Abstract
Introduction
Expert interpretation of modern noninvasive neuroimaging such as CTA or MRA should detect nearly all aneurysms responsible for an isolated third nerve palsy. Whether a catheter angiogram should still be obtained in cases with negative CTA or MRA remains debated, and mostly relies on whether the noninvasive study was correctly performed and interpreted. The aim of our study was to review the diagnostic strategies used to evaluate patients with isolated aneurysmal third nerve palsy at a large academic center.
Methods
Retrospective review of all cases with posterior communicating artery (PCom A) aneurysmal third nerve palsies seen at our institution since 2001.
Results
We identified 417 cases with third nerve palsy, aneurysm, or subarachnoid hemorrhage, among which 17 presented with an acute isolated painful third nerve palsy related to an ipsilateral PCom A aneurysm (mean age 52; range 33–83 years). Patients were classified into 3 groups based on the results of the noninvasive imaging obtained at initial presentation. Group I included 4 cases with subarachnoid hemorrhage on initial non-contrast head CT initially obtained in an emergency department for evaluation of their isolated third nerve palsy. Group II included 5 cases with isolated third nerve palsy and normal non-contrast head CT at presentation, immediately correctly diagnosed with a PCom A aneurysm at the referring institution. Group III included the 8 remaining cases who all had aneurysms that were missed on noninvasive studies at outside institutions. Review of these outside studies at our institution showed a PCom A aneurysm, confirming misinterpretation of these tests by the outside radiologists, rather than inadequate technique. Absence of specific training in neuroradiology and inaccurate clinical information provided to the interpreting radiologist were associated with test misinterpretation at the outside institutions. The average size of PCom A aneurysms causing an isolated third nerve palsy across all 3 groups was 7.3 mm, and was similar in each group.
Conclusion
Our study suggests that aside from an accurate history, the training and experience of the interpreting radiologist is probably the most important factor in determining the reliability of a noninvasive scan in patients with isolated third nerve palsies.
Introduction
Isolated third nerve palsy can be the sentinel sign of an aneurysm at the junction of the internal carotid artery and posterior communicating artery (PCom A), and evaluation of isolated third nerve palsies remains one of the most challenging situations in neuro-ophthalmology.1–9 Expert interpretation of modern noninvasive neuroimaging such as computed tomographic angiography (CTA) and magnetic resonance angiography (MRA), should detect nearly all aneurysms responsible for an isolated third nerve palsy.9–12 Most clinicians prefer CT/CTA for the initial study in this clinical setting because of CTA’s easy accessibility and rapid acquisition time, although this varies depending on the institution.8,9,11,12 Whether a catheter angiogram should still be obtained in cases of isolated third nerve palsy with negative CTA or MRA remains a difficult decision.8,9 Although recent studies13 have reported a risk of neurologic complications close to zero for diagnostic cerebral angiographies performed within a high volume neuro-interventional practice, the risk of neurologic complications following catheter cerebral angiography was once between 0.9 and 4%14 and, therefore, algorithms trying to avoid routine catheter angiography, especially as a screening test, have been proposed in the past.1,2,5 Recent publications have emphasized the importance of having presumed negative noninvasive vascular imaging studies reviewed by a skilled neuroradiologist before aneurysm is rejected as the cause of the third nerve palsy or before the patient undergoes catheter angiography.9,12
The aim of our study was to review the diagnostic strategies used to evaluate patients with isolated third nerve palsy at our tertiary academic center since 2001, and to examine details of those cases with false negative noninvasive neurovascular imaging studies.
Methods
We performed a retrospective review of all cases with PCom A aneurysmal third nerve palsy seen at our institution from 2001 to 2010. Cases seen prior to 2001 were excluded from this study because high quality noninvasive vascular imaging was not routinely performed prior to that date. Our database was searched for diagnosis codes of third nerve palsy, aneurysm, and subarachnoid hemorrhage and these charts were reviewed in detail. Reports from outside radiology tests were obtained and outside films were reviewed with our neuroradiologists whenever possible. We specifically recorded the clinical information provided to the interpreting radiologist on the initial imaging report. We also inquired whether the interpreting radiologist was specifically trained in neuroradiology or not.
We included all cases that initially presented with a nontraumatic isolated third nerve palsy and were later found to have an ipsilateral PCom A aneurysm. Patients who presented initially with symptoms and signs of subarachnoid hemorrhage or who developed a third nerve palsy postoperatively were excluded. Aneurysms in locations elsewhere (such as the intracavernous internal carotid artery, basilar tip, or cerebellar arteries) usually presented with other neurological findings and were thus excluded. The study was approved by our Institutional Review Board.
Results
Four hundred seventeen cases with third nerve palsy, aneurysm, or subarachnoid hemorrhage were identified. Of the 417 cases reviewed, 17 presented with an acute isolated painful third nerve palsy related to an ipsilateral PCom A aneurysm (mean age 52; range 33–83 years). The characteristics of these 17 patients are detailed in Table 1. Patients were classified into 3 groups based on the results of the noninvasive imaging obtained at initial presentation.
Table 1.
