Summary
The Hedgehog signaling pathway plays critical roles in metazoan development and in cancer. How the Hedgehog ligand is secreted and spreads to distant cells is unclear, given its covalent modification with a hydrophobic cholesterol molecule, which makes it stick to membranes. We demonstrate that Hedgehog ligand secretion from vertebrate cells is accomplished via two distinct and synergistic cholesterol-dependent binding events, one mediated by the membrane protein Dispatched and the other by a Scube family member, secreted proteins essential for Hedgehog signaling in zebrafish. Cholesterol modification is sufficient for a heterologous protein to interact with Scube, and to be secreted in a Scube-dependent manner. Dispatched and Scube recognize different structural aspects of cholesterol, similar to how Niemann-Pick disease proteins 1 and 2 interact with cholesterol, suggesting a hand-off mechanism for transferring Hedgehog from Dispatched to Scube. Thus, Dispatched and Scube cooperate to dramatically enhance secretion and solubility of the cholesterol-modified Hedgehog ligand.
Introduction
The Hedgehog (Hh) signaling pathway has fundamental roles in embryonic development, adult stem cell maintenance and carcinogenesis (Lum and Beachy, 2004). Hh signaling is triggered by binding of the secreted Hh ligand to its membrane receptor, Patched (Ptch), setting in motion signal transduction events that ultimately lead to the specific transcriptional output of the Hh pathway. The Hh ligand is generated from a precursor protein, which is translocated into the endoplasmic reticulum (ER), undergoes signal sequence cleavage and then is modified covalently with two lipids: 1) a palmityl residue is attached at the N-terminus by the palmityl transferase Skinny hedgehog (Chamoun et al., 2001); and 2) a cholesteryl residue is attached at the C-terminus by autocatalytic modification (Porter et al., 1996). The cholesterol modification reaction relies on the intein activity of the C-terminal domain of the Hh precursor, and generates an N-terminal fragment (the cholesterol-modified Hh ligand) and a C-terminal fragment that is disposed of by ER-associated degradation (Chen et al., 2011). The two lipid modifications of the Hh ligand occur independently (Chamoun et al., 2001) and are both essential for normal Hh signaling (Chamoun et al., 2001; Traiffort et al., 2004).
The Hh ligand is strongly hydrophobic and hence membrane-associated, which raises the critical question of how it is secreted and how it reaches cells located at a distance from the signaling cell. Genetic analysis identified Dispatched (Disp) and the Scube family of proteins as essential for long-range Hh signaling. Disp is a multi-spanning membrane protein required for long-range Hh signaling in Drosophila (Burke et al., 1999), mouse (Ma et al., 2002) and zebrafish (Nakano et al., 2004). Disp belongs to the RND family of transporters (Tseng et al., 1999) and contains a sterol-sensing domain (SSD), a sequence of 5 consecutive membrane-spanning helices found in several membrane proteins involved in cholesterol homeostasis (Kuwabara and Labouesse, 2002). Disp is specifically required for secretion of cholesterol-modified Hh, as the N-terminal fragment of Hh without the cholesterol modification can be released in the absence of Disp. The Scube family (Grimmond et al., 2000) consists of the secreted proteins Scube 1, 2 and 3, and is required for long-range Hh signaling in zebrafish (Johnson et al., 2012). Scube2 was first identified in zebrafish (Hollway et al., 2006; Kawakami et al., 2005; Woods and Talbot, 2005) as playing a non-cell autonomous role in long-range Hh signaling. Epistatic analysis led to the proposal that Scube2 is involved in the transport or stability of Hh ligand in the extracellular space (Hollway et al., 2006; Kawakami et al., 2005; Woods and Talbot, 2005).
For both Disp and Scube proteins, the mechanism by which they promote long-range Hh signaling is unknown. Although Disp is required for Hh secretion, there is no direct evidence that Disp participates in Hh release from cells. Additionally, it is unclear how the Hh ligand is kept soluble in the extracellular space, and how it is delivered to responding cells. Regarding Scube proteins, it is unclear if they are involved in Hh biosynthesis, secretion or in another aspect of Hh function outside the producing cell.
Here we dissect the mechanism of Hh secretion in vertebrate cells. We show that the vertebrate homologue, Dispatched-A (DispA) interacts with human Sonic hedgehog (hShh) via its cholesterol anchor, and that this interaction is necessary for hShh secretion. Interestingly, an inactive DispA mutant binds hShh more strongly than wild-type DispA, suggesting that dissociation of hShh from DispA is important for efficient secretion. However, DispA alone is not sufficient to release hShh from cells, indicating that additional factors are required to overcome the insolubility conferred by cholesterol modification. We demonstrate that a Scube family member, Scube2, synergizes with DispA to cause a dramatic increase in hShh secretion. Scube2 binds the cholesterol anchor of hShh and this interaction is required for promoting hShh secretion. Cholesterol modification is sufficient for a heterologous protein to bind Scube2 and to be secreted in a Scube2-dependent manner. Importantly, DispA and Scube2 recognize different aspects of the cholesterol anchor of hShh. Our results support a model in which membrane-associated hShh is secreted by being handed off from DispA to Scube2, in a manner reminiscent of the transport of free cholesterol by the Niemann-Pick disease proteins NPC1 and NPC2 (Infante et al., 2008b). Thus a relay mechanism involving DispA and Scube2 promotes the release of cholesterol-modified hShh from the plasma membrane of producing cells.
Results
Dispatched-A interacts with hShh in a cholesterol-dependent manner
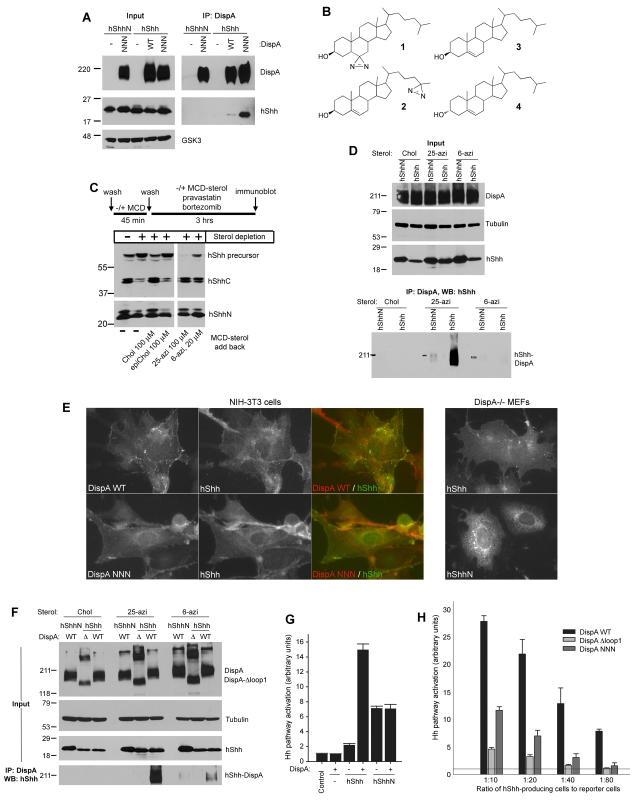
It was suggested that Dispatched (Disp) might bind cholesterol-modified Hh (Burke et al., 1999); however, such an interaction has not been demonstrated. We first tested if mouse Dispatched-A (DispA) binds cholesterol-modified human Sonic Hedgehog (hShh) by co-immunoprecipitation. DispA and hShh were stably co-expressed in 293T cells, followed by detergent solubilization and immunoprecipitation. Under these conditions wild-type DispA showed modest but reproducible binding to cholesterol-modified hShh (Figure 1A), consistent with the expectation of a transient DispA-hShh interaction. To probe the cholesterol dependence of the interaction, we expressed a construct that encodes the N-terminal fragment of hShh (hShhN, amino acids 1-198), which generates a protein lacking cholesterol but which is still palmitylated (Chamoun et al., 2001; Chen et al., 2004). DispA did not bind hShhN (Figure 1A), indicating that binding to hShh is cholesterol-dependent. Interestingly, the inactive DispA mutant, DispA-NNN (Ma et al., 2002) showed significantly stronger hShh binding compared to wild-type DispA (Figure 1A); the same result was obtained with the inactive DispA-AAA mutant. DispA-NNN and DispA-AAA are triple point mutants in which 3 aspartate residues located in transmembrane helices 4 and 10 are mutated to asparagines or alanines; one of these aspartates is conserved in RND family proteins and is required for their transporter function. Our results suggest that DispA-NNN and DispA-AAA are defective in hShh secretion perhaps because they bind hShh too tightly, thus interfering with its release from cells.
