Abstract
Atherosclerosis, the major cause of myocardial infarction and stroke, is a chronic arterial disease characterized by lipid deposition and inflammation in the vessel wall. Cholesterol, in low-density lipoprotein (LDL), plays a critical role in the pathogenesis of atherosclerosis. Vinpocetine, a derivative of the alkaloid vincamine, has long been used as a cerebral blood flow enhancer for treating cognitive impairment. Recent study indicated that vinpocetine is a potent inflammatory agent. However, its role in the pathogenesis of atherosclerosis remains unexplored. In the present study, we show that vinpocetine significantly reduced atherosclerotic lesion formation in ApoE knockout mice fed with a high-fat diet. In cultured murine macrophage RAW264.7 cells, vinpocetine markedly attenuated oxidized LDL (ox-LDL) uptake and foam cell formation. Moreover, vinpocetine greatly blocked the induction of ox-LDL receptor 1 (LOX-1) in cultured macrophages as well as in the LOX-1 level in atherosclerotic lesions. Taken together, our data reveal a novel role of vinpocetine in reduction of pathogenesis of atherosclerosis, at least partially through suppressing LOX-1 signaling pathway. Given the excellent safety profile of vinpocetine, this study suggests vinpocetine may be a therapeutic candidate for treating atherosclerosis
Keywords: Vinpocetine, Atherosclerosis, oxidized LDL, Macrophage
Introduction
Atherosclerosis is the major trigger of myocardial infarction and stroke, the leading causes of morbidity and mortality in the developed countries. Cholesterol deposition in the artery wall plays a critical role in atherosclerosis [1; 2; 3]. Elevated plasma lipid, particular the low density lipoprotein (LDL) cholesterol, is an important risk factor of atherosclerosis [1; 4], and the clinical benefit of statins in atherosclerosis management is primarily dependent on the cholesterol lowering effect [5]. LDL can pass through endothelium of vessel wall and reside in sub-endothelial space, where LDL can be oxidatively modified to become oxidized LDL (ox-LDL). ox-LDL is the primary trigger of endothelial dysfunction and vascular inflammation, playing a key role in the development and progression of atherosclerosis [2; 6]. Scavenger receptor such as lectin-like oxidized LDL receptor-1 (LOX-1) is a major ox-LDL receptor [6; 7]. LOX-1 is expressed at low levels in healthy vascular cells and up-regulated by many pro-atherogenic stimuli and its agonist ox-LDL [6; 7]. Macrophages in the subendothelial space ingest ox-LDL, become foam cells, and cause fatty streak formation, which represents major pathological characteristics at the early stage of atherogenesis [2; 6].
Vinpocetine is produced by slightly altering the vincamine molecule, an alkaloid extracted from the periwinkle plant, Vinca minor. Vinpocetine was originally discovered and marketed in 1978 under the trade name Cavinton (Hungary). Since then, vinpocetine has been widely used in many countries for the prevention of cerebrovascular disorders and cognitive impairment, including stroke, senile dementia, and memory disturbances [8]. For instance, different types of vinpocetine-containing memory enhancers (Intelectol in Europe and Memolead in Japan) are currently used as dietary supplements worldwide. To date, there have been no reports of its significant side effects, toxicity, or contraindications at the reported therapeutic doses [9]. Vinpocetine is a cerebral vasodilator that improves brain blood flow [10], and a cerebral metabolic enhancer by enhancing oxygen and glucose uptake and increasing neuronal ATP production [11]. Vinpocetine appears to have multiple cellular targets, including cyclical nucleotide phosphodiesterase (PDE1), and voltage-dependent Na+ channels and Ca2+ channels [12]. We have recently found that IKK is a new cellular target of vinpocetine and vinpocetine attenuates TNF-α-induced NF-κB activation and the subsequent induction of proinflammatory mediators in multiple cell types, including vascular smooth muscle cells, endothelial cells and macrophages [13]. More recently, we have demonstrated that vinpocetine is able to suppress intimal hyperplasia by inhibiting vascular smooth muscle cell proliferation and migration [14]. In the present study, we provide evidence to demonstrate that vinpocetine attenuates lipid deposition and atherosclerotic lesion development in a mouse model of atherosclerosis, at least partially through down-regulating LOX-1 expression, inhibiting ox-LDL uptake, and preventing macrophage foam cell formation.
