Abstract
Cytochrome P450 enzymes primarily catalyze mixed-function oxidation reactions, plus some reductions and rearrangements of oxygenated species, e.g. prostaglandins. Most of these reactions can be rationalized in a paradigm involving Compound I, a high-valent iron-oxygen complex (FeO3+), to explain seemingly unusual reactions, including ring couplings, ring expansion and contraction, and fusion of substrates. Most P450s interact with flavoenzymes or iron-sulfur proteins to receive electrons from NAD(P)H. In some cases, P450s are fused to protein partners. Other P450s catalyze non-redox isomerization reactions. A number of permutations on the P450 theme reveal the diversity of cytochrome P450 form and function.
Keywords: Cytochrome P450, Enzyme Catalysis, Enzyme Mechanisms, Flavin, Heme, Natural Products, Oxidation-Reduction
General Aspects of Catalytic Mechanisms
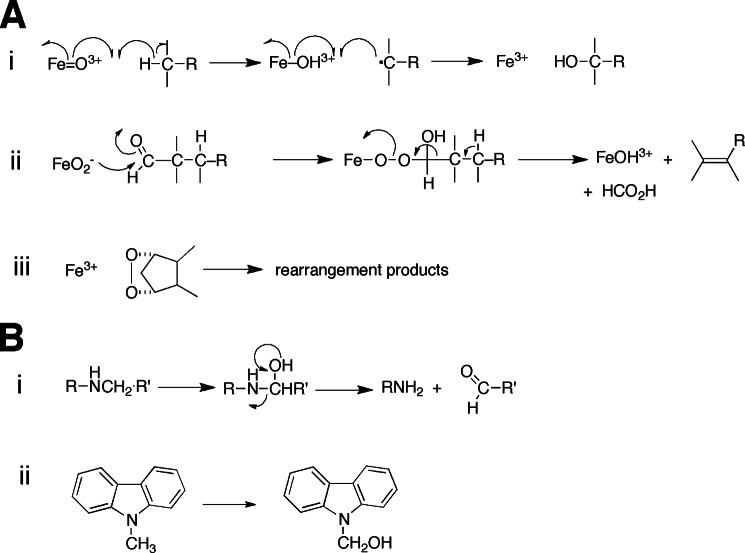
Details of the cytochrome P450 catalytic mechanism are described in the accompanying minireview by Green and co-workers (1). Most of the P450 reactions can be rationalized in the context of the intermediate FeO3+, which corresponds to what was first described as “Compound I” in peroxidase chemistry (Fig. 1A, i). A variant mechanism has been proposed for some P450 reactions (Fig. 1A, ii), particularly those regarding aldehydes (4). Another mode of P450 reactions is the rearrangement of some oxygenated substrates (e.g. prostaglandins) (Fig. 1A, iii), as exemplified by the P450s CYP5A1 and CYP8A1, the respective prostacyclin and thromboxane synthases (see below). Another mechanistic aspect is the distinction of reactions regarding low- and high-spin FeO3+, proposed by Shaik et al. (7) to explain multiple reactions catalyzed by a P450. Although intriguing, this hypothesis has been examined only at the theoretical level. The experimental approaches described by Green and co-workers (1, 8) can be applied to the questions posed by the spin hypothesis.
FIGURE 1.
A, major modes of oxidation reactions catalyzed by P450 enzymes. i, Compound I (FeO3+) with hydrogen abstraction and oxygen rebound. A variant on this is the initial abstraction of a non-bonded electron from a heteroatom, followed by base-catalyzed rearrangement of the aminium radical (N+·) to a carbon radical prior to oxygen rebound (2, 3). ii, reaction of an iron peroxy anion (normally an intermediate in the production of FeO3+) with an aldehyde, probably the best documented example of this kind of chemistry (4). The process leads to an alkene or aromatic ring, e.g. estrogen synthesis in the aromatase (CYP19A1) reaction (see Fig. 2B). iii, rearrangement of an oxidized entity, exemplified here by the P450 CYP58A1 formation of TXA2 from PGH2. B, rearrangements. i, formation and non-enzymatic rearrangement of a carbinolamine and a gem-halohydrin. ii, a stable carbinolamine formed from N-methylcarbazole in P450 oxidations (5, 6).
Oxidations
Most of the unusual reactions of P450s can be understood in the context of rearrangements, either of the reaction products (due to instability) or within an enzymatic reaction intermediate. Examples of both will be considered. For a more extensive collection of unusual P450 reactions, see previous reviews (4, 9–11). The literature of these unusual reaction products is dominated by contributions from the fields of drug metabolism and plant biochemistry (which are not unrelated, in that many drugs are plant secondary metabolites). Most drugs are complex molecules, and regulatory agencies require elucidation of chemical structures of metabolites prior to registration (12). Plants have large numbers of P450 genes (hundreds per species) and utilize these in the synthesis of complex secondary metabolites, e.g. alkaloids, modified terpenes, flavonoids, etc.
Rearrangements of Oxidized Products
Many examples are known of α-hydroxy heteroatom products that break down, e.g. carbinolamines, hemiacetals, and gem-halohydrins (Fig. 1B). However, some stable carbinolamines (5, 6) and hemiaminals (13) are known. In many cases, the initial products have not been observed directly, but indirect approaches have been used to demonstrate their existence, e.g. oxygenated halogen atoms (haloso compounds) (supplemental Fig. S1) (14, 15).
Sometimes, alcohol products dehydrate to yield olefins. However, olefins can also be formed directly by an oxidative mechanism resembling those of recognized desaturases (9, 16).
Arene oxides (epoxides) rearrange to phenols (17). In addition, vinyl compounds with good leaving groups can form epoxides that rearrange, e.g. to α-halocarbonyls (18, 19).
In more complex (cellular) settings, leaving groups may be attached to the alcohols generated by P450s. Accordingly, the elimination or nucleophilic addition products may be recovered and identified. The latter type of reaction with a macromolecule (i.e. DNA and protein) is a major issue in chemical carcinogenesis and toxicity (20).
A dramatic example of rearrangement in a product involves the drug candidate BMS-690514 (supplemental Fig. S2). The reaction is rationalized by an electrophilic attack of a P450 (CYP3A4?) on a pyrrole ring to form a hydroxypyrrole, which rearranges to open the pyrrole ring and eventually involves reaction of a neighboring aniline ring to form a stable carbinolamine product (21).
Rearrangements Involving Enzyme Intermediates
In general, rearrangements of enzyme intermediates are more complex, more interesting, and often more difficult to rationalize. A brief discussion of kinetics and mechanism is in order before consideration of examples.
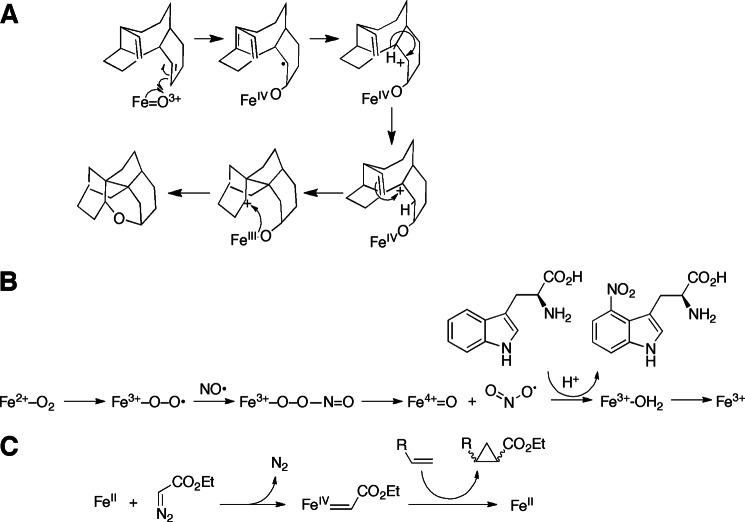
Strained cycloalkyl entities have been utilized as “radical clocks” (22, 23). Abstraction of a hydrogen atom is an energetically unfavorable reaction, but, once formed, the “oxygen rebound” step (Fig. 1A, i) is very rapid, as recently demonstrated in direct experiments with P450 119A1 Compound I by Green and co-workers (1, 8). The rates of rearrangement of some cycloalkyl radical systems are known (in solution) and can be used to estimate the rates of rearrangement and oxygen rebound (radical recombination, i.e. reaction of FeOH3+ + •C) in enzymes. Although this field has not been without controversy (24), rates of recombination of ∼109 s−1 are considered to occur in several P450s (25). Such studies have caveats regarding rates of rearrangement, in that atoms of these molecules are undoubtedly restrained in active sites through various bonding forces, and this can change the rates compared with those measured in simple chemical systems (26).