Imaging strategies in 17 patients with isolated third nerve palsy related to an ipsilateral posterior communicating aneurysm. Group I: SAH on initial non-contrast CT; Group II: no SAH but aneurysm correctly initially diagnosed; Group III: no SAH and initial diagnosis missed.
| Patient | Age/ Sex |
First imaging modalities |
SAH on CT |
Initial diagnosis missed |
Clinical indication on radiology report |
Read by neuroradiologist initially |
How correct diagnosis was made |
Side and size of aneurysm in mm* |
|---|---|---|---|---|---|---|---|---|
| I-1 | 60 F | Non-contrast Head CT, then angiogram | Yes | No | “Headache” | No | Initial imaging (angiogram) | Right 6 × 5 × 4 |
| I-2 | 46 M | CT/CTA, then angiogram | Yes | No | “Right pupil dilation, suspicion of aneurysm” | Yes | Initial imaging (CTA) | Right 5.4 × 3.6 |
| I-3 | 59 F | CT/CTA, then angiogram | Yes | No | “Head/retroorbital-paralyzed left eye” | NA | Initial imaging (CTA) | Left 10 × 5 × 4 |
| I-4 | 83 F | CT, MRI/MRA, then angiogram | Yes | No | “Headache with new right III nerve palsy. Evaluate for possible aneurysm” | Yes | Initial imaging (MRI/MRA) | Right 6 × 4.5 × 4.5 |
| II-5 | 70 F | CT/CTA | No | No | “Left CN III palsy” | Yes | Initial imaging (CTA) | Left 6.2 × 3 × 2.7 |
| II-6 | 53 M | MRI/MRA | No | No | NA | NA | Initial imaging (MRI/MRA) | Right 8 × 5 |
| II-7 | 41 F | Non-contrast Head CT, MRI/MRA | No | No | “Stroke, assess for aneurysm” | Yes | Initial imaging (MRI/MRA) | Right 10 × 8 × 7 |
| II-8 | 64 F | Non-contrast Head CT, MRI/MRA | No | No | “Right IIIrd nerve palsy” | Yes | Initial imaging (MRI/MRA) | Right 5.5 × 4.5 × 4.45 |
| II-9 | 54 M | Non-contrast Head CT, then angiogram (artifact from prior surgical clip) | No | No | “Headache with history of cerebral aneurysm” | Yes | Angiogram | Left 9.6 × 8.1 × 5.8 |
| III-10 | 55 F | Non-contrast head CT; MRI/MRA | No | Yes (misread) | "Headache with right Horner" | No | Review of outside MRI/MRA showed aneurysm on source images | Right 5 × 3 × 3 |
| III-11 | 53 F | Non-contrast head CT, then MRI/MRA | No | Yes (misread) | NA | NA | Review of outside MRI/MRA | Right NA |
| III-12 | 50 F | CT/CTA, MRI/MRA | No | Yes (misread) | "Headaches; evaluate for possible aneurysm" | Yes (but mostly performs general radiology) | Review of outside CT/CTA. Outside MRI poor quality. | Left 6 × 3 |
| III-13 | 39 F | Non-contrast head CT; MRI/MRA | No | Yes (misread) | “R/o aneurysm right eyelid droop and right-sided headache” | No | Review of outside MRI/MRA showed aneurysm on source images | Right Bilobed: 9.4 total 7.1 × 4.4 × 3.6 2.3 × 2 × 1.8 |
| III-14 | 40 F | Non-contrast head CT; then MRI/MRA | No | Yes (misread) | “TIA, hypertension” | No | Review of outside MRI/MRA | Left Bilobed: 12 total 3 × 3 8 × 6 |
| III-15 | 33 F | Non-contrast head CT; MRI/MRA | No | Yes | “Right-sided headache” | No | Outside MRI/MRA not available for review. Diagnosis on angiogram | Right 6 × 3 |
| III-16 | 46 F | MRI; then CT with contrast; then angiogram | No | Yes (misread) | “Migraine headache” and “CVA” | No | Review of initial imaging (contrast CT). | Left 3.5 × 2.5 × 2.5 |
| III-17 | 37 F | CT with and without contrast, MRI/MRA; then CTA | No | Yes (misread) | “Headache, Horner syndrome” | No | Review of outside MRI/MRA. | Left 7.7 × 4.7.4.2 |
R/o: rule out; CT: computed tomography. CTA: CT-angiography; MRI: magnetic resonance imaging; MRA: magnetic resonance angiography; CN: cranial nerve; PCOM: posterior communicating artery; TIA: transient ischemic attack; CVA: cerebrovascular accident. NA: not available.
Size of aneurysm in mm, as measured on catheter angiography (angiogram refers to catheter angiography).
