stage of metastatic development are suggested as ammunition for future anti-metastatic therapies.
Limitations and unanswered questions
There are considerable limitations to the studies described above, and many questions remain unanswered. Examining truly premetastatic tissues in animal models is limited by the sensitivity and accuracy of tumour cell detection techniques. An even greater challenge lies is corroborating these data and confirming validity in the human setting, for which obtaining premetastatic and micrometastatic human tissue samples is required.
The vast majority of studies of metastasis have focused on the lung as a metastatic organ with little data available on other target sites such as bone marrow, liver and brain. Furthermore, a wide variety of in vivo experimental models of metastasis are employed in the studies described (Box 2), and each of these approaches carries specific limitations that need to be considered when interpreting the data. And, due to its highly complex cellular and molecular architecture, recapitulating the metastatic niche for in vitro studies is very difficult.
Box 2. In vivo models of metastasis.
| Model | Benefits and disadvantages |
|---|---|
| Spontaneous animal tumour models | Considered the ‘gold standard’ approach to model all stages in tumourigenesis and metastasis but limited availability of tumour subtypes |
| Orthotopic flank inoculations of syngeneic tumour cell lines | Enables study of spontaneous metastasis from a primary tumour in immunocompetent animals |
| Intravascular tumour inoculation | Bypasses primary tumour growth, invasion and intravasation but may be employed to specifically study migratory and invasive patterns of circulating tumour cells |
| Human cancer cell lines in immunodeficient mice | Directly examines human tumours but requires use of immunocompromized animals which may affect host response to tumour |
Several outstanding issues require further clarification. For example, what are the implications of the metastatic niche model for metastatic tumour dormancy? What determines the specific localization of these niches within an organ? Are they newly-initiated, or do pre-existing ‘inducible niches’ exist at certain sites; if so, are these related to physiological stem cell niches (Box 3) and do differences in genetic make-up of the host influence the number, capacity, location, or efficiency of these niches?
Box 3. Relevance to other physiological and pathological systems.
Interesting comparisons can be drawn between the cellular, molecular and functional phenotype of the metastatic niche and physiological or pathological niches that occur in non-malignant conditions. For example in reproductive physiology, the uterine wall is “primed” to accept the incoming, fertilized ovum, which for successful implantation must navigate the fallopian tubes and uterus in a migratory and invasive “tumour-like” fashion. Implantation (invasion) of the developing blastocyst in the uterine wall requires extensive communication between the blastocyst and endometrium through interactions between surface integrins such as α5β1 and extracellular matrix proteins such as fibronectin93, 94. In pathology, the anatomy of the focal inflammatory plaques seen in multiple sclerosis, atherosclerosis and rheumatoid arthritis also bear many similarities to that of the primary tumour microenvironment and the metastatic niche, with recruitment of cells and molecules known to be involved in metastasis, such as VLA4+ monocytes, MMPs and osteopontin and microvasculature changes. Consideration of these analogous “niches” may suggest areas for study in metastasis research.
| Permissive microenvironments in development and disease | |
|---|---|
| Physiological | Germ cells and gametogenesis95 |
| Implantation of the blastocyst in the uterine endometrium93, 94 | |
| Maintenance of organ-specific stem cells in the adult such as neuronal stem cells96 | |
| Pathological | Multiple Sclerosis97 |
| Atherosclerotic plaques98, 99 | |
| Rheumatoid arthritis98, 100 | |
We are just beginning to understand the complexities involved in the evolution of the metastatic niche, and many aspects discussed in this article remain speculative. Clearly, substantial progress is required before specific therapies that target the metastatic microenvironment are successfully employed in the clinical arena. However, the preliminary insights highlighted here are integral steps towards identifying molecular and cellular targets for therapeutic development.
Acknowledgments
The authors thank Hector Peinado for his artistic assistance with Figure 1. B.P received research support from a Kay Kendall Leukaemia Fund Travelling Fellowship and a Fulbright Scholarship in Cancer Research. DL receives grants from the NCI (RO1CA098234); Susan G. Komen for the Cure; National Foundation for Cancer Research; Emerald Foundation; Malcolm Hewitt Wiener Foundation; Nancy C. and Daniel P. Paduano Foundation; AHEPA; Charles and Meryl Witmer Family Foundation; Butler Foundation and the Children's Cancer and Blood Foundation.
