Abstract
Purpose
Glioblastoma (GBM) is the most aggressive human primary brain tumor and is currently incurable. Immunotherapies have the potential to target GBM stem cells, which are resistant to conventional therapies. Human epidermal growth factor receptor 2 (HER2) is a validated immunotherapy target and we determined if HER2-specific T cells can be generated from patients with disease that will target autologous HER2-positive GBMs and their CD133-positive stem cell compartment.
Experimental design
HER2-specific T cells from 10 consecutive GBM patients were generated by transduction with a retroviral vector encoding a HER2-specific chimeric antigen receptor (CAR). The effector function of HER2-specific T cells against autologous GBM cells, including CD133-positive stem cells, was evaluated in vitro and in an orthotopic murine xenograft model.
Results
Stimulation of HER2-specific T cells with HER2-positive autologous GBM cells resulted in T-cell proliferation and secretion of IFN-γ and IL-2 in a HER2-dependent manner. Patients’ HER2-specific T cells killed CD133-positive and CD133-negative cells derived from primary HER2-positive GBMs, whereas HER2-negative tumor cells were not killed. Injection of HER2-specific T cells induced sustained regression of established autologous GBM xenografts established in the brain of SCID mice.
Conclusions
Gene transfer allows the reliable generation of HER2-specific T cells from GBM patients, which have potent antitumor activity against autologous HER2-positive tumors including their putative stem cells. Hence, the adoptive transfer of HER2-redirected T-cells may be a promising immunotherapeutic approach for GBM.
Keywords: Glioblastoma multiforme, GBM, immunotherapy, stem cells, progenitors, HER2/neu, ERBB2, chimeric antigen receptor
Introduction
Glioblastoma (GBM) is the most aggressive primary brain tumor in adults (1). Currently, the best therapy consists of gross total surgical resection, which is frequently not possible due to location in eloquent areas of the brain, followed by radiotherapy and chemotherapy. Long-term survival is occasionally seen in rare cases that occur in young adults, generally under age 30. However in the majority of cases, which occur in middle and old age, radiotherapy and chemotherapy only slows, but do not stop tumor growth, resulting in five-year survival rates of less than 4% (2,3). The recent identification of chemo- and radiotherapy resistant CD133-positive tumor stem cells in GBMs (4,5) may help explain why conventional therapies are ineffective. While the exact mechanism of tumor stem cell resistance to conventional therapies remains elusive, their quiescent state, and increased capacity to eliminate cytotoxic drugs and repair damaged DNA are thought to be key contributing factors (4,5). Immunotherapy may be able to benefit GBM patients since immune-mediated killing relies neither on tumor cell proliferation nor the aforementioned cytotoxic pathways.
Results from completed Phase I/II immunotherapy clinical trials with tumor cell or dendritic cell (DC) vaccines were encouraging, demonstrating disease stabilization and suggesting prolonged patient survival (6-9). However, these trials have also highlighted some of the limitations of DC vaccines (10) particularly their failure to reproducibly and effectively expand tumor antigen-specific T cells, which may be present at low frequency or are anergized. One means of overcoming this limitation is adoptive T-cell transfer, in which tumor-specific T cells are prepared ex vivo and then transferred to affected individuals. Genetic modification of T cells with chimeric antigen receptors (CARs) can reliably generate tumor-specific T cells ex vivo for clinical use (11,12). CARs are synthetic molecules that consist of an extracellular antigen binding domain that usually contains the heavy and light chain variable regions of a monoclonal antibody joined to transmembrane and cytoplasmic signaling domains derived from the CD3-ζ chain and from costimulatory molecules such as CD28. CARs recognize antigens expressed on the surface of tumor cells, and in this study we targeted the human epidermal growth factor receptor 2 (HER2), a tumor associated antigen that is expressed by up to 80% of GBMs but not by normal postnatal neurons or glia (13-16).
We now show that T cells from GBM patients can readily be modified with HER2-specific CARs to produce effector cells which release immunostimulatory cytokines in response to, and readily kill, autologous primary HER2-positive GBM tumor cells, including CD133-positive GBM stem cells. These HER2-specific T cells had a potent antitumor activity against autologous tumors in an orthotopic xenogeneic SCID mouse model.
Methods
Blood donors, primary tumor cells and cell lines
Blood samples and primary tumor cells were obtained from subjects with GBM on a protocol approved by the Institutional Review Board of Baylor College of Medicine at Baylor College of Medicine and The Methodist Hospital. The GBM and medulloblastoma cell lines (U373 and Daoy) and the breast cancer cell line (MDA-MB-468) were purchased from the American Type Culture Collection (ATCC; Manassas, VA). All cell lines were grown in DMEM (Invitrogen, Carlsbad, CA) with 10% fetal calf serum (FCS; HyClone, Logan, UT), supplemented with 2 mM GlutaMAX-I, 1.5 g/L sodium bicarbonate, 0.1 mMol/L nonessential amino acids, and 1.0 mMol/L sodium pyruvate (all media supplements from Invitrogen). T cells were maintained in RPMI 1640 with 10% FCS containing 2 mMol/L GlutaMAX-I.
Tumor tissues from patients with GBM undergoing surgical resection were processed aseptically, and primary cell cultures were initiated using DMEM high glucose medium (Invitrogen), supplemented with 15% heat inactivated FCS, 2 mM GlutaMAX-I, 1% Insulin-Transferrin-Selenium-X supplement, and 1% Penicillin-Streptomycin mixture (all media supplements from Invitrogen). Cells were used within 7 days of plating or established as primary cell lines.
Immunohistochemistry
Mice were euthanized by CO2 inhalation and fixed with intra-cardiac perfusion of 4% paraformaldehyde. The brain tissue was post-fixed overnight and embedded in paraffin, and histology was performed on 10-μm serial horizontal sections. Tissue sections obtained from mouse xenografts and from paraffin-embedded surgical excision samples were stained by a standard hematoxylin and eosin technique. HER2 expression in GBM xenografts was detected by phospho-HER2 immunohistochemistry as previously described (17).