Materials and Methods
Animals
All animals were used in accordance with the guidelines of the National Institutes of Health and American Heart Association for the care and use of laboratory animals. The procedures were performed in accordance with experimental protocols that were approved by the University Committee on Animal Resources at the University of Rochester. Male C57BL/6J ApoE knockout mice (Jackson Laboratories) at age of 8 weeks were fed with the normal chow diet or high-fat diet containing 1.25% cholesterol (Research Diets D12108C) for 16 weeks. Vinpocetine (5 mg/kg of body weight) or same volume of vehicle was administrated via an i.p. route once every other day for 16 weeks as previously described [14].
Atherosclerotic lesion assessment
Atherosclerotic lesion area was quantified by En face Oil-red O staining as described previously [15]. Briefly, mice were anesthetized by i.p. injection with 80 mg kg−1 ketamine and 5 mg kg−1 xylazine, and perfused with saline and 10% neutral buffered formalin (10% NBF), then fixed overnight with 10%NBF. The heart and aortas were carefully removed and cleaned under a dissecting microscope. All peripheral fat and connective tissue was removed. The vessels were then cut open longitudinally in PBS. Aortas were rinsed with 60% isopropanol for 5 min and stained with oil red O solution for 15 min. Vessels were then rinsed with 60% isopropanol for 15 min, followed by several rinse with distilled water. After staining, vessels were precisely kept open with entire luminal surface area faced up on a microscope slide. Images were captured by MZ12.5 microscope (Leica) with a SPOT camera (SPOT Insight 4; Diagnostic Instruments, Inc.). The lesion area was quantified using Image-Pro 6.2 software (Media Cybernetics). For aortic valve lesion quantification, heart was fixed in 10% and incubated with two changes of 30% sucrose-PBS within 48 h at 4°C and embedded into OCT. The cross cryostat sections (6 mm) were cut at 100 μm intervals. Slides were stained with hematoxylin and eosin (H&E) and Images were captured with microscope (BX41, Olympus) and with digital camera (Spot Insight 2, Diagnostic Instruments, Inc.). The lesion area (remodeling area) was quantified using Image-Pro 6.2 software (Media Cybernetics) and 4 sections from each animal were examined.
Blood pressure, serum cholesterol measurement
Blood pressures were measured using a non-invasive tail-cuff procedure and Visitech BP-2000 blood pressure analysis system as described previously [16]. The cholesterol of serum LDL and HDL was measured using HDL and LDL/VLDL Cholesterol Assay Kit (Abcam) according to the manufacturer’s instructions.
Cell culture
Murine RAW264.7 macrophage cell line (ATCC, Rockville, MD) were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS) in a humidified incubator (37°C, 5% CO2).
RNA isolation and RT-PCR
Total cellular RNA was isolated from RAW264.7 cells using an RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. cDNA was synthesized by reverse transcription using Taqman reverse transcription kit (Applied Biosystems) following the manufacturer’s instructions. The real time PCR was performed using iQ™ SYBR Green supermix (BIO-RAD) with LOX-1 primers: 5′-CAAGATGAAGCCTGCGAATGA (forward) and 5′-ACCTGGCGTAATTGTGTCCAC (reverse). The relative quantities of mRNAs were obtained by normalizing with glyceraldehydes-3-phosphate dehydrogenase (GAPDH).