Biographies
Bethan Psaila completed her medical degree and clinical training at Cambridge University and University College of London Hospital, U.K. and she is currently a research fellow at Imperial College School of Medicine, London and Weill-Cornell Medical College of Cornell University, New York. Her research interests include the bone marrow microenvironment, megakaryocytes, platelets, and metastasis.
David Lyden holds the Stavros S. Niarchos Chair and is Associate Professor of Pediatrics and Cell and Developmental Biology at Weill-Cornell Medical Center, New York. He is also a pediatric neuro-oncologist at Memorial Sloan-Kettering Cancer Center, New York and an Adjunct Professor in Metastasis Prevention at the Champalimaud Foundation, Lisbon, Portugal. His research interests include the study of bone marrow-derived cells and their contribution to tumor angiogenesis and the pre-metastatic/metastatic niche.
References
- 1.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- 2.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 3.Virchow R. In: Cellularpathologie. 4th. Hirschwalkd A, editor. Berlin: 1858. [Google Scholar]
- 4.Ewing J. In: Neoplastic diseases. 6th. Saunders W, editor. Philadelphia, PA: 1928. [Google Scholar]
- 5.Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–5. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- 6.Hart IR, Fidler IJ. Role of organ selectivity in the determination of metastatic patterns of B16 melanoma. Cancer Res. 1980;40:2281–7. [PubMed] [Google Scholar]
- 7.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66:4553–7. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 8.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Li L. Stem cell niche: microenvironment and beyond. J Biol Chem. 2008;283:9499–503. doi: 10.1074/jbc.R700043200. [DOI] [PubMed] [Google Scholar]
- 10.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 11.Sneddon JB, Werb Z. Location, location, location: the cancer stem cell niche. Cell Stem Cell. 2007;1:607–11. doi: 10.1016/j.stem.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Psaila B, Kaplan RN, Port ER, Lyden D. Priming the ‘soil’ for breast cancer metastasis: the pre-metastatic niche. Breast Dis. 2006;26:65–74. doi: 10.3233/bd-2007-26106. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 14.Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin Cancer Biol. 2008;18:311–21. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joyce JA, Hanahan D. Multiple roles for cysteine cathepsins in cancer. Cell Cycle. 2004;3:1516–619. doi: 10.4161/cc.3.12.1289. [DOI] [PubMed] [Google Scholar]
- 16.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–75. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 19.Hiratsuka S, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008 doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 20.Peinado H, Rafii S, Lyden D. Inflammation joins the “niche”. Cancer Cell. 2008;14:347–9. doi: 10.1016/j.ccr.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyden D, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 22.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97:2293–9. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 24.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 25.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan RN, Psaila B, Lyden D. Niche-to-niche migration of bone-marrow-derived cells. Trends Mol Med. 2007;13:72–81. doi: 10.1016/j.molmed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Alix-Panabieres C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res. 2008;14:5013–21. doi: 10.1158/1078-0432.CCR-07-5125. [DOI] [PubMed] [Google Scholar]
- 28.Kim S, et al. Carcinoma produced factors activate myeloid cells via TLR2 to stimulate metastasis. Nature. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiratsuka S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 30.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–8. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Moses HL. Transforming growth factor beta: tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 2008;68:9107–11. doi: 10.1158/0008-5472.CAN-08-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103–18. doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- 34.Bellahcene A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer. 2008;8:212–26. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 36.Yang L, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avecilla ST, et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 38.Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–56. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 39.Jin DK, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–67. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Italiano JE, Jr, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–33. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rafii DC, Psaila B, Butler J, Jin DK, Lyden D. Regulation of vasculogenesis by platelet-mediated recruitment of bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2008;28:217–22. doi: 10.1161/ATVBAHA.107.151159. [DOI] [PubMed] [Google Scholar]