Figure 1. Cholesterol-dependent binding of hShh to DispA is required for hShh secretion.
(A) 293T cells stably co-expressing hShh or hShhN, and myc-tagged wild-type DispA (WT) or DispA-NNN (NNN) were lysed with detergent and analyzed by immunoprecipitation with anti-myc antibodies, followed by SDS-PAGE and immunoblotting with anti-hShh antibodies.
(B) Structures of the photoreactive sterols, 6-azicholestanol (1) and 25-azicholesterol (2). Also shown are the structures of cholesterol (3) and the inactive diastereomer, epicholesterol (4).
(C) HShh, HA-tagged at the C-terminus (hShh-HA) was stably expressed in 293T cells. The cells were sterol-depleted with methyl-β-cyclodextrin (MCD), after which cholesterol (Chol), epicholesterol (epiChol), 6-azicholestanol (6-azi) or 25-azicholesterol (25-azi) were added back as soluble MCD complexes, for 3 hours. Lysates were analyzed by SDS-PAGE and immunoblotting with HA antibodies (to detect the hShh precursor and the C-terminal fragment, hShhC) and hShh antibodies (to detect the hShh ligand).
(D) 293T cells stably co-expressing myc-tagged DispA-WT and hShh or hShhN were labeled with the indicated sterols, as in (B). After incubation for 6 hours, the cells were UV-irradiated and DispA-hShh photocrosslinking was analyzed by denaturing immunoprecipitation with myc antibodies, followed by SDS-PAGE and immunoblotting for hShh. Immunoblotting for tubulin served as loading control.
(E) Left panels: immunofluorescence microscopy of hShh stably co-expressed with myc-tagged DispA-WT or DispA-NNN in NIH-3T3 cells. Cells were stained with rabbit anti-hShh and mouse anti-myc antibodies, followed by goat anti-rabbit Alexa488 and goat anti-mouse Alexa594 secondary antibodies. Right panels: localization by immunofluorescence microscopy of hShh and hShhN expressed in DispA-/- MEFs.
(F) As in (C) but with 293T cells stably co-expressing myc-tagged DispA or the mutant missing the first extracellular loop, DispA-Δloop1 (Δ), together with hShh or hShhN.
(G) DispA-/- MEFs stably expressing hShh or hShhN, rescued by lentiviral expression of mCherry-tagged DispA or not, were co-cultured with Hh-responsive Shh Light II cells at a 1:20 ratio. Luciferase measurements were normalized to reporter cells grown alone (control). All experiments were performed in triplicate. Error bars represent the standard deviation of the mean.
(H) DispA-/- MEFs stably expressing hShh, transduced with lentiviruses expressing mCherry-tagged DispA-WT, DispA-Δloop1 or DispA-NNN were analyzed as in (G), at four different ratios of hShh-producing cells to reporter cells.
A potential problem with our binding assay is that detergent solubilization could have a negative effect on the native conformation of DispA and/or might disrupt the DispA-hShh interaction. We thus developed a strategy to examine the DispA-hShh interaction in intact cells, using two different photoreactive cholesterol derivatives: 1) 6-azicholestanol (compound 1 in Figure 1B), a photoreactive sterol that carries a diazirine group on the B ring of the molecule (Thiele et al., 2000), and 2) 25-azicholesterol (compound 2 in Figure 1B), a novel photoreactive sterol that we synthesized, which carries a diazirine group at the end of the isooctyl tail. We reasoned that having the photoreactive group in two locations of the sterol molecule would increase the chance of detecting a potential interaction with DispA. We first asked if the two photoreactive sterols modify hShh in cells. When cells stably expressing hShh (Chen et al., 2011) were depleted of sterols by acute treatment with methyl-β-cyclodextrin (MCD), hShh processing was strongly inhibited, causing the accumulation of the unprocessed hShh precursor (Figure 1C). Processing was rescued by adding back cholesterol (compound 3 in Figure 1B) but not the diastereomer epicholesterol (compound 4 in Figure 1B), which is inactive in modifying Hh proteins (Mann and Beachy, 2004). Adding back either of the two photoreactive sterols fully rescued hShh processing (Figure 1C). We next used this strategy to generate hShh modified with photoreactive sterols by labeling cells that stably express DispA and hShh. The cells were then UV-irradiated, and formation of a covalent DispA-hShh bond was tested by denaturing immunoprecipitation of DispA followed by immunoblotting for hShh. DispA showed crosslinking to hShh modified with photoreactive sterol (Figure 1D), but not to hShhN, demonstrating that DispA binds hShh in a cholesterol-dependent manner in intact cells. Interestingly, DispA was photocrosslinked much more efficiently when hShh was modified with 25-azicholesterol than with 6-azicholestanol (Figure 1D and 1F), suggesting that the isooctyl tail of cholesterol is a feature recognized by DispA during its interaction with hShh. Consistent with a DispA-hShh interaction in cells, DispA (wild-type and the NNN mutant) and hShh are both present at the plasma membrane by immunofluorescence microscopy (Figure 1E). Importantly, not all hShh puncta co-localize with DispA at the membrane, as expected from a transient interaction between the two proteins. In contrast to hShh, hShhN is found predominantly in intracellular vesicles and not at the plasma membrane (Figure 1E), consistent with the efficient release of hShhN from the cell surface.
We next asked if binding of DispA to hShh is important for hShh secretion. To generate a DispA mutant that cannot bind hShh, we deleted the first extracellular loop of DispA. This choice was based on the role that the first extracellular loop plays in two Disp-related proteins: it is required in the SREBP cleavage-activating protein (SCAP) for binding cholesterol (Motamed et al., 2011), and in Ptch for binding hShh (Marigo et al., 1996). Indeed, DispA-Δloop1 did not bind hShh in our in vivo photocrosslinking assay (Figure 1F). We turned to a cellular assay that relies on the function of DispA in hShh secretion and long-range signaling. DispA-/- mouse embryonic fibroblasts (MEFs) (Ma et al., 2002) were co-cultured with the Hh reporter cells, Shh Light II (Taipale et al., 2000). Stable expression of hShh in DispA-/- MEFs caused a slight activation of the Hh pathway in reporter cells (Figure 1G) because short-range Hh signaling does not require DispA. Co-expression of mCherry-tagged DispA in DispA-/- MEFs strongly increased the response of reporter cells, indicating that DispA rescued hShh secretion (Figure 1G). As expected, DispA did not affect signaling by DispA-/- MEFs expressing hShhN (Figure 1G), because DispA is only required for secretion of cholesterol-modified hShh. Similar results were obtained by overexpressing DispA in NIH 3T3 cells (Figure S1). In contrast to wild-type DispA, mCherry-tagged DispA-Δloop1 or DispA-NNN did not rescue signaling by DispA-/- MEFs expressing hShh, a result more obvious at a lower ratio of signaling cells to reporter cells (Figure 1H). The fact that DispA-Δloop1 is inactive suggests that cholesterol-dependent binding of hShh to DispA is required for hShh secretion.