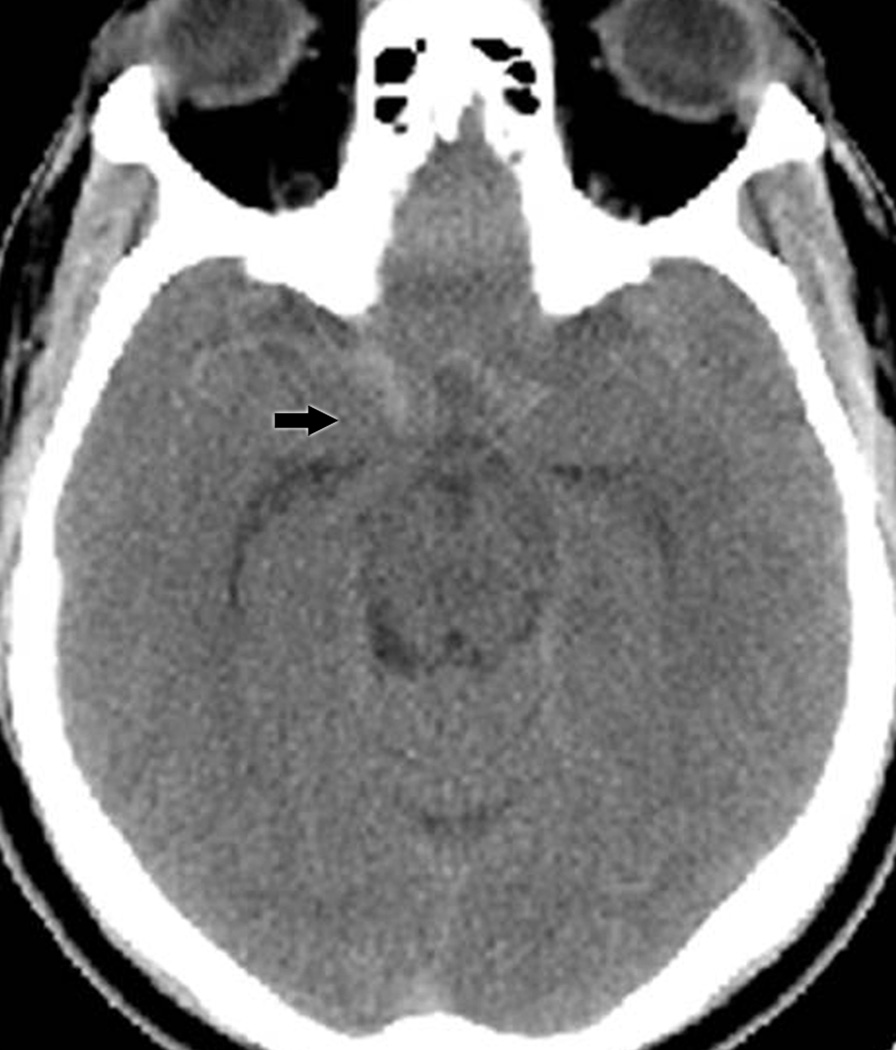
Group I included 4 cases that were found to have evidence of subarachnoid hemorrhage on initial non-contrast head CT obtained in an emergency department for evaluation of their isolated third nerve palsy (Table 1, patients I-1 to I-4; Figure 1A). The presence of subarachnoid hemorrhage on CT facilitated the immediate correct diagnosis of aneurysmal third nerve palsy in all 4 patients. The mean age of these 4 subarachnoid hemorrhage patients was 59.5 years (range 46–83 years). The size range (greatest dimension, as measured on catheter angiography or intra-operatively) of aneurysms in Group I was 5.4 to 10 mm.
Figure 1.
A: Non-contrast head CT showing subarachnoid hemorrhage (arrow) in a patient with an isolated painful right third nerve palsy (Group I).
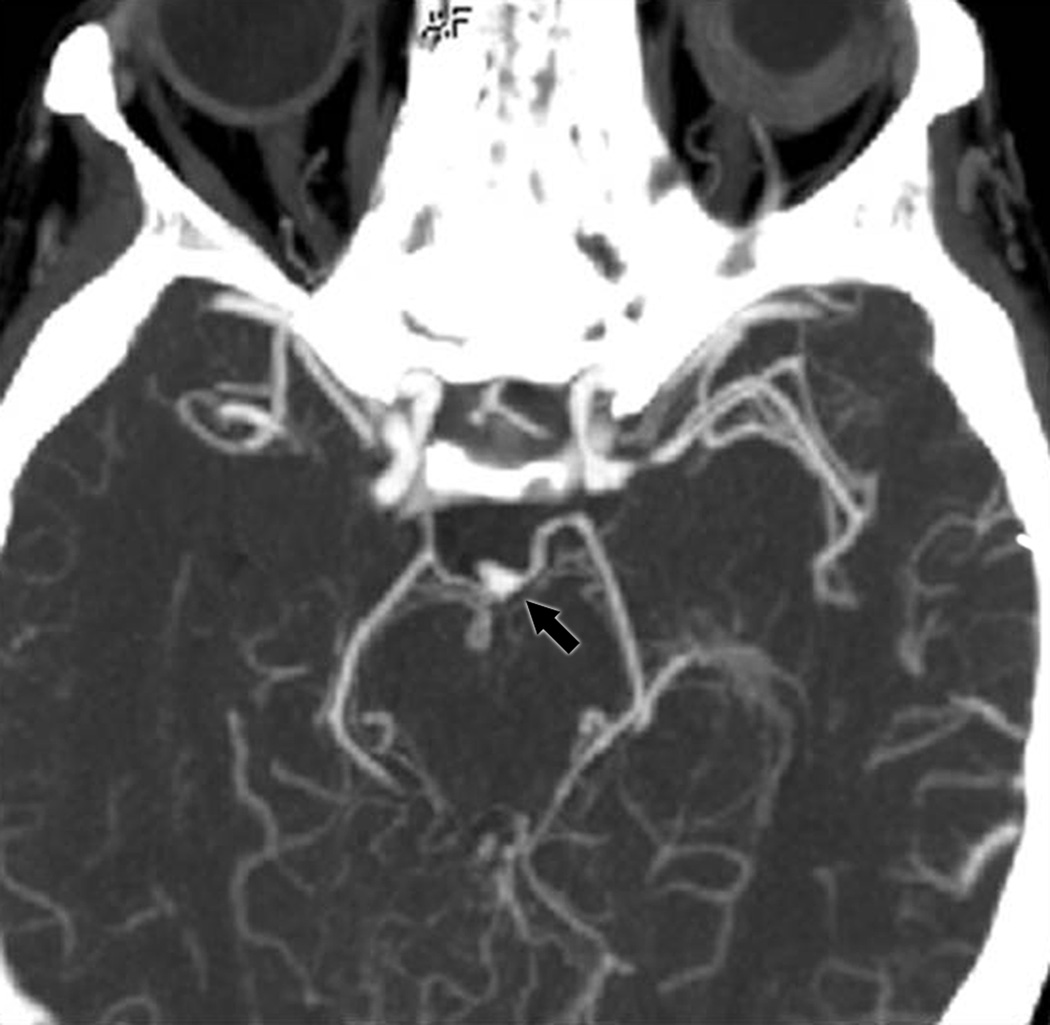
B: CTA demonstrating a left posterior communicating artery aneurysm (arrow) in a patient from Group II.