- 42.Jain S, et al. Platelet glycoprotein Ib alpha supports experimental lung metastasis. Proc Natl Acad Sci U S A. 2007;104:9024–8. doi: 10.1073/pnas.0700625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kucia M, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–94. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 44.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 45.Erler JT, et al. Hypoxia-induced lysyl oxidase is a critifcal mediator of bone marrow-derived cell recruitment to form the pre-metastatic niche. Cancer Cell. 2008 doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Deventer HW, et al. C-C chemokine receptor 5 on pulmonary fibrocytes facilitates migration and promotes metastasis via matrix metalloproteinase 9. Am J Pathol. 2008;173:253–64. doi: 10.2353/ajpath.2008.070732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 48.Cheng JD, Weiner LM. Tumors and their microenvironments: tilling the soil. Commentary re: A. M. Scott et al., A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin. Cancer Res., 9: 1639-1647, 2003. Clin Cancer Res. 2003;9:1590–5. [PubMed] [Google Scholar]
- 49.Olaso E, et al. Tumor-dependent activation of rodent hepatic stellate cells during experimental melanoma metastasis. Hepatology. 1997;26:634–642. doi: 10.1002/hep.510260315. [DOI] [PubMed] [Google Scholar]
- 50.Olaso E, et al. Proangiogenic role of tumor-activated hepatic stellate cells in experimental melanoma metastasis. Hepatology. 2003;37:674–685. doi: 10.1053/jhep.2003.50068. [DOI] [PubMed] [Google Scholar]
- 51.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol. 2008;19:2282–7. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghajar CM, Bissell MJ. Extracellular matrix control of mammary gland morphogenesis and tumorigenesis: insights from imaging. Histochem Cell Biol. 2008;130:1105–18. doi: 10.1007/s00418-008-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Du R, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Denko NC, et al. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene. 2003;22:5907–14. doi: 10.1038/sj.onc.1206703. [DOI] [PubMed] [Google Scholar]
- 55.Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–6. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 56.Astrof S, et al. Direct test of potential roles of EIIIA and EIIIB alternatively spliced segments of fibronectin in physiological and tumor angiogenesis. Mol Cell Biol. 2004;24:8662–70. doi: 10.1128/MCB.24.19.8662-8670.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alcaraz J, et al. Laminin and biomimetic extracellular elasticity enhance functional differentiation in mammary epithelia. EMBO J. 2008;27:2829–38. doi: 10.1038/emboj.2008.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cross M, Alt R, Niederwieser D. The case for a metabolic stem cell niche. Cells Tissues Organs. 2008;188:150–9. doi: 10.1159/000114206. [DOI] [PubMed] [Google Scholar]
- 59.Gao D, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–8. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 60.McDonbald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Research. 2002;62:5381–5385. [PubMed] [Google Scholar]
- 61.Dvorak HF, Nagy JA, Dvorak JT, Dvorak AM. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am J Pathol. 1988;133:95–109. [PMC free article] [PubMed] [Google Scholar]
- 62.Padua D, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ribatti D, Nico B, Vacca A, Roncali L, Dammacco F. Endothelial cell heterogeneity and organ specificity. J Hematother Stem Cell Res. 2002;11:81–90. doi: 10.1089/152581602753448559. [DOI] [PubMed] [Google Scholar]
- 64.LeCouter J, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–84. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 65.LeCouter J, Lin R, Ferrara N. Endocrine gland-derived VEGF and the emerging hypothesis of organ-specific regulation of angiogenesis. Nat Med. 2002;8:913–7. doi: 10.1038/nm0902-913. [DOI] [PubMed] [Google Scholar]
- 66.Minn AJ, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding L, et al. In vivo evaluation of the early events associated with liver metastasis of circulating cancer cells. Br J Cancer. 2001;85:431–8. doi: 10.1054/bjoc.2001.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Biancone L, Araki M, Araki K, Vassalli P, Stamenkovic I. Redirection of tumor metastasis by expression of E-selectin in vivo. J Exp Med. 1996;183:581–7. doi: 10.1084/jem.183.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirakawa S, et al. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood. 2007;109:1010–7. doi: 10.1182/blood-2006-05-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirakawa S, et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–99. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rinderknecht M, Detmar M. Tumor lymphangiogenesis and melanoma metastasis. J Cell Physiol. 2008;216:347–54. doi: 10.1002/jcp.21494. [DOI] [PubMed] [Google Scholar]
