Background: Protein synthesis control is important for β-cell fate during ER stress.
Results: Increased protein synthesis during chronic ER stress in β-cells involves the transcriptional induction of an amino acid transporter network.
Conclusion: Increased amino acid uptake in β-cells during ER stress promotes apoptosis.
Significance: Induced expression of a network of amino acid transporters in islets can contribute to chronic ER stress-induced diabetes.
Keywords: Amino Acid Transport, Aminoacyl tRNA Synthetase, Beta Cell, Diabetes, ER Stress, Glutamine
Abstract
Endoplasmic reticulum (ER) stress-induced responses are associated with the loss of insulin-producing β-cells in type 2 diabetes mellitus. β-Cell survival during ER stress is believed to depend on decreased protein synthesis rates that are mediated via phosphorylation of the translation initiation factor eIF2α. It is reported here that chronic ER stress correlated with increased islet protein synthesis and apoptosis in β-cells in vivo. Paradoxically, chronic ER stress in β-cells induced an anabolic transcription program to overcome translational repression by eIF2α phosphorylation. This program included expression of amino acid transporter and aminoacyl-tRNA synthetase genes downstream of the stress-induced ATF4-mediated transcription program. The anabolic response was associated with increased amino acid flux and charging of tRNAs for branched chain and aromatic amino acids (e.g. leucine and tryptophan), the levels of which are early serum indicators of diabetes. We conclude that regulation of amino acid transport in β-cells during ER stress involves responses leading to increased protein synthesis, which can be protective during acute stress but can lead to apoptosis during chronic stress. These studies suggest that the increased expression of amino acid transporters in islets can serve as early diagnostic biomarkers for the development of diabetes.
Introduction
Type 2 diabetes mellitus (T2DM)4 begins with insulin resistance of peripheral tissues (1, 2). To compensate for this resistance, pancreatic β-cells respond with increased insulin synthesis and proliferation. Increased insulin synthesis in the ER causes ER stress that triggers the UPR (3, 4). Prolonged activation of the UPR in β-cells leads to apoptosis, which limits circulating insulin levels and leads to T2DM (5). The mechanism(s) involved in the progression of β-cell dysfunction in T2DM are poorly understood.
The UPR involves transcriptional and translational reprogramming of the stressed cells (2). During the early response, PERK kinase phosphorylates the initiation factor eIF2α, which represses the translation of most mRNAs and limits the accumulation of unfolded proteins. This phosphorylation also induces translation of mRNAs encoding two key regulators of the stress response program: the transcription factor ATF4 and the phosphatase subunit GADD34 (6). During prolonged stress, GADD34, which is also induced by transcriptional mechanisms, dephosphorylates eIF2α, enabling the translation of stress-induced mRNAs (2). This translational recovery during the UPR needs to be tightly regulated to ensure that the synthesized proteins do not exceed the folding capacity of the ER. The current view is that translational recovery is mediated by controlling the extent of eIF2α phosphorylation via GADD34 (1, 2, 7).
Several mouse models with mutations in the UPR pathway confirm its importance in β-cell fate (2, 8, 9). In humans, mutant INS gene-induced diabetes of youth is caused by an insulin (Ins) gene mutation that induces autosomal dominant insulin-deficient diabetes, consistent with induction of the UPR in β-cells by the mutant insulin (10). We tested the hypothesis that translational recovery during chronic UPR in β-cells involves mechanisms additional to the actions of GADD34. We used Min6 insulinoma and Akita mice, which have a mutation (C96Y) in the Ins2 gene that causes proinsulin misfolding, leading to UPR-induced β-cell apoptosis (11). These mice develop hyperglycemia and diabetes without obesity or peripheral insulin resistance (12).
We report the identification of a cohort of ATF4-induced anabolic genes that promote protein synthesis during prolonged ER stress in Min6 cells and Akita islets in vivo. Induction of protein synthesis involved a sensing mechanism of extracellular nutrients that included regulation of AA flux and increased charging of a select group of tRNAs. This was accompanied by increased expression of AA transport systems L (branched chain and aromatic AAs) and A (small neutral AAs) and the corresponding aminoacyl-tRNA synthetases. Protein synthesis recovery during chronic ER stress correlated with β-cell apoptosis and development of diabetes. These studies identify the expression of transport systems L and A as early biomarkers in the development of diabetes. These data also suggest that increased expression of system L AA transporters in β-cells may promote development of diabetes in individuals with elevated plasma levels of system L substrates.
EXPERIMENTAL PROCEDURES
Chemicals and Cell Culture
Tg (Sigma) was used at 400 nm. Sal003 was from Tocris. Min6 cells (passages 30–42) were cultured as described (13). Adenoviral particles expressing shRNA against ATF4 (Vector Biolabs) or a mock shRNA were propagated in HEK293T cells. The expression of vector-encoded GFP was monitored to ensure similar levels of infection.
Transport Assays
AA transport was performed at 37 °C in Earle's balanced salt solution (EBSS) or EBSS with NaCl replaced by choline chloride as follows: (i) system A: 0.1 mm [14C]MeAIB (4 μCi/ml) for 2 min; (ii) system L: 0.01 mm [3H]Leu or [3H]Met (3 μCi/ml) for 2 min, (iii) system y+: 0.1 mm [3H]Arg (8 μCi/ml) for 30 s in the presence of 2 mm Leu, which prevents uptake through system y+L; and (iv) 0.05 mm [3H]Gln (5 μCi/ml). Gln efflux was measured after preloading cells with 0.25 mm [3H]Gln (10 μCi/ml) for 10 min followed by incubation with the indicated AAs (0.1 mm) for 1 min. All radiochemicals were from PerkinElmer Life Sciences.
Animal Studies
Experimental protocols were approved by the Case Western Reserve University Institutional Animal Care and Use Committee. Mice from Jackson Laboratory were bred at Case Western Reserve University and were fed standard lab chow (LabDiet) and housed under 12:12-h light/dark cycle with free access to food and water at 23 ± 1 °C. Tu (Sigma) in 150 mm dextrose was injected intraperitoneally (2 μg/g of body weight). C57BL/6J and C57BL/6-Ins2+/Akita mice were used for experiments. Fractional protein synthesis rates in vivo were measured as described (14). Pancreatic islets were isolated as described (15).
mRNA Analysis
Islets from four to six mice were pooled and cultured for 2 h in RPMI 1640 medium. 70–80 islets were picked and used for RNA isolation. Islets were treated with QIAshredder (Qiagen), and RNA was purified using the RNeasy Plus Micro kit (Qiagen). RNA from whole pancreas and Min6 cells was isolated using TRIzol (Invitrogen). cDNA synthesis and qPCR analysis of RNA was performed as described previously (16). Primers used in the study are listed in Table 1.
TABLE 1.
Primers used for qPCR
| Primer name | Sequence |
|---|---|
| EIF2B4_tot_For | TCAACGGCAAGACCCAATCA |
| EIF2B4_tot_Rev | AGTTCTGCCTTACTCCGGC |
| EIF2B4_v1_For | CGGAGCCTGTCTGGCTCACT |
| EIF2B4_v1_Rev | GTCAACTCCCGCCCTGCTG |
| EIF2B4_v2_For | ATGGCTGCGGTGGCGGTGGCT |
| EIF2B4_v2_Rev | CATCTCGGATCTCGATTC |
| Slc1a5_For | TCAACCATGGTCCAGCTTCT |
| Slc1a5_Rev | CGGGTGCGTACCACATAATC |
| Slc3a2_For | CATGAGCCAGGACACCGAAG |
| Slc3a2_Rev | TCCTCCGCCACCTTGATCTT |
| Slc7a1_For | ATCGGTACTTCAAGCGTGGC |
| Slc7a1_Rev | CCATGGCTGACTCCTTCACG |
| Slc7a5_For | CTGCTACAGCGTAAAGGC |
| Slc7a5_Rev | AACACAATGTTCCCCACGTC |
| Slc38A2_For | TAATCTGAGCAATGCGATTGTGG |
| Slc38A2_Rev | AGATGGACGGAGTATAGCGAAAA |
| Slc38A3_For | GAGAGACCGGGGAGAAAAC |
| Slc38A3_Rev | CCTCGAAATCGGTGAAGTGT |
| Slc38A5_For | TGGCACACACTGGAGTCATC |
| Slc38A5_Rev | ACGGATGCCTACAACACTGG |
| GRP78(BIP)_Rev | ATCGCCAATCAGACGCTCC |
| GRP78(BIP)_For | ACTTGGGGACCACCTATTCCT |
| p58IPK_For | CCGTTCCTGCTGGTCCTGGTG |
| p58IPK_Rev | GCTTCTCCACATCCGCATTTACTCC |
| CHOP_For | CTGGAAGCCTGGTATGAGGAT |
| CHOP_Rev | CAGGGTCAAGAGTAGTGAAGGT |
| EPRS_For | GATGAAGGCGGAACGTGAAC |
| EPRS_Rev | CAGGACTGACCAAACTGGCT |
| GARS_For | GGAAGGCGCTATGCAAGAAC |
| GARS_Rev | GAGTCTCGGTCCCTCAGAGT |
| LARS_For | GCACCCCTGACGTGCTATAA |
| LARS_Rev | CTGCAAGATCATCCGGGGAA |
| MARS_For | GCAAGGTATTGTCGCCTTCG |
| MARS_Rev | CCAGCGGTAGATGTCAGCAT |
| SARS_For | CGTGACACCCGTGGTATCTT |
| SARS_Rev | AGGGATCCCCAAAGACTGGT |
| 18S_rRNA_For | TTGACGGAAGGGCACCACCAG |
| 18S_rRNA_Rev | GCACCACCACCCACGGAATCG |
| GAPDH_For | CGCCTGGAGAAACCTGCCAAGTATG |
| GAPDH_Rev | GGTGGAAGAATGGGAGTTGCTGTTG |
| INS1_For | CAACTGGAGCTGGGAGGAAG |
| INS1_Rev | GCTGGTAGAGGGAGCAGATG |
| Amy2_For | GCAACAATGTTGGTGTCCGTAT |
| Amy2_Rev | AATTCCCTGTTATTTGGATTGAGG |
Other Methods
The following techniques were performed as described: cell extraction and Western blotting (17), using antibodies listed in the figure legends; incorporation of [35S]Met/Cys using EXPRE35S35S Protein Labeling Mix (18); eIF2B-GEF activity (19); and flow cytometry (17). Charged and total tRNA species were quantified as described (20). Statistical significance was determined by Student's t test and ANOVA.