Generation of retroviral constructs
The HER2-specific CAR with a CD28.ζ signaling domain was constructed by subcloning the HER2-specific single chain variable fragment FRP5 into a SFG.CD28.ζ retroviral vector as previously described (18). A retroviral vector encoding the fusion protein eGFP-Firefly Luciferase (eGFP.FFLuc) was used to generate firefly luciferase expressing GBM cells for the in vivo studies (18).
Retrovirus production and transduction of T cells
To produce retroviral supernatant, 293T cells were co-transfected with an FRP5.CD28.ζ retroviral vector containing plasmid, Peg-Pam-e plasmid encoding the sequence for MoMLV gag-pol, and plasmid pMEVSVg containing the sequence for VSV-G, using GeneJuice transfection reagent (EMD Biosciences, San Diego, CA) (19). Supernatants containing the retrovirus were collected 48 and 72 hours later. VSV-G pseudotyped viral particles were used to transduce the PG-13 producer cell line for the production of viral particles.
OKT3/CD28 activated T cells were transduced with retroviral vectors as described (19). Briefly, peripheral blood mononuclear cells (PBMC) were isolated by Lymphoprep (Greiner Bio-One, Monroe, NC) gradient centrifugation. 5×105 PBMC per well in a 24-well plate were activated with OKT3 (OrthoBiotech, Raritan, NJ) and CD28 monoclonal antibodies (BD Biosciences, Palo Alto, CA) at a final concentration of 1 μg/mL. On day 2, recombinant human IL-2 (Chiron, Emeryville, CA) was added at a final concentration of 100 units/mL, and on day 3, cells were harvested for retroviral transduction. For transduction, we pre-coated a non-tissue culture treated 24-well plate with a recombinant fibronectin fragment (FN CH-296; Retronectin; Takara Bio USA, Madison, WI). Wells were washed with phosphate-buffered saline (PBS; Sigma, St. Louis, MO) and incubated twice for 30 minutes with retrovirus. Subsequently, 3×105 T cells per well were transduced with retrovirus in the presence of 100 units IL-2 per mL. After 48-72 hours cells were removed and expanded in the presence of 50-100 units IL-2 per mL for 10-15 days prior to use.
Flow cytometry
We used a FACScalibur instrument (BD, Becton Dickinson, Mountain View, CA) and CellQuest software (BD) for all flow-cytometric analyses, analyzing >10,000 events; in all cases negative controls included isotype antibodies. Cells were washed once with PBS containing 2% FBS and 0.1% sodium azide (Sigma; FACS buffer) prior to addition of antibodies. After 15 to 30 minutes of incubation at 4°C in the dark the cells were washed once and fixed in 0.5% paraformaldehyde/FACS buffer prior to analysis.
T cells were analyzed with anti-CD8 FITC, -CD4 PE, and -CD3 PerCP, and tumor cell lines with anti-HER2 PE. All monoclonal antibodies (except CD133, Miltenyi Biotec, Bergisch Gladbach, Germany) were obtained from BD Biosciences, Palo Alto, CA. To determine cell surface expression of the HER2 CAR transgene, a recombinant HER2-Fc fusion protein (R&D Systems, Minneapolis, MN) was used. Bound HER2-FC was detected with a goat anti-Fc FITC secondary antibody (Chemicon, Temecula, CA) (19).
Cytotoxicity assays
Cytotoxicity assays were performed as previously described (20). Briefly, 1×106 target cells were labeled with 0.1 mCi (3.7MBq) 51Cr and mixed with decreasing numbers of effector cells to give effector to target ratios of 40:1, 20:1, 10:1 and 5:1. Target cells incubated in complete medium alone or in 1% Triton X-100 were used to determine spontaneous and maximum 51Cr release, respectively. After 4 hours we collected supernatants and measured radioactivity in a gamma counter (Cobra Quantum; PerkinElmer; Wellesley; MA). The mean percentage of specific lysis of triplicate wells was calculated according to the following formula: [test release − spontaneous release] / [maximal release − spontaneous release] × 100.
Analysis of cytokine production and T-cell expansion
Effector T cells (FRP5. CD28.ζ.CAR expressing T cells or non-transduced T cells) from GBM patients or healthy volunteers were co-cultured with primary autologous GBM cells in short-term culture (<14 days) HER2-positive and HER2-negative cell lines at a 1:1 effector to target ratio were plated in a 24 well plate. After 24 to 48 hours incubation, culture supernatants were harvested and the presence of IFN-γ and IL-2 was determined by ELISA as per the manufacturer’s instructions (R&D Systems, Minneapolis, MN). T-cell expansion was determined by counting viable cells (trypan blue exclusion) seven days after stimulation.
Whole-genome gene expression profiling
The genome-wide expression analysis was performed by the Dan L Duncan Center Genomics and Proteomics Core Laboratory at Texas Children’s Hospital using the Illumina’s HumanWG-6 v3 BeadChips (Illumina, Inc. San Diego, CA). This array contains > 48K transcript probes (representing 42,620 genes). Briefly, RNA was isolated from primary tumors and the corresponding primary cell lines established in vitro. Total RNA concentrations were measured using the Nanodrop 1000 spectrophotometer (Nanodrop Scientific, Wilmington, DE). RNA integrity was checked in electrophoresis using 2% agarose gel with 6% formaldehyde. ½ μg total RNA was used to synthesize biotinylated cRNA using Totalprep RNA amplification kit (Ambion Inc, Austin, TX), and 1.5 μg of biotinylated cRNA was applied to the HumanWG-6 v3 Beadchips and processed according to the vendor’s instruction. The Beadchips were scanned using Beadstation 500 GX scanner. The image files were imported into the Bead Studio software version 3.2.7 (Illumina Inc.) and the data was processed using the quantile normalization algorithm. This method assumes that the distribution of the expression values does not change dramatically between arrays. All arrays were adjusted so that they had an almost identical overall intensity distribution. All 42,620 genes were used to calculate the correlation coefficient between samples in the Beadstudio software. All samples that contained genes with detection p value less than 0.001 were subjected to a differential analysis using Illumina’s own test with false discovery correction.