DispA is not sufficient to release cholesterol-modified hShh from cells
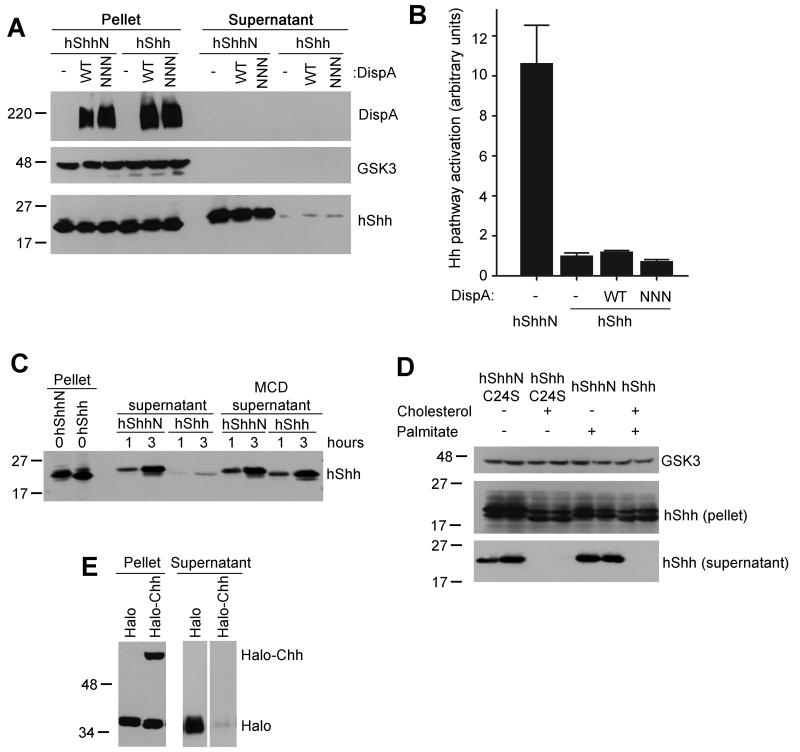
Although the co-culture system provides a good functional assay for DispA, it measures hShh secretion only indirectly. We next tested directly if DispA is sufficient for hShh release from membranes, by examining secretion of hShh from 293T cells that stably over-express DispA. Since we observed that serum releases hShh in a non-specific, DispA-independent manner, we took care to remove residual serum from the cells by repeated washes with serum-free medium. Under serum-free conditions, no hShh was released into the medium, in the absence or presence of overexpressed DispA, as assayed by Western blotting (Figure 2A) or by Hh activity assays (Figure 2B). These cells expressed large amounts of hShh, which was properly processed (Figure 2A) and strongly localized to plasma membrane, as determined by immunofluorescence microscopy. In contrast, stably expressed hShhN was efficiently secreted into serum-free medium and was active; as expected, DispA had no effect on hShhN secretion (Figure 2A and B). These results indicate that DispA alone is not sufficient to release hShh, perhaps because another factor is required to solubilize it in the media; in the absence of such a factor, hShh remains membrane-associated.
Figure 2. DispA is not sufficient for release of cholesterol-modified hShh.
(A) 293T cells stably expressing hShh and myc-tagged DispA constructs were washed of serum and were incubated with serum-free media for 6 hours. Secreted proteins were precipitated with trichloroacetic acid (TCA). Pellet and supernatant fractions were analyzed by SDS-PAGE and immunoblotting with anti-myc and anti-hShh antibodies. Blotting for GSK3 served as loading control.
(B) Supernatants collected as in (A) were diluted 1:2 with serum-free media and hShh activity was assayed using Shh Light II reporter cells. Measurements were performed in triplicate and error bars represent standard deviation of the mean.
(C) 293T cells expressing hShhN or hShh were incubated with serum-free media, with or without methyl-β-cyclodextrin (MCD, 100 μM), and supernatants were harvested after 1 or 3 hours. Secretion of hShh and hShhN was analyzed as in (A).
(D) 293T cells transiently expressing hShh, hShhN, hShh with a palmitylation site mutation (hShhC24S), or hShhN with a palmitylation site mutation (hShhN-C24S) were incubated in serum-free media for 4 hours, and secreted proteins were TCA-precipitated. Pellet and supernatant fractions were analyzed as in (A). The samples in this panel were loaded in duplicate.
(E) A secreted HA-tagged Halotag protein was fused to amino acids 190-462 of hShh (Halo-Chh); autocatalytic processing of Halo-Chh generates Halotag fused to amino acids 190-198 of hShh, modified with cholesterol. Secreted HA-Halotag fused to amino acids 190-198 of hShh (Halo) is not cholesterol-modified and serves as negative control. The Halotag constructs were expressed in 293T cells, and secreted proteins were collected into serum free media for 4 hours. Pellet and supernatant fractions were analyzed by SDS-PAGE and immunoblotting with anti-HA antibodies.
Cholesterol is the main determinant of hShh membrane association
Given that hShhN is efficiently secreted, we tested if the cholesterol anchor is responsible for the strong association of hShh with membranes. HShh was quickly released from cells when media were supplemented with methyl-β-cyclodextrin (MCD) (Figure 2C), which can be explained by MCD binding the cholesterol anchor and promoting hShh solubilization. In contrast to hShh, secretion of hShhN was not further enhanced by MCD (Figure 2C).
We next asked if the other hydrophobic modification of hShh, palmitylation, plays a role in hShh association with membranes. Like wild-type hShh, a mutant of hShh that cannot be palmitylated but is still modified with cholesterol (hShhC24S, (Chamoun et al., 2001)) was membrane-associated and was not secreted into serum-free medium (Figure 2D). As expected, hShhN and a mutant that lacks both the cholesteryl and palmityl moieties (hShhN-C24S) were efficiently secreted (Figure 2D).
Finally, we asked if cholesterol modification of an unrelated soluble protein is sufficient to recapitulate the strong membrane attachment of hShh. We used a construct encoding a secreted version of the Halotag protein fused to the C-terminal domain of hShh (Halotag-Chh); in cells, this fusion undergoes autocatalytic processing to generate cholesterol-modified Halotag. HA-tagged Halotag-Chh was expressed in 293T cells and secretion into serum-free medium was measured by immunoblotting with HA antibodies. Under these conditions, cholesterol-modified Halotag was not secreted and remained membrane-associated (Figure 2E); in contrast, Halotag without the cholesterol modification was soluble (Figure 2E).
Together, these results indicate that the cholesterol anchor is necessary and sufficient for hShh membrane attachment, while the palmityl moiety plays a less important role, if any. Thus in order to secrete hShh, cells must find a way to solubilize its cholesterol anchor.
Scube2 dramatically increases secretion of cholesterol-modified hShh
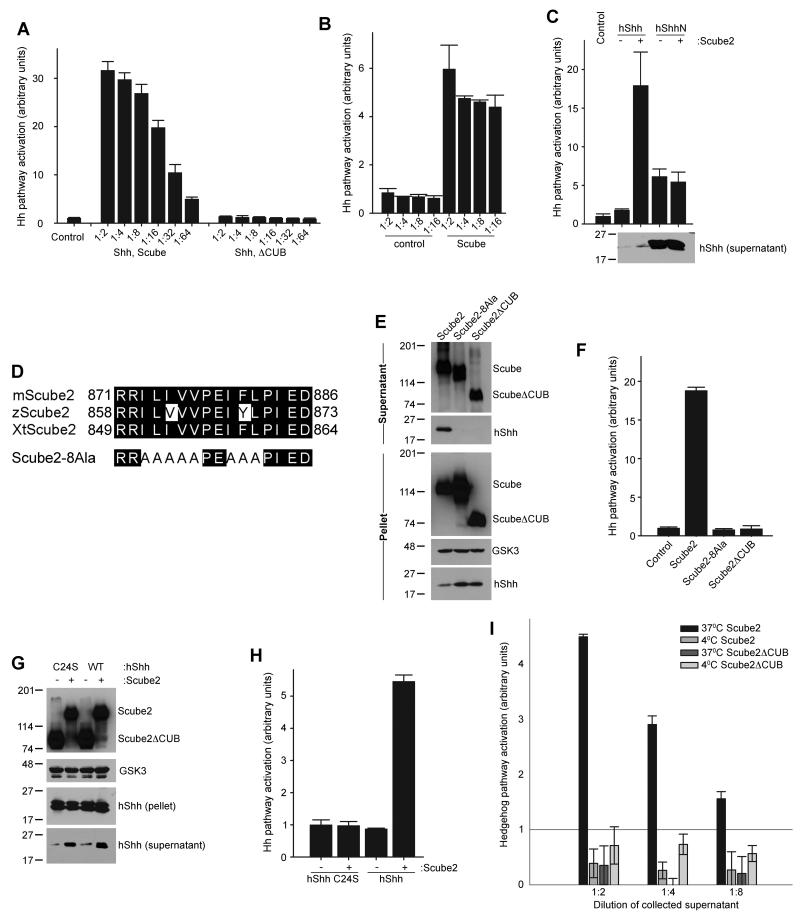
The experiments above suggest the existence of an extracellular protein that helps the secretion of hShh by overcoming its cholesterol-dependent insolubility. The Scube family of secreted proteins is required for long-range Hh signaling in zebrafish (Johnson et al., 2012), but its mechanism is unknown. We examined if the first Scube protein implicated in Hh signaling, Scube2 (Hollway et al., 2006; Kawakami et al., 2005; Woods and Talbot, 2005), is involved in releasing hShh from cells. When Scube2 was expressed in 293T cells stably expressing hShh, it caused a dramatic increase in secretion of active hShh (Figure 3A). As negative control we used Scube2ΔCUB, a truncation mutant of Scube2 that lacks a C-terminal portion of the protein, including a cysteine-rich domain and a CUB domain; this loss-of-function mutation was identified in the initial cloning of zebrafish Scube2 (Woods and Talbot, 2005). Scube2ΔCUB was efficiently secreted but failed to stimulate hShh secretion (Figure 3A). Scube2 supplied exogenously also released hShh into serum-free media, indicating that Scube2 and hShh do not have to be co-expressed (Figure 3B). Scube2 had no effect on the secretion of hShhN, indicating that it acts specifically to release cholesterol-modified hShh (Figure 3C). Interestingly, although the amount of released hShh was significantly smaller than that of hShhN, the signaling activity of hShh was much higher than that of hShhN (Figure 3C), suggesting that cholesterol modification greatly enhances the potency of the hShh ligand.