With this background, we can consider some of the rearrangements. Another aspect to consider is one already mentioned, that of desaturation resulting from the abstraction of a second hydrogen atom to achieve a net 2-electron loss.
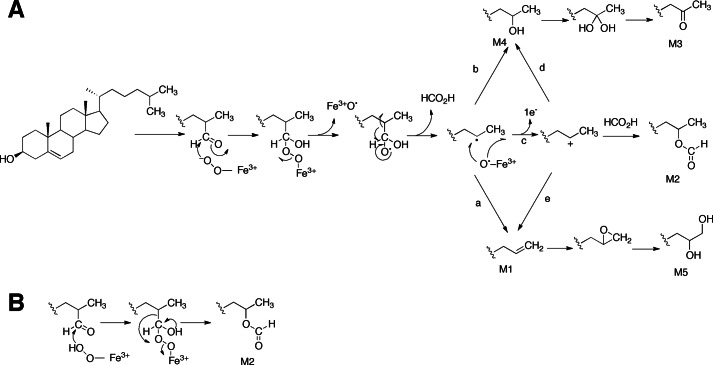
One recent example of an unusual rearrangement in a P450 complex involves the P450 enzyme CYP125 (from Mycobacterium tuberculosis and rhodococci) and the oxidation of cholesterol (Fig. 2). An unusual product is the formate ester (M2 in Fig. 2A). The reaction is shown as releasing formate in the proposed mechanism in Fig. 2A, presumably without extrusion into the medium (equilibration with any formate in the medium was not examined.) An alternative concerted Baeyer-Villiger reaction is shown in Fig. 2B.
FIGURE 2.
Possible mechanisms for steroid deformylation reactions catalyzed by the P450 CYP125. A, the important steps involve addition of the ferric-peroxo anion (FeO2−) of CYP125 to the C-26 carbonyl and subsequent radical fragmentation of the peroxyhemiacetal adduct (27). The radical fragmentation of the peroxyhemiacetal adduct leads to formation of an alkene (M1; arrow a) or a one-carbon deficient alcohol (M4; arrow b). The Compound I-catalyzed oxidation of M1 generates a diol (M5) via the acid-catalyzed ring opening of an epoxide intermediate. A C-25 cation may also derive from the single-electron oxidation of the C-25 radical (arrow c). Trapping of the cation by formate or water (arrow d) results in the formation of the C-25 oxyformyl (M2) or the one-carbon reduced alcohol (M4), respectively. Loss of a proton from the C-25 cation may also generate M1 (arrow e). The Compound I-catalyzed oxidation of M4 produces a gem-diol intermediate that dehydrates to a keto compound (M3). B, a possible alternative mechanism involving Baeyer-Villiger oxidation with the ferric-peroxo anion (FeO2−) of CYP125 to yield M2 (see Fig. 1B, ii).
Many other P450 reactions are known in which rearrangements undoubtedly occur with a P450 enzyme-intermediate complex. Notable examples are the conversion of vinyl halides to α-haloacetaldehydes (18, 28). A recent similar example involves the “direct” conversion of 7,8-dehydrocholesterol to 7-ketocholesterol by the human P450 CYP7A1 (29). Strained cycloalkyl rings undergo rearrangements (2, 30). Other examples in which cleavage of a substrate occurs in such a mechanism involve the ipso cleavage of bisphenol A (supplemental Fig. S3) (31) and the conversion of nabumetone to 6-methoxy-2-naphthylacetic acid by the human P450 CYP1A2 (supplemental Fig. S4) (32, 33).
Coupling Reactions
P450-catalyzed C–C and C–O bond couplings are common in plant biosynthetic pathways (e.g. alkaloid biosynthesis) and in bacteria (e.g. antibiotic synthesis in Actinomycetes). These reactions are often critical in the biosynthesis of plant secondary metabolites. An interesting example from Taxol biosynthesis (plus a possible mechanism) is shown in Fig. 3A (34).
FIGURE 3.
Some unusual P450 reactions. A, proposed mechanism for rearrangement of taxa-4(5),11(12)-diene to 5(12)-oxa-3(11)-cyclotaxane, catalyzed by the P450 CYP725A4 from yew trees (34). B, proposed pathway for nitration of tryptophan (35). C, cyclopropanation by P450BM-3 (carbene transfer) (36).
Some other interesting examples include C–O bond coupling in the biosynthesis of grayanic acid (supplemental Fig. S5) (37), coupling in the synthesis of isoquinoline alkaloids (supplemental Fig. S6) (38), and oxidative coupling in early steps of morphine biosynthesis (supplemental Fig. S7) (39, 40). These coupling reactions, generally believed to involve radical chemistry, are not restricted to plants and bacteria. Human P450s have been shown to also catalyze steps in morphine synthesis (supplemental Fig. S8) (41). Most of these reactions are internal couplings, in which rings are in juxtaposition to fuse if a radical pathway is initiated. The coupling of two flaviolin molecules together has been observed in a Streptomyces coelicolor CYP158A2 reaction, in which the two molecules are both bound in the active site (42). A dimer of capsaicin is formed during its oxidation by human liver microsomes (although which P450 is involved remains unknown) (43). The general reaction supports a view of an aromatic radical pathway but is rare among oxidations of unlinked substrates. Recently, the environmental contaminant BDE-47, a polybrominated bis-phenyl ether flame retardant, was reported to be converted to a (brominated) p-dioxin molecule by the introduction of a second ether oxygen (supplemental Fig. S9) (44).
Nitration
A highly unusual reaction is involved in the synthesis of a phytotoxin, thaxtomin, in Streptomyces turgidiscabies (Fig. 3B) (35). The FeO22+ form of the enzyme is proposed to react with nitric oxide to form an iron peroxynitrite-like species that can produce a nitrating species capable of nitrating tryptophan.
Cyclopropanation
This is not a physiological reaction but nevertheless demonstrates the versatility of P450s (this is not an oxidation per se but is included here in the context of mechanistic similarity). The ferrous form of a mutant of bacterial CYP102A1 (P450BM-3) reacted with ethyl diazoacetate to convert styrene to a cyclopropyl derivative (Fig. 3C) (36). Multiple turnovers were observed, and the stereochemistry was distinct from that seen in reactions with only free heme.
Methane Hydroxylation
Although P450s are well known catalysts of alkane hydroxylations, the oxidation of methane presents a special challenge due to the strong C–H bond strength (104 kcal mol−1). Two groups have employed a strategy of “activating” CYP102A1 by using short perfluorinated fatty acids (with no C–H bonds available for oxidation) to “activate” the enzyme and then also adding short alkanes, which can be oxidized as substrates (45, 46). The activation process includes both a spin-state change in the iron and a constriction in the size of the active site. Watanabe and co-workers (46) were not able to oxidize methane or ethane in their system, but Reetz and co-workers (45) were able to achieve multiple turnovers of methane, an unusual feat.
Reductions
Although the vast majority of P450 reactions are oxidations, reductions are also known. Most are observed more readily under anaerobic or hypobaric conditions. For rather unclear reasons, the substrates for such reactions accept electrons from ferrous P450 at a rate that is competitive with the rates of binding and reduction of oxygen. These reactions can occur in cells with low oxygen tension, e.g. those most distant from capillary arteries in animals. The electrons must be transferred one at a time.
The nature of the 1-electron reduced intermediates and, in some ways, the fate of an oxygen in a substrate remain unclear. One unusual case is the conversion of benzo[a]pyrene epoxides to the polycyclic hydrocarbon itself, i.e. benzo[a]pyrene (47). For reasons that are not clear, the only reactions of the human “orphan” P450 CYP2S1 that have been reproducibly observed to date are all reductions (48–50).
Non-redox Reactions
At least three non-redox P450 reactions have been reported, including a phospholipase D-type hydrolysis by several mammalian P450s (51), pyrophosphatase (hydrolytic) activity of S. coelicolor CYP170A1 (52), and the rearrangement of a bicyclic pentaenone to an oxetane by CYP154A1 from the same bacterium (53). Those reactions are likely to involve elements of acid-base chemistry, but the details are unknown. In the case of CYP170A1, an alternative active site for the hydrolytic reaction has been identified (the P450 also catalyzes oxidative reactions).