Immunofluorescent staining
Immunostaining were performed as described previously [14]. Briefly, frozen sections were fixed with 4% paraformaldehyde and permeabilized in 0.2% Triton X-100. The sections were blocked with Dako serum-free blocking solution (M0841, Dako) and incubated with primary antibody. The primary antibodies were MAC-2 (CL8942AP, Cedarlane) and LOX-1 (sc-11653, Santa Cruz). The sections were then incubated with fluorescence conjugated secondary antibodies. The nuclear was stained with DAPI. Images were captured with an Olympus (BX-51) fluorescent microscope. The Oil-red O positive area, LOX-1 expression and Mac-2 positive area were quantified using Image-Pro 6.2 software (Media Cybernetics).
Ox-LDL accumulation
To measure fluorescence-labeled ox-LDL (Dil-ox-LDL) uptake and accumulation, RAW264.7 cells were pretreated with different doses of vinpocetine for 24 h in DMEM containing 0.1% FBS, then loaded with 10 μg/ml Dil-ox-LDL (Biomedical Technologies, Inc.) for an additional 4 h. Cells were then washed with PBS and fixed with formalin. Nuclear was stained with DAPI. Images were taken with a BX-51 Olympus fluorescent microscope. To measure regular ox-LDL uptake and accumulation, cells were treated with vinpocetine as described above, then loaded with 50 μg/ml ox-LDL (Biomedical Technologies, Inc.) for 24 h. Cells were then washed, fixed and stained with Oil-red O solution. The Dil-oxLDL and Oil-red O staining intensities were quantified using Image-Pro 6.2 software (Media Cybernetics) and five fields were examined for each sample.
Statistical analysis
Quantitative results are expressed as mean ± SEM or mean ± SD as indicated. All results shown were confirmed by at least three independent experiments. Data were analyzed by one-way ANOVA. *P-values <0.05 was considered statistically significant.
Results
Vinpocetine ameliorates atherosclerosis formation
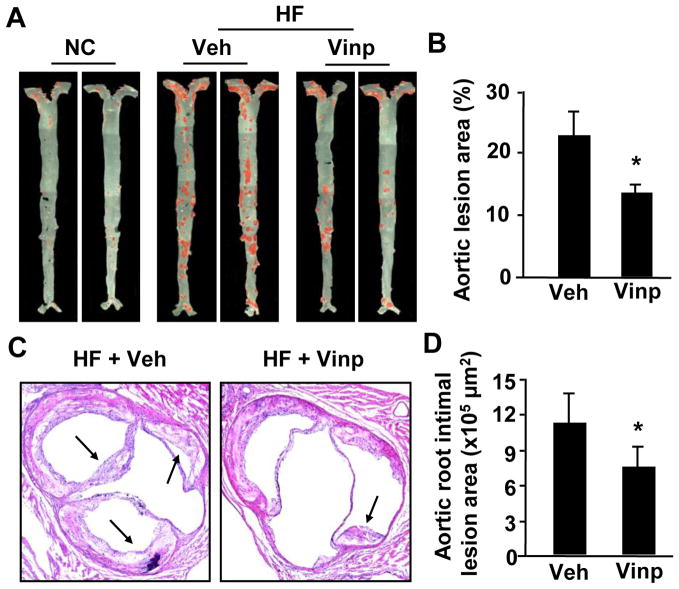
To assess effects of vinpocetine on atherosclerotic formation, we utilized hyperlipidemia-induced atherosclerosis in ApoE knockout mouse, a well-established mouse model of atherosclerosis [17]. ApoE−/− mice were fed with normal chow diet or Western high-fat diet for 16 weeks, and received 5mg/kg vinpocetine or equal volume of vehicle once every two days via i.p, as previously described [14]. The atherosclerotic lesions were evaluated by morphometric analyses of en face arterial trees stained with Oil-red O for lipids. The lesion size was quantified by the percentage of Oil-red O positive areas. As shown in Figure 1, there was a marked decrease in total aortic atherosclerotic lesions in mice fed with high-fat diet compared to normal chow diet (Fig. 1A and B). Vinpocetine treatment significantly reduced Oil-red O stained areas (Fig. 1A and B). In addition, we evaluated the intimal lesion area of aortic sinus by histological analyses and found that vinpocetine also reduced aortic sinus intimal lesions (Fig. 1C and D). These data suggest that vinpocetine ameliorates formation of atherosclerotic lesion.