Figure 3. The extracellular protein Scube2 stimulates secretion of cholesterol-modified hShh.
(A) Scube2 or Scube2ΔCUB was transfected into 293T cells stably expressing hShh, and 24 hours later secreted proteins were collected into serum-free media for 4 hours. HShh activity in serial dilutions of the supernatant was measured by luciferase assay in Shh Light II reporter cells. All experiments were performed in triplicate. Error bars represent the standard deviation of the mean.
(B) Scube2 or Scube2ΔCUB was added in serum-free media to 293T cells stably expressing hShh, for 4 hours. Activity of secreted hShh was measured as in (A).
(C) As in (A) but with 293T cells stably expressing hShh or hShhN. Secreted hShh and hShhN were collected for 6 hours, and were analyzed by reporter assay in Shh Light II cells and by immunoblotting with anti-hShh antibodies. Note the much higher amount of secreted hShhN compared to hShh.
(D) Sequence of the conserved hydrophobic patch in the CUB domain of vertebrate Scube2 orthologs. Also shown is the sequence of the Scube2-8Ala mutant, in which 8 hydrophobic residues are mutated to alanines.
(E) HA-tagged Scube2, Scube2-8Ala, or Scube2ΔCUB were transfected into 293T cells stably expressing hShh. Secreted proteins were collected 24 hours later, for 6 hours into serum-free media. Aliquots of the cell pellet and supernatants were analyzed by SDS-PAGE and immunoblotting for HA and hShh.
(F) HShh activity in the supernatants collected in (E) was measured as in (A).
(G) HShh or the palmitylation-defective mutant hShhC24S were co-expressed in 293T cells with HA-tagged Scube2 or Scube2ΔCUB. HShh and hShhC24S secretion was analyzed as in (E).
(H) HShh and hShhC24S activity in the supernatants collected in (G) was measured as in (A).
(I) Scube2 or Scube2ΔCUB was added in serum-free medium to 293T cells stably expressing hShh, for 1 hour at 37°C or 4°C. HShh activity in the supernatants was measured as in (A).
It is known that hShh can be released from cells by serum (Chen et al., 2011) and by high levels of heparin. HShh released with either serum or heparin was inactive in signaling assays, in contrast to hShh released by Scube2 (Figure S2). Thus unlike other factors that can release hShh, release by Scube2 is physiological.
All Scube2 orthologs contain a conserved hydrophobic stretch in the middle of the CUB domain (Figure 3D), while the rest of the protein shows little clustering of hydrophobic amino acids outside the signal sequence. We reasoned that this hydrophobic stretch might be important for release of hShh. Indeed, a Scube2 mutant in which 8 amino acid residues in the hydrophobic stretch are mutated to alanines (Scube2-8Ala) is secreted (Figure 3E) but is completely inactive in releasing hShh (Figure 3E, F). This indicates that the conserved hydrophobic sequence in the CUB domain is necessary for Scube2 activity.
We next asked if palmitylation of hShh is required for release by Scube2. Like wild-type hShh, the non-palmitylated mutant hShhC24S was released by Scube2 but not by Scube2ΔCUB (Figure 3G), indicating that the palmitylation is not required for Scube-mediated hShh secretion. The Scube2-released hShhC24S was inactive in Hh signaling assays (Figure 3H), consistent with the requirement of the palmityl modification for Hh activity (Chamoun et al., 2001).
We also performed an experiment to determine the effect of temperature on the release of hShh from cells by Scube2. While added Scube2 released a significant amount of hShh during 1 hour at 37°C, no hShh was released at 4°C (Figure 3I). This is consistent with Scube2 having to overcome the hydrophobic interaction between the cholesterol anchor of hShh and the membrane, an interaction of increased strength at lower temperature.
Scube2 does not affect hShh processing in producing cells or signaling in responding cells
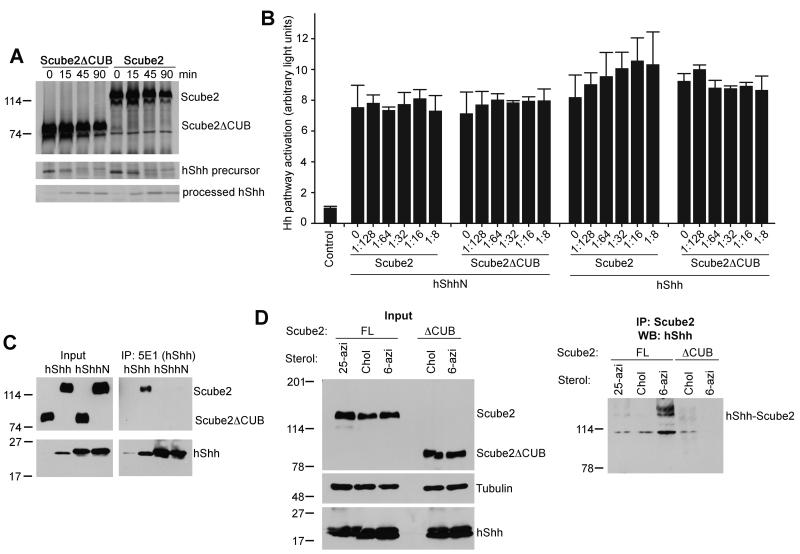
One possible explanation for the enhanced secretion of hShh is that Scube2 stimulates hShh processing in producing cells. Radioactive pulse-chase analysis of hShh processing showed that it proceeded with the same kinetics in the presence of co-expressed Scube2 or the inactive Scube2ΔCUB mutant (Figure 4A). This result indicates that Scube2 does not affect hShh processing.
Figure 4. Cholesterol-dependent binding of Scube2 to hShh is required for hShh secretion.
(A) HA-tagged Scube2 or Scube2ΔCUB was expressed in 293T cells stably expressing hShh. The cells were pulsed with 35S-methionine for 3 min and were chased with media containing unlabeled methionine for the indicated times. HShh processing was analyzed by immunoprecipitation with the 5E1 antibody, which recognizes full-length and processed hShh. Scube2 and Scube2ΔCUB were immunoprecipitated with anti-HA antibodies. The precipitated protein was analyzed by SDS-PAGE and fluorography. The same number of cells was processed for each condition.
(B) The two graphs on the left show that Scube2 does not affect hShhN activity. HShhN was mixed with HA-tagged Scube2 or Scube2ΔCUB, in the indicated ratios, and its activity was measured in Shh Light II reporter cells. The two graphs on the right show Scube2 does not affect the activity of hShh pre-released with Scube2. 293T cells co-expressing hShh and HA-tagged Scube2 were used to generate serum-free hShh-Scube2 conditioned media. This media was mixed with HA-tagged Scube2 or Scube2ΔCUB, in the indicated ratios, and its activity was measured in Shh Light II reporter cells. All experiments were performed in triplicate. Error bars represent the standard deviation of the mean.
(C) 293T cells co-expressing hShh or hShhN, and HA-tagged Scube2 or Scube2ΔCUB were incubated in serum-free media for 4 hours. HShh and hShhN were immunoprecipitated from the supernatant with 5E1 antibodies. A portion of the supernatant was TCA-precipitated to serve as input. All samples were analyzed by SDS-PAGE and immunoblotting with anti-HA and anti-hShh antibodies.
(D) HShh or hShhN was stably co-expressed with HA-tagged Scube2 or Scube2ΔCUB in 293T cells. The cells were labeled with the indicated sterols, followed by UV irradiation. Photocrosslinked Scube2-hShh was analyzed by denaturing immunoprecipitation with HA antibodies, followed by SDS-PAGE and immunoblotting for hShh.