Unusual P450 Enzymes
The “classical” eukaryotic P450 enzymes are membrane-associated P450s that interact either with the NADPH-dependent diflavin enzyme cytochrome P450 reductase (CPR2; for Class II microsomal P450s) or with the flavoprotein adrenodoxin reductase (ADR) and the iron-sulfur protein adrenodoxin (ADx; for Class I mitochondrial P450s) (Fig. 4). Typical prokaryotic Class I systems have similar flavoprotein reductase and ferredoxin (FDx) partners (60). Interestingly, some of the mammalian microsomal P450s are also imported in the mitochondria and use ADx (an FDx) and its ADR (64). Recent years have seen the identification of several distinct types of P450s that do not fall into the general Class I/II types, many of which have been identified through characterization of P450 enzymes identified from genome sequencing projects.
FIGURE 4.
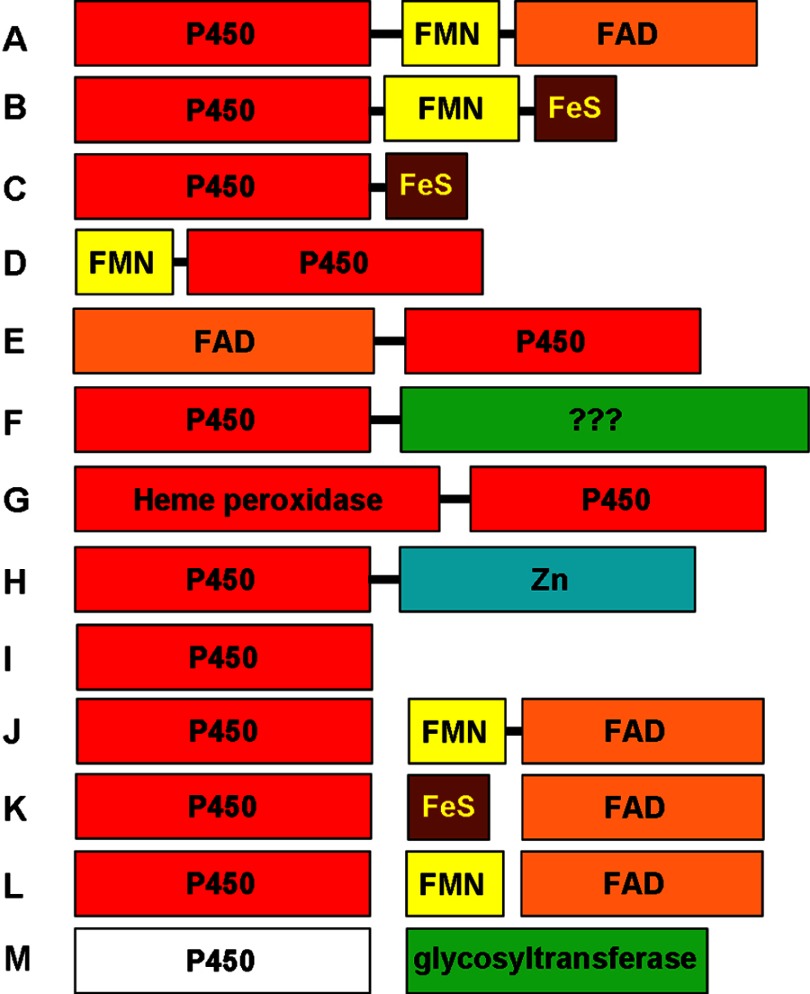
Diversity of P450 redox systems and P450 fusion proteins. A selection of distinct types of P450 enzymes and (where relevant) their redox partner systems is shown. The sizes of the boxes are indicative of the lengths of the protein modules. Bound prosthetic groups are indicated in the color-coded domains. A, P450BM-3 (CYP102A1)-type P450-CPR fusion, also seen for fungal P450foxy (CYP505)-type systems (54). B, CYP116B-type P450-phthalate dioxygenase reductase fusion (55). C, M. capsulatus P450-FDx fusion CYP51FX (56). D, R. rhodochrous P450-flavodoxin fusion XplA, involved in reductive degradation of explosives (57). E, Pseudomonas fluorescens PfO-1 acyl-CoA dehydrogenase-P450 fusion CYP222A1. This protein is depicted with FAD bound in its N-terminal domain, but there is no report to date of characterization of this protein. F, Mimivirus CYP5253A1, with a P450 fused to a C-terminal domain of uncertain function but containing several potential sites for post-translational modification. G, PpoA dioxygenase/peroxidase-P450 fusion enzyme from A. nidulans, involved in Psi factor production (58). H, P450-hydrolase fusion CYP631B5, involved in mycophenolic acid production (59). I, “stand-alone” P450 that acts without partner proteins, typified by P450nor (CYP55A)-type nitric-oxide reductase enzymes that interact directly with NAD(P)H, peroxygenase CYP152 P450s that use H2O2 to oxidize substrates, P450s that isomerize substrates (e.g. CYP5A1/8A1), and allene oxide synthase (CYP74A) dehydratase P450s. J, typical eukaryotic Class II P450 systems with separate membrane-associated P450 and a CPR partner. K, Class I (mitochondrial) P450 system that interacts with the iron-sulfur protein ADx, which is in turn reduced by ADR. Most bacterial systems use a similar redox apparatus (60). L, variation on system K, in which a flavodoxin replaces the iron-sulfur protein. This type of system supports CYP176A1 (P450cin); enables Citrobacter braakii to catabolize cineole; and can also reduce CYP107H1 (P450BioI), involved in B. subtilis biotin synthesis (61, 62). M, heme-free EryCII P450-like protein devoid of a cysteine proximal ligand. EryCII is an allosteric activator of the glycosyltransferase EryCIII in the production of erythromycin D in S. erythraea (63).
P450-Redox Partner Fusion Proteins
P450BM-3
The discovery of a high-activity fatty acid hydroxylase in Bacillus megaterium led Narhi and Fulco (65) to identify the first P450 linked to its redox partner. P450BM-3 (CYP102A1) has a eukaryote-like CPR fused to the C terminus of the P450. Both domains lack the membrane anchor regions typical of their eukaryotic relatives, and P450BM-3 was the first example of a prokaryotic CPR (54). The P450-CPR fusion arrangement of P450BM-3 facilitates rapid electron transfer from NADPH to the P450 heme, and the CPR portion (of P450BM-3) is also reduced by NADPH much faster than are eukaryotic CPRs (66), leading to P450BM-3 having the fastest substrate oxidation rate yet reported for a P450 enzyme, ∼250 s−1 (67). The catalytic proficiency of P450BM-3 has aroused great interest in its capacity for oxychemical synthesis, and notable successes in re-engineering P450BM-3 include transforming the enzyme from a fatty acid (ω-1, ω-2, and ω-3) hydroxylase into variants capable of oxidizing short chain alkanes and alkenes as well as steroids (68, 69). P450BM-3 is a dimeric enzyme, and intermonomeric electron transfer occurs in this system, as also seen for the structurally related eukaryotic nitric-oxide synthases (70–72). Several other P450BM-3-type P450-CPR enzymes are now known (e.g. see Ref. 73). The physiological function of P450BM-3 still remains unclear, although a role in bacterial quorum sensing mediated by oxidative inactivation of acylhomoserine lactones has been proposed (74). Similar types of P450-CPR fusion enzymes have been found in lower eukaryotes, most notably the Fusarium oxysporum membrane-associated fatty acid hydroxylase P450foxy (CYP505) (75).