Figure 1.
Effects of vinpocetine on atherosclerotic lesion formation. (A) Representative En face images of Oil-red O stained aorta from ApoE knockout mice fed with normal chow (NC) or a high-fat diet (HF) for 16 weeks with i.p injection of 5mg/kg/day vinpocetine (Vinp) or vehicle saline (Veh). (B) Quantitative data showing the percentage of atherosclerotic lesion in entire aorta. (C) Representative images of the aortic sinus stained with H&E from mice fed with high-fat diet and injected with vinpocetine or vehicle. (D) Quantitative data of showing intimal lesion areas in the aortic root. Values are means ± SEM (n=7 for both vinpocetine and vehicle groups). *P<0.05 compared with vehicle group.
Moreover, ApoE−/− mice fed with high-fat diet suffered from sickness and weight reduction, which was significantly improved by vinpocetine (Table 1). Importantly, vinpocetine treatment also decreased the death rate from 27% to 8% (Table 1). The protective effects of vinpocetine are not attributed to change of the lipid metabolism because the plasma HLD, LDL, and VLDL levels were not altered by vinpocetine (Table 1). High-fat diet induced blood pressure increase in ApoE−/− mice (Table 1), similarly to previous reported findings [18; 19]. Vinpocetine suppressed blood pressure elevation, which is in line with the vasodilatory effect that vinpocetine has [20]. However, blood pressure does not account for the atherosclerosis development in ApoE−/− mice [19]. Thus the anti-atherogenic effect of vinpocetine is likely mediated by its action on vessel walls.
Table 1.
Serum Cholesterol, Blood Pressure, Body Weight and Death Rate
| NC | Vehicle | Vinpocetine | p | |
|---|---|---|---|---|
| LDL cholesterol (mg/dL) | 215.9 ± 23.9 (7) | 223.8 ± 18.9 (7) | NS | |
| HDL cholesterol (mg/dL) | 6.6 ± 1.3 (7) | 7.0 ± 1.1 (7) | NS | |
| Blood pressure (mmHg) | 113.0 ±2.5 (4) | 124.3 ±1.7 (8) | 116.1 ±2.2 (9) | P<0.05 |
| Body weight (g) | 31.5 ±2.2 (4) | 24.3 ±0.7 (8) | 26.8 ±0.9 (9) | P<0.05 |
| Death rate (%) | 0% | 27.2% | 8.3% |
NS: no significant difference. The digitals in brackets represent animal numbers. Values are means ± SEM. P<0.05 compared with vehicle group.
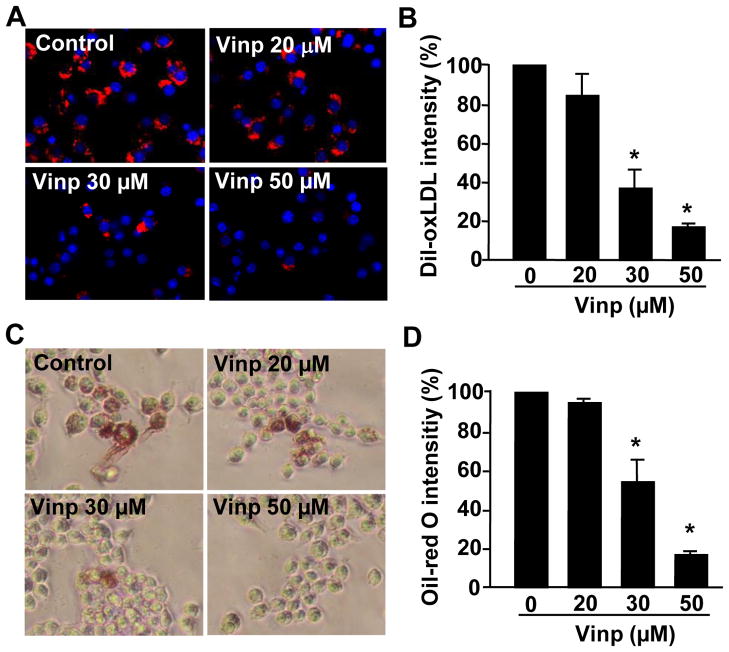
Vinpocetine inhibits ox-LDL uptake and accumulation in macrophages