To determine if Scube2 has an effect on Hh signal transduction, we performed two experiments. First, we asked if Scube2 affects signaling by hShhN. Increasing concentrations of Scube2 added to a fixed concentration of hShhN had no effect on Hh pathway stimulation (Figure 4B, left side of the graph). In a second experiment, we asked if excess Scube2 affects signaling by hShh. When increasing amounts of Scube2 were added to hShh released into Scube2-containing media, there was no effect on Hh pathway stimulation (Figure 4B, right side of the graph); furthermore, Scube2 had no effect on Hh signaling on its own. Although it was proposed that Scube2 promotes Hh signaling at the level of the responding cell, possibly via its interaction with Ptch (Tsai et al., 2009), our Scube2 titration experiment argues against such a model. Our data, however, cannot exclude the possibility that the hShh-Scube2 complex (see below) is the active species in long-range Hh signaling.
Scube2 binds hShh in a cholesterol-dependent manner
It seemed likely that Scube2 might stimulate hShh secretion by direct binding. We first tested this hypothesis by immunoprecipitation of hShh secreted into serum-free medium by cells co-expressing hShh and HA-tagged Scube2, followed by immunoblotting for HA. Under these conditions we detected binding of Scube2 to hShh (Figure 4C). Scube2 did not bind hShhN, indicating that Scube2 binds hShh in a cholesterol-dependent manner (Figure 4C). As expected, the inactive mutant Scube2ΔCUB did not release or bind hShh, suggesting that binding to Scube2 is required for hShh secretion.
In the experiment above, hShh was immunoprecipitated with the 5E1 monoclonal antibody, which blocks binding of hShh to Ptch and to the antagonist Hedgehog-interacting protein (HIP), due to the very similar binding interfaces between hShh and 5E1, HIP, and Ptch (Maun et al., 2010). In contrast, the 5E1 antibody did not block binding of hShh to Scube2, indicating that the hShh-Scube2 interface is distinct and thus hShh binding to Scube2 might allow hShh interaction with the downstream components Ptch and HIP.
Finally, we examined the interaction between Scube2 and hShh in cells by photocrosslinking. Cells stably expressing HA-tagged Scube2 and hShh were labeled with photoreactive sterols, and were then UV-irradiated. Lysates were subjected to denaturing immunoprecipitation with HA antibodies followed by immunoblotting for hShh. We detected crosslinking between hShh modified with 6-azicholestanol and Scube2; as expected, Scube2ΔCUB was not crosslinked to Scube2 (Figure 4D). Interestingly, hShh modified with 25-azicholesterol showed much less crosslinking to Scube2 (Figure 4D), in contrast to DispA-hShh crosslinking, which occurred preferentially with 25-azicholesterol. This differential crosslinking suggests that Scube2 and DispA recognize different structural aspects of the cholesterol molecule, in a manner that might facilitate the hand-off of hShh from DispA to Scube2.
DispA synergizes with Scube2 to promote hShh secretion
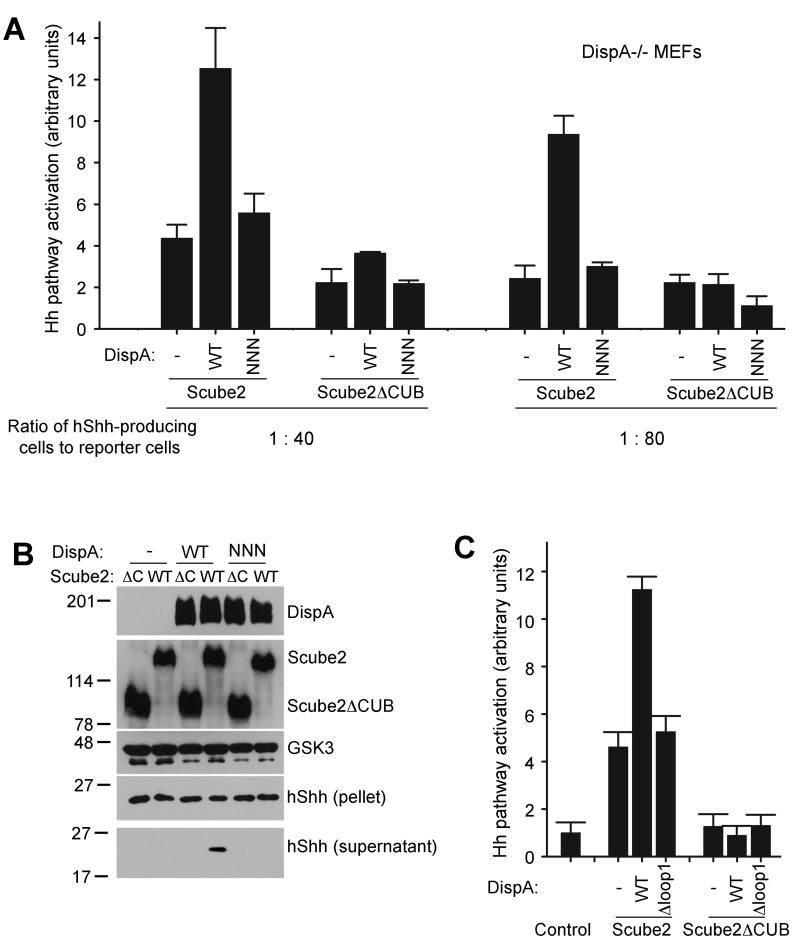
We next tested if DispA and Scube2 act synergistically to promote hShh secretion, first in the co-culture system of DispA-/- MEFs and Hh reporter cells. DispA-/- MEFs expressing hShh and DispA were plated at different ratios with Hh reporter cells, and the co-cultures were incubated with Scube2 in serum-free media, followed by Hh activity assays. Under these conditions, Scube2 strongly synergized with wild-type DispA to cause hShh secretion and Hh pathway activation (Figure 5A). As expected, Scube2 did not synergize with DispA-NNN and had no effect on signaling by hShhN; also, Scube2ΔCUB had no effect on hShh (Figure 5A). Interestingly, at a higher ratio of hShh-producing cells to reporter cells, Scube2 was able to release some hShh even in the absence of DispA (Figure 5A); this is consistent with a model in which hShh partitions between the membrane and Scube2 in the media.
Figure 5. Scube2 synergizes with DispA to stimulate secretion of cholesterol-modified hShh.
(A) DispA-/- MEFs stably expressing hShh, transduced with lentiviruses expressing mCherry-tagged wild-type DispA or DispNNN, were plated with Shh Light II reporter cells at 1:40 and 1:80 ratios. After 12 hours, Scube2 or Scube2ΔCUB was added in serum-free media. Luciferase measurements were performed 30 hours later and were normalized to untreated reporter cells. All experiments were done in triplicate. Error bars represent the standard deviation of the mean.
(B) HA-tagged Scube2 or Scube2ΔCUB was transfected into 293T cells that stably express hShh, hShh and myc-tagged DispA-WT, or hShh and myc-tagged DispA-NNN. Secreted proteins were collected into serum-free media 24 hours later, for 4 hours. Aliquots of the cellular pellets and supernatants were analyzed by SDS-PAGE and immunoblotting with HA, myc and hShh antibodies. Blotting for GSK3 served as loading control.
(C) As in (B), but with 293T cells expressing DispA-Δloop1 instead of DispA-NNN. Activity of secreted hShh was measured as in (A).
Similar results were obtained when we measured hShh secretion in 293T cells, in the absence or presence of co-expressed myc-tagged DispA. Addition of Scube2 to the cells synergized with wild-type DispA to release hShh into the supernatant, as assayed by immunoblotting (Figure 5B). As expected, Scube2 did not synergize with DispA-NNN, and Scube2ΔCUB had no effect on hShh secretion, irrespective of the presence of DispA (Figure 5B). Similarly, Scube2 added to 293T cells synergized with DispA but not with DispA-Δloop1, to cause secretion of hShh, as measured by Hh reporter assays (Figure 5C). Together, these data show that DispA and Scube2 act synergistically to promote hShh secretion.