The CYP116B Family
Several years elapsed between the discovery of P450BM-3 and the characterization of the next type of P450-redox partner fusion enzyme. Genome sequence data led to the identification of a small number of bacterial P450s fused to partners resembling phthalate dioxygenase reductases (76). Heterologous expression of two CYP116B family members (CYP116B1 and CYP116B2) enabled their purification and the demonstration that the reductase component binds FMN and 2Fe-2S iron-sulfur prosthetic groups and that P450 turnover is driven by NAD(P)H (55, 77). In the case of CYP116B1, the enzyme was shown to be catalytically active in the oxidation of thiocarbamate herbicides, consistent with the sequence similarity between its P450 domain and the herbicide-degrading Class I P450 CYP116A1 from Rhodococcus sp. strain NI86/21 (78). The CYP116B-type model has recently been mimicked in artificial P450 fusion enzymes in efforts to enhance eukaryotic P450 activities and produce metabolites of interest (e.g. see Ref. 79). Although electron transfer kinetics in the CYP116B-type reductase may be ∼30-fold slower than in the P450BM-3 reductase, fusing exogenous P450s to this CYP116B type of reductase may circumvent the problem of dimerization of the P450BM-3 reductase and provide a more robust fusion system for P450 engineering.
Other P450-Redox Partner Fusion Protein Systems
The P450BM-3 (CYP102A)- and CYP116B-type P450-partner fusions are catalytically self-sufficient in terms of having all protein partners required for function covalently linked together, thus requiring only an electron donor (NAD(P)H) and a P450 substrate for oxidative catalysis. A different type of self-sufficient system was described in Rhodococcus ruber strain DSM 44319, composed of FDx, FMN-containing flavoprotein reductase, and P450 modules and able to catalyze NADPH-dependent oxidation of polycyclic aromatic hydrocarbons (80). However, other systems have been characterized in which “partial” fusions decrease the number of redox partners required to reconstitute P450 function. In Methylococcus capsulatus, a CYP51-FDx fusion was shown to be composed of a P450 linked to a 3Fe-4S FDx at its C terminus. The enzyme (CYP51FX) was demonstrated to catalyze NADPH-dependent 14α-demethylation of lanosterol when spinach NADPH-FDx reductase was added to reconstitute a complete Class I-like system (56). A soluble biotechnologically relevant P450 enzyme from Rhodococcus rhodochrous strain 11Y (XplA) has an FDx module fused to the P450 at its N terminus. XplA receives electrons from an NADPH-dependent flavoprotein reductase (XplB) and catalyzes the reductive degradation of the explosive hexahydro-1,3,5-trinitro-1,3,5-triazine, producing nitrite as a final product (57).
A flavodoxin-like module in CPR is ultimately responsible for electron delivery to mammalian microsomal P450s, and flavodoxins were also shown to support electron transfer to the bacterial P450s involved in biotin synthesis and cineole catabolism, replacing ferredoxins in Class I-like systems (62, 81). XplA has unusual properties, including unusually weak binding of the FMN cofactor and the absence of a phylogenetically conserved Ser/Thr residue in the P450 I helix. The latter structural deviation is consistent with a primarily reductive role for XplA because the Ser/Thr residue is implicated in hydrogen bonding and/or proton transfer to iron-bound oxygen in the normal P450 catalytic cycle (82).
P450s Acting Independently of Redox Partner Proteins
Although the classical P450 catalytic cycle requires the timed delivery of 2 consecutive electrons to the heme iron (1) to allow (i) formation of ferrous iron, enabling O2 binding and formation of the ferrous-oxy (FeO22+) state, and (ii) reduction of the FeO22+ form (followed by further oxygen activation), a number of P450 enzymes bypass this mechanism. In doing so, these P450s either use an alternative route to achieve substrate oxidation or exploit the P450 scaffold for unconventional catalytic functions.
P450 Peroxygenases
The “peroxide shunt” is a long-used chemical procedure for driving P450 oxidase reactions in the absence of NAD(P)H-dependent redox partners. The reaction of H2O2 or organic peroxides with a ferric P450 can result in its direct conversion to the ferric-hydroperoxo (Compound 0) intermediate (FeO2−) (Fig. 1A, ii), protonation of which leads to loss of a water molecule and formation of the reactive Compound I species (ferryl-oxo with a porphyrin π cation radical, FeO3+; see above) that oxidizes the substrate (4, 83). The peroxide is rarely efficient and is often highly destructive in oxidizing the protein and the heme prosthetic group (non-physiological high-valent iodine compounds, e.g. iodosylbenzene (84, 85), can give high rates but are also destructive). However, this class of P450s uses this mechanism and avoids excessive oxidative damage during the process. Notable P450 peroxygenases are the Sphingomonas paucimobilis P450SPα (CYP152B1) and the Bacillus subtilis P450BSβ (CYP152A1), which catalyze long chain fatty acid hydroxylation primarily at the α- and β-carbons, respectively (86, 87). A more recently discovered member of the CYP152 P450 family is the OleT P450 from Jeotgalicoccus ATCC 8456, which uses the peroxygenase route to decarboxylate a range of long chain fatty acids to form the n-1 alkenes. OleT thus has potential applications for biofuel and fine chemical synthesis. A speculative mechanism for the reaction involves abstraction of a hydrogen atom from the α- or β-position of the fatty acid. Subsequent oxygen rebound chemistry would lead to hydroxylation at the α- or β-position (as seen for P450SPα and P450BSβ), but, in the case of OleT, the abstraction of a further proton from the β-position would lead to formation of a molecule of H2O and an unstable carbocation that spontaneously decarboxylates to form the terminal alkene (88).
Nitric-oxide Reductases
In a purely reductive reaction, the fungal nitric-oxide reductase P450s (P450nor enzymes) bind NAD(P)H in the P450 active site and catalyze an unusual hydride transfer from the nicotinamide cofactor to a heme iron-bound NO• molecule. This intermediate reacts with a second molecule of NO• to form N2O and H2O (89). There are separate cytosolic and mitochondrial isoforms of P450nor found in F. oxysporum (CYP55A1) and other fungi (60, 89). The CYP55A1 gene is nitrite/nitrate-inducible and is part of an energy-producing pathway that reduces these molecules to N2O. Reduction of NO• in mitochondria may also serve to prevent its action as a respiratory chain inhibitor. Bypassing the need for electron transfer reactions involving partner proteins helps to enable the fast reaction rates of NO• reduction (>103 s−1) observed for CYP55A1 (90).
Molecular Rearrangements
Prostaglandin Reactions
Selected P450s are known to catalyze isomerization reactions. Mammalian examples of this class include the eicosanoid-transforming thromboxane synthase (CYP5A1) that converts prostaglandin (PG) H2 into thromboxane A2 (TXA2). CYP5A1 also catalyzes molecular rearrangements of PGH1, PGH2, and PGG2. TXA2 causes vasoconstriction and induces platelet aggregation. CYP8A1 (prostacyclin synthase) also isomerizes PGH2, in this case, generating prostacyclin, which is vasodilatory and inhibits platelet aggregation (91). These reactions thus have competing regulatory functions. In both cases, the mechanism is proposed to involve homolytic cleavage of the PG endoperoxide, with a ferryl P450 iron-bonded to one or the other of the oxygen atoms (depending on whether CYP5A1 or CYP58A1 is the catalyst and the particular binding mode of the substrate in the P450 active site) (i.e. Fig. 1A, iii) and a radical on the other oxygen. Radical migration to a substrate carbon and electron transfer to the iron with carbocation formation on intermediates precede their final rearrangement to form either TXA2 or prostacyclin (92).
Allene Oxide Synthases
Plant allene oxide synthases (the CYP74A family) catalyze dehydration of fatty acid hydroperoxides to form the respective allene oxides (93), which are reactive epoxides that are further transformed into the plant hormone jasmonic acid. Jasmonic acid and its metabolites are crucial in plant growth and development and defense. Flax CYP74A1 converts (13S)-hydroperoxylinolenic acid to its allene oxide at a rate of >103 s−1 (94). Related CYP74 family members utilize the same fatty acid hydroperoxide substrates to catalyze carbon chain cleavage and the formation of aldehydes (hydroperoxide lyases CYP74B and CYP74C) (95) or the formation of fatty divinyl ethers (divinyl ether synthase CYP74D) (96).
Non-redox Partner-P450 Fusion and Partner Proteins
Genome sequencing projects have led to the identification of a number of novel P450 enzymes covalently attached to protein modules that are not obvious NAD(P)H-dependent redox partners or domains thereof. In several cases, these modules are homologs of structurally/biochemically characterized enzymes, although the functions of these fused enzymes are unknown in most cases. Many of these P450 fusions likely catalyze consecutive reactions or otherwise interact productively with the P450s to perform important physiological functions.