Orthotopic xenogeneic SCID mouse model of GBM
All animal experiments were conducted on a protocol approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. Recipient NOD-SCID mice were purchased from Taconic (C.B-Igh-1b/IcrTac-Prkdcscid; FOX CHASE CB-17 SCID™ ICR; Taconic, Hudson, NY). Male 9- to 12-week-old mice were anesthetized with rapid sequence inhalation isofluorane (Abbot Laboratories, England) followed by an intraperitoneal injection of 225-240 mg/kg Avertin® solution and then maintained on isofluorane by inhalation throughout the procedure. The head was shaved, then the mice were immobilized in a Cunningham™ Mouse/Neonatal Rat Adaptor (Stoelting, Wood Dale, IL) stereotactic apparatus fitted into an E15600 Lab Standard Stereotaxic Instrument (Stoelting), then scrubbed with 1% povidone-iodine. A 10 mm skin incision was made along the midline. The tip of a 31G ½ inch needle mounted on a Hamilton syringe (Hamilton, Reno, NV) served as the reference point. A 1mm burr-hole was drilled into the skull, 1 mm anterior to and 2 mm to the right of the bregma. Firefly-luciferase expressing primary GBM cells (5 × 104 in 2.5 μL) from patients 2, 3 or 5 were injected 3 mm deep to the bregma, corresponding to the center of the right caudate nucleus over 5 minutes. The needle was left in place for 3 minutes, to avoid tumor cell extrusion, and then withdrawn over 5 minutes. All GBMs engrafted and started to grow as judged by exponential increments in their bioluminescence signals. Six days after tumor cell infection, mice containing malignant cells derived from each individual patient were randomly assigned to receive no therapy, HER2-specific T cells or non-transduced T cells. T-cell therapy consisted of 2 × 106 autologous HER2-specific or non-transduced T cells injected locally in 5 μL to the same tumor coordinates. The incision was closed with 2-3 interrupted 7.0 Ethicon® sutures (Ethicon, Inc. Somerville, NJ). A subcutaneous injection of 0.03-0.1 mg/kg buprenorphine (Buprenex® RBH, Hull, England) was given for pain control. For the experiment with CD133-positive GBM stem cells, cells were isolated by high-speed cell sorting and injected into mice as described above (1 × 104 in 2.5 μL cells per mouse). On day 8 mice were treated with 2 × 106 autologous HER2-specific or non-transduced T cells in 5 μL to the same tumor coordinates.
Bioluminescence imaging
Isofluorane anesthetized animals were imaged using the IVIS® system (IVIS, Xenogen Corp., Alameda, CA) 10 minutes after 150 mg/kg D-luciferin (Xenogen) was injected intraperitoneally. The photons emitted from luciferase-expressing cells within the animal body and transmitted through the tissue were quantified using “Living Image”, a software program provided by the same manufacturer. A pseudo-color image representing light intensity (blue least intense and red most intense) was generated and superimposed over the grayscale reference image. Animals were imaged every other day for one week after injections, then twice weekly for two weeks then weekly thereafter. They were regularly examined for any neurological deficits, weight loss or signs of stress and euthanized according to pre-set criteria, in accordance the Baylor College of Medicine’s Center for Comparative Medicine guidelines.
Statistical analysis
For the bioluminescence experiments, intensity signals were log-transformed and summarized using mean ± SD at baseline and multiple subsequent time points for each group of mice. Changes in intensity of signal from baseline at each time point were calculated and compared using paired t-tests or Wilcoxon signed-ranks test.
Results
Generation of functional HER2-specific T cells from subjects with GBM
To redirect patients’ lymphocytes to HER2, we used a second generation HER2-specific CAR consisting of an extracellular domain derived from the high-affinity HER2-monoclonal antibody, FRP5 (21), and signaling domains from the costimulatory molecule CD28 and the CD3 ζ-chain (FRP5.CD28.ζ CAR; Supplemental Figure 1A). CD3/CD28 activated T cells from ten consecutive, newly-diagnosed GBM patients were transduced with RD114-pseudotyped retroviral vectors encoding FRP5.CD28.ζ CAR, and 4 to 7 days after transduction, we determined the expression of HER2-specific CARs by FACS analysis. On average, 79% (SD +/−15%) of GBM patients’ T cells expressed HER2-specific CARs, and both CD4- and CD8-positive T cells were transduced (Supplemental Figure 1B and 1C).
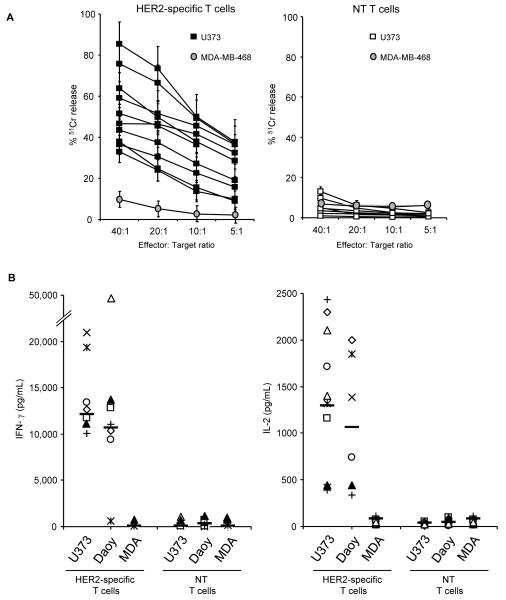
To evaluate the in vitro function of patients’ HER2-specific T cells we used standard cytotoxicity assays and co-culture experiments with both HER2-positive targets (GBM cell line U373 and medulloblastoma cell line Daoy) and HER2-negative controls (MDA-MB-468). All patients’ HER2-specific T cells killed HER2-positive target cells and secreted immunostimulatory Th1 cytokines (IFN-γ and IL-2), whereas non-transduced T cells from the same patients did not (Figure 1A, B). In addition, HER2 negative target cells were not killed and did not induce cytokine production. Hence, genetic modification of T cells from GBM patients allows rapid and reliable generation of functional HER2-specific T cells.
Figure 1. Functionality of HER2-specific T cells from subjects with GBM.