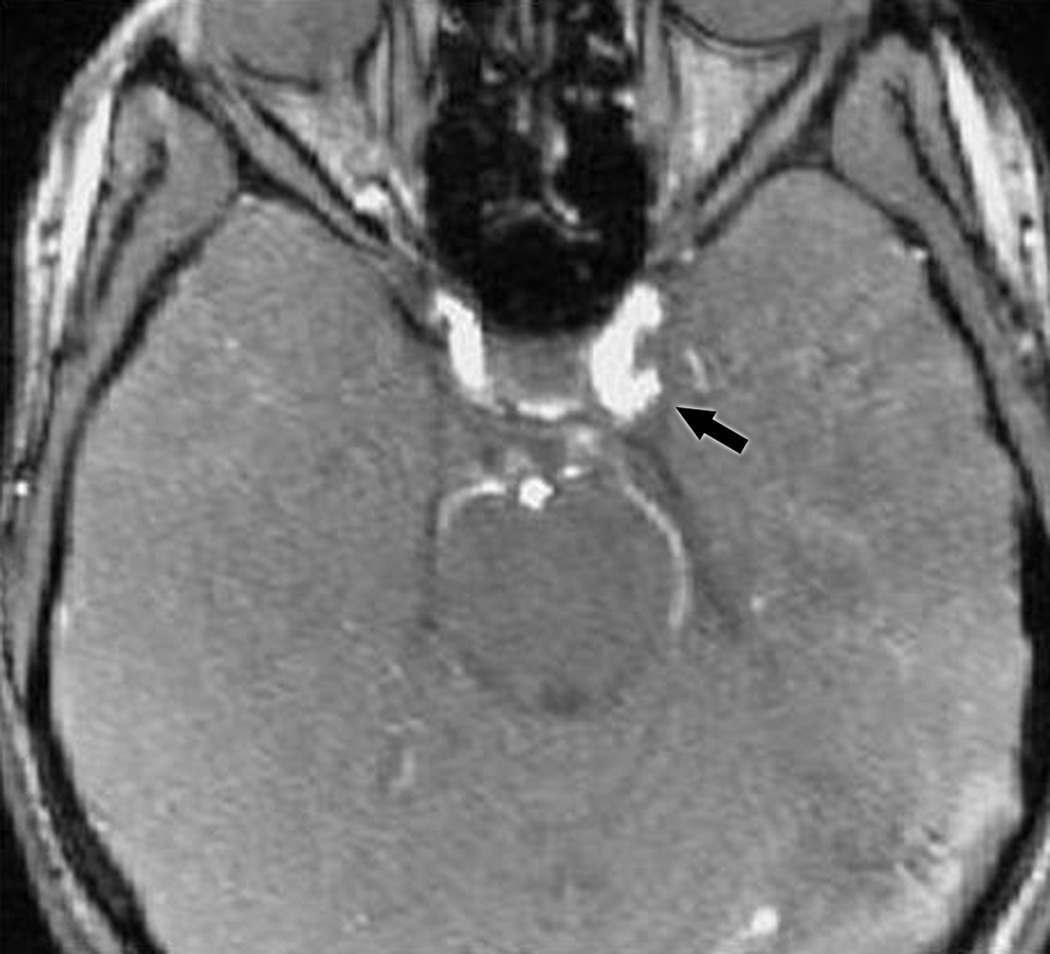
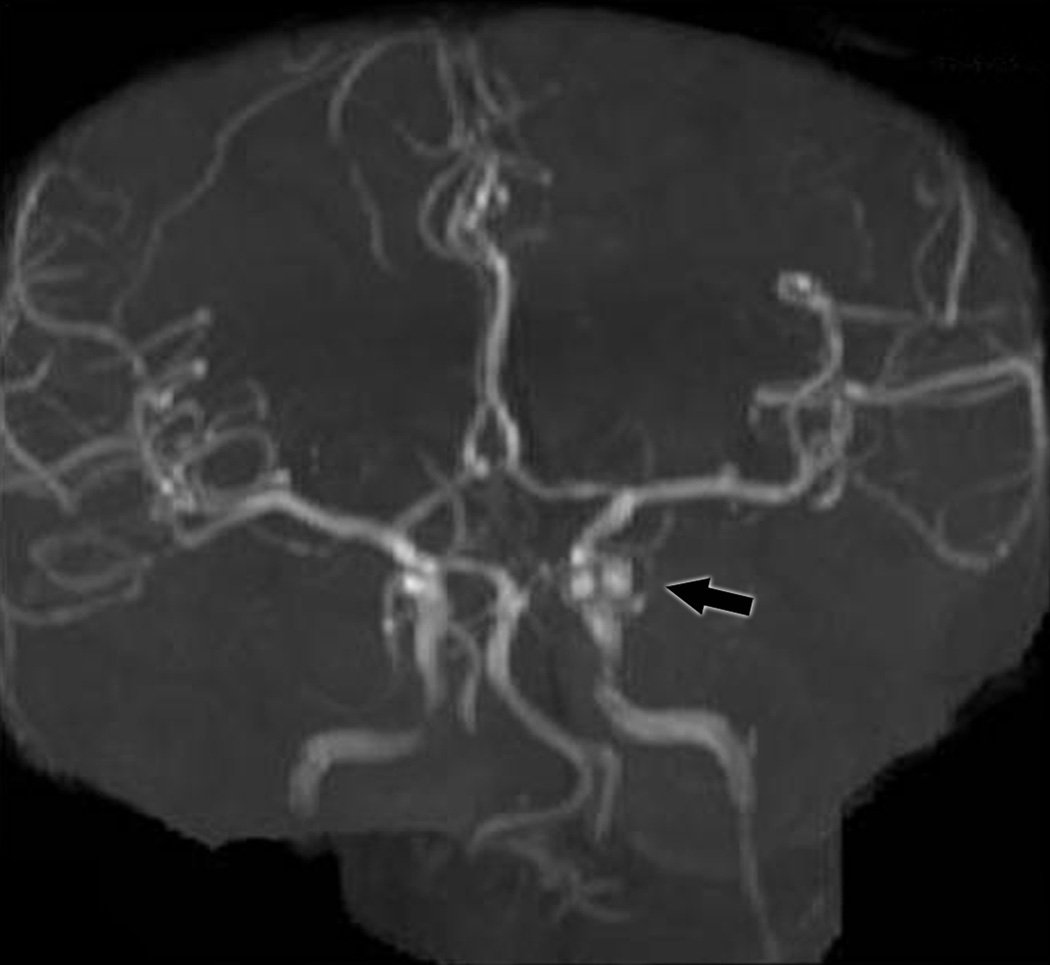
C: (top) MRA reformatted image from a patient in Group III showing a posterior communicating artery aneurysm (arrow); (bottom) MRA source image from the same patient showing the aneurysm (arrow).
Group II included 5 cases with isolated third nerve palsy and normal head CT without contrast at presentation, immediately correctly diagnosed with a PCom A aneurysm (Table 1, patients II-5 to II-9; Figure 1B). Of these 5 patients, 4 patients required only noninvasive vascular imaging for immediate correct diagnosis of aneurysm (1 CTA, 3 MRI/MRA). The one patient diagnosed by direct catheter angiography could not have a brain MRI because of previous history of anterior communicating artery aneurysmal clipping, which also created an artifact, making the CT difficult to interpret. The mean age of these 5 patients was 56.4 years (range 41–70 years). Aneurysms in Group II ranged in size from 5.5 to 10 mm.
Group III included the 8 remaining cases (Table 1, patients III-10 to III-17; Figure 1C) who all had aneurysms that were missed on noninvasive studies. These 8 patients were all initially evaluated at outside institutions. In one case (# III-15, Table 1), the patient was transferred from an outside hospital because of high clinical suspicion of aneurysm despite “negative” noninvasive vascular imaging. The outside studies were not available for review and a catheter angiogram was performed immediately upon arrival to our institution, which revealed a PCom A aneurysm. In all 7 other cases (# III-10 to III-14; III-16, and III-17, Table 1), review of the outside noninvasive vascular studies by our institutional personnel, including neuro-ophthalmologists, neurologists, neuroradiologists