The PpoA Dioxygenase/Peroxidase-P450 Fusion
The filamentous fungus Aspergillus nidulans encodes a P450 fused at the C terminus of a heme-binding dioxygenase/peroxidase domain. Characterization of this PpoA enzyme demonstrated that the peroxidase domain catalyzed oxidation of linoleic acid to form (8R)-hydroperoxyoctadecadienoic acid, with this product then being isomerized by the P450 domain to form 5,8-dihydroxyoctadecadienoic acid. Isomerization is predicted to occur via a ferryl heme iron intermediate, as in the case of CYP5A1 and CYP58A1 (97). The Ppo enzymes, which have now been detected in a number of fungi (97), are responsible for production of Psi (precocious sexual inducer) factors, which are important for controlling the balance between sexual and asexual life cycles.
Fungal Mycophenolic Acid Synthesis
The fungal secondary metabolite mycophenolic acid has immunosuppressant activity and may also have other useful properties (e.g. antiviral and antibacterial). Initial steps in its synthesis in Penicillium brevicompactum are catalyzed by the MpaDE fusion protein, composed of a P450 (CYP631B5) with a hydrolase fused to its C terminus (59). The substrate 5-methylorsellinic acid undergoes MpaD P450-dependent hydroxylation on a methyl group to generate 4,6-dihydroxy-2-(hydroxymethyl)-3-methylbenzoic acid. A lactonization reaction is then likely catalyzed by the MpaE domain of MpaDE, forming 5,7-dihydroxy-4-methylphthalide, which is then acted on by later pathway enzymes. Other fungal genomes have separate mpaD/E genes, and thus, the gene fusion in P. brevicompactum may provide kinetic advantages in product formation.
Other P450 Fusions of Unspecified Function
Among several uncharacterized P450 fusions identifiable from database searches are enzymes fused to modules related to acyl-CoA dehydrogenase (CYP221A1), cinnamyl alcohol dehydrogenase, and F-box motif-containing proteins. The first viral P450 (CYP5253A1) was discovered in the Mimivirus genome and is fused at its C terminus to a protein of unknown function but containing several post-translational modification sites (phosphorylation, myristoylation, and glycosylation) (98, 99). Recent studies showed that the Saccharopolyspora erythraea glycosyltransferase enzyme EryCIII, which produces erythromycin D (from the substrates thiamine diphosphate-d-desosamine and 3-α-mycarosylerythronolide B), is activated by the addition of the protein EryCII, with which it forms a heterotetrameric (2:2) complex (63). EryCII is homologous to P450s but lacks the cysteinate ligand to the heme iron and was shown to be heme-free in the structure of the complex. It appears that EryCII has evolved to become an allosteric activator of EryCIII, allowing the enzyme binding sites for mycarosylerythronolide B and thiamine diphosphate-d-desosamine to interact productively (63).
Conclusions and Future Considerations
Characterization of P450 enzymes has revealed considerable diversity in the types of protein arrangements that can be used for electron transfer and catalysis. In one sense, the chemical mechanisms have a great deal of commonality (Fig. 1), but these are manifested in a wide variety of reactions because of the natures of the substrates. Today, we have insight into the physiological roles of a small minority of the >18,000 P450 genes whose sequences have been obtained, and the future promises to provide more interesting vignettes into this important superfamily.
Acknowledgments
We thank Drs. F. K. Yoshimoto, D. Kim, and A. R. Brash for comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R37 CA090426 and P30 ES000267. This is the first article in the Thematic Minireview Series on Cytochrome P450.

This article contains supplemental Figs. S1–S10 and additional references.
- CPR
- cytochrome P450 reductase
- ADR
- adrenodoxin reductase
- ADx
- adrenodoxin
- FDx
- ferredoxin
- PG
- prostaglandin
- TXA2
- thromboxane A2.
REFERENCES
- 1. Krest C. M., Onderko E. L., Yosca T. H., Calixto J. C., Karp R. F., Livada J., Rittle J., Green M. T. (2013) Reactive intermediates in cytochrome P450 catalysis. J. Biol. Chem. 288, 17074–17081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Macdonald T. L., Zirvi K., Burka L. T., Peyman P., Guengerich F. P. (1982) Mechanism of cytochrome P-450 inhibition by cyclopropylamines. J. Am. Chem. Soc. 104, 2050–2052 [Google Scholar]
- 3. Okazaki O., Guengerich F. P. (1993) Evidence for specific base catalysis in N-dealkylation reactions catalyzed by cytochrome P450 and chloroperoxidase. Differences in rates of deprotonation of aminium radicals as an explanation for high kinetic hydrogen isotope effects observed with peroxidases. J. Biol. Chem. 268, 1546–1552 [PubMed] [Google Scholar]
- 4. Ortiz de Montellano P. R., De Voss J. J. (2005) Substrate oxidation by cytochrome P450 enzymes. in Cytochrome P450: Structure, Mechanism, and Biochemistry (Ortiz de Montellano P. R., ed) 3rd Ed., pp. 183–245, Kluwer Academic/Plenum Publishers, New York [Google Scholar]
- 5. Kedderis G. L., Dwyer L. A., Rickert D. E., Hollenberg P. F. (1983) Source of the oxygen atom in the product of cytochrome P-450-catalyzed N-demethylation reactions. Mol. Pharmacol. 23, 758–760 [PubMed] [Google Scholar]
- 6. Shea J. P., Valentine G. L., Nelson S. D. (1982) Source of oxygen in cytochrome P-450 catalyzed carbinolamine formation. Biochem. Biophys. Res. Commun. 109, 231–235 [DOI] [PubMed] [Google Scholar]
- 7. Shaik S., Kumar D., de Visser S. P., Altun A., Thiel W. (2005) Theoretical perspective on the structure and mechanism of cytochrome P450 enzymes. Chem. Rev. 105, 2279–2328 [DOI] [PubMed] [Google Scholar]
- 8. Rittle J., Green M. T. (2010) Cytochrome P450 compound I: capture, characterization, and C-H bond activation kinetics. Science 330, 933–937 [DOI] [PubMed] [Google Scholar]
- 9. Guengerich F. P. (2001) Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 14, 611–650 [DOI] [PubMed] [Google Scholar]
- 10. Isin E. M., Guengerich F. P. (2007) Complex reactions catalyzed by cytochrome P450 enzymes. Biochim. Biophys. Acta 1770, 314–329 [DOI] [PubMed] [Google Scholar]
- 11. Guengerich F. P., Isin E. M. (2014) Unusual metabolic reactions and pathways. in Handbook of Metabolic Pathways of Xenobiotics (Prakash C., Gau L., Zhong D., Aizawa H., Lee P., eds) Vol. 1, John Wiley & Sons, Inc., New York, in press [Google Scholar]
- 12. Humphreys W. G. (2008) Drug metabolism research as an integral part of the drug discovery process. in Drug Metabolism in Drug Design and Development (Zhang D., Zhu M., Humphreys W. G., eds) pp. 239–260, John Wiley & Sons, Inc., Hoboken, NJ [Google Scholar]
- 13. Ross D., Farmer P. B., Gescher A., Hickman J. A., Threadgill M. D. (1983) The metabolism of a stable N-hydroxymethyl derivative of a N-methylamide. Life Sci. 32, 597–604 [DOI] [PubMed] [Google Scholar]
- 14. He X., Cryle M. J., De Voss J. J., Ortiz de Montellano P. R. (2005) Calibration of the channel that determines the ω-hydroxylation regiospecificity of cytochrome P450 4A1. Catalytic oxidation of 12-halododecanoic acids. J. Biol. Chem. 280, 22697–22705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guengerich F. P. (1989) Oxidation of halogenated compounds by cytochrome P-450, peroxidases, and model metalloporphyrins. J. Biol. Chem. 264, 17098–17205 [PubMed] [Google Scholar]