HER2-specific and non-transduced (NT) T cells, generated from newly-diagnosed GBM patients, were co-cultured with HER2-positive (U373 or Daoy) or HER2-negative (MDA-MB-468) cells. (A) Only HER2-specific T cells killed HER2 positive targets, in a 4 hour 51Cr release cytotoxicity assay; NT T cells did not. The HER2-negative cell line MDA-MB-468 (MDA) was not killed by HER2-specific or NT T cells (mean and standard deviation for all patients are shown). Results from experiments done for 9 GBM patients in triplicates are shown. (B) in coculture assays performed at a 1:1 T-cell to tumor cell ratio, the IFN-γ and IL-2 concentration was determined in the coculture supernatant 24 to 48 hours post stimulation. Only HER2-specific T cells produced IFN-γ and IL-2 after exposure to HER2-positive cells in comparison to NT T cells. Results from experiments done in duplicates are shown.
HER2-specific T cells recognize and kill autologous GBM cells
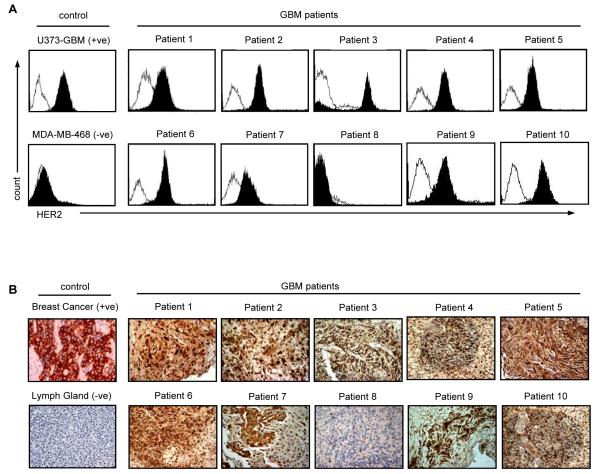
To investigate if patients’ HER2-specific T cells recognize and kill autologous HER2-positive GBM cells, we used short-term-cultured, autologous GBM cells as targets. Nine out of 10 tumor cell lines expressed HER2 on the cell surface as judged by FACS analysis (Figure 2A). HER2 expression of the corresponding primary tumor was confirmed by immunohistochemistry (IHC; Figure 2B). While we observed moderate heterogeneity of HER2 expression within individual GBMs, there was concordance between FACS analysis and IHC, with one tumor having no detectable HER2 protein expression using both methods (patient 8).
Figure 2. HER2 protein expression on primary GBM.
(A) FACS analysis: primary GBM cells from freshly excised tumors in short term culture were stained for HER2 expression (isotype control: open curves; HER2: solid curve. Nine out of ten tumor cell lines expressed HER2 on the cell surface. (B) Using the HER2-specific mouse monoclonal antibody NCL-L-CB11 (NovocastraTM Newcastle upon Tyne, UK), HER2 expression was confirmed on the corresponding paraffin-embedded sections. One tumor had no detectable HER2 protein expression using both methods (patient 8). Magnification 200x.
In standard 4-hour 51Cr-release assays there was always an increase of cytolytic activity of HER2-specific T cells in comparison to non-transduced (NT) T cells against autologous HER2-positive GBMs, confirming HER2-specificity (Figure 3A). Besides tumor cell killing, cytokine production of T cells is critical for their activation and sustained antitumor activity. In coculture experiments, HER2-specific T cells from 8 out of 9 patients with HER2-positive GBMs secreted substantial amounts of Th1 cytokines (IFN-γ and IL-2) denoting T-cell activation (Figure 3B). No IFN-γ or IL-2 production was detected when HER2-specific T cells were cultured with HER2-negative targets (MDA-MB-468) or from cocultures with autologous non-transduced T cells. These results indicate that HER2-specific T cells recognize and kill autologous, HER2-positive GBM cells in a HER2-specific manner.
Figure 3. HER2-specific T cells kill autologous HER2-positive GBM and are activated in coculture.
(A) The cytolytic activity of T cells expressing HER2 CAR was determined in a standard 4 hour chromium release assay. There was always an increase of cytolytic activity of HER2-specific T cells above background (non-transduced (NT) T cells) against autologous HER2-positive GBMs. As controls the HER2-positive GBM cell lines, U373, and the HER2 negative MDA-MB-468 (MDA) were used. HER2-specific T cells from all patients killed U373 cells where as MDA was not killed (shown for patient 4). (B) HER2-specific T cells (solid bars) or non-transduced (NT-T cells; open bars) from GBM patients were cocultured with autologous tumor cells or HER2-negative control cells (MDA-MB-468; hatched bars) in a 1:1 ratio. 24 to 48 hours after stimulation the cytokine concentration in the media was determined by ELISA. HER2-specific T cells produced IFN-γ and IL-2 after stimulation with 8 out of 9 HER2-positive tumor samples. No cytokine release was seen with NT T cells. Median cytokine levels for all patients are shown for U373 (HER2-positive control) and MDA-MB-468 (HER2-negative control). Results of experiments done in duplicates are shown.
HER2-specific T cells kill autologous CD133-positive GBM stem cells
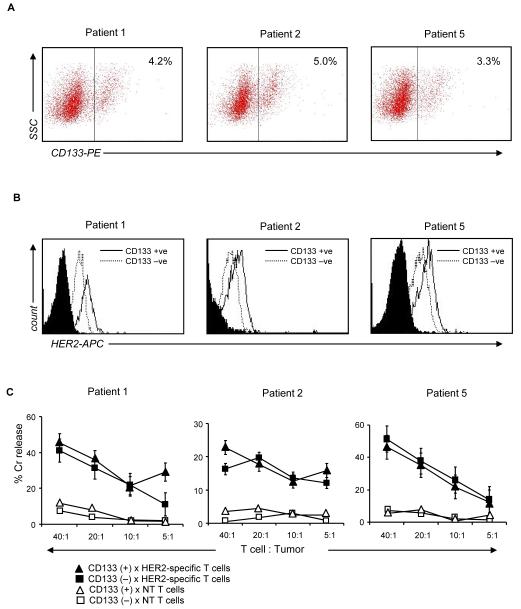
We next evaluated if HER2-specific T cells also kill CD133-positive GBM stem cells, which are resistant to current standard therapies including radiation- and chemo-therapy. We separated the CD133-positive and CD133-negative cell populations from primary GBM cell lines of three patients by high-speed cell sorting. 3-5% of the total cell population was CD133-positive (Figure 4A). This GBM stem cell population expressed 2.8- to 4-fold higher levels of HER2 in comparison to their CD133-negative counterparts (Figure 4B). In a 4 hour 51Cr release assay, HER2-specific T cells of all 3 patients killed autologous CD133-positive as well as CD133-negative cells (Figure 4C). Autologous non-transduced T cells induced no significant killing of either cell populations. These results demonstrate that HER2-specific T cells are able to target the treatment-resistant CD133-positive compartment, which may contribute to tumor recurrence in GBM.