or neurosurgeons, allowed identification of a PCom A aneurysm, confirming misinterpretation of these tests by the outside radiologists, rather than inadequate technique. Two of these patients had normal-appearing reconstructed MRA images, but an aneurysm was detected on the source images. Five of the seven misread noninvasive imaging studies were performed and read by general radiologists who had no neuroradiology training. In one case, the interpreting radiologist had received neuroradiology training 20 years prior, but had been practicing mostly as a general radiologist for many years. We could not determine the training of the radiologist in one case (III-11), but the test had been performed at a general radiology center with no special expertise in neuroradiology. Review of the indications for these misinterpreted studies in the patients’ medical records revealed that in 6/7 cases, the radiologist performing and interpreting the test was given vague, or wrong, clinical history. Misleading clinical indications included “headache, Horner”, “transient ischemic attack, hypertension”, “headache”, and “rule out aneurysm” without mention of the side or location of the suspected aneurysm (Table 1). The mean age for Group III was 44.1 years (range 33–55 years). The size range for the aneurysms that were initially missed (Group III) was 3.5 mm (case III-16) to a bilobed 12 mm aneurysm (case III-14) (Table 1).
The average greatest dimension of the aneurysms in each group was 6.9 mm for Group I, 7.9 mm for Group II, and 7.1 mm for Group III. A one way ANOVA test showed no statistically significant difference of aneurysm size among the 3 groups (p=0.33). The average size of PCom A aneurysms causing an isolated third nerve palsy across all 3 groups was 7.3 mm.