- 16. Rettie A. E., Rettenmeier A. W., Howald W. N., Baillie T. A. (1987) Cytochrome P-450 catalyzed formation of δ4-VPA, a toxic metabolite of valproic acid. Science 235, 890–893 [DOI] [PubMed] [Google Scholar]
- 17. Guroff G., Daly J. W., Jerina D. M., Renson J., Witkop B., Udenfriend S. (1967) Hydroxylation-induced migration: the NIH shift. Science 157, 1524–1530 [DOI] [PubMed] [Google Scholar]
- 18. Liebler D. C., Guengerich F. P. (1983) Olefin oxidation by cytochrome P-450: evidence for group migration in catalytic intermediates formed with vinylidene chloride and trans-1-phenyl-1-butene. Biochemistry 22, 5482–5489 [DOI] [PubMed] [Google Scholar]
- 19. Cai H., Guengerich F. P. (1999) Mechanism of aqueous decomposition of trichloroethylene oxide. J. Am. Chem. Soc. 121, 11656–11663 [Google Scholar]
- 20. Rendic S., Guengerich F. P. (2012) Contributions of human enzymes in carcinogen metabolism. Chem. Res. Toxicol. 25, 1316–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hong H., Caceres-Cortes J., Su H., Huang X., Roongta V., Bonacorsi S., Jr., Hong Y., Tian Y., Iyer R. A., Humphreys W. G., Christopher L. J. (2011) Mechanistic studies on a P450-mediated rearrangement of BMS-690514: conversion of a pyrrolotriazine to a hydroxypyridotriazine. Chem. Res. Toxicol. 24, 125–134 [DOI] [PubMed] [Google Scholar]
- 22. Austin R. N., Deng D., Jiang Y., Luddy K., van Beilen J. B., Ortiz de Montellano P. R., Groves J. T. (2006) The diagnostic substrate bicyclohexane reveals a radical mechanism for bacterial cytochrome P450 in whole cells. Angew. Chem. Int. Ed. Engl. 45, 8192–8194 [DOI] [PubMed] [Google Scholar]
- 23. Auclair K., Hu Z., Little D. M., Ortiz de Montellano P. R., Groves J. T. (2002) Revisiting the mechanism of P450 enzymes with the radical clocks norcarane and spiro[2,5]octane. J. Am. Chem. Soc. 124, 6020–6027 [DOI] [PubMed] [Google Scholar]
- 24. Toy P. H., Newcomb M., Hollenberg P. F. (1998) Hypersensitive mechanistic probe studies of cytochrome P450-catalyzed hydroxylation reactions. Implications for the cationic pathway. J. Am. Chem. Soc. 120, 7719–7729 [Google Scholar]
- 25. Cooper H. L., Groves J. T. (2011) Molecular probes of the mechanism of cytochrome P450. Oxygen traps a substrate radical intermediate. Arch. Biochem. Biophys. 507, 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frey P. A. (1997) Radicals in enzymatic reactions. Curr. Opin. Chem. Biol. 1, 347–356 [DOI] [PubMed] [Google Scholar]
- 27. Sivaramakrishnan S., Ouellet H., Matsumura H., Guan S., Moënne-Loccoz P., Burlingame A. L., Ortiz de Montellano P. R. (2012) Proximal ligand electron donation and reactivity of the cytochrome P450 ferric-peroxo anion. J. Am. Chem. Soc. 134, 6673–6684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller R. E., Guengerich F. P. (1982) Oxidation of trichloroethylene by liver microsomal cytochrome P-450: evidence for chlorine migration in a transition state not involving trichloroethylene oxide. Biochemistry 21, 1090–1097 [DOI] [PubMed] [Google Scholar]
- 29. Shinkyo R., Xu L., Tallman K. A., Cheng Q., Porter N. A., Guengerich F. P. (2011) Conversion of 7-dehydrocholesterol to 7-ketocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J. Biol. Chem. 286, 33021–33028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bondon A., Macdonald T. L., Harris T. M., Guengerich F. P. (1989) Oxidation of cycloalkylamines by cytochrome P-450. Mechanism-based inactivation, adduct formation, ring expansion, and nitrone formation. J. Biol. Chem. 264, 1988–1997 [PubMed] [Google Scholar]
- 31. Kolvenbach B., Schlaich N., Raoui Z., Prell J., Zühlke S., Schäfter A., Guengrich F. P., Corvini P. F. X. (2007) Degradation of bisphenol A: does ipso substitution apply to phenols containing a quartenary C-α structure in the para positions? Appl. Environ. Microbiol. 73, 4476–4784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haddock R. E., Jeffery D. J., Lloyd J. A., Thawley A. R. (1984) Metabolism of nabumetone (BRL 14777) by various species including man. Xenobiotica 14, 327–337 [DOI] [PubMed] [Google Scholar]
- 33. Turpeinen M., Hofmann U., Klein K., Mürdter T., Schwab M., Zanger U. M. (2009) A predominate role of CYP1A2 for the metabolism of nabumetone to the active metabolite, 6-methoxy-2-naphthylacetic acid, in human liver microsomes. Drug Metab. Dispos. 37, 1017–1024 [DOI] [PubMed] [Google Scholar]
- 34. Rontein D., Onillon S., Herbette G., Lesot A., Werck-Reichhart D., Sallaud C., Tissier A. (2008) CYP725A4 from yew catalyzes complex structural rearrangement of taxa-4(5),11(12)-diene into the cyclic ether 5(12)-oxa-3(11)-cyclotaxane. J. Biol. Chem. 283, 6067–6075 [DOI] [PubMed] [Google Scholar]
- 35. Barry S. M., Kers J. A., Johnson E. G., Song L., Aston P. R., Patel B., Krasnoff S. B., Crane B. R., Gibson D. M., Loria R., Challis G. L. (2012) Cytochrome P450-catalyzed l-tryptophan nitration in thaxtomin phytotoxin biosynthesis. Nat. Chem. Biol. 8, 814–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coelho P. S., Brustad E. M., Kannan A., Arnold F. H. (2013) Olefin cyclopropanation via carbene transfer catalyzed by engineered cytochrome P450 enzymes. Science 339, 307–310 [DOI] [PubMed] [Google Scholar]
- 37. Culberson C. F., Armaleo D. (1992) Induction of a complete secondary-product pathway in a cultured lichen fungus. Exp. Mycol. 16, 52–63 [Google Scholar]
- 38. Ikezawa N., Iwasa K., Sato F. (2009) CYP719A subfamily of cytochrome P450 oxygenases and isoquinoline alkaloid biosynthesis in Eschscholzia californica. Plant Cell Rep. 28, 123–133 [DOI] [PubMed] [Google Scholar]
- 39. Ikezawa N., Iwasa K., Sato F. (2008) Molecular cloning and characterization of CYP80G2, a cytochrome P450 that catalyzes an intramolecular C-C phenol coupling of (S)-reticuline in magnoflorine biosynthesis, from cultured Coptis japonica cells. J. Biol. Chem. 283, 8810–8821 [DOI] [PubMed] [Google Scholar]
- 40. Gesell A., Rolf M., Ziegler J., Díaz Chávez M. L., Huang F. C., Kutchan T. M. (2009) CYP719B1 is salutaridine synthase, the C-C phenol-coupling enzyme of morphine biosynthesis in opium poppy. J. Biol. Chem. 284, 24432–24442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grobe N., Zhang B., Fisinger U., Kutchan T. M., Zenk M. H., Guengerich F. P. (2009) Mammalian cytochrome P450 enzymes catalyze the phenol-coupling step in endogenous morphine biosynthesis. J. Biol. Chem. 284, 24425–24431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao B., Guengerich F. P., Bellamine A., Lamb D. C., Izumikawa M., Lei L., Podust L. M., Sundaramoorthy M., Kalaitzis J. A., Reddy L. M., Kelly S. L., Moore B. S., Stec D., Voehler M., Falck J. R., Shimada T., Waterman M. R. (2005) Binding of two flaviolin substrate molecules, oxidative coupling, and crystal structure of Streptomyces coelicolor A3(2) cytochrome P450 158A2. J. Biol. Chem. 280, 11599–11607 [DOI] [PubMed] [Google Scholar]