Figure 4. HER2-specific T cells target primary GBM stem cells.
(A) Primary GBM cells from three patients (GBM patient 2, 3 and 5) were stained for CD133 and isolated using high speed sorting. Approximately 3-5% of the total primary GBM cell population was CD133-positive. (B) This CD133-positive cell compartment was uniformly HER2-positive. Moreover, in all three tumors analyzed, the CD133 positive GBM stem cells expressed higher levels of HER2 in comparison to the CD133-negative tumor cell population. (C) In a 4 hour 51Cr release assay, HER2-specific T cells from these 3 patients killed autologous CD133-positive cells as well as their CD133-negative counterparts. Autologous non-transduced T cells induced no appreciable killing.
Regression of primary GBM xenografts after administration of autologous HER2-specific T cells
To determine the in vivo antitumor activity of HER2-specific T cells we used an orthotopic, autologous GBM xenograft model. Primary GBM cell lines from GBM patients were established in vitro and their identity with the primary tumor was confirmed using a 48,000 probe gene chip array (Pearson correlation coefficient (r2)=0.77-0.83; Supplemental Figure 2). To allow serial bioluminescence imaging in vivo, we transduced primary GBM cell lines from 3 patients with a retroviral vector encoding an eGFP-firefly luciferase fusion gene. All cells were GFP positive as judged by FACS analysis and firefly luciferase expression was confirmed in vitro (data not shown). The tumorigenicity of the 3 patients’ eGFP.FFLuc expressing GBM cell lines was confirmed by injecting 5×104 cells stereotacticaly into the right frontal cortex of SCID mice. Bioluminescence imaging of tumor xenografts demonstrated progressive and exponential growth of tumors in all experimental animals (Supplemental Figure 3). Untreated animals had a median survival of 17 days (range 14-22).
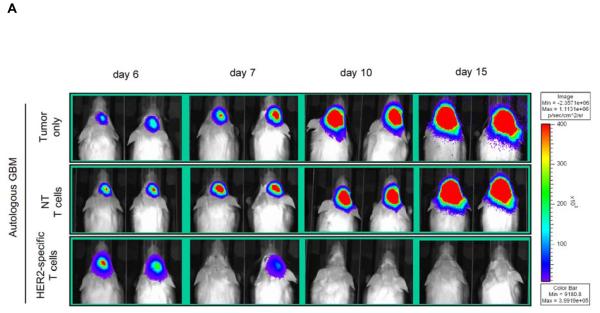
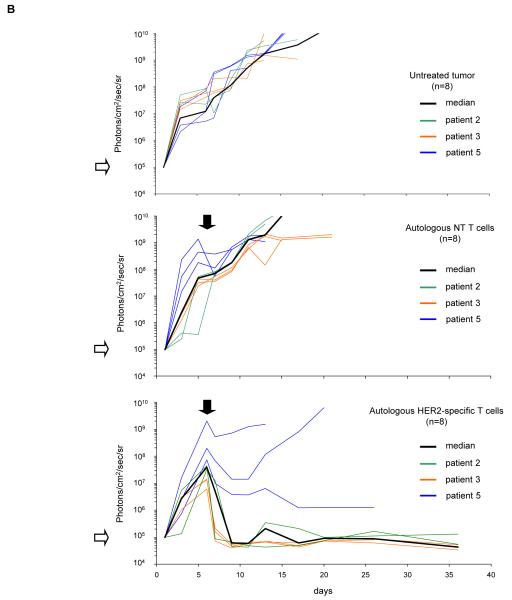
To test the effector function of autologous HER2-specific T cells, 5×104 eGFP.FFLuc expressing GBM cells from all three patients were injected stereotactically into the right frontal cortex of SCID mice. On day 6 after tumor-cell injection, mice received an intratumoral injection of 2×106 autologous HER2-specific T cells or non-transduced T cells. A subset of animals was not treated. We quantified tumor growth by serial bioluminescence. In untreated animals, and in animals treated with autologous non-transduced T cells, (Figure 5A), tumors grew exponentially over time. In contrast, photon emission decreased significantly in all 8 mice after autologous HER2-specific T-cell injection, indicating tumor regression (Figure 5B). GBM xenografts from patients 2 and 3 regressed completely, while the xenografts from patient 5 had a partial response. The light signal continued to be undetectable after 6 months in 4 of 8 animals indicating tumor eradication. This was confirmed by histological examination.
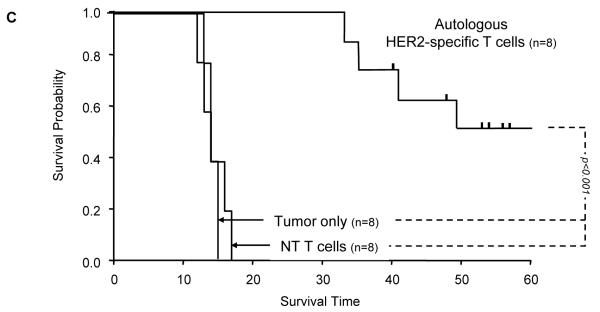
Figure 5. Adoptively transferred HER2-specific T cells induce regression of autologous GBM xenografts in vivo.