Cholesterol modification is sufficient for interaction with Scube2 and Scube2-dependent secretion
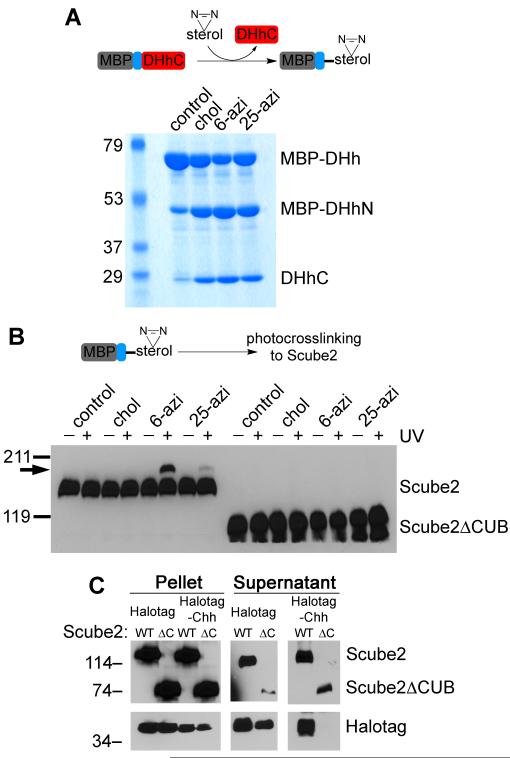
We asked if cholesterol modification is sufficient for interaction with Scube2, by photocrosslinking in vitro. To this end, we used an unrelated protein, maltose-binding protein (MBP) that we modified with photoreactive sterols by in vitro processing (Chen et al., 2011) (Figure 6A). Sterol-modified MBP fusions were then incubated with HA-tagged Scube2, followed by UV irradiation and immunoblotting with HA antibodies, to detect an increase in Scube2 size due to photocrosslinking. Under these conditions, Scube2 was crosslinked to MBP modified with 6-azicholestanol (Figure 6B), and much less efficiently to MBP modified with 25-azicholesterol. This is the same preference for 6-azicholestanol over 25-azicholesterol that we observed for hShh-Scube2 photocrosslinking in cells (Figure 4D). The size of the electrophoretic mobility shift in Scube2 crosslinked to MBP (Figure 6B) is consistent with the addition of one molecule of MBP (40 kDa), indicating a 1:1 binding ratio between Scube2 and sterol-modified MBP. As expected, Scube2ΔCUB was not crosslinked to any of the sterol-modified MBP proteins. These results suggest that cholesterol modification is sufficient for binding to Scube2.
Figure 6. Cholesterol modification is sufficient for interaction with and secretion by Scube2.
(A) Purified maltose-binding protein (MBP) fused to amino acids 244-471 of Drosophila Hedgehog (MBP-DHh) was used in in vitro processing reactions to generate MBP modified with either cholesterol (control), 6-azicholestanol or 25-azicholesterol. The samples were analyzed by SDS-PAGE and Coomassie staining. MBP-DHhN and DHh-C are the two fragments generated by in vitro processing.
(B) The processing reactions in (A) were incubated with HA-tagged Scube2 or Scube2ΔCUB, followed by UV irradiation to induce photocrosslinking. The samples were analyzed by reducing SDS-PAGE and immunoblotting with HA antibodies. The arrow indicates the position of the photocrosslinked Scube2-MBP species.
(C) Constructs that generate Halotag protein modified with cholesterol (Halotag-Chh) or not (Halotag) were co-expressed in 293T cells with myc-tagged Scube or Scube2ΔCUB. Secreted proteins were collected in serum-free media for 6 hours, followed by TCA precipitation and immunoblotting with HA and myc antibodies.
Finally, we asked if cholesterol modification is sufficient for Scube2-dependent secretion. For this purpose we used cholesterol-modified Halotag, which is membrane-associated (Figure 2E). HA-tagged Halotag-Chh was co-expressed in 293T cells with myc-tagged Scube2, and secretion into serum-free medium was measured by immunoblotting. As shown in figure 6C, cholesterol-modified Halotag was secreted only in the presence of Scube2, while Halotag without a cholesterol anchor was secreted independently of Scube2. As expected, Scube2ΔCUB did not release cholesterol-modified Halotag. These data demonstrate that a cholesterol anchor is sufficient for an unrelated soluble protein to become membrane-associated, to bind Scube2, and to be secreted in a Scube2-dependent manner.
Discussion
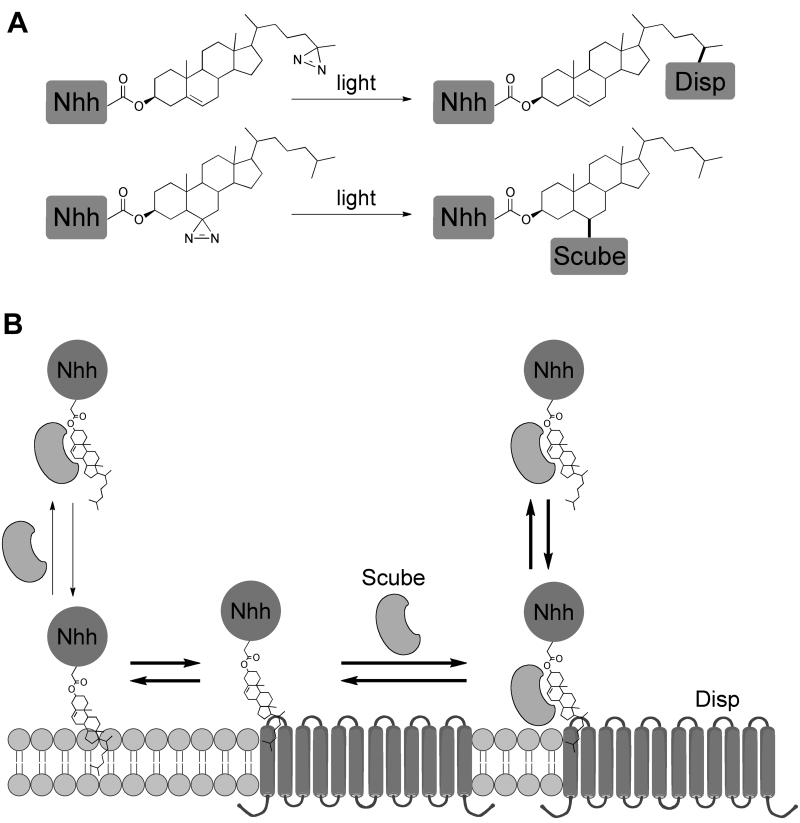
The present study suggests a mechanism for how hShh is released from cells, in spite of its strong association with membranes. HShh has two lipid modifications, a palmityl residue at the N-terminus and a cholesteryl residue at the C-terminus. The cholesteryl moiety is mainly responsible for the insolubility of hShh, which has to be overcome to allow hShh mobilization and long-range Hh signaling. We now show that the multi-spanning membrane protein Dispatched-A (DispA), and the secreted protein Scube2 cooperate to accomplish hShh secretion. We demonstrate that during secretion hShh uses its unique cholesterol modification to interact with DispA and Scube2 via two distinct and synergistic binding events, and that these two interactions are required for hShh secretion. Interestingly, DispA and Scube2 recognize different structural aspects of the cholesterol molecule (Figure 7A), suggesting a hand-off mechanism for transferring hShh from DispA to Scube2. The advantage of such a mechanism is that it ensures hShh is never free in the aqueous environment, thus preventing its precipitation. According to this model, DispA would act in the hShh-synthesizing cell, whereas Scube2 could be provided either by the producing cell or by another cell.
Figure 7. Mechanism of hShh secretion by the DispA and Scube2.
(A) Summary of the results for photocrosslinking of hShh to DispA and to Scube2 in cells. DispA is crosslinked to hShh modified with 25-azicholesterol and Scube2 is crosslinked to hShh modified with 6-azicholestanol, suggesting that DispA and Scube2 recognize different aspects of the cholesterol molecule.
(B) The hShh ligand associates with membranes due to its hydrophobic cholesterol anchor. HShh binds DispA in a cholesterol-dependent manner, and this interaction is required for hShh secretion, perhaps by lowering the activation energy for hShh extraction from the lipid bilayer. From DispA, hShh is transferred to the secreted protein Scube2. The fact that DispA and Scube2 recognize different features of the cholesterol molecule suggests a hand-off mechanism for this transfer, such that the cholesterol anchor of hShh never contacts directly the aqueous environment, and is thus kept soluble. This mechanism is reminiscent of the transfer of free cholesterol between the Niemann-Pick disease proteins NPC1 and NPC2 during cholesterol egress from late endosomes/lysosomes.
Our results are in agreement with previous genetic evidence that showed that long-range Hh signaling absolutely requires Disp in vertebrates (Ma et al., 2002; Nakano et al., 2004) and in insects (Burke et al., 1999), and the Scube family of secreted proteins in zebrafish (Hollway et al., 2006; Johnson et al., 2012; Kawakami et al., 2005; Woods and Talbot, 2005). Both Disp and Scube are not required for short-range Hh signaling, consistent with their demonstrated role in hShh secretion, rather than signal transduction. In addition, consistent with our results, genetic analysis shows that Scube2 acts non-cell autonomously, in contrast to Disp. So far, an essential role for Scube proteins in Hh signaling has only been demonstrated in zebrafish (Johnson et al., 2012), but sequence conservation suggests that the function of Scube is conserved in mammalian Hh signaling.