- 43. Reilly C. A., Henion F., Bugni T. S., Ethirajan M., Stockmann C., Pramanik K. C., Srivastava S. K., Yost G. S. (2013) Reactive intermediates produced from the metabolism of the vanilloid ring of capsaicinoids by P450 enzymes. Chem. Res. Toxicol. 26, 55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Feo M. L., Gross M. S., McGarrigle B. P., Eljarrat E., Barceló D., Aga D. S., Olson J. R. (2013) Biotransformation of BDE-47 to potentially toxic metabolites is predominantly mediated by human CYP2B6. Environ. Health Perspect. 121, 440–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zilly F. E., Acevedo J. P., Augustyniak W., Deege A., Häusig U. W., Reetz M. T. (2011) Tuning a P450 enzyme for methane oxidation. Angew. Chem. Int. Ed. Engl. 50, 2720–2724 [DOI] [PubMed] [Google Scholar]
- 46. Kawakami N., Shoji O., Watanabe Y. (2011) Use of perfluorocarboxylic acids to trick cytochrome P450BM3 into initiating the hydroxylation of gaseous alkanes. Angew. Chem. Int. Ed. Engl. 50, 5315–5318 [DOI] [PubMed] [Google Scholar]
- 47. Sugiura M., Yamazoe Y., Kamataki T., Kato R. (1980) Reduction of epoxy derivatives of benzo(a)pyrene by microsomal cytochrome P-450. Cancer Res. 40, 2910–2914 [PubMed] [Google Scholar]
- 48. Nishida C. R., Lee M., Ortiz de Montellano P. R. (2010) Efficient hypoxic activation of the anticancer agent AQ4N by CYP2S1 and CYP2W1. Mol. Pharmacol. 78, 497–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xiao Y., Shinkyo R., Guengerich F. P. (2011) Cytochrome P450 2S1 is reduced by NADPH-cytochrome P450 reductase. Drug Metab. Dispos. 39, 944–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang K., Guengerich F. P. (2012) Oxidation of fluorinated 2-aryl-benzothiazole antitumor molecules by human cytochromes P450 1A1 and 2W1. Deactivation by cytochrome P450 2S1. Chem. Res. Toxicol. 25, 1740–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yun C.-H., Ahn T., Guengerich F. P., Yamazaki H., Shimada T. (1999) Phospholipase D activity of cytochromes P450 in human liver endoplasmic reticulum. Arch. Biochem. Biophys. 367, 81–88 [DOI] [PubMed] [Google Scholar]
- 52. Zhao B., Lei L., Vassylyev D. G., Lin X., Cane D. E., Kelly S. L., Yuan H., Lamb D. C., Waterman M. R. (2009) Crystal structure of albaflavenone monooxygenase containing a moonlighting terpene synthase active site. J. Biol. Chem. 284, 36711–36719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cheng Q., Lamb D. C., Kelly S. L., Lei L., Guengerich F. P. (2010) Cyclization of a cellular dipentaenone by Streptomyces coelicolor cytochrome P450 154A1 without oxidation-reduction. J. Am. Chem. Soc. 132, 15173–15175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Munro A. W., Leys D. G., McLean K. J., Marshall K. R., Ost T. W., Daff S., Miles C. S., Chapman S. K., Lysek D. A., Moser C. C., Page C. C., Dutton P. L. (2002) P450 BM3: the very model of a modern flavocytochrome. Trends Biochem. Sci. 27, 250–257 [DOI] [PubMed] [Google Scholar]
- 55. Warman A. J., Robinson J. W., Luciakova D., Lawrence A. D., Marshall K. R., Warren M. J., Cheesman M. R., Rigby S. E., Munro A. W., McLean K. J. (2012) Characterization of Cupriavidus metallidurans CYP116B1–a thiocarbamate herbicide oxygenating P450-phthalate dioxygenase reductase fusion protein. FEBS J. 279, 1675–1693 [DOI] [PubMed] [Google Scholar]
- 56. Jackson C. J., Lamb D. C., Marczylo T. H., Warrilow A. G., Manning N. J., Lowe D. J., Kelly D. E., Kelly S. L. (2002) A novel sterol 14α-demethylase/ferredoxin fusion protein (MCCYP51FX) from Methylococcus capsulatus represents a new class of the cytochrome P450 superfamily. J. Biol. Chem. 277, 46959–46965 [DOI] [PubMed] [Google Scholar]
- 57. Rylott E. L., Jackson R. G., Sabbadin F., Seth-Smith H. M., Edwards J., Chong C. S., Strand S. E., Grogan G., Bruce N. C. (2011) The explosive-degrading cytochrome P450 XplA: biochemistry, structural features and prospects for bioremediation. Biochim. Biophys. Acta 1814, 230–236 [DOI] [PubMed] [Google Scholar]
- 58. Brodhun F., Göbel C., Hornung E., Feussner I. (2009) Identification of PpoA from Aspergillus nidulans as a fusion protein of a fatty acid heme dioxygenase/peroxidase and a cytochrome P450. J. Biol. Chem. 284, 11792–11805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hansen B. G., Mnich E., Nielsen K. F., Nielsen J. B., Nielsen M. T., Mortensen U. H., Larsen T. O., Patil K. R. (2012) Involvement of a natural fusion of a cytochrome P450 and a hydrolase in mycophenolic acid biosynthesis. Appl. Environ. Microbiol. 78, 4908–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Munro A. W., Girvan H. M., McLean K. J. (2007) Variations on a (t)heme–novel mechanisms, redox partners and catalytic functions in the cytochrome P450 superfamily. Nat. Prod. Rep. 24, 585–609 [DOI] [PubMed] [Google Scholar]
- 61. Hawkes D. B., Slessor K. E., Bernhardt P. V., De Voss J. J. (2010) Cloning, expression and purification of cindoxin, an unusual FMN-containing cytochrome P450 redox partner. ChemBioChem 11, 1107–1114 [DOI] [PubMed] [Google Scholar]
- 62. Lawson R. J., von Wachenfeldt C., Haq I., Perkins J., Munro A. W. (2004) Expression and characterization of the two flavodoxin proteins of Bacillus subtilis, YkuN and YkuP: biophysical properties and interactions with cytochrome P450 BioI. Biochemistry 43, 12390–12409 [DOI] [PubMed] [Google Scholar]
- 63. Moncrieffe M. C., Fernandez M. J., Spiteller D., Matsumura H., Gay N. J., Luisi B. F., Leadlay P. F. (2012) Structure of the glycosyltransferase EryCIII in complex with its activating P450 homologue EryCII. J. Mol. Biol. 415, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bajpai P., Sangar M. C., Singh S., Tang W., Bansal S., Chowdhury G., Cheng Q., Fang J.-K., Martin M. V., Guengerich F. P., Avadhani N. G. (2013) Metabolism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by mitochondria-targeted cytochrome P450 2D6. Implications for Parkinson disease. J. Biol. Chem. 288, 4436–4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Narhi L. O., Fulco A. J. (1987) Identification and characterization of two functional domains in cytochrome P-450BM-3, a catalytically self-sufficient monooxygenase induced by barbiturates in Bacillus megaterium. J. Biol. Chem. 262, 6683–6690 [PubMed] [Google Scholar]
- 66. Munro A. W., Lindsay J. G. (1996) Bacterial cytochromes P-450. Mol. Microbiol. 20, 1115–1125 [DOI] [PubMed] [Google Scholar]
- 67. Noble M. A., Miles C. S., Chapman S. K., Lysek D. A., MacKay A. C., Reid G. A., Hanzlik R. P., Munro A. W. (1999) Roles of key active-site residues in flavocytochrome P450 BM3. Biochem. J. 339, 371–379 [PMC free article] [PubMed] [Google Scholar]
- 68. Glieder A., Farinas E. T., Arnold F. H. (2002) Laboratory evolution of a soluble, self-sufficient, highly active alkane hydroxylase. Nat. Biotechnol. 20, 1135–1139 [DOI] [PubMed] [Google Scholar]
- 69. Kille S., Zilly F. E., Acevedo J. P., Reetz M. T. (2011) Regio- and stereoselectivity of P450-catalysed hydroxylation of steroids controlled by laboratory evolution. Nat. Chem. 3, 738–743 [DOI] [PubMed] [Google Scholar]
- 70. Neeli R., Girvan H. M., Lawrence A., Warren M. J., Leys D., Scrutton N. S., Munro A. W. (2005) The dimeric form of flavocytochrome P450 BM3 is catalytically functional as a fatty acid hydroxylase. FEBS Lett. 579, 5582–5588 [DOI] [PubMed] [Google Scholar]