Macrophage-derived foam cell formation is the hallmark of early stage of atherogenesis. Therefore, we examined the effects of vinpocetine on fluorescence-labeled Dil-ox-LDL as well as regular ox-LDL uptake and accumulation in murine RAW264.7 macrophages. As shown in Figure 2, when macrophages were loaded with Dil-ox-LDL for 4 hours, the red fluorescent intensities reflecting Dil-ox-LDL levels were significantly increased in macrophages, which was dose-dependently attenuated by vinpocetine (Fig. 2A and 2B). Similarly, ox-LDL loading for 24 hours also significantly increased the overall Oil-red O staining, indicating the increased ox-LDL uptake and accumulation in macrophages. Vinpocetine, however, dose-dependently decreased ox-LDL levels in macrophages.
Figure 2.
Effects of vinpocetine on ox-LDL uptake and accumulation in macrophages (A–B). Representative images (A) and quantitative data (B) show that vinpocetine inhibits Dil-ox-LDL uptake and accumulation in RAW264.7 cells. RAW264.7 cells were treated with different doses of vinpocetine for 24 h in DMEM containing 0.1% FBS, then loaded with 10 μg/ml Dil-ox-LDL for an additional 4 h. After nuclear staining with DAPI, cellular Dil-ox-LDL was monitored with a fluorescent microscope. Red: Dil-ox-LDL. Blue: DAPI. (C–D) Representative images (C) and quantitative data (D) show that vinpocetine inhibits regular ox-LDL uptake and accumulation as assessed by Oil-red O staining. Cells were observed with bright-field microscopy. Values are means ± SD from at least three independent experiments. *P<0.05 vs. control with vehicle (zero vinpocetine).
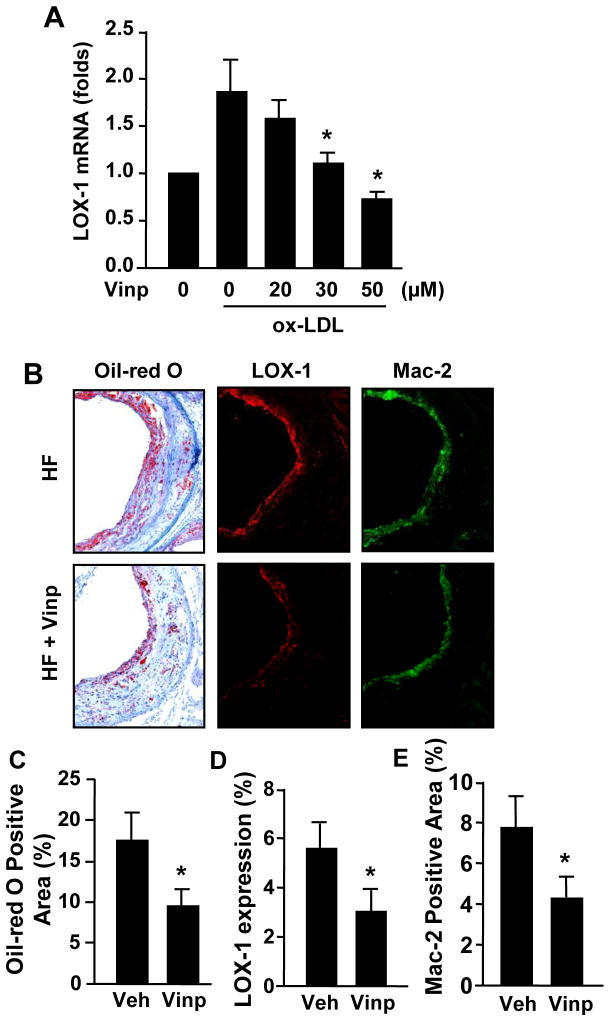
Vinpocetine attenuates LOX-1 expression in vitro and in vivo
Ox-LDL is taken up via scavenger receptors such as LOX-1 and LOX-1 has been shown to play key roles in foam cell formation and in the progression of atherosclerosis [6]. Therefore, we examined the effect of vinpocetine on LOX-1 expression in macrophages. As shown in Figure 3A, LOX-1 mRNA level, as measured by qPCR, was significantly up-regulated by ox-LDL in macrophages, which was blocked by vinpocetine in a dose-dependent manner. In addition, we found that LOX-1 immunostaining intensities were significantly reduced in aortic sinus areas treated with vinpocetine compared to vehicle control (Fig. 3B and D), which was accompanied with decreased Oil-red O staining (Fig. 3B and C) and macrophages marker staining (Fig. 3B and E).