Discussion
Our study confirms that interpretation of noninvasive neurovascular imaging is not easy and that a negative result can only be trusted after verifying that the interpreting radiologist is aware of the correct clinical indication for the study and has appropriate training and experience. Many radiology centers in the United States are equipped with state of the art CT scanners and MRI machines, but only a few benefit from experienced technologists able to appropriately manipulate the raw data, and from neuroradiologists specifically trained to interpret noninvasive neurovascular imaging studies.12 In centers without skilled personnel, patients with an isolated non-traumatic third nerve palsy, should likely undergo a catheter angiogram, which remains the gold standard for the diagnosis of intracranial aneurysms. Unfortunately, it is likely that these centers are also the ones with the highest risk of catheter angiography-related complications because of their lack of experience with invasive neurovascular imaging.13,14 Intracranial aneurysms presenting with an isolated third nerve palsy are relatively rare,9–11,15 and only high volume centers with experienced neuroradiologists, interventional neuroradiologists and vascular neurosurgeons have enough experience to efficiently “rule out” an aneurysm on noninvasive vascular imaging. Even at our highly specialized center, we could only identify 17 cases with isolated aneurysmal third nerve palsy over a period of 9 years. Many more aneurysmal third nerve palsies presented to our Emergency and Neurosurgery Departments over the same time period, but these were not isolated, manifesting the more typical symptoms and signs of aneurysmal rupture.
In theory, noninvasive vascular imaging (CTA or MRA) should be sensitive enough to detect nearly all aneurysmal third nerve palsies.9–12 Indeed, the smallest PCom A aneurysm reported to presumably cause a third nerve palsy was 3 mm, and was missed initially on MRA.16 With this case and our case III-16 as very rare exceptions, most reports have maintained that a PCom A aneurysm needs to be at least 4 mm to cause a third nerve palsy, within the range of highest sensitivity for both current MRA and CTA machines.3,4,6,9–11,17,18 The sensitivity of 1.5 T MRA for aneurysms larger than 5 mm has improved to at least 95%, whereas the sensitivity of 1.5T MRA has been reported to be much lower (around 50%) for smaller aneurysms,10,19 the latter unlikely to cause a third nerve palsy. The sensitivity of 3T MRA is only slightly better than with 1.5 T MRA.19 Interestingly, MRA of 7 T might not significantly increase the sensitivity over the standard 1.5 T MRA in diagnosing smaller aneurysms.20 Although the sensitivity for aneurysm detection itself is not significantly improved, however, 3 T and even 7 T MRA will likely be more desirable for evaluation of aneurysms in the future, especially once more widely available, because of the higher image quality and improved signal to noise, higher blood-to-background contrast, and more rapid acquisition times.20 The availability of 64-section multidetector CTA in recent years, compared with the previously used 4- or 16-section CTA, has dramatically improved the quality of the CTA examination, and in turn, the sensitivity of CTA in the detection of small aneurysms.11,21,22 With improved spatial and temporal resolution and reduced slice thickness, the sensitivity of CTA for aneurysms above 3 mm is 99–100%.23 In addition, rapid acquisition time makes CTA practical for emergency evaluation of cerebral aneurysms. However, unless the interpreting physician improves his/her ability for aneurysm detection, these advancements in imaging will not be beneficial.
In 4/17 of our cases (Group I, Table 1), there was evidence of subarachnoid hemorrhage on the initial non-contrast CT obtained emergently, and the diagnosis of aneurysm was easily made. Indeed, in the presence of subarachnoid hemorrhage, the index of clinical suspicion for intracranial aneurysm is very high, which improves the chance that the correct sequence of imaging studies will be performed, and that the radiologist will meticulously evaluate the images in the expected location and identify the aneurysm. The lack of subarachnoid hemorrhage makes the identification of an aneurysm more difficult. The correct diagnosis was immediately made on initial noninvasive vascular imaging in 5/13 cases without subarachnoid hemorrhage (Group II). Some of these studies were performed at various institutions (including ours) and nothing differentiated these cases from those that were missed. Interestingly, the patients in Group II (correctly diagnosed) were older than those in Group III (missed), and perhaps should have been less suspected of harboring an aneurysm and more suspected to suffer a microvascular third nerve palsy. The aneurysms’ sizes were similar in Groups II and III.
All 8 patients with an initial misread as negative noninvasive neurovascular study (Group III) had studies performed at outside institutions. When considering these 8 patients with missed aneurysms, our first reaction was to blame the technical quality of the test obtained. However, we were able to easily identify the missed aneurysm on all reviewed outside imaging studies (on a CD, or plain films, without the ability to reformat the images), suggesting that in all reviewed cases, noninvasive imaging (both CTA and MRA) performed at various institutions was of adequate quality to correctly identify the PCom A aneurysms causing a compressive third nerve palsy. Because of this finding, we chose not to evaluate or report in detail the techniques used to perform the CTAs and MRAs at outside institutions, but rather focused on the interpretation of the studies themselves.