- 71. Kitazume T., Haines D. C., Estabrook R. W., Chen B., Peterson J. A. (2007) Obligatory intermolecular electron-transfer from FAD to FMN in dimeric P450BM-3. Biochemistry 46, 11892–11901 [DOI] [PubMed] [Google Scholar]
- 72. Girvan H. M., Dunford A. J., Neeli R., Ekanem I. S., Waltham T. N., Joyce M. G., Leys D., Curtis R. A., Williams P., Fisher K., Voice M. W., Munro A. W. (2011) Flavocytochrome P450 BM3 mutant W1046A is a NADH-dependent fatty acid hydroxylase: implications for the mechanism of electron transfer in the P450 BM3 dimer. Arch. Biochem. Biophys. 507, 75–85 [DOI] [PubMed] [Google Scholar]
- 73. Gustafsson M. C., Roitel O., Marshall K. R., Noble M. A., Chapman S. K., Pessegueiro A., Fulco A. J., Cheesman M. R., von Wachenfeldt C., Munro A. W. (2004) Expression, purification, and characterization of Bacillus subtilis cytochromes P450 CYP102A2 and CYP102A3: flavocytochrome homologues of P450 BM3 from Bacillus megaterium. Biochemistry 43, 5474–5487 [DOI] [PubMed] [Google Scholar]
- 74. Chowdhary P. K., Keshavan N., Nguyen H. Q., Peterson J. A., González J. E., Haines D. C. (2007) Bacillus megaterium CYP102A1 oxidation of acyl homoserine lactones and acyl homoserines. Biochemistry 46, 14429–14437 [DOI] [PubMed] [Google Scholar]
- 75. Kitazume T., Takaya N., Nakayama N., Shoun H. (2000) Fusarium oxysporum fatty-acid subterminal hydroxylase (CYP505) is a membrane-bound eukaryotic counterpart of Bacillus megaterium cytochrome P450BM3. J. Biol. Chem. 275, 39734–39740 [DOI] [PubMed] [Google Scholar]
- 76. De Mot R., Parret A. H. (2002) A novel class of self-sufficient cytochrome P450 monooxygenases in prokaryotes. Trends Microbiol. 10, 502–508 [DOI] [PubMed] [Google Scholar]
- 77. Roberts G. A., Celik A., Hunter D. J., Ost T. W., White J. H., Chapman S. K., Turner N. J., Flitsch S. L. (2003) A self-sufficient cytochrome P450 with a primary structural organization that includes a flavin domain and a [2Fe-2S] redox center. J. Biol. Chem. 278, 48914–48920 [DOI] [PubMed] [Google Scholar]
- 78. Nagy I., Schoofs G., Compernolle F., Proost P., Vanderleyden J., De Mot R. (1995) Degradation of the thiocarbamate herbicide EPTC (S-ethyl dipropylcarbamothioate) and biosafening by Rhodococcus sp. strain NI86/21 involve an inducible cytochrome P-450 system and aldehyde dehydrogenase. J. Bacteriol. 177, 676–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Schückel J., Rylott E. L., Grogan G., Bruce N. C. (2012) A gene-fusion approach to enabling plant cytochromes P450 for biocatalysis. ChemBioChem 13, 2758–2763 [DOI] [PubMed] [Google Scholar]
- 80. Liu L., Schmid R. D., Urlacher V. B. (2006) Cloning, expression, and characterization of a self-sufficient cytochrome P450 monooxygenase from Rhodococcus ruber DSM 44319. Appl. Microbiol. Biotechnol. 72, 876–882 [DOI] [PubMed] [Google Scholar]
- 81. Meharenna Y. T., Li H., Hawkes D. B., Pearson A. G., De Voss J., Poulos T. L. (2004) Crystal structure of P450cin in a complex with its substrate, 1,8-cineole, a close structural homologue to d-camphor, the substrate for P450cam. Biochemistry 43, 9487–9494 [DOI] [PubMed] [Google Scholar]
- 82. Bui S. H., McLean K. J., Cheesman M. R., Bradley J. M., Rigby S. E., Levy C. W., Leys D., Munro A. W. (2012) Unusual spectroscopic and ligand binding properties of the cytochrome P450-flavodoxin fusion enzyme XplA. J. Biol. Chem. 287, 19699–19714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Denisov I. G., Makris T. M., Sligar S. G., Schlichting I. (2005) Structure and chemistry of cytochrome P450. Chem. Rev. 105, 2253–2277 [DOI] [PubMed] [Google Scholar]
- 84. Gustafsson J. Å., Rondahl L., Bergman J. (1979) Iodosylbenzene derivatives as oxygen donors in cytochrome P-450 catalyzed steroid hydroxylations. Biochemistry 18, 865–870 [DOI] [PubMed] [Google Scholar]
- 85. Yamazaki H., Ueng Y.-F., Shimada T., Guengerich F. P. (1995) Roles of divalent metal ions in oxidations catalyzed by recombinant cytochrome P450 3A4 and replacement of NADPH-cytochrome P450 reductase with other flavoproteins, iron-sulfur proteins, and oxygen surrogates. Biochemistry 34, 8380–8389 [DOI] [PubMed] [Google Scholar]
- 86. Fujishiro T., Shoji O., Nagano S., Sugimoto H., Shiro Y., Watanabe Y. (2011) Crystal structure of H2O2-dependent cytochrome P450SPα with its bound fatty acid substrate. Insight into the regioselective hydroxylation of fatty acids at the α position. J. Biol. Chem. 286, 29941–29950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lee D. S., Yamada A., Sugimoto H., Matsunaga I., Ogura H., Ichihara K., Adachi S., Park S. Y., Shiro Y. (2003) Substrate recognition and molecular mechanism of fatty acid hydroxylation by cytochrome P450 from Bacillus subtilis. Crystallographic, spectroscopic, and mutational studies. J. Biol. Chem. 278, 9761–9767 [DOI] [PubMed] [Google Scholar]
- 88. Rude M. A., Baron T. S., Brubaker S., Alibhai M., Del Cardayre S. B., Schirmer A. (2011) Terminal olefin (1-alkene) biosynthesis by a novel P450 fatty acid decarboxylase from Jeotgalicoccus species. Appl. Environ. Microbiol. 77, 1718–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Daiber A., Shoun H., Ullrich V. (2005) Nitric oxide reductase (P450nor) from Fusarium oxysporum. J. Inorg. Biochem. 99, 185–193 [DOI] [PubMed] [Google Scholar]
- 90. Oshima R., Fushinobu S., Su F., Zhang L., Takaya N., Shoun H. (2004) Structural evidence for direct hydride transfer from NADH to cytochrome P450nor. J. Mol. Biol. 342, 207–217 [DOI] [PubMed] [Google Scholar]
- 91. Guengerich F. P. (2005) Human cytochrome P450 enzymes. in Cytochrome P450: Structure, Mechanism, and Biochemistry (Ortiz de Montellano P. R., ed) 3rd Ed., pp. 377–530, Kluwer Academic/Plenum Publishers, New York [Google Scholar]
- 92. Hecker M., Ullrich V. (1989) On the mechanism of prostacyclin and thromboxane A2 biosynthesis. J. Biol. Chem. 264, 141–150 [PubMed] [Google Scholar]
- 93. Brash A. R. (2009) Mechanistic aspects of CYP74 allene oxide synthases and related cytochrome P450 enzymes. Phytochemistry 70, 1522–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Song W. C., Brash A. R. (1991) Purification of an allene oxide synthase and identification of the enzyme as a cytochrome P-450. Science 253, 781–784 [DOI] [PubMed] [Google Scholar]
- 95. Matsui K., Shibutani M., Hase T., Kajiwara T. (1996) Bell pepper fruit fatty acid hydroperoxide lyase is a cytochrome P450 (CYP74B). FEBS Lett. 394, 21–24 [DOI] [PubMed] [Google Scholar]
- 96. Itoh A., Howe G. A. (2001) Molecular cloning of a divinyl ether synthase. Identification as a CYP74 cytochrome P450. J. Biol. Chem. 276, 3620–3627 [DOI] [PubMed] [Google Scholar]
- 97. Brodhun F., Feussner I. (2011) Oxylipins in fungi. FEBS J. 278, 1047–1063 [DOI] [PubMed] [Google Scholar]
- 98. Lamb D. C., Waterman M. R. (2013) Unusual properties of the cytochrome P450 superfamily. Phil. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lamb D. C., Lei L., Warrilow A. G., Lepesheva G. I., Mullins J. G., Waterman M. R., Kelly S. L. (2009) The first virally encoded cytochrome P450. J. Virol. 83, 8266–8269 [DOI] [PMC free article] [PubMed] [Google Scholar]