Figure 3.
Effects of vinpocetine on LOX-1 expression in vitro and in vivo. (A) Vinpocetine inhibits ox-LDL-induced LOX-1 expression in macrophages. RAW264.7 cells were starved in 0.1% FBS-starved for 24 h, pretreated with indicated doses of vinpocetine for 1 h, followed by treatment with 50 μg/ml ox-LDL for 24 h, the mRNA levels were measured by real time RT-PCR. Values are means ± SD from at least three independent experiments. *P<0.05 vs. ox-LDL with zero vinpocetine. (B–E) Vinpocetine inhibits LOX-1 expression in vivo. Representative images (B) stained with Oil-red O (left panels), LOX-1 antibody (middle panels), or macrophage marker Mac-2 (right panels), in aortic sinus of ApoE knockout mice fed with a high-fat diet (HF) for 16 weeks with i.p injection of 5 mg/kg/day vinpocetine (Vinp) or vehicle (Veh). (C–E) Quantitative data of Oil-red O, LOX-1 and MAC-2 positive areas. Values are means ± SEM from at least five animals. *P<0.05 compared with saline i.p injection. Magnification: ×100.
Discussion
In the present study, we demonstrate that vinpocetine, when applied systematically, reduces high-fat diet induced atherosclerosis formation in ApoE knockout mice in vivo. We also show that vinpocetine is capable of attenuating ox-LDL uptake and accumulation in macrophages, likely through downregulating ox-LDL receptor LOX-1 expression. These results suggest that vinpocetine-mediated inhibition of LOX-1 expression is at least one of mechanisms by which vinpocetine antagonizes lipid accumulation and atherosclerosis formation. Given that vinpocetine has been proven safe for long-term use, our findings suggest that vinpocetine can be potentially used as attractive therapeutic candidate for atherosclerosis.
Atherogenesis is also a chronic inflammatory process in the vessel wall because atherosclerotic lesions are filled with immune cells that orchestrate and regulate inflammatory responses [2; 3]. Vascular cells such as ECs and VSMCs produce significant amounts of cytokines, chemokines, and adhesion molecules that facilitate immune cell adhesion, migration, and proliferation. In the early stage of atherogenesis, leukocytes are recruited to the vessel wall dependent upon endothelium derived adhesion molecules such as E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1). The adhesion stage is then followed by transmigration of the leukocytes through the endothelial cell layer and accumulation in the subendothelial area, which is governed by chemotactic factors, e.g. monocyte chemotactic peptide-1 (MCP-1) produced in the subendothelial layer derived from multiple cell types including ECs, VSMCs, and intimal monocytes/macrophages. We have previously found that vinpocetine attenuates NF-κB–dependent inflammatory gene expression by inhibiting IκB kinase (IKK), and subsequently suppresses TNF-α–induced proinflammatory mediator expression in multiple cell types, including ECs, VSMCs and macrophages [13]. Specifically we showed that vinpocetine inhibits TNF-α-induced expression of ICAM and VCAM in ECs; VCAM and MCP-1 in VSMCs; as well as IL-1β and MIP2 in macrophages [13]. Moreover, vinpocetine inhibited the monocyte adhesion to ECs and monocyte chemotaxis to VSMCs [13], which are critical in atherogenesis. Therefore, the anti-inflammatory function of vinpocetine may also contribute to its inhibitory role in atherosclerosis.