Detection of intracranial aneurysm on noninvasive neurovascular studies requires not only expertise but also time. It is crucial to carefully examine the source data in addition to the post-processed reconstructed images. Manual alteration of the window levels and widths on an interpreting workstation can also improve aneurysmal detection. These steps can only be performed if the interpreting radiologist was given the correct clinical history (e.g., third nerve palsy) and knew the side of the suspected third nerve palsy. Interestingly, review of the indications for the misinterpreted studies in the patients’ medical records revealed that in 6/7 cases, the radiologist performing and interpreting the test was given vague, or wrong, clinical history. Misleading clinical indications included “headache, Horner”, “transient ischemic attack, hypertension”, and “headache”, without mention of an acute third nerve palsy, or “rule out aneurysm”, without mention of the side or location of the suspected aneurysm (Group III, Table 1). It is not surprising that when the history is vague or inaccurate, the radiologist may not focus as carefully on the expected location of the aneurysm and may miss aneurysms that are sometimes seen only on one CTA slice or MRA source image.
Aside from an accurate history, the training and experience of the interpreting radiologist is probably the most important factor in determining the reliability of a noninvasive scan interpretation.12 Most (5/7) misread noninvasive imaging studies were interpreted by general radiologists who had no formal neuroradiology training. In one case, the interpreting radiologist had received neuroradiology training 20 years prior, but had been practicing mostly as a general radiologist for many years. White et al24 compared the detection of intracranial aneurysms on CTA and MRA by neuroradiologists versus “observers”, which included a neurosurgeon, general radiologist and radiographer with experience in looking at the neuraxis, but no formal neuroradiology training. Neuroradiologists were consistently more accurate. For aneurysms larger than 5 mm on CTA, accuracy for neuroradiologists was 100%, whereas accuracy for the observers was 86–93%. For MRA, accuracy was 93–100% for neuroradiologists and 86–100% for the observers. In another study25 in which neuroradiologists reinterpreted head and neck imaging in a multidisciplinary cancer center, a change in interpretation occurred in 41% of images, altering management in 98% and prognosis in 95%, most with a worse prognosis, confirming that neuroradiology certification improves interpretation skills of head imaging dramatically. Currently in the United States, neuroradiology expertise is gained through a one year Accreditation Council for Graduate Medical Education (ACGME) certified neuroradiology fellowship that follows the traditional 4 year radiology residency. Most larger academic radiology departments offer a one year neuroradiology fellowship. Radiology residents usually apply for these fellowships in the third year of the four year radiology residency.
Despite its small sample, our study confirms that while technical improvements in noninvasive neurovascular imaging techniques, including CTA and MRA, have substantially increased their sensitivity in detecting intracranial aneurysms, the most important step in imaging remains interpretation, which is entirely dependent on the training, skills and experience of the interpreting radiologist. Appropriate communication of the correct clinical information and level of suspicion of an aneurysmal third nerve palsy is essential. Numerous previous reports have emphasized the paucity of trained technologists capable of advanced manipulation of the raw data, and of certified neuroradiologists to interpret the images.12 Trobe9 recently stated that determining whether a negative report is reliable is the hard part when evaluating a patient with isolated third nerve palsy. Our study suggests that to avoid diagnostic mistakes, all negative noninvasive studies should be reviewed with an experienced neuroradiologist before rejecting aneurysmal compression as the cause of the third nerve palsy, or before ordering a catheter angiogram in cases with high clinical suspicion for an aneurysmal third nerve palsy. This is obviously institution dependent, and delays resulting from patient or image transfer to a specialized institution must be balanced against the risks of immediate catheter angiogram.
Acknowledgements
This study was supported in part by a departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc. New York, NY, and by NIH grants KL2-RR025009 (Dr. Bruce) and UL1-RR025008 (Drs. Bruce and Biousse). Dr. Newman is the recipient of a Research to Prevent Blindness Lew R. Wasserman Merit Award.
Footnotes
Disclosure: The authors report no proprietary or commercial interest in the topics discussed.
References
- 1.Trobe JD. Isolated pupil-sparing third nerve palsy. Ophthalmology. 1985;92:58–61. doi: 10.1016/s0161-6420(85)34067-8. [DOI] [PubMed] [Google Scholar]
- 2.Trobe JD. Third nerve palsy and the pupil. Footnotes to the rule. Arch Ophthalmol. 1988;106:601–602. doi: 10.1001/archopht.1988.01060130655019. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson DM, Trobe JD. The emerging role of magnetic resonance angiography in the management of patients with third cranial nerve palsy. Am J Ophthalmol. 1999;128:94–96. doi: 10.1016/s0002-9394(99)00107-5. [DOI] [PubMed] [Google Scholar]