5×104 primary GBM cells from patients 2 (2 mice per group), 3 (3 mice per group) and 5 (3 mice per group) were injected stereotactically into the caudate nucleus of 9 to 12 week old SCID mice followed by intratumoral injection of 2×106 autologous HER2-specific or non-transduced T cells (NT-T cells) 6 days after tumor inoculation. (A) Tumors grew progressively in untreated mice as shown for two representative animals (upper row) and in mice receiving non-transduced T cells (middle row), while tumors regressed over a period of 2-5 days in response to a single injection of autologous HER2-specific T cells generated from the same patient (lower row). (B) Quantitative bioluminescence imaging: Autologous HER2-specific T cells induced tumor regression when compared to NT-T cells (two-tailed P value = 0.002, Mann-Whitney U test). Solid arrows: time of T-cell injection; open arrows: background luminescence (mean~105 photon/sec/cm2/sr); n=number of animals tested in each group. (C) Kaplan-Meier survival curve: Survival analysis performed 60 days after tumor establishment. Mice treated with autologous HER2-specific T cells had a significantly longer survival probability (p<0.001) in comparison to untreated mice and mice that received NT-T cells. (D) 1×104 CD133-positive GBM cells from patient 2 were injected as described above followed by intratumoral injection of 2×106 autologous HER2-specific or NT-T cells 8 days after tumor inoculation. While tumors in animals treated with NT T cells (n=4) continued to grow exponentially, all of the animals treated with autologous HER2 T cell (n=4) regressed with two of these animals having no detectable tumors with in 6 days after T-cell injection.
Kaplan-Meier survival studies 60 days after tumor injection showed that untreated mice and mice receiving autologous non-transduced T cells had a median survival of 14 and 15 days respectively. In contrast, mice treated with autologous HER2-specific T cells had a median survival of 90 days with 50% of the mice surviving > 100 days (p<0.001; Figure 5C).
To provide direct evidence that HER2-specific T cells also kill autologous CD133-positive GBM stem cells in vivo, CD133-positive cells from the eGFP.FFLuc expressing GBM cell lines of patient 2 were sorted and injected stereotactically into the right frontal cortex of SCID mice (1 ×104 cells; n=8). Mice were imaged daily until the bioluminescence signal was comparable to that of xenografts obtained from unsorted cells. On day 8, mice were injected with autologous HER2-specific T cells or non-transduced T cells. While tumors in animals treated with non-transduced T cells continued to grow exponentially, all animals treated with HER2-specific T cells had a measurable response, including 2 complete remissions, on bioluminescence imaging (Figure 5D). This result further supports the conclusions of our in vitro experiments (Figure 4) that HER2-specific T cells have antitumor activity against CD133-positive glioma cells.
Discussion
In this study we investigated HER2 targeted T-cells as a potential therapeutic agent for GBM and showed that T cells from GBM patients can be readily genetically engineered to be rendered HER2-specific. These effector cells recognized autologous HER2-positive GBMs including their CD133-positive stem cells in vitro and had potent antitumor activity in an orthotopic, xenograft model.
It has proved difficult to generate tumor-specific T cells in cancer patients using DC vaccines, most likely because tumor specific T cells are present at low frequency and in an anergic state due to the immunosuppressive environment induced by malignancies such as GBMs (10,22). Adoptive T-cell transfer of ex vivo expanded tumor-specific T cells may overcome these limitations and forced expression of antigen-specific CARs or transgenic α/β T-cell receptors (TCR) have been used to generate such effector T lymphocytes (23-26). CARs combine the antigen-binding property of monoclonal antibodies with the lytic and self-renewal capacities of T cells and have several advantages over transgenic α/β TCR receptors (11,12). CAR-expressing T cells recognize and kill tumor cells in an MHC nonrestricted fashion, so that target cell recognition by CAR T cells is unaffected by some of the major mechanisms by which tumors avoid MHC-restricted T-cell (α/β TCR) recognition, such as downregulation of HLA class I molecules and defective antigen processing (11,12). In addition, most tumors do not express costimulatory molecules so that α/β TCR engagement is followed by incomplete T-cell activation. We (27) and others (28,29) have shown that CARs can overcome this limitation if they incorporate costimulatory endodomains within the chimeric receptor sequence or if they are expressed in virus specific cytotoxic T lymphocytes. Our current HER2-specific CAR with a CD28.ζ signaling domain induces immunostimulatory cytokine release, including IL-2, from transduced cells of 8 of 9 GBM patients following CAR engagement.
Several cell surface antigens have been identified as potential targets for GBM directed CAR T-cell therapies including HER2, IL-13Rα2, the EPH receptor A2 (EphA2) and EGFRvIII (14,30-32). We chose HER2 since this antigen is expressed by a high percentage of tumors (>70%) and signaling through HER2 deregulates cell proliferation, inhibits apoptosis, and increases the metastatic potential of cancer cells (33,34). Moreover, HER2 expression increases with the degree of anaplasia in glial tumors, and mutations in the HER2 signaling pathway have been identified in gliomas (35) Our own results confirmed a high frequency of HER2 expression, showing the antigen on 9 of 10 GBM tumors by two independent methods (IHC and FACS analysis). As with other tumor associated antigens expressed by GBMs, there was moderate heterogeneity in the expression pattern of HER2 within individual tumors (36,37), and optimum killing in a clinical setting may require targeting more than one tumor associated antigen or the induction of epitope spreading beyond the original targets to prevent immune escape (20,38).
Despite the biological significance of HER2 signaling, the expression of HER2 on GBMs is low in comparison to breast cancer, rendering HER2 monoclonal antibodies ineffective (19,30). HER2-specific T cells allow targeting of the relatively low levels of HER2 expressed by GBMs because the overall avidity of receptors arrayed on a T-cell is greater than the avidity of a bivalent antibody, and engagement of even a limited number of T cell receptor molecules is sufficient to trigger a cytotoxic effector response (39,40). One concern of targeting HER2-expressing malignancies with T cells are “ off target” effects since the administration of HER2 monoclonal antibodies has been associated with side effects, the most concerning of which is the poorly understood cardiac toxicity. If HER2-specific T cells are long lived, this problem could be accentuated in severity and persistence. Potential cardiac toxicities are difficult to model in mice since T cells that are specific for human HER2 do not recognize murine HER2. Nonetheless, we have shown that HER2-negative cells are not killed by HER2-specific T cells. In addition, primary endothelial and epithelial cells (Supplemental Figure 4) do not activate HER2-specific T cells and 5 patients who received HER2-specific T cells had no dose limiting toxicities (41,42). Finally, HER2 vaccines are well tolerated, and no cardiac toxicities were observed in patients, who developed HER2-specific T-cell responses (43).