A great deal of evidence also indicates that ox-LDL and its primary receptor LOX-1 play key roles in the development and progression of atherosclerosis. In addition, LOX-1 is also involved in EC dysfunction, leukocyte adhesion/infiltration, SMC proliferation/apoptosis, and platelet activation, all of which contribute significantly to the pathogenesis of atherosclerosis. For example, LOX-1 deficiency reduced, whereas LOX-1 overexpression augmented atherosclerosis in ApoE deficient mice, respectively [21; 22]. Therefore, LOX-1 represents an attractive therapeutic target for treating atherosclerosis. We showed that vinpocetine is able to inhibit LOX-1 expression in macrophages and VSMCs (data not shown), which at least partially accounts for the anti-atherogenic effect of vinpocetine. The molecular mechanism by which vinpocetine regulates LOX-1 expression is still not fully understood. We have previous shown that vinpocetine blocks NF-kB signaling by acting as an IKK inhibitor [13]. It has been found that NF-kB is an important transcription factor in regulating LOX-1 gene expression [23]. Thus, it is also likely that vinpocetine inhibits IKK/NF-kB signaling and subsequently blocks LOX-1 induction.
Vinpocetine attenuates hyperlipidemia–induced atherosclerosis in a mouse model
Vinpocetine antagonizes ox–LDL uptake and accumulation in macrophages
Vinpocetine blocks the induction of ox–LDL receptor LOX–1 in vitro and in vivo
Acknowledgments
We thank Dr. Jun-ichi Abe (University of Rochester, Rochester, NY) for assistance with real-time PCR. This study was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL111291; HL088400] (to C.Y.); the American Heart Association [Grant 12GRNT2060014] (to C.Y.); and a Shanghai Oriental Scholarship (to C.Y.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. INFLAMMATION OR ATHEROGENESIS. New England Journal of Medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 4.Ballantyne CM. Low-density lipoproteins and risk for coronary artery disease. Am J Cardiol. 1998;82:3Q–12Q. doi: 10.1016/s0002-9149(98)00769-3. [DOI] [PubMed] [Google Scholar]
- 5.Halcox JP, Deanfield JE. Beyond the laboratory: clinical implications for statin pleiotropy. Circulation. 2004;109:II42–8. doi: 10.1161/01.CIR.0000129500.29229.92. [DOI] [PubMed] [Google Scholar]
- 6.Mitra S, Goyal T, Mehta JL, Oxidized LDL. LOX-1 and atherosclerosis. Cardiovasc Drugs Ther. 2011;25:419–29. doi: 10.1007/s10557-011-6341-5. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimoto R, Fujita Y, Kakino A, Iwamoto S, Takaya T, Sawamura T. The discovery of LOX-1, its ligands and clinical significance. Cardiovasc Drugs Ther. 2011;25:379–91. doi: 10.1007/s10557-011-6324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagoly E, Fehér EG, Szapáry L. The role of vinpocetine in the treatment of cerebrovascular diseases based in human studies. Orvosi Hetilap. 2007;148:1353–1358. doi: 10.1556/OH.2007.28115. [DOI] [PubMed] [Google Scholar]
- 9.Balestreri R, Fontana L, Astengo F. A double-blind placebo controlled evaluation of the safety and efficacy of vinpocetine in the treatment of patients with chronic vascular senile cerebral dysfunction. J Am Geriatr Soc. 1987;35:425–30. doi: 10.1111/j.1532-5415.1987.tb04664.x. [DOI] [PubMed] [Google Scholar]
- 10.Tamaki N, Matsumoto S. Agents to improve cerebrovascular circulation and cerebral metabolism--vinpocetine. Nippon Rinsho. 1985;43:376–8. [PubMed] [Google Scholar]