While this manuscript was under review, another study (Creanga et al., 2012) was published, showing that Scube2 mediates release of cholesterol- and palmityl-modified hShh. Both this study and ours agree that cholesterol but not palmitate is strictly required for hShh release by Scube2; however Creanga et al. show that palmitylation enhances release of hShh by Scube2, which we did not observe in our experiments. The reason for this discrepancy remains to be elucidated. It should be pointed out that Scube-released hShh that lacks the palmitate modification is completely inactive in signaling assays (Creanga et al., 2012).
Our data support the following sequence of events in hShh biosynthesis (Figure 7B). Following autocatalytic cholesterol modification in the endoplasmic reticulum and palmitylation, hShh reaches the plasma membrane, to which it remains strongly attached via its cholesterol anchor. HShh then binds DispA, a cholesterol-dependent interaction that lowers the activation energy required for hShh extraction from the bilayer. Alternatively, hShh could associate with DispA earlier in the secretory pathway, and the two proteins might travel together to the plasma membrane. Binding to DispA is necessary but not sufficient for hShh secretion, because an extracellular binding partner is needed for hShh solubility. Additionally, if DispA binding is too strong, hShh secretion is impaired, as seen with some inactive DispA mutants. In the next step, hShh is transferred from DispA to the secreted protein Scube2, which is required for long-range Hh signaling in zebrafish. Scube2 interacts with the cholesterol anchor of hShh and dramatically enhances hShh secretion by promoting its solubility. Cholesterol modification is sufficient, as demonstrated by the fact that an unrelated protein can be secreted in a Scube2-dependent manner. Although it is possible that Scube and Shh associate before reaching the plasma membrane when synthesized in the same cell, it is clear that this is not required for hShh secretion, as exogenous Scube2 can release hShh displayed on the cell surface.
It should be emphasized that the steps in hShh secretion outlined above represent partitioning equilibria of cholesterol-modified hShh, so that high concentrations of Scube2 solubilize hShh from membranes even without DispA (the equilibrium on the left of Figure 7B). HShh secretion however is greatly enhanced by DispA, as demonstrated by the strong synergy we observed between DispA and Scube2. How DispA synergizes with Scube2 remains to be elucidated. One possibility is that the orientation of the cholesterol anchor of hShh in the membrane favors its transfer to DispA, and is less favorable for a direct transfer to Scube2. Alternatively, Scube2 might bind DispA, which could facilitate the transfer of hShh between the two proteins. Based on homology to RND transporters, it was postulated that DispA acts as a pump (Ma et al., 2002). We speculate that the pumping activity of DispA might be involved in the transfer of hShh to Scube2. It will be important to elucidate the source of energy that DispA uses to pump hShh.
The interaction between Scube2 and hShh is expected to be dynamic, to allow hShh release. Such a transient interaction raises the question of how Scube2 keeps hShh soluble. One model is that hShh is kept soluble by an excess of Scube2, similar to sterol solubilization by complexation with methyl-β-cyclodextrin (MCD), in which sterol-MCD binding is dynamic and a several-fold molar excess of MCD is usually required for keeping the sterol soluble in aqueous solution. In a more complicated model, Scube2 might chaperone hShh and help it form a soluble multimeric species in which the cholesterol anchors are shielded from the aqueous environment by interactions between hShh monomers. A more detailed biochemical and structural analysis of the hShh-Scube interaction will be needed to answer these questions.
Our results suggest that Scube2 is not simply a solubilizing factor. HShh can be released by other agents, such as heparin and serum, but this requires very high concentrations and the released hShh is inactive in signaling assays. Thus, Scube2 interacts with hShh in a specific and efficient manner that maintains hShh activity. This raises the possibility that the hShh-Scube complex is the active species that signals to responding cells. For example, Scube2 might interact with Ptch, a possibility suggested by our finding that the 5E1 antibody, which blocks hShh binding to Ptch does not block hShh binding to Scube2. Alternatively, Scube2 might interact with one of the hShh co-receptors Gas1, Cdo and Boc. Any such interaction could facilitate the delivery of hShh to responding cells.
While cholesterol modification of Hh and the role of Disp are conserved between invertebrates and vertebrates, Scube proteins are only present in vertebrates. How then do invertebrates secrete Hh? A likely possibility is that the role of Scube is fulfilled in invertebrates by another secreted protein or by lipoprotein particles. Lipoproteins have been implicated in systemic transport of Hh in Drosophila (Panakova et al., 2005). It is unclear, however, if lipoproteins are present in the extracellular space of early embryonic tissues, and thus if they play a role in Hh signaling at that stage. A biochemical approach will be required to determine if a secreted protein and/or lipoproteins function in invertebrates in a manner similar to Scube in vertebrates.
The proposed mechanism of hShh secretion is reminiscent of cholesterol trafficking by the Niemann-Pick disease proteins NPC1 and NPC2 (Infante et al., 2008b), which are involved in the egress of cholesterol from late endosomes/lysosomes. Cholesteryl esters are hydrolyzed in the lumen of the lysosome, releasing free cholesterol that binds NPC2, a soluble luminal cholesterol-binding protein. NPC2 transfers cholesterol to NPC1, a multispanning membrane protein in the limiting membrane of the lysosome. Cholesterol is then released from NPC1 into the lysosomal membrane, followed by its transport to other cellular membranes, perhaps through the cytosolic face of the membrane.
A comparison of hShh secretion to cholesterol egress from lysosomes reveals similarities as well as some important differences. DispA and NPC1 are both members of the RND family of transporters, and DispA binds the cholesterol anchor of hShh as NPC1 binds cholesterol (Infante et al., 2008a). While there is no homology between Scube2 and NPC2, they localize to topologically equivalent spaces (the extracellular space for Scube2 and the lumen of the late endosome/lysosome for NPC2), and Scube2 binds the cholesterol anchor of hShh as NPC2 binds cholesterol (Infante et al., 2008b). Importantly, DispA and Scube2 recognize different aspects of the cholesterol molecule (Figure 7A), which parallels the two distinct binding modes of NPC1 and NPC2 to cholesterol (Kwon et al., 2009). However, while DispA appears to recognize the isooctyl tail of cholesterol, NPC1 binds cholesterol with the 3β-hydroxy buried and the isooctyl tail exposed (Kwon et al., 2009). Also, Scube2 appears to not bind the isooctyl tail of cholesterol, while NPC2 binds the isooctyl tail (Kwon et al., 2009). Finally, one important difference is the direction of transport: DispA and Scube2 move hShh from the membrane to the extracellular space, while NPC1 and NPC2 transport cholesterol in reverse, from the lumen of the lysosome into the limiting membrane.
In summary, our results demonstrate a mechanism for hShh secretion that is reminiscent of the egress of free cholesterol from late endosomes/lysosomes. Both processes rely on the recognition of unique aspects of the cholesterol molecule and in both cases a similar mechanism is used to achieve solubility of the hydrophobic ligand. As mutations in NPC proteins cause disease, it seems possible that Disp and Scube could be involved in the pathogenesis of Hh signaling defects, such as those causing holoprosencephaly.
Experimental Procedures
Chemicals
Synthesis of the two photoreactive sterols used in this study is described in the Supplemental Information.
Cell culture
Details of cell culture conditions, DNA constructs, immunofluorescence microscopy, pulse-chase assays, immunoprecipitation, and production of Scube2-condition media are provided in Supplemental Information. To determine if photoreactive sterols modify hShh in vivo, 293T cells stably expressing hShh-HA (Chen et al., 2011) were sterol-depleted with 1% methyl-β-cyclodextrin (MCD) in Dulbecco’s Modified Eagle’s Medium (DMEM) for 45 minutes. Sterols were then added back as soluble MCD complexes. After 3 hours, the cells were harvested and hShh processing was assayed by immunoblotting.
Photocrosslinking in cells
Human 293T cells stably expressing myc-tagged DispA and hShh constructs were sterol-depleted, followed by incubation with sterol-MCD complexes (75 μM for cholesterol and 25-azicholesterol, and 25 μM for 6-azicholestanol) in OptiMEM (Invitrogen), for 2 hours. After washing with OptiMEM, cells were incubated for 6 hours, followed by UV irradiation for 10 minutes on ice. The cells were harvested and DispA constructs were subjected to denaturing immunoprecipitation with anti-myc antibodies (9E10) followed by SDS-PAGE and immunoblotting with rabbit anti-hShhN antibodies (Cell Signaling). A similar protocol was used to detect interaction of hShh to HA-tagged Scube2 in cells.