- 4.Lee AG, Hayman LA, Brazis PW. The evaluation of isolated third nerve palsy revisited: an update on the evolving role of magnetic resonance, computed tomography, and catheter angiography. Surv Ophthalmol. 2002;47:137–157. doi: 10.1016/s0039-6257(01)00303-4. [DOI] [PubMed] [Google Scholar]
- 5.Bruce BB, Biousse V, Newman NJ. Third nerve palsies. Semin Neurol. 2007;27:257–268. doi: 10.1055/s-2007-979681. [DOI] [PubMed] [Google Scholar]
- 6.Schultz KL, Lee AG. Diagnostic yield of the evaluation of isolated third nerve palsy in adults. Can J Ophthalmol. 2007;42:110–115. [PubMed] [Google Scholar]
- 7.Vaphiades MS, Cure J, Kline L. Management of intracranial aneurysm causing a third cranial nerve palsy: MRA, CTA or DSA? Semin Ophthalmol. 2008;23:143–150. doi: 10.1080/08820530801978534. [DOI] [PubMed] [Google Scholar]
- 8.Lee AGBP. Clinical evaluation for aneurysm in patients with third cranial nerve palsy. Expert Rev Ophthalmol. 2009;4:547–552. [Google Scholar]
- 9.Trobe JD. Searching for brain aneurysm in third cranial nerve palsy. J Neuroophthalmol. 2009;29:171–173. doi: 10.1097/WNO.0b013e3181b4a2f7. [DOI] [PubMed] [Google Scholar]
- 10.Kupersmith MJ, Heller G, Cox TA. Magnetic resonance angiography and clinical evaluation of third nerve palsies and posterior communicating artery aneurysms. J Neurosurg. 2006;105:228–234. doi: 10.3171/jns.2006.105.2.228. [DOI] [PubMed] [Google Scholar]
- 11.Mathew MR, Teasdale E, McFadzean RM. Multidetector computed tomographic angiography in isolated third nerve palsy. Ophthalmology. 2008;115:1411–1415. doi: 10.1016/j.ophtha.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhary N, Davagnanam I, Ansari SA, Pandey A, Thompson BG, Gemmete JJ. Imaging of intracranial aneurysms causing isolated third cranial nerve palsy. J Neuroophthalmol. 2009;29:238–244. doi: 10.1097/WNO.0b013e3181b415f4. [DOI] [PubMed] [Google Scholar]
- 13.Thiex R, Norbash AM, Frerichs KU. The safety of dedicated-team catheter-based diagnostic cerebral angiography in the era of advanced nonivasive imaging. AJNR. 2010;31:230–234. doi: 10.3174/ajnr.A1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann TJ, Huston J, 3rd, Mandrekar JN, Schleck CD, Thielen KR, Kallmes DF. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology. 2007;243:812–819. doi: 10.1148/radiol.2433060536. [DOI] [PubMed] [Google Scholar]
- 15.Richards BW, Jones FR, Jr, Younge BR. Causes and prognosis in 4,278 cases of paralysis of the oculomotor, trochlear, and abducens cranial nerves. Am J Ophthalmol. 1992;113:489–493. doi: 10.1016/s0002-9394(14)74718-x. [DOI] [PubMed] [Google Scholar]
- 16.Ross JS, Masaryk TJ, Modic MT, Ruggieri PM, Haacke EM, Selman WR. Intracranial aneurysms: evaluation by MR Angiography. AJNR. 1990;11:449–456. [PMC free article] [PubMed] [Google Scholar]
- 17.Yanaka K, Matsumaru Y, Mashiko R, Hyodo A, Sugimoto K, Nose T. Small unruptured cerebral aneurysms presenting with oculomotor nerve palsy. Neurosurgery. 2003;52:553–557. doi: 10.1227/01.neu.0000047816.02757.39. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 18.Friedman JA, Piepgras DG, Pichelmann MA, Hansen KK, Brown RD, Jr, Wiebers DO. Small cerebral aneurysms presenting with symptoms other than rupture. Neurology. 2001;57:1212–1216. doi: 10.1212/wnl.57.7.1212. [DOI] [PubMed] [Google Scholar]
- 19.Li MH, Cheng YS, Li YD, et al. Large-cohort comparison between three-dimensional time-of-flight magnetic resonance and rotational digital subtraction angiographies in intracranial aneurysm detection. Stroke. 2009;40:3127–3129. doi: 10.1161/STROKEAHA.109.553800. [DOI] [PubMed] [Google Scholar]
- 20.Monninghoff C, Maderwald S, Theysohn JM, et al. Evaluation of intracranial aneurysms with 7 T versus 1.5 T time-of-flight MR angiography - initial experience. Rofo. 2009;181:16–23. doi: 10.1055/s-2008-1027863. [DOI] [PubMed] [Google Scholar]
- 21.McKinney AM, Palmer CS, Truwit CL, Karagulle A, Teksam M. Detection of aneurysms by 64-section multidetector CT angiography in patients acutely suspected of having an intracranial aneurysm and comparison with digital subtraction and 3D rotational angiography. AJNR Am J Neuroradiol. 2008;29:594–602. doi: 10.3174/ajnr.A0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKinney AM, Truwit CL, Palmer CS, Teksam M. Intracranial aneurysms: is the diagnostic accuracy rate of multidetector CT angiography equivalent to that of three-dimensional rotational conventional angiography? Radiology. 2008;246:982. doi: 10.1148/radiol.2463071392. author reply -3. [DOI] [PubMed] [Google Scholar]
- 23.Li Q, Lv F, Li Y, Luo T, Li K, Xie P. Evaluation of 64-section CT angiography for detection and treatment planning of intracranial aneurysms by using DSA and surgical findings. Radiology. 2009;252:808–815. doi: 10.1148/radiol.2523081911. [DOI] [PubMed] [Google Scholar]
- 24.White PM, Wardlaw JM, Lindsay KW, Sloss S, Patel DK, Teasdale EM. The noninvasive detection of intracranial aneurysms: are neuroradiologists any better than other observers? Eur Radiol. 2003;13:389–396. doi: 10.1007/s00330-002-1520-1. [DOI] [PubMed] [Google Scholar]
- 25.Loevner LA, Sonners AI, Schulman BJ, et al. Reinterpretation of cross-sectional images in patients with head and neck cancer in the setting of a multidisciplinary cancer center. AJNR Am J Neuroradiol. 2002;23:1622–1626. [PMC free article] [PubMed] [Google Scholar]