RESULTS
Translational Recovery in Response to ER Stress in β-Cells Has a Component Independent of eIF2α Dephosphorylation
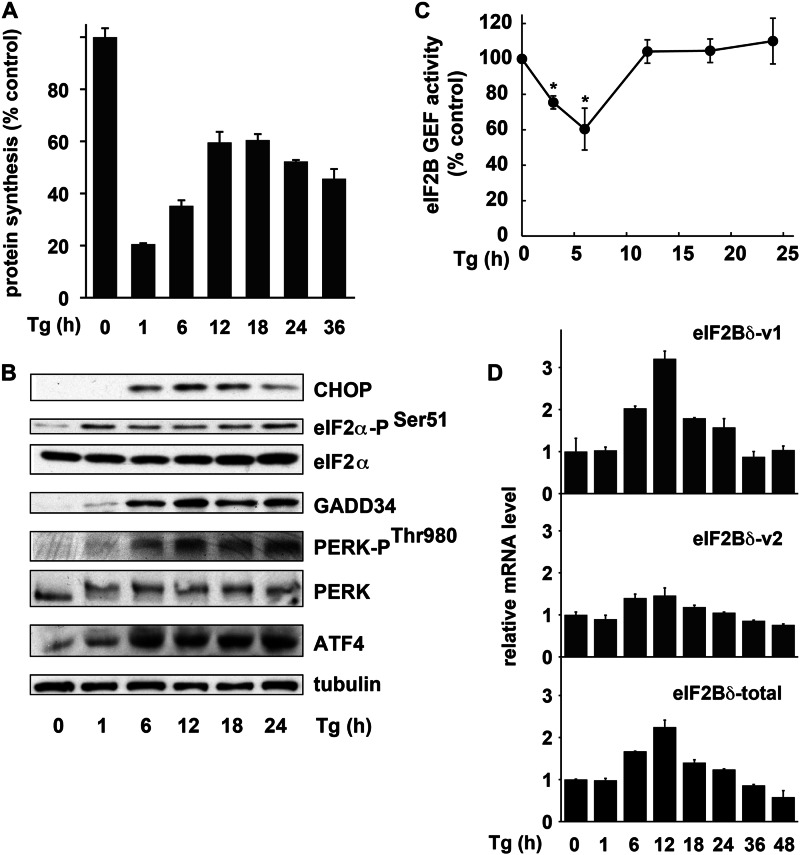
Uncontrolled protein synthesis in β-cells leads to apoptosis and development of diabetes (3, 21). We used Tg-treated Min6 cells as a model to study the mechanisms that regulate protein synthesis in β-cells during ER stress. Protein synthesis was measured by [35S]Met/Cys incorporation into proteins. Translational inhibition at 1 h of stress was followed by translational recovery at 6–18 h (Fig. 1A). Translational recovery did not correlate well with GADD34 induction and eIF2α dephosphorylation (Fig. 1B), the major drivers of translational recovery during ER stress (22, 23). The persistence of eIF2α phosphorylation was in agreement with the sustained activation of PERK via its phosphorylation at Thr980 (Fig. 1B). These data suggested that there is an alternative pathway of translational recovery during stress in β-cells, which may be less sensitive to inhibition of protein synthesis by eIF2α-P.
FIGURE 1.
Translational recovery in Min6 cells during prolonged ER stress. A, [35S]Met/Cys incorporation into proteins in Min6 cells treated with Tg for the indicated times. Results are the mean of triplicate determinations. All Tg-treated samples are significantly less than the untreated control p < 0.01. B, Western blot analysis of extracts from cells treated with Tg for the indicated times. Antibodies for the listed antigens were from the following vendors: eIF2a-PSer51, PERK-PThr980, and PERK from Cell Signaling; CHOP, GADD34, ATF4, and eIF2α from Santa Cruz Biotechnology; tubulin from Sigma. C, eIF2B-GEF activity measured in extracts from cells treated with Tg for the indicated times. Data are expressed as mean ± S.E. (error bars) of three independent experiments. *, significantly different from the untreated control (p < 0.01). D, qPCR analysis of mRNA isolated from cells treated with Tg for the indicated times. qPCR primers detect the indicated variants or total eIF2Bδ mRNA. Results of duplicate analyses were normalized to 18S rRNA and shown as -fold change over untreated cells. One-way ANOVA showed significant changes for each of the mRNAs over time (p < 0.01).
eIF2α-P inhibits the guanine nucleotide exchange activity (GEF) of eIF2B, an essential step in ternary complex recycling and translation initiation (24). We showed that eIF2B-GEF activity decreased early in the stress response, but it was completely restored during translational recovery (Fig. 1C). Because some cancer cells have decreased sensitivity to eIF2α-P by up-regulating expression of eIF2Bδ variant-1 (25), we tested whether ER stress in β-cells induces expression of this variant. mRNA for eIF2Bδ v-1, but not the constitutively expressed v-2, increased with kinetics similar to eIF2B-GEF activity (Fig. 1, C and D). eIF2Bδ v-1 was the predominant eIF2Bδ mRNA species during stress, as determined by qPCR analysis using primers that detect a common region of the two variants (Fig. 1D).
ER Stress in β-Cells Induces AA Transport
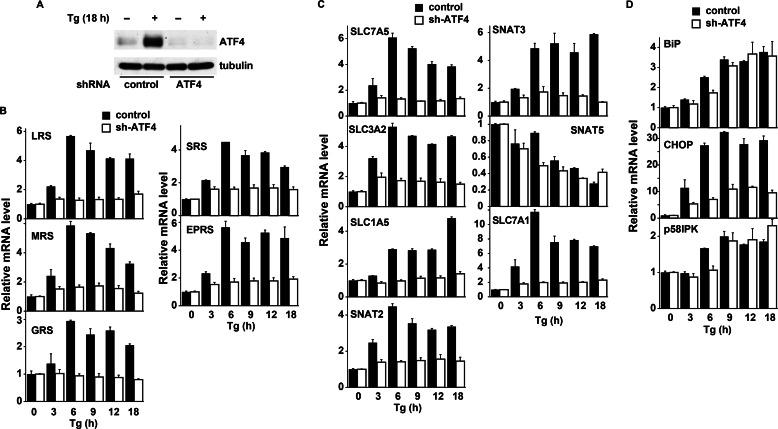
We hypothesized that protein synthesis recovery involves increased expression of genes encoding tRNA synthetases and AA transporters that can positively affect mRNA translation via increased tRNA charging. We used qPCR to measure the ER stress-induced changes in mRNA levels. Because ATF4 was previously implicated in this regulation (26–28), we examined this stress response in control and ATF4-depleted cells (Fig. 2A). With Tg treatment, the levels of several tRNA synthetase mRNAs increased (Fig. 2B), including MRS (Met), LRS (Leu), SRS (Ser), GRS (Gly) and EPRS (Glu-Pro). The AAs that are used by these tRNA synthetases are substrates for AA transport systems A (Ser, Gly, Pro) and L (Leu and Met). Both systems are known to be up-regulated in anabolic conditions and to promote cell growth (27, 29). Interestingly, the expression of AA transporters of systems L (light chain SLC7A5 and heavy chain SLC3A2) and A (SNAT2/SLC38A2) were also increased in an ATF4-dependent manner (Fig. 2C). For system L to mediate increased net uptake of branched chain AAs during stress, another transport system must provide intracellular substrate AAs for efflux (30, 31). Transport systems that could supply these intracellular AAs were also induced in an ATF4-dependent manner. These include systems ASC (SLC1A5), A (SNAT2/SLC38A2), and N (SNAT3/SLC38A3) (Fig. 2C). Expression of the system y+ cationic AA transporter (Cat1/SLC7A1) was also increased during stress by ATF4 (32). These data suggest that ATF4 coordinately induces expression of AA transporter genes and aminoacyl-tRNA synthetases in β-cells during ER stress. This expression suggests that translational recovery during ER stress may involve a nutrient sensing mechanism that is regulated by AA flux. The latter was tested next in Tg-treated Min6 cells by measuring AA uptake.