CD133 expression has identified a population with stem-cell-like properties in normal and cancerous tissues of the central nervous system (4,5). In GBMs these cells are chemo- and radiotherapy resistant and most likely contribute to the ineffectiveness of conventional therapies. For example, while temozolomide was reported to preferentially deplete CD133-positive stem cells in primary GBMs in vitro, this effect was absent in O(6)-methylguanine-DNA-methyltransferase (MGMT)-non-methylated tumors which represent 50-70% of primary GBMs and carry a worse prognosis (44). The use of temozolomide in a randomized prospective clinical trial in patients with GBM has thus only resulted in a marginal survival advantage (14.6 versus 12.1 months) (3). We show here that CD133-positive GBM stem cells express HER2 and are killed by autologous HER2-specific T cells in vitro and in vivo similar to CD133-negative GBM cells. Hence immune targeted therapies may eradicate malignant stem cells that are resistant to conventional therapy.
Several studies have reported the infusion of activated T lymphocytes systemically or into resection cavities of recurrent or progressive malignant gliomas (45-47). These infusions were safe and resulted in disease stabilization and in some instances in partial regression. We injected HER2-specific T cells directly into autologous GBM xenografts in tumor bearing mice. Tumors from patients 2 and 3 regressed completely without the need for exogenous cytokines, whereas untreated tumors and tumors treated with non-transduced T cells continued to grow. Tumors from patient 5 only had a partial response. Initial analysis revealed no significant differences in the level of HER2 expression as well as the in vitro effector function of HER2-specific T cells from these three patients. We are now investigating other biological differences beteween the tumors that may account for variability in effector cell sensitivity.
Tumors recurred in a number of animals most likely due to limited T cell persistence in vivo. Kahlon et al. reported complete regression and no recurrence of U87 gliomas in an orthotopic, xenogeneic SCID model after intratumoral injection of T cells expressing an IL-13Rα2-specific CAR (48). In their model, the U87 glioma cell line was genetically modified to secrete IL-2, a cytokine critical for T-cell survival and expansion in vivo. Tumor recurrence in our model may be due to inadequate persistence of human HER2-specific T cells in the xenograft, a known limitation of SCID mouse models.
In summary, this study shows that HER2-specific T cell can be readily generated from GBM patients by gene transfer with HER2-specific CARs. HER2-specific T cells recognized and killed autologous HER2-positive GBM, including CD133-positive stem cells, ex vivo, and induced regression of experimental GBMs in vivo. Hence, the adoptive transfer of HER2-redirected T-cells may be an attractive immunotherapeutic approach for GBM.
Supplementary Material
Statement of Translational Relevance.
The outcome for patients with GBM remains unchanged over the last 30 years. Thus, there is a need for new targeted therapies, and T-cell immunotherapy clearly has the potential to fulfill this need. We show here that T cells from GBM patients can readily be modified with HER2-specific chimeric antigen receptors to produce effector cells which release immunostimulatory cytokines in response to, and kill, autologous primary HER2-positive GBM tumor cells, including CD133-positive GBM stem cells. These HER2-specific T cells also had a potent antitumor activity against autologous tumors in an orthotopic xenogeneic SCID mouse model. Hence, the adoptive transfer of HER2-redirected T-cells may be an attractive immunotherapeutic approach for GBM.
Acknowledgements
We thank Dr. Malcolm K. Brenner for the helpful discussion and advice, Awateef Akrabi for assistance with FACS analysis and Christopher Threeton for assistance with flow sorting. We also would like to thank The Methodist Hospital Neurosurgeons, Drs. Richard L. Harper and James Rose.
Support: The authors were supported by grants from the Clayton Foundation for Research, the Dana Foundation, and the American Brain Tumor Association. H.E.H. is the recipient of a Doris Duke Distinguished Clinical Scientist Award.
References
- 1.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 2.Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30:10–14. doi: 10.1053/j.seminoncol.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, Van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Altaner C. Glioblastoma and stem cells. Neoplasma. 2008;55:369–374. [PubMed] [Google Scholar]
- 5.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 6.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 7.Okada H, Lieberman FS, Walter KA, et al. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas. J Transl Med. 2007;5:67. doi: 10.1186/1479-5876-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider T, Gerhards R, Kirches E, Firsching R. Preliminary results of active specific immunization with modified tumor cell vaccine in glioblastoma multiforme. J Neurooncol. 2001;53:39–46. doi: 10.1023/a:1011856406683. [DOI] [PubMed] [Google Scholar]
- 9.Wheeler CJ, Black KL, Liu G, et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 2008;68:5955–5964. doi: 10.1158/0008-5472.CAN-07-5973. [DOI] [PubMed] [Google Scholar]
- 10.Fine HA. Toward a glioblastoma vaccine: promise and potential pitfalls. J Clin Oncol. 2004;22:4240–4243. doi: 10.1200/JCO.2004.06.927. [DOI] [PubMed] [Google Scholar]
- 11.Pule M, Finney H, Lawson A. Artificial T-cell receptors. Cytotherapy. 2003;5:211–226. doi: 10.1080/14653240310001488. [DOI] [PubMed] [Google Scholar]
- 12.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 13.Liu G, Ying H, Zeng G, et al. HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res. 2004;64:4980–4986. doi: 10.1158/0008-5472.CAN-03-3504. [DOI] [PubMed] [Google Scholar]