- 72.Podsypanina K, et al. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–4. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chin L. The genetics of malignant melanoma: lessons from mouse and man. Nat Rev Cancer. 2003;3:559–70. doi: 10.1038/nrc1145. [DOI] [PubMed] [Google Scholar]
- 74.Weiss L. Metastatic inefficiency. Adv Cancer Res. 1990;54:159–211. doi: 10.1016/s0065-230x(08)60811-8. [DOI] [PubMed] [Google Scholar]
- 75.Weiss L. Cancer cell traffic from the lungs to the liver: an example of metastatic inefficiency. Int J Cancer. 1980;25:385–92. doi: 10.1002/ijc.2910250313. [DOI] [PubMed] [Google Scholar]
- 76.Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev. 2007;28:297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 77.Minn AJ, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest. 2005;115:44–55. doi: 10.1172/JCI22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minn AJ, et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc Natl Acad Sci U S A. 2007;104:6740–5. doi: 10.1073/pnas.0701138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lyden D, et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature. 1999;401:670–7. doi: 10.1038/44334. [DOI] [PubMed] [Google Scholar]
- 80.Gupta GP, et al. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc Natl Acad Sci U S A. 2007;104:19506–11. doi: 10.1073/pnas.0709185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nat Rev Cancer. 2003;3:55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- 82.Vaida KS, et al. Breast cancer metastasis suppressor-1 differentially modulates growth factor signaling. Journal of Biological Chemistry. 2008;283:28354–28360. doi: 10.1074/jbc.M710068200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lapidot T, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 84.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pawelek JM, Chakraborty AK. The cancer cell--leukocyte fusion theory of metastasis. Adv Cancer Res. 2008;101:397–444. doi: 10.1016/S0065-230X(08)00410-7. [DOI] [PubMed] [Google Scholar]
- 87.Pawelek JM, Chakraborty AK. Fusion of tumour cells with bone marrow-derived cells: a unifying explanation for metastasis. Nat Rev Cancer. 2008;8:377–86. doi: 10.1038/nrc2371. [DOI] [PubMed] [Google Scholar]
- 88.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–58. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 89.Yu Q, Toole BP, Stamenkovic I. Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med. 1997;186:1985–96. doi: 10.1084/jem.186.12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–53. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 91.Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006;5:1779–87. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 92.Yamamoto M, et al. TSU68 prevents liver metastasis of colon cancer xenografts by modulating the premetastatic niche. Cancer Res. 2008;68:9754–62. doi: 10.1158/0008-5472.CAN-08-1748. [DOI] [PubMed] [Google Scholar]
- 93.Wang J, Armant DR. Integrin-mediated adhesion and signaling during blastocyst implantation. Cells Tissues Organs. 2002;172:190–201. doi: 10.1159/000066970. [DOI] [PubMed] [Google Scholar]
- 94.Wang J, Mayernik L, Armant DR. Integrin signaling regulates blastocyst adhesion to fibronectin at implantation: intracellular calcium transients and vesicle trafficking in primary trophoblast cells. Dev Biol. 2002;245:270–9. doi: 10.1006/dbio.2002.0644. [DOI] [PubMed] [Google Scholar]
- 95.Hess RA, Cooke PS, Hofmann MC, Murphy KM. Mechanistic insights into the regulation of the spermatogonial stem cell niche. Cell Cycle. 2006;5:1164–70. doi: 10.4161/cc.5.11.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taupin P. Adult neural stem cells, neurogenic niches, and cellular therapy. Stem Cell Rev. 2006;2:213–9. doi: 10.1007/s12015-006-0049-0. [DOI] [PubMed] [Google Scholar]
- 97.Steinman L. Nuanced roles of cytokines in three major human brain disorders. J Clin Invest. 2008;118:3557–63. doi: 10.1172/JCI36532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Libby P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am J Med. 2008;121:S21–31. doi: 10.1016/j.amjmed.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 99.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–15. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 100.Szekanecz Z, Koch AE. Mechanisms of Disease: angiogenesis in inflammatory diseases. Nat Clin Pract Rheumatol. 2007;3:635–43. doi: 10.1038/ncprheum0647. [DOI] [PubMed] [Google Scholar]