- 11.Szobor A, Klein M. Ethyl apovincaminate therapy in neurovascular diseases. Arzneimittelforschung. 1976;26:1984–9. [PubMed] [Google Scholar]
- 12.Bonoczk P, Gulyas B, Adam-Vizi V, Nemes A, Karpati E, Kiss B, Kapas M, Szantay C, Koncz I, Zelles T, Vas A. Role of sodium channel inhibition in neuroprotection: effect of vinpocetine. Brain Res Bull. 2000;53:245–54. doi: 10.1016/s0361-9230(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 13.Jeon KI, Xu X, Aizawa T, Lim JH, Jono H, Kwon DS, Abe JI, Berk BC, Li JD, Yan C. Vinpocetine inhibits NF-kappaB-dependent inflammation via an IKK-dependent but PDE-independent mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9795–800. doi: 10.1073/pnas.0914414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Y, Knight WE, Guo S, Li JD, Knight PA, Yan C. Vinpocetine suppresses pathological vascular remodeling by inhibiting vascular smooth muscle cell proliferation and migration. J Pharmacol Exp Ther. 2012;343:479–88. doi: 10.1124/jpet.112.195446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nigro P, Satoh K, O’Dell MR, Soe NN, Cui Z, Mohan A, Abe J, Alexis JD, Sparks JD, Berk BC. Cyclophilin A is an inflammatory mediator that promotes atherosclerosis in apolipoprotein E-deficient mice. Journal of Experimental Medicine. 2010 doi: 10.1084/jem.20101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller CL, Oikawa M, Cai Y, Wojtovich AP, Nagel DJ, Xu X, Xu H, Florio V, Rybalkin SD, Beavo JA, Chen YF, Li JD, Blaxall BC, Abe J, Yan C. Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ Res. 2009;105:956–64. doi: 10.1161/CIRCRESAHA.109.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Q. Mouse models of arteriosclerosis: from arterial injuries to vascular grafts. Am J Pathol. 2004;165:1–10. doi: 10.1016/S0002-9440(10)63270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang R, Powell-Braxton L, Ogaoawara AK, Dybdal N, Bunting S, Ohneda O, Jin H. Hypertension and endothelial dysfunction in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 1999;19:2762–8. doi: 10.1161/01.atv.19.11.2762. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Kuhlencordt PJ, Astern J, Gyurko R, Huang PL. Hypertension does not account for the accelerated atherosclerosis and development of aneurysms in male apolipoprotein e/endothelial nitric oxide synthase double knockout mice. Circulation. 2001;104:2391–4. doi: 10.1161/hc4501.099729. [DOI] [PubMed] [Google Scholar]
- 20.Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, Munzel T, Beavo JA, Berk BC, Yan C. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation. 2001;104:2338–43. doi: 10.1161/hc4401.098432. [DOI] [PubMed] [Google Scholar]
- 21.Mehta JL, Sanada N, Hu CP, Chen J, Dandapat A, Sugawara F, Satoh H, Inoue K, Kawase Y, Jishage K, Suzuki H, Takeya M, Schnackenberg L, Beger R, Hermonat PL, Thomas M, Sawamura T. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100:1634–42. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- 22.White SJ, Sala-Newby GB, Newby AC. Overexpression of scavenger receptor LOX-1 in endothelial cells promotes atherogenesis in the ApoE(-/-) mouse model. Cardiovasc Pathol. 2011;20:369–73. doi: 10.1016/j.carpath.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Liu Y, Liu H, Hermonat PL, Mehta JL. Molecular dissection of angiotensin II-activated human LOX-1 promoter. Arterioscler Thromb Vasc Biol. 2006;26:1163–8. doi: 10.1161/01.ATV.0000209998.73303.b5. [DOI] [PubMed] [Google Scholar]