FIGURE 2.
Induction of aminoacyl-tRNA synthetase and AA transporter genes during ER stress in Min6 cells involves the transcription factor ATF4. A, Western blot analysis of the ATF4 protein in Min6 cells infected with adenovirus expressing control and shRNA against ATF4. B–D, 5 days after infection, cells were treated with Tg for the indicated times and used for qPCR analysis. B, aminoacyl-tRNA synthetase genes. C, plasma membrane AA transporter genes. D, stress-induced marker genes. As expected, induction of the ER chaperone genes (BiP and p58IPK) was independent of ATF4, and induction of CHOP mRNA was severely inhibited in cells depleted of ATF4. Two-way ANOVA showed significant time-dependent changes for all samples and significant effects for the shRNA in all samples except BiP and p58IPK (p < 0.01).
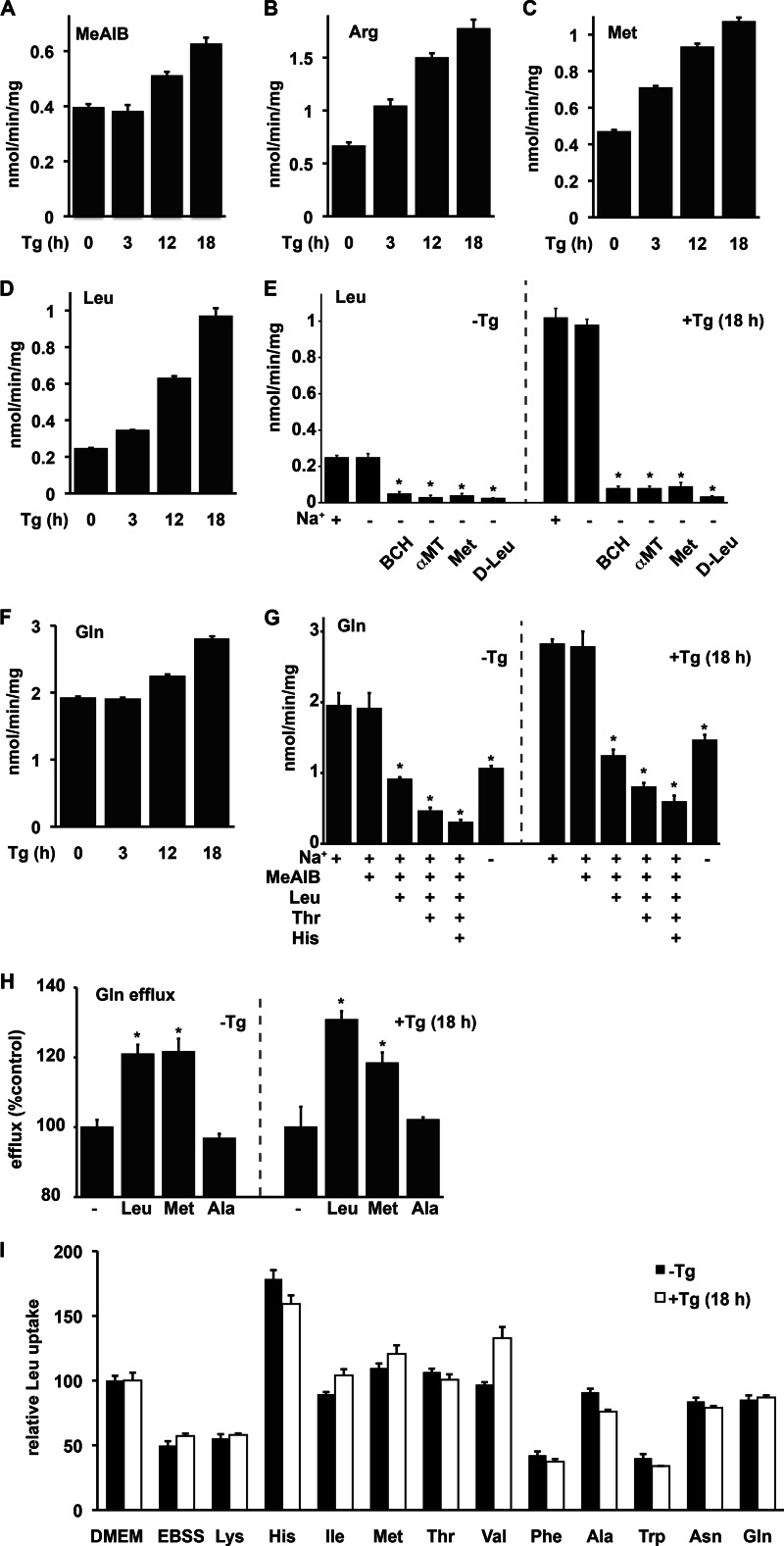
System A-mediated uptake of MeAIB increased in a manner that paralleled the expression of the gene SNAT2/SLC38A2 (Figs. 2C and 3A). Because system A transport is trans-inhibited by the intracellular accumulation of its substrates (33), the increased AA uptake was lower than the 3-fold increase in the levels of the transporter mRNA. As previously reported in other cell types (34), system y+ activity, measured as Arg uptake, increased during ER stress (Fig. 3B), consistent with the increased levels of the SLC7A1 mRNA (Fig. 2C).
FIGURE 3.
Regulation of AA flux in Min6 cells during ER stress. A–D, uptake of MeAIB by system A (A), Arg by system y+ (B), and Met and Leu by system L (C and D) tested in unstressed cells and after Tg treatment for the indicated times. E, effect of Na+ ions and inhibitors of system L (BCH and α-methyltryptophan (α-MT)) and Met or d-Leu (5 mm) on Leu uptake in unstressed and Tg-treated cells. F, Na+-dependent uptake of Gln in cells treated with Tg for the indicated times. G, effect of Na+ ions and AA competitors (5 mm) on Gln uptake in untreated and Tg-treated cells. H, Gln efflux in the presence of 0.1 mm indicated AAs, in untreated or Tg-treated cells. I, Leu uptake in untreated and Tg-treated cells depleted of AAs (10-min incubation in EBSS), followed by 5 min re-feeding with individual AAs (5 mm). Data were normalized to Leu uptake by cells in DMEM. Results are expressed as mean ± S.E. (error bars) of three independent experiments. Tg caused significant increases in the uptake of MeAIB, Arg, Met, and Gln (A–D and F; one-way ANOVA (p < 0.01)). G and H, significant effects of added compounds (p < 0.05) are indicated (*). I, Leu uptake in all media was significantly different (p < 0.01) from EBSS, except media with Lys and Phe.
System L is known to mediate the sodium-independent exchange of branched chain and aromatic AAs (31). Met is a substrate for system L in some cell types (30). We therefore measured the sodium-independent uptake of Leu (l-Leu) and Met in Tg-treated Min6 cells. Induction of Met uptake was observed earlier than induction of Leu uptake (Fig. 3, C and D). Uptake of Leu (preferred substrate) increased at 18 h of treatment (3.5-fold) in good correlation with increased mRNA levels for SLC7A5 and SLC3A2. Although maximum induction of the mRNA levels was observed between 6 and 9 h of treatment (∼6-fold, Fig. 2C), Leu uptake at 9 h increased only by 2-fold (data not shown). The delayed response can be explained by the inhibition of translation for the transporter mRNA early in the stress response due to eIF2α-P and the need of translational recovery for the efficient translation of the system L transporter mRNAs. This was in contrast to the uptake of Arg, which increased with kinetics that paralleled SLC7A1 mRNA levels. The latter is in agreement with our finding that translation of the SLC7A1 mRNA is IRES-mediated and is not inhibited by eIF2α-P (34).
Because there is a strong correlation between dietary Leu and development of diabetes (35, 36), we further characterized the AA transport systems that are involved in the uptake of Leu in control and stressed Min6 cells. Leu uptake was sodium-independent and was inhibited by the system L inhibitors BCH and α-methyltryptophan (37) and in both control and Tg-treated Min6 cells (Fig. 3E). We also tested the effect of d-Leu, a known competitor of system L (38, 39), on the uptake of l-Leu in Min6 cells. d-Leu inhibited Leu uptake in control and Tg-treated Min6 cells (Fig. 3E). High concentrations of Met also inhibited Leu uptake, suggesting that Leu and Met are transported by system L (Fig. 3E). These data suggest that system L is the major AA transport system for Leu in β-cells. In addition, SLC7A5 (also known as LAT1) was the only system L transporter that was regulated by stress (data not shown).
As mentioned previously, net Leu uptake by system L depends on other transporters that provide efflux substrates (30, 31). It was proposed that Gln is an efflux substrate for Leu uptake in cancer cells (40). Gln uptake was relatively high compared with other tested AAs and uptake increased during ER stress (Fig. 3F). Because several AA transport systems can facilitate Gln uptake, we determined the characteristics for Gln uptake (Fig. 3G): (i) in both untreated and Tg-treated Min6 cells, Gln uptake was not inhibited by MeAIB, suggesting a minor contribution of system A; (ii) Gln uptake in the absence of Na+ and its inhibition by Leu revealed system L as a Gln exchanger; (iii) further inhibition of Gln uptake by Thr and His suggested the involvement of systems ASC (SLC1A5), and N (SNAT3/SLC38A3), respectively; and (iv) increased Gln uptake in Tg-treated cells was observed for all identified transport systems. Finally, the remaining Gln uptake that was not inhibited by the four competitors (MeAIB, Leu, Thr, and His) was induced during Tg treatment, indicating that additional ER stress-induced AA transport systems may also contribute to Gln flux.
To further support the idea that Gln is an efflux substrate for system L-mediated Leu uptake, we measured Gln efflux in the presence of extracellular Leu or Met (system L substrates) or Ala (system A substrate). Cells were preloaded with [3H]Gln for 10 min to minimize metabolic turnover of the labeled AA. System L substrate AAs increased release of Gln from cells in control and Tg-treated Min6 cells (Fig. 3H). In contrast, the substrate for system A did not stimulate Gln efflux (Fig. 3H).
We next tested whether other AAs serve as efflux substrates for system L, as a means of maximizing Leu uptake by β-cells. Cells were incubated for 10 min in complete medium (DMEM) or medium depleted of AAs (EBSS). Leu uptake via system L decreased by ∼50% in AA-depleted cells (Fig. 3I). Refeeding (5 min) with Lys, Phe, or Trp did not restore Leu uptake, suggesting that either they are not good efflux substrates for system L and/or the cells did not have transporters to increase their intracellular pool (Fig. 3I). Supplementation with other AAs increased Leu uptake. Gln or Asn restored Leu uptake to values of 85% of the AA-fed cells (Fig. 3I). Surprisingly, Met, Thr, Val, or His stimulated uptake significantly more than Gln (p < 0.05). This suggests that other AA transport systems cause the concentration of these AAs in Min6 cells and/or they are better substrates for system L-mediated efflux than Gln (Fig. 3I). For example, (i) His is a system N substrate and expression of the system N gene, SNAT3/SLC38A3, increased during stress (Fig. 2C) and (ii) Met is a good substrate for the net accumulation by system A and a good exchange substrate for system L. Similar results were obtained in control and Tg-treated Min6 cells, suggesting that a similar set of transporters is expressed in both conditions, although the levels increased during stress. Taken together these data suggest that the regulation of several AA transport systems contributes to increased Leu uptake in β-cells during ER stress.
ER Stress in β-Cells Increases tRNA Charging for AAs That Are Substrates of Transport Systems L and A
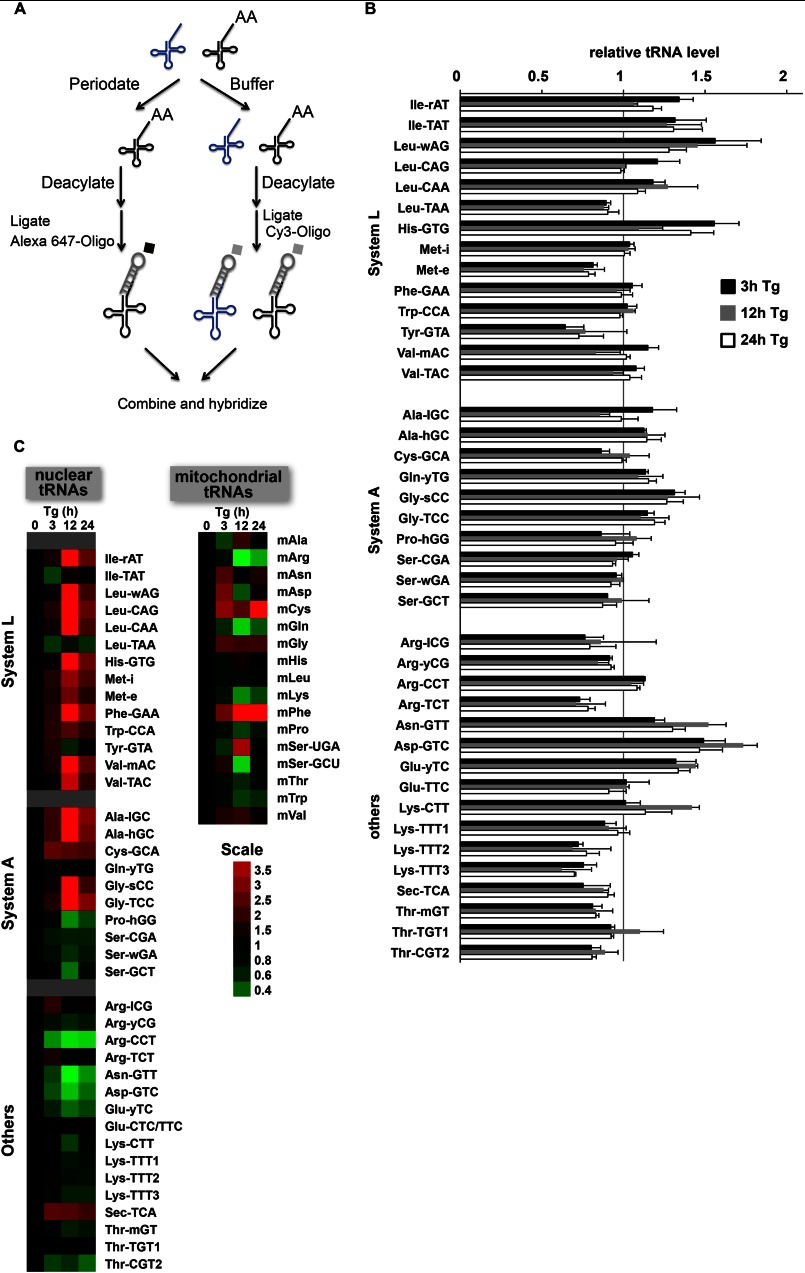
We hypothesized that translational recovery during ER stress involves a global increase of tRNA charging. We tested changes in the levels and charging of tRNAs using custom made microarrays (Fig. 4A). The total levels of the tRNAs did not change significantly (Fig. 4B). In contrast, we observed increased levels of charged tRNAs that carry AAs which are substrates for systems L and A (Fig. 4C). tRNAiMet, which is part of the ternary complex (eIF2/GTP/Met-tRNAiMet) of translation initiation, was one of the tRNAs with increased charging. We also evaluated changes in charging of mitochondria-encoded tRNAs (Fig. 4C). Interestingly, charging of some mitochondrial tRNAs increased at 12 h of Tg treatment whereas others decreased. Changes in the levels of charged tRNAs were likely the result of increased availability of the corresponding AAs in the cytosol and mitochondria. Because the intracellular levels of most AAs increased in Min6 cells during Tg treatment (data not shown), it is possible that sequestration of AAs in subcellular compartments such as the lysosomes limits their availability for tRNA charging. Increased charging was unlikely a reflection of increased aminoacyl-tRNA synthetases. Although we observed increased expression of several tRNA synthetases, only a subset of tRNAs showed increased charging (Fig. 4A). In conclusion, we show for the first time that ER stress in Min6 cells induced charging of a subset of tRNAs with neutral AAs that are known substrates of the growth-promoting AA transport systems A and L.
FIGURE 4.
Genome-wide analysis of tRNA charging in Min6 cells during ER stress. A, tRNA was extracted from untreated and Tg-treated cells under acidic conditions to retain aminoacylated-tRNAs. One portion of each sample was treated with periodate, which oxidizes the 3′-acceptor stem of uncharged tRNAs. A second sample was kept in buffer solution. The tRNAs in both samples were deacylated and ligated to fluorescently tagged oligonucleotides with a stable stem-loop and a region complementary to the 3′-CCA sequence that is conserved among all tRNAs (Integrated DNA Technologies). This allowed the labeling of one sample with Cy3 and the other with Alexa Fluor 647. Samples were combined and hybridized to tRNA microarrays containing complementary probes for 40 nuclear encoded mouse tRNAs and 18 mouse mitochondria-encoded tRNAs. Microarrays were custom printed by Microarray Inc. (Nashville, TN). B, abundance of total tRNA in cells treated with Tg for the indicated times. The average of two independent experiments is shown. C, levels of charged tRNA shown as a TreeView image. All values are relative to unstressed cells. Assays were performed with Alexa Fluor 647-labeled charged tRNA and Cy3-labeled total tRNA or with Cy3-labeled charged tRNA and Alexa Fluor 647-labeled total tRNA. Data are the average from these two experiments. Green indicates a lower and red a higher level of charged tRNA during stress relative to control.
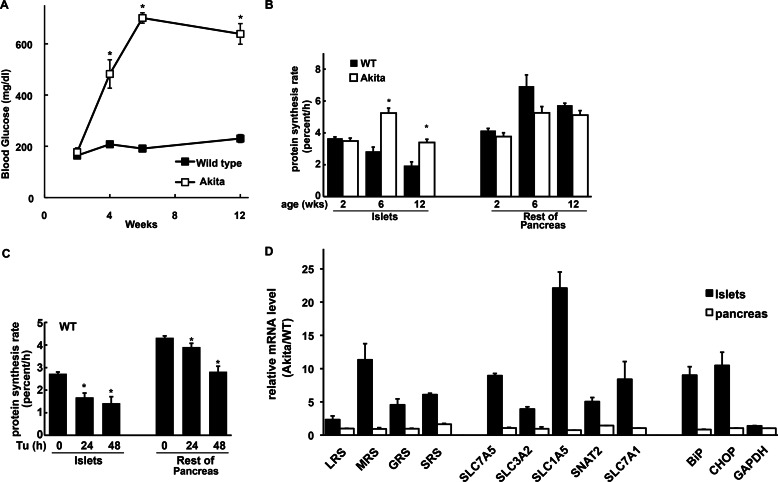
Protein Synthesis and Induction of the Anabolic Program in Pancreatic Islets under ER Stress
In the Akita mouse, misfolded mutant proinsulin induces ER stress in β-cells leading to apoptosis (10, 11). Akita male mice had elevated blood glucose levels, starting at 4 weeks (Fig. 5A). By studying incorporation of deuterium from 2H2O (14), we quantified fractional protein synthesis rates in islets and the leftover pancreata from 2–12-week-old WT and Akita mice. ER stress begins in the Akita islets upon birth due to formation of aggregates between mutant and WT proinsulin in the ER. It would therefore be expected that stress in 2-week-old Akita islets to cause a decrease in protein synthesis compared with WT littermates. At 2 weeks, WT and mutant mice had normal blood glucose levels and had similar fractional protein synthesis rates in islets (Fig. 5B), suggesting that translational recovery has started before development of diabetes. In agreement with this conclusion, induction of CHOP mRNA was prominent in 2-week-old Akita islets (data not shown). In contrast, protein synthesis was higher than WT in 6- and 12-week-old Akita islets (Fig. 5B), but the rates in the remaining pancreata were similar in mutant and WT (Fig. 5B). These data suggest that chronic ER stress in β-cells correlated with higher fractional protein synthesis rates in islets, β-cell loss, and development of diabetes.
FIGURE 5.
Regulation of protein synthesis and anabolic gene expression in pancreatic islets from Akita mice. A, blood glucose levels from Akita and WT (C57BL/6J) mice (n = 8). B, fractional protein synthesis rates measured as [2H]Ala enrichment in proteins from islets and rest of pancreas (remaining pancreatic tissue after removal of islets) in 2-, 6-, and 12-week-old male Akita (n = 6–8) and age/sex-matched WT littermates (n = 4–8). C, fractional protein synthesis rates in islets of 6-week-old male WT mice (n = 4) measured as [2H]Ala enrichment in proteins from islets and rest of pancreas after Tu injection (2 μg/g of body weight). A–C, *, significantly different from WT (p < 0.01). D, qPCR analysis of RNA from islets and whole pancreas from 6-week-old Akita male mice (n = 6) and age/sex-matched WT littermates (n = 4). The ratio of signals in Akita and WT mice is shown. For islets, all of the signals from Akita mice were significantly higher than WT (p < 0.05) for all mRNAs except GAPDH. No significant differences between Akita and WT were seen in the remaining pancreatic tissue.
We next determined the effect of acute ER stress on islet protein synthesis rates in WT mice injected with the ER stressor Tu. Acute ER stress decreased protein synthesis in both islets and leftover pancreata (Fig. 5C). The combined data from the chronic (Akita) and acute (WT mice injected with Tu) induction of ER stress in islets (Fig. 5, B and C), suggested that in chronic ER stress there is a loss of the translational inhibition that is present during acute stress.
We next determined the expression of the anabolic genes that can contribute to translational recovery in the islets of 6-week-old mice. The purity of the isolated islets was determined by evaluating mRNA levels for genes that are expressed only in islets or acinar cells. There was a ∼700-fold enrichment of the β-cell marker Ins2 mRNA in islets over whole pancreas and a ∼12,000-fold depletion of mRNA for the acinar cell marker amylase 2. The mRNA levels of the tested aminoacyl-tRNA synthetases (LRS, MRS, SRS, and GRS) were higher in Akita than in WT islets (Fig. 5D). In addition, expression of the AA transporters known to assist Leu uptake (SLC7A5, SLC3A2, and SLC1A5) showed significant induction in Akita islets (Fig. 5D). Induced expression of the mRNAs for systems A (SNAT2/SLC38A2) and y+ (SLC7A1) was also observed, further establishing similar responses to chronic ER stress in Akita islets and Min6 cells (Figs. 2C and 5D).
The specificity of the induction of protein synthesis-promoting genes during chronic ER stress in the β-cells of Akita islets (<2% of the pancreatic tissue) is demonstrated by the fact that we did not observe increased mRNA levels for any of the anabolic genes in Akita pancreata (Fig. 5D). In addition, as expected, stress-induced marker genes such as BiP and the transcription factor CHOP showed increased mRNA levels in Akita islets but not in Akita pancreata (Fig. 5D). GAPDH mRNA levels were similar in islets and pancreata (Fig. 5D). Thus, either this transcription program is not the direct result of hyperglycemia in the Akita mice (given the absence of induction of anabolic genes in Akita pancreata), or the islets are selectively sensitive to hyperglycemia in ways that do not affect the remaining pancreas. However, given that Tg treatment of Min6 cells produced similar changes under conditions where extracellular glucose was held constant, it seems unlikely that these effects require changes in extracellular glucose. We therefore propose that the changes in the transcription of anabolic genes in Akita islets are a response to chronic ER stress in β-cells due to accumulation of misfolded proinsulin.
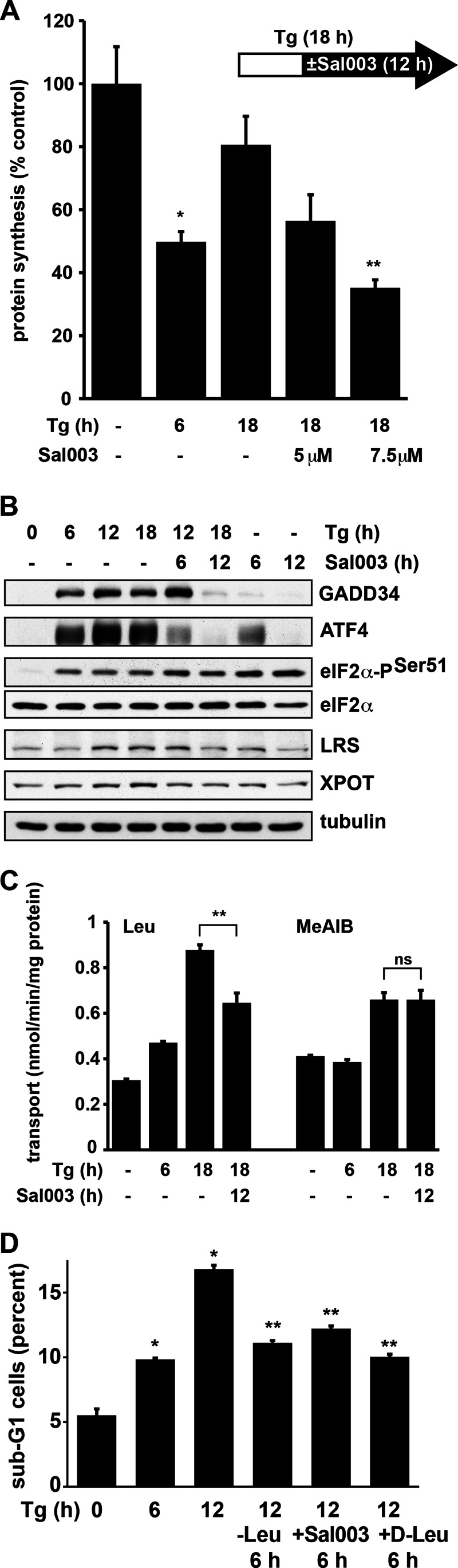
Attenuation of Protein Synthesis Recovery during ER Stress Improves Cell Survival
Our data show that ATF4 is an important contributor to the anabolic program that promotes translational recovery (Fig. 2). Because ATF4 induction during ER stress promotes expression of genes involved in both survival and apoptosis (26, 41), it is difficult to evaluate the molecular mechanisms that mediate cell fate during ER stress by depleting cells from ATF4. In addition, ATF4 promotes transcription of GADD34, which contributes to protein synthesis recovery via eIF2α dephosphorylation (6, 23). ATF4 depletion is expected to decrease protein synthesis due to the accumulation of eIF2α-P. We have shown that Tg treatment of Min6 cells sustained eIF2α-P during the recovery of protein synthesis between 6 and 18 h of stress (Fig. 1B). We attributed the recovery of protein synthesis to three parallel cellular responses: (i) increased eIF2B activity via induction of an eIF2Bδ subunit variant (Fig. 1, C and D); (ii) induction of GADD34, which contributed to partial eIF2α dephosphorylation; and (iii) ATF4-mediated induction of genes that promote protein synthesis. Disturbing the balance of any of these responses should affect translational recovery and β-cell fate during ER stress. We next tested the relationship between translational recovery and β-cell fate.
We used the salubrinal derivative Sal003, an inhibitor of the PP1 phosphatase (42), to attenuate protein synthesis during the 6–18 h of translational recovery during ER stress (Fig. 6A). We first determined the concentration of Sal003 that attenuated protein synthesis at 12 h, when added to cells 6 h after starting Tg treatment. This design (i) allowed the induction of the ATF4-mediated transcription program during the first 6 h of Tg treatment (Fig. 1B) and (ii) tested the requirement of protein synthesis recovery on sustaining translation of the ATF4 mRNA and synthesis of the anabolic proteins. Sal003 treatment of Min6 cells in the presence of Tg decreased ATF4 levels in a time-dependent manner (Fig. 6B). Similar decreases in ATF4 protein levels were observed in cells treated with Sal003 in the absence of stress (Fig. 6B). This suggests that chronic exposure of cells to eIF2α-P diminishes the available active ternary complex, leading to decreased translation of ATF4 mRNA. Decreased ATF4 levels, in turn, will attenuate the stress-induced transcription program. A similar regulation was observed for GADD34 (Fig. 6B), which is translated via mechanisms similar to ATF4 (43). In agreement with our hypothesis that translational recovery promotes the anabolic program of AA uptake and sensing, expression of the anabolic gene LRS downstream of ATF4 also decreased during Sal003 treatment (Fig. 6B). To further support our hypothesis, we found that Leu uptake decreased during Sal003 treatment in the presence of Tg (Fig. 6C). Interestingly system A-mediated uptake of MeAIB did not change significantly (Fig. 6C), in agreement with translation of the SNAT2 mRNA via an IRES that is insensitive to increased eIF2α-P (42, 44).
FIGURE 6.
Attenuation of translational recovery during ER stress in Min6 cells promotes survival. A, [35S]Met/Cys incorporation into proteins in cells treated with Tg and Sal003 for the indicated times. Sal003 was added 6 h after initiation of Tg treatment as indicated by the scheme. B, Western blot analysis of the indicated proteins from cells treated with Tg and Sal003 (7.5 μm for the last 6 or 12 h of Tg treatment). Antibodies against LRS and XPOT were from Abcam; others are listed in Fig. 1. C, Leu and MeAIB uptake in cells treated with Tg and Sal003 (7.5 μm) for the indicated times. D, cells were treated as indicated and apoptosis was assessed by measuring the percentage of sub-G1 cells by flow cytometry of propidium iodide-stained cells. Leu starvation was performed by replacing the media after 6 h of Tg treatment with Tg-containing Leu-free medium. d-Leu (40 mm) or Sal003 (7.5 μm) was added for the last 6 h of Tg treatment. Significant differences (p < 0.01) from untreated cells (*) or the appropriate Tg-treated cells (**) are indicated. Error bars, S.E.
Finally, we determined the effect of translational recovery on the fate of Min6 cells during Tg treatment. The percentage of apoptotic cells increased during translational recovery between 6 and 12 h of treatment (Fig. 6D). Sal003, when added during translational recovery (6–12 h of Tg treatment), caused a decrease in apoptosis (Fig. 6D). Similarly, depletion of cells of the essential AA Leu, during 6–12 h of Tg treatment decreased apoptosis (Fig. 6D). We further supported the hypothesis that increased system L-mediated Leu uptake contributes to β-cell apoptosis during chronic ER stress by inhibiting system L-transporter activity with d-Leu (Fig. 3E). We show that d-Leu decreased apoptosis in Tg-treated Min6 cells (Fig. 6D). These findings further support our hypothesis that up-regulation of the AA transporter network and translational recovery during unresolved ER stress in β-cells promotes apoptosis.
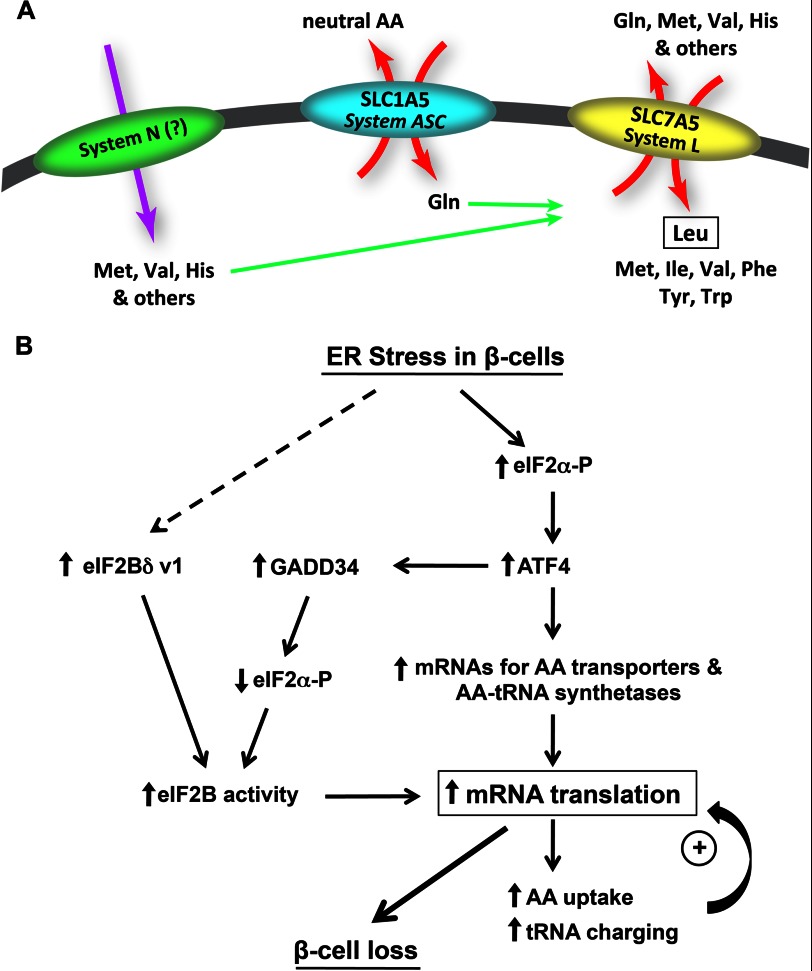
DISCUSSION
Uncontrolled protein synthesis in β-cells leads to apoptosis (3). During ER stress, protein synthesis is regulated by modulating the phosphorylation of eIF2α (6) via the activities of PERK and PP1 (with GADD34 as the regulatory subunit). The interplay between PERK and PP1 favors repression of protein synthesis via increased eIF2α-P during acute (early) stress and translational recovery via dephosphorylation during chronic (adaptive) stress (8). We show here that the efficiency of translational recovery in β-cells during ER stress is an important factor for their survival. We report novel mechanisms in addition to the PERK/GADD34 interplay that promote translational recovery. These mechanisms (Fig. 7B) involve the following: (i) translational recovery associated with increased eIF2B GEF activity and increased expression of eIF2Bδ v-1, which is not inhibited by eIF2α-P, (ii) induction of an anabolic program with increased aminoacyl-tRNA synthetase and AA transporter gene expression under the control of the transcription factor ATF4, (iii) increased AA uptake by systems L and A and increased tRNA charging with the AA substrates of these systems (both systems known to promote protein synthesis and growth). In agreement with our findings, proinsulin synthesis in Akita islets increased compared with WT islets (45). These mechanisms allow for translational recovery in β-cells even though they have elevated eIF2α-P levels and PERK activation during chronic stress.
FIGURE 7.
Working model of the AA network and its contribution in regulation of protein synthesis in β-cells during ER stress. A, proposed mechanism for the increased uptake of Leu via system L during chronic ER stress. B, proposed model by which translational recovery leads to apoptosis during chronic ER stress.
Induction of the ATF4-dependent expression of AA transporters and tRNA synthetases has been reported during insulin-mediated anabolism (27). This is also supported by the lower levels of mRNAs for tRNA synthetases and AA transporters in tissues from ATF4-deficient mice (46). The same anabolic genes were induced during stress in different cell types (26). These earlier studies assumed that increased expression of the anabolic genes would increase global tRNA charging (47). Instead, we found increased tRNA charging in β-cells only for tRNAs charged with AAs that are substrates for systems L and A. We propose that the ATF4 transcription program induces expression of specific AA transporters and tRNA synthetases that provides increased AAs for protein synthesis. The mechanism of the selective increase in tRNA charging is not known. However, we observed that ATF4 also increased the levels of the nuclear export receptor for tRNAs (XPOT, Fig. 6B, and data not shown), which introduces a novel hypothesis on how selective tRNA charging may occur during stress.
Our studies suggest a novel regulatory mechanism in the β-cell response to ER stress. It is well established that Leu uptake or feeding increases protein synthesis in cells and tissues via mechanisms that activate mTORC1 (48, 49). The increased system L-mediated AA transport we observed may signal through this pathway to increase protein synthesis. Recently the Leu-sensing mechanism for mTORC1 activation was shown to involve leucyl-tRNA synthetase (50). It will therefore be interesting to determine whether translational recovery in β-cells involves increased mTORC1 activity and translation of TOP mRNAs downstream of the ATF4 transcription program which controls the intracellular AA pool. Because TOP mRNAs encode proteins of the translation machinery (51), their efficient translation is expected to be a significant contributor to translational recovery during ER stress. The latter is supported by the finding that ATF4-depleted cells have a lower pool of free AAs (27).
We conclude that during chronic ER stress β-cells induce a self-defeating prosurvival program. This program involves induction of tRNA synthetases and a network of AA transporters that lead to increased tRNA charging with system L and A substrate AAs (Fig. 7A). These mechanisms contribute to translational recovery, which leads to β-cell apoptosis. In agreement with the idea that the transporter activity contributes to cell fate of β cells during chronic stress was the finding that inhibition of system L-mediated Leu uptake, in late stress decreased apoptosis (Fig. 6D). Induction of apoptosis is probably the result of increased oxidative stress in the cytosol and mitochondria due to high rates of protein synthesis and limited protein folding capacity in the ER (3, 7, 23, 41). Similar to our findings, earlier reports concluded that attenuation of protein synthesis protected cells from apoptosis (42).
Our studies lead us to speculate that increased AA uptake by β-cells may be a mechanism contributing to development of diabetes in humans. A recent report showed that increased plasma levels of five AAs (Ile, Leu, Val, Tyr and Phe, all system L substrates) in normoglycemic human subjects, was a good predictor for development of T2DM (36). Higher plasma levels of specific AAs in these individuals may increase the activity of exchangers on the β-cell plasma membrane, resulting in increased system L-mediated uptake of AAs with the consequence of increased protein synthesis. In agreement with this idea, our studies provide a mechanistic explanation for this finding: In the prediabetic state which involves peripheral tissue insulin resistance, chronic demand for insulin induces ER stress in β-cells, which activates the ATF4-mediated anabolic program. The presence of higher plasma levels of system L substrate AAs may drive the exchange of extracellular Leu with intracellular substrates, thus promoting protein synthesis, increasing ER stress, and promoting the development of diabetes. Future studies will determine whether increased AA uptake in β-cells in vivo promotes β-cell dysfunction and early development of diabetes.
Because our data showed induced expression of the amino acid transporters in islets and not in the total pancreatic tissue of the Akita mice (Fig. 5D), we speculate that amino acid transporters in islets can serve as early diagnostic biomarkers for the detection of diabetes using positron emission tomography (PET). PET has been used to detect increased rates of AA uptake in tumors using 18F-labeled AAs for systems L, A, Gln, and others (52). In addition, islets and specifically β cells have been visualized by PET imaging in transgenic mouse models (53). We can therefore envision that we can image islets in Akita mice, via the utilization of 18F-labeled AA analogues that are system L substrates (52). These studies are currently under investigation, and they can potentially result in a diagnostic tool for the early diagnosis of diabetes in humans.
Acknowledgments
We thank Jing Wu and Scott Becka, Case Western Reserve University, and Lydia Kutzler, The Pennsylvania State University College of Medicine, for technical assistance and Dr. M. McManus, University of California San Francisco, for providing the Min6 cells.
This work was supported, in whole or in part, by National Institutes of Health Grants R37-DK60596 and R01-DK53307 (to M. H.), DK48280 (to P. A.), R37DK042394 and R01DK088227 (to R. J. K.), DK15658 (to S. K.), and MMPC U24 DK76174 (to Case Western Reserve University).
- T2DM
- type 2 diabetes mellitus
- AA
- amino acid
- ANOVA
- analysis of variance
- BCH
- 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid
- EBSS
- Earle's balanced salt solution
- ER
- endoplasmic reticulum
- GEF
- guanine nucleotide exchange factor
- MeAIB
- methyl-aminoisobutyrate
- qPCR
- quantitative PCR
- PERK
- PKR-like endoplasmic reticulum kinase
- Sal003
- cell-permeable salubrinal analog
- Tg
- thapsigargin
- Tu
- tunicamycin
- UPR
- unfolded protein response.
REFERENCES
- 1. Samuel V. T., Shulman G. I. (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148, 852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Back S. H., Kaufman R. J. (2012) Endoplasmic reticulum stress and type 2 diabetes. Annu. Rev. Biochem. 81, 767–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Back S. H., Scheuner D., Han J., Song B., Ribick M., Wang J., Gildersleeve R. D., Pennathur S., Kaufman R. J. (2009) Translation attenuation through eIF2α phosphorylation prevents oxidative stress and maintains the differentiated state in β- ‘cells. Cell Metab. 10, 13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Papa F. R. (2012) Endoplasmic reticulum stress, pancreatic β-cell degeneration, and diabetes. Cold Spring Harb. Perspect. Med. 2, a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oslowski C. M., Urano F. (2011) The binary switch that controls the life and death decisions of ER-stressed β-cells. Curr. Opin. Cell Biol. 23, 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ron D., Harding H. P. (2007) in Translational Control in Biology and Medicine (Mathews M. B., Sonenberg N., Hershey J. W. B., eds) pp. 345–368, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 7. Tabas I., Ron D. (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 13, 184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaufman R. J., Back S. H., Song B., Han J., Hassler J. (2010) The unfolded protein response is required to maintain the integrity of the endoplasmic reticulum, prevent oxidative stress and preserve differentiation in β-cells. Diabetes Obes. Metab. 12, Suppl. 2, 99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaufman R. J. (2011) β-Cell failure, stress, and type 2 diabetes. N. Engl. J. Med. 365, 1931–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu M., Hodish I., Haataja L., Lara-Lemus R., Rajpal G., Wright J., Arvan P. (2010) Proinsulin misfolding and diabetes: mutant INS gene-induced diabetes of youth. Trends Endocrinol. Metab. 21, 652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang J., Takeuchi T., Tanaka S., Kubo S. K., Kayo T., Lu D., Takata K., Koizumi A., Izumi T. (1999) A mutation in the insulin 2 gene induces diabetes with severe pancreatic β-cell dysfunction in the MODY mouse. J. Clin. Invest. 103, 27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oyadomari S., Koizumi A., Takeda K., Gotoh T., Akira S., Araki E., Mori M. (2002) Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J. Clin. Invest. 109, 525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ku G. M., Pappalardo Z., Luo C. C., German M. S., McManus M. T. (2012) An siRNA screen in pancreatic β-cells reveals a role for Gpr27 in insulin production. PLoS Genet. 8, e1002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yuan C. L., Sharma N., Gilge D. A., Stanley W. C., Li Y., Hatzoglou M., Previs S. F. (2008) Preserved protein synthesis in the heart in response to acute fasting and chronic food restriction despite reductions in liver and skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 295, E216–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li D. S., Yuan Y. H., Tu H. J., Liang Q. L., Dai L. J. (2009) A protocol for islet isolation from mouse pancreas. Nat. Protoc. 4, 1649–1652 [DOI] [PubMed] [Google Scholar]
- 16. Majumder M., Huang C., Snider M. D., Komar A. A., Tanaka J., Kaufman R. J., Krokowski D., Hatzoglou M. (2012) A novel feedback loop regulates the response to endoplasmic reticulum stress via the cooperation of cytoplasmic splicing and mRNA translation. Mol. Cell. Biol. 32, 992–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chiribau C. B., Gaccioli F., Huang C. C., Yuan C. L., Hatzoglou M. (2010) Molecular symbiosis of CHOP and C/EBP β isoform LIP contributes to endoplasmic reticulum stress-induced apoptosis. Mol. Cell. Biol. 30, 3722–3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bevilacqua E., Wang X., Majumder M., Gaccioli F., Yuan C. L., Wang C., Zhu X., Jordan L. E., Scheuner D., Kaufman R. J., Koromilas A. E., Snider M. D., Holcik M., Hatzoglou M. (2010) eIF2α phosphorylation tips the balance to apoptosis during osmotic stress. J. Biol. Chem. 285, 17098–17111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tuckow A. P., Vary T. C., Kimball S. R., Jefferson L. S. (2010) Ectopic expression of eIF2Bϵ in rat skeletal muscle rescues the sepsis-induced reduction in guanine nucleotide exchange activity and protein synthesis. Am. J. Physiol. Endocrinol. Metab. 299, E241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zaborske J. M., Narasimhan J., Jiang L., Wek S. A., Dittmar K. A., Freimoser F., Pan T., Wek R. C. (2009) Genome-wide analysis of tRNA charging and activation of the eIF2 kinase Gcn2p. J. Biol. Chem. 284, 25254–25267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scheuner D., Song B., McEwen E., Liu C., Laybutt R., Gillespie P., Saunders T., Bonner-Weir S., Kaufman R. J. (2001) Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7, 1165–1176 [DOI] [PubMed] [Google Scholar]
- 22. Novoa I., Zeng H., Harding H. P., Ron D. (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153, 1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marciniak S. J., Yun C. Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H. P., Ron D. (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pathak V. K., Schindler D., Hershey J. W. (1988) Generation of a mutant form of protein synthesis initiation factor eIF-2 lacking the site of phosphorylation by eIF-2 kinases. Mol. Cell. Biol. 8, 993–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin L., Kimball S. R., Gardner L. B. (2010) Regulation of the unfolded protein response by eif2Bδ isoforms. J. Biol. Chem. 285, 31944–31953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harding H. P., Zhang Y., Zeng H., Novoa I., Lu P. D., Calfon M., Sadri N., Yun C., Popko B., Paules R., Stojdl D. F., Bell J. C., Hettmann T., Leiden J. M., Ron D. (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 [DOI] [PubMed] [Google Scholar]
- 27. Adams C. M. (2007) Role of the transcription factor ATF4 in the anabolic actions of insulin and the anti-anabolic actions of glucocorticoids. J. Biol. Chem. 282, 16744–16753 [DOI] [PubMed] [Google Scholar]
- 28. Kilberg M. S., Shan J., Su N. (2009) ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol. Metab. 20, 436–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hundal H. S., Taylor P. M. (2009) Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am. J. Physiol. Endocrinol. Metab. 296, E603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bröer S. (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 88, 249–286 [DOI] [PubMed] [Google Scholar]
- 31. Meier C., Ristic Z., Klauser S., Verrey F. (2002) Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J. 21, 580–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hatzoglou M., Fernandez J., Yaman I., Closs E. (2004) Regulation of cationic amino acid transport: the story of the CAT-1 transporter. Annu. Rev. Nutr. 24, 377–399 [DOI] [PubMed] [Google Scholar]
- 33. Franchi-Gazzola R., Dall'Asta V., Sala R., Visigalli R., Bevilacqua E., Gaccioli F., Gazzola G. C., Bussolati O. (2006) The role of the neutral amino acid transporter SNAT2 in cell volume regulation. Acta Physiol. 187, 273–283 [DOI] [PubMed] [Google Scholar]
- 34. Fernandez J., Yaman I., Sarnow P., Snider M. D., Hatzoglou M. (2002) Regulation of internal ribosomal entry site-mediated translation by phosphorylation of the translation initiation factor eIF2α. J. Biol. Chem. 277, 19198–19205 [DOI] [PubMed] [Google Scholar]
- 35. Melnik B. C. (2012) Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World J. Diabetes 3, 38–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang T. J., Larson M. G., Vasan R. S., Cheng S., Rhee E. P., McCabe E., Lewis G. D., Fox C. S., Jacques P. F., Fernandez C., O'Donnell C. J., Carr S. A., Mootha V. K., Florez J. C., Souza A., Melander O., Clish C. B., Gerszten R. E. (2011) Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karunakaran S., Umapathy N. S., Thangaraju M., Hatanaka T., Itagaki S., Munn D. H., Prasad P. D., Ganapathy V. (2008) Interaction of tryptophan derivatives with SLC6A14 (ATB0,+) reveals the potential of the transporter as a drug target for cancer chemotherapy. Biochem. J. 414, 343–355 [DOI] [PubMed] [Google Scholar]
- 38. Tomi M., Mori M., Tachikawa M., Katayama K., Terasaki T., Hosoya K. (2005) L-type amino acid transporter 1-mediated l-leucine transport at the inner blood-retinal barrier. Invest. Ophthalmol. Vis. Sci. 46, 2522–2530 [DOI] [PubMed] [Google Scholar]
- 39. Shennan D. B., Thomson J. (2008) Inhibition of system L (LAT1/CD98hc) reduces the growth of cultured human breast cancer cells. Oncol. Rep. 20, 885–889 [PubMed] [Google Scholar]
- 40. Nicklin P., Bergman P., Zhang B., Triantafellow E., Wang H., Nyfeler B., Yang H., Hild M., Kung C., Wilson C., Myer V. E., MacKeigan J. P., Porter J. A., Wang Y. K., Cantley L. C., Finan P. M., Murphy L. O. (2009) Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 136, 521–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li G., Mongillo M., Chin K. T., Harding H., Ron D., Marks A. R., Tabas I. (2009) Role of ERO1-α-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J. Cell Biol. 186, 783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Boyce M., Bryant K. F., Jousse C., Long K., Harding H. P., Scheuner D., Kaufman R. J., Ma D., Coen D. M., Ron D., Yuan J. (2005) A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307, 935–939 [DOI] [PubMed] [Google Scholar]
- 43. Lee Y. Y., Cevallos R. C., Jan E. (2009) An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2α phosphorylation. J. Biol. Chem. 284, 6661–6673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gaccioli F., Huang C. C., Wang C., Bevilacqua E., Franchi-Gazzola R., Gazzola G. C., Bussolati O., Snider M. D., Hatzoglou M. (2006) Amino acid starvation induces the SNAT2 neutral amino acid transporter by a mechanism that involves eukaryotic initiation factor 2α phosphorylation and cap-independent translation. J. Biol. Chem. 281, 17929–17940 [DOI] [PubMed] [Google Scholar]
- 45. Liu M., Hodish I., Rhodes C. J., Arvan P. (2007) Proinsulin maturation, misfolding, and proteotoxicity. Proc. Natl. Acad. Sci. U.S.A. 104, 15841–15846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seo J., Fortuno E. S., 3rd, Suh J. M., Stenesen D., Tang W., Parks E. J., Adams C. M., Townes T., Graff J. M. (2009) Atf4 regulates obesity, glucose homeostasis, and energy expenditure. Diabetes 58, 2565–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Malmberg S. E., Adams C. M. (2008) Insulin signaling and the general amino acid control response: two distinct pathways to amino acid synthesis and uptake. J. Biol. Chem. 283, 19229–19234 [DOI] [PubMed] [Google Scholar]
- 48. Efeyan A., Zoncu R., Sabatini D. M. (2012) Amino acids and mTORC1: from lysosomes to disease. Trends Mol. Med. 18, 524–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang X., Proud C. G. (2006) The mTOR pathway in the control of protein synthesis. Physiology 21, 362–369 [DOI] [PubMed] [Google Scholar]
- 50. Han J. M., Jeong S. J., Park M. C., Kim G., Kwon N. H., Kim H. K., Ha S. H., Ryu S. H., Kim S. (2012) Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 149, 410–424 [DOI] [PubMed] [Google Scholar]
- 51. Thoreen C. C., Chantranupong L., Keys H. R., Wang T., Gray N. S., Sabatini D. M. (2012) A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 485, 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang C., McConathy J. (2013) Fluorine-18-labeled amino acids for oncologic imaging with positron emission tomography. Curr. Top Med. Chem., in press [DOI] [PubMed] [Google Scholar]
- 53. McGirr R., Hu S., Yee S. P., Kovacs M. S., Lee T. Y., Dhanvantari S. (2011) Towards PET imaging of intact pancreatic β-cell mass: a transgenic strategy. Mol. Imaging Biol. 13, 871–891 [DOI] [PubMed] [Google Scholar]