Photocrosslinking in vitro
A fragment of Drosophila Hedgehog comprising amino acids 244-471 was expressed and purified from bacteria as a soluble maltose-binding protein (MBP) fusion (Chen et al., 2011), and was used to generate photoreactive sterol-modified MBP by in vitro processing. Sterol-modified MBP was incubated for 1 hour with HA-tagged Scube2 or Scube2ΔCUB, followed by UV irradiation for 10 minutes on ice. The samples were analyzed by SDS-PAGE and immunoblotting with HA antibodies.
Secretion assays
293T cells stably expressing hShh were washed several times with DMEM to remove serum, and were incubated in DMEM, with or without the indicated factors. At the indicated time, the culture medium was harvested and secreted protein was analyzed by either precipitation with trichloroacetic acid followed by SDS-PAGE and immunoblotting, or by Hh reporter assays.
Reporter assays
Hh activity was assayed in Shh Light II cells (Taipale et al., 2000). After incubation for 30 hours, firefly luciferase (expressed under control of a Hh-responsive promoter) and Renilla luciferase (expressed under control of a constitutive promoter) were measured using the Dual-Glo kit (Promega). Hh pathway activity was calculated as the firefly/Renilla ratio, normalized to 1 for unstimulated cells. Each experiment was performed in triplicate, and error bars represent standard deviation of the mean. For co-culture assays, 293T cells or DispA-/- MEFs expressing various hShh, DispA and Scube2 constructs were plated together with Shh Light II cells. After 12 hours, serum was washed off and the cells were incubated for 30 hours in serum-free media, followed by luciferase reporter assays.
Supplementary Material
Highlights.
- Hedgehog secretion must overcome insolubility due to modification with cholesterol
- Hedgehog binds Disp and Scube proteins in a cholesterol-dependent manner
- Differential cholesterol recognition by Disp and Scube suggests hand-off mechanism
- Hedgehog secretion similar to sterol trafficking by Niemann-Pick disease proteins
Acknowledgements
We thank P. Beachy for DispA-/- mouse embryonic fibroblasts and T. Rapoport for critical reading of the manuscript. A.S. is supported in part by NIH grant RO1 GM092924.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information Supplemental Information includes Supplemental Experimental Procedures and two figures.
The authors declare no competing financial interests.
References
- Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, Dickson BJ, Basler K. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell. 1999;99:803–815. doi: 10.1016/s0092-8674(00)81677-3. [DOI] [PubMed] [Google Scholar]
- Chamoun Z, Mann RK, Nellen D, von Kessler DP, Bellotto M, Beachy PA, Basler K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–2084. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- Chen MH, Li YJ, Kawakami T, Xu SM, Chuang PT. Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev. 2004;18:641–659. doi: 10.1101/gad.1185804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Tukachinsky H, Huang CH, Jao C, Chu YR, Tang HY, Mueller B, Schulman S, Rapoport TA, Salic A. Processing and turnover of the Hedgehog protein in the endoplasmic reticulum. J Cell Biol. 2011;192:825–838. doi: 10.1083/jcb.201008090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creanga A, Glenn TD, Mann RK, Saunders AM, Talbot WS, Beachy PA. Scube/You activity mediates release of dually lipid-modified Hedgehog signal in soluble form. Genes Dev. 2012;26:1312–1325. doi: 10.1101/gad.191866.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmond S, Larder R, Van Hateren N, Siggers P, Hulsebos TJ, Arkell R, Greenfield A. Cloning, mapping, and expression analysis of a gene encoding a novel mammalian EGF-related protein (SCUBE1) Genomics. 2000;70:74–81. doi: 10.1006/geno.2000.6370. [DOI] [PubMed] [Google Scholar]
- Hollway GE, Maule J, Gautier P, Evans TM, Keenan DG, Lohs C, Fischer D, Wicking C, Currie PD. Scube2 mediates Hedgehog signalling in the zebrafish embryo. Dev Biol. 2006;294:104–118. doi: 10.1016/j.ydbio.2006.02.032. [DOI] [PubMed] [Google Scholar]
- Infante RE, Abi-Mosleh L, Radhakrishnan A, Dale JD, Brown MS, Goldstein JL. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J Biol Chem. 2008a;283:1052–1063. doi: 10.1074/jbc.M707943200. [DOI] [PubMed] [Google Scholar]
- Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci U S A. 2008b;105:15287–15292. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Hall T, Dyson J, Sonntag C, Ayers K, Berger S, Gautier P, Mitchell C, Hollway GE, Currie PD. Scube activity is necessary for Hedgehog signal transduction in vivo. Dev Biol. 2012 doi: 10.1016/j.ydbio.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Kawakami A, Nojima Y, Toyoda A, Takahoko M, Satoh M, Tanaka H, Wada H, Masai I, Terasaki H, Sakaki Y, Takeda H, Okamoto H. The zebrafish-secreted matrix protein you/scube2 is implicated in long-range regulation of hedgehog signaling. Curr Biol. 2005;15:480–488. doi: 10.1016/j.cub.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Kuwabara PE, Labouesse M. The sterol-sensing domain: multiple families, a unique role? Trends Genet. 2002;18:193–201. doi: 10.1016/s0168-9525(02)02640-9. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- Ma Y, Erkner A, Gong R, Yao S, Taipale J, Basler K, Beachy PA. Hedgehog-mediated patterning of the mammalian embryo requires transporter-like function of dispatched. Cell. 2002;111:63–75. doi: 10.1016/s0092-8674(02)00977-7. [DOI] [PubMed] [Google Scholar]
- Mann RK, Beachy PA. Novel lipid modifications of secreted protein signals. Annu Rev Biochem. 2004;73:891–923. doi: 10.1146/annurev.biochem.73.011303.073933. [DOI] [PubMed] [Google Scholar]
- Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the Hedgehog receptor. Nature. 1996;384:176–179. doi: 10.1038/384176a0. [DOI] [PubMed] [Google Scholar]
- Maun HR, Wen X, Lingel A, de Sauvage FJ, Lazarus RA, Scales SJ, Hymowitz SG. Hedgehog pathway antagonist 5E1 binds hedgehog at the pseudo-active site. J Biol Chem. 2010;285:26570–26580. doi: 10.1074/jbc.M110.112284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamed M, Zhang Y, Wang ML, Seemann J, Kwon HJ, Goldstein JL, Brown MS. Identification of luminal Loop 1 of Scap protein as the sterol sensor that maintains cholesterol homeostasis. J Biol Chem. 2011;286:18002–18012. doi: 10.1074/jbc.M111.238311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Kim HR, Kawakami A, Roy S, Schier AF, Ingham PW. Inactivation of dispatched 1 by the chameleon mutation disrupts Hedgehog signalling in the zebrafish embryo. Dev Biol. 2004;269:381–392. doi: 10.1016/j.ydbio.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Panakova D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, Beachy PA. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- Thiele C, Hannah MJ, Fahrenholz F, Huttner WB. Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles. Nat Cell Biol. 2000;2:42–49. doi: 10.1038/71366. [DOI] [PubMed] [Google Scholar]
- Traiffort E, Dubourg C, Faure H, Rognan D, Odent S, Durou MR, David V, Ruat M. Functional characterization of sonic hedgehog mutations associated with holoprosencephaly. J Biol Chem. 2004;279:42889–42897. doi: 10.1074/jbc.M405161200. [DOI] [PubMed] [Google Scholar]
- Tsai MT, Cheng CJ, Lin YC, Chen CC, Wu AR, Wu MT, Hsu CC, Yang RB. Isolation and characterization of a secreted, cell-surface glycoprotein SCUBE2 from humans. Biochem J. 2009;422:119–128. doi: 10.1042/BJ20090341. [DOI] [PubMed] [Google Scholar]
- Tseng TT, Gratwick KS, Kollman J, Park D, Nies DH, Goffeau A, Saier MH., Jr. The RND permease superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. Journal of molecular microbiology and biotechnology. 1999;1:107–125. [PubMed] [Google Scholar]
- Woods IG, Talbot WS. The you gene encodes an EGF-CUB protein essential for Hedgehog signaling in zebrafish. PLoS biology. 2005;3:e66. doi: 10.1371/journal.pbio.0030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.