- 14.Zhang JG, Eguchi J, Kruse CA, et al. Antigenic profiling of glioma cells to generate allogeneic vaccines or dendritic cell-based therapeutics. Clin Cancer Res. 2007;13:566–575. doi: 10.1158/1078-0432.CCR-06-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JG, Kruse CA, Driggers L, et al. Tumor antigen precursor protein profiles of adult and pediatric brain tumors identify potential targets for immunotherapy. J Neurooncol. 2008;88:65–76. doi: 10.1007/s11060-008-9534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozaki M, Kishigami S, Yano R. Expression of receptors for neuregulins, ErbB2, ErbB3 and ErbB4, in developing mouse cerebellum. Neurosci Res. 1998;30:351–354. doi: 10.1016/s0168-0102(98)00013-3. [DOI] [PubMed] [Google Scholar]
- 17.Gilbertson RJ, Pearson AD, Perry RH, Jaros E, Kelly PJ. Prognostic significance of the c-erbB-2 oncogene product in childhood medulloblastoma. Br J Cancer. 1995;71:473–477. doi: 10.1038/bjc.1995.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed N, Salsman VS, Yvon E, et al. Immunotherapy for Osteosarcoma: Genetic Modification of T cells Overcomes Low Levels of Tumor Antigen Expression. Mol Ther. 2009;17(10):1779–87. doi: 10.1038/mt.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed N, Ratnayake M, Savoldo B, et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007;67:5957–5964. doi: 10.1158/0008-5472.CAN-06-4309. [DOI] [PubMed] [Google Scholar]
- 20.Gottschalk S, Ng CYC, Smith CA, et al. An Epstein-Barr virus deletion mutant that causes fatal lymphoproliferative disease unresponsive to virus-specific T cell therapy. Blood. 2001;97:835–843. doi: 10.1182/blood.v97.4.835. [DOI] [PubMed] [Google Scholar]
- 21.Moritz D, Wels W, Mattern J, Groner B. Cytotoxic T lymphocytes with a grafted recognition specificity for ERBB2-expressing tumor cells. Proc Natl Acad Sci U S A. 1994;91:4318–4322. doi: 10.1073/pnas.91.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marras C, Mendola C, Legnani FG, DiMeco F. Immunotherapy and biological modifiers for the treatment of malignant brain tumors. Curr Opin Oncol. 2003;15:204–208. doi: 10.1097/00001622-200305000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JR, Digiusto DL, Slovak M, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 25.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pule MA, Straathof KC, Dotti G, et al. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 28.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 29.Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koka V, Potti A, Forseen SE, et al. Role of Her-2/neu overexpression and clinical determinants of early mortality in glioblastoma multiforme. Am J Clin Oncol. 2003;26:332–335. doi: 10.1097/01.COC.0000020922.66984.E7. [DOI] [PubMed] [Google Scholar]
- 31.Hatano M, Eguchi J, Tatsumi T, et al. EphA2 as a glioma-associated antigen: a novel target for glioma vaccines. Neoplasia. 2005;7:717–722. doi: 10.1593/neo.05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bullain SS, Sahin A, Szentirmai O, et al. Genetically engineered T cells to target EGFRvIII expressing glioblastoma. J Neurooncol. 2009;94:373–382. doi: 10.1007/s11060-009-9889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernan R, Fasheh R, Calabrese C, et al. ERBB2 up-regulates S100A4 and several other prometastatic genes in medulloblastoma. Cancer Res. 2003;63:140–148. [PubMed] [Google Scholar]
- 34.Lane HA, Beuvink I, Motoyama AB, et al. ErbB2 potentiates breast tumor proliferation through modulation of p27(Kip1)-Cdk2 complex formation: receptor overexpression does not determine growth dependency. Mol Cell Biol. 2000;20:3210–3223. doi: 10.1128/mcb.20.9.3210-3223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarboe JS, Johnson KR, Choi Y, Lonser RR, Park JK. Expression of interleukin-13 receptor alpha2 in glioblastoma multiforme: implications for targeted therapies. Cancer Res. 2007;67:7983–7986. doi: 10.1158/0008-5472.CAN-07-1493. [DOI] [PubMed] [Google Scholar]
- 37.Saikali S, Avril T, Collet B, et al. Expression of nine tumour antigens in a series of human glioblastoma multiforme: interest of EGFRvIII, IL-13Ralpha2, gp100 and TRP-2 for immunotherapy. J Neurooncol. 2007;81:139–148. doi: 10.1007/s11060-006-9220-3. [DOI] [PubMed] [Google Scholar]
- 38.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 40.Unanue ER, Harding CV, Luescher IF, Roof RW. Antigen-binding function of class II MHC molecules. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):383–392. doi: 10.1101/sqb.1989.054.01.047. [DOI] [PubMed] [Google Scholar]
- 41.Bernhard H, Neudorfer J, Gebhard K, et al. Adoptive transfer of autologous, HER2-specific, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol Immunother. 2008;57:271–280. doi: 10.1007/s00262-007-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Disis M, Salazar LG, Coveler A, et al. Phase I study of infusion of HER2/neu (HER2) specific T cells in patients iwth advanced-stage HER2 overexpressing cancers who have received a HER2 vaccine. J Clin Oncol. 2009;27:3000. [Google Scholar]
- 43.Mittendorf EA, Holmes JP, Ponniah S, Peoples GE. The E75 HER2/neu peptide vaccine. Cancer Immunol Immunother. 2008;57:1511–1521. doi: 10.1007/s00262-008-0540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glas M, Happold C, Rieger J, et al. Long-term survival of patients with glioblastoma treated with radiotherapy and lomustine plus temozolomide. J Clin Oncol. 2009;27:1257–1261. doi: 10.1200/JCO.2008.19.2195. [DOI] [PubMed] [Google Scholar]
- 45.Tsuboi K, Saijo K, Ishikawa E, et al. Effects of local injection of ex vivo expanded autologous tumor-specific T lymphocytes in cases with recurrent malignant gliomas. Clin Cancer Res. 2003;9:3294–3302. [PubMed] [Google Scholar]
- 46.Plautz GE, Miller DW, Barnett GH, et al. T cell adoptive immunotherapy of newly diagnosed gliomas. Clin Cancer Res. 2000;6:2209–2218. [PubMed] [Google Scholar]
- 47.Sloan AE, Dansey R, Zamorano L, et al. Adoptive immunotherapy in patients with recurrent malignant glioma: preliminary results of using autologous whole-tumor vaccine plus granulocyte-macrophage colony-stimulating factor and adoptive transfer of anti-CD3-activated lymphocytes. Neurosurg Focus. 2000;9:e9. doi: 10.3171/foc.2000.9.6.10. [DOI] [PubMed] [Google Scholar]
- 48.Kahlon KS, Brown C, Cooper LJ, et al. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